Adaptation and plasticity in insular evolution of the house mouse mandible
Adaptation et plasticité dans l’évolution insulaire de la mandibule de souris domestique
Abstract
enMorphometric methods allow the quantification of directions of phenotypic changes and their statistical comparison in a morphometric space. We applied this approach to investigate several candidate factors to explain changes in mandible shape occurring in house mice (Mus musculus domesticus, Mammalia, Rodentia) in Corsica and a nearby islet. The role of niche widening and of the concomitant change in diet was evaluated by comparing the micro-evolutionary insular change to the macro-evolutionary difference between omnivorous and herbivorous rodents. Phenotypic plasticity, which may contribute to rapid insular evolution, was assessed by breeding laboratory mice on hard versus soft food. The related change in mandible shape was compared with differences between continental and insular populations. The role of allometry was evaluated by assessing shape change related to size within the continental population and comparing this direction of change with differences on islands. Finally, evolution may be facilitated along the direction of the greatest phenotypic variance. This hypothesis was tested by computing in wild populations vectors corresponding to this direction and by comparing these vectors with those corresponding to estimates of shape changes related to plasticity, micro- and macro-evolutionary processes. In Corsica, the congruence in directions of macro- and micro-evolutionary phenotypic vectors (Corsican/continental mice versus omnivorous/herbivorous rodents) supports the hypothesis of adaptation in mandible shape evolution. By contrast, on the islet, phenotypic divergence follows directions of plastic response to food consistency as well as within-population allometry. Thus, results suggest differences in the relative importance of processes which may influence rodent mandibular shape depending on the size of the islands they colonized. Faster evolution and plasticity may be more evident in small and often ephemeral populations living on small islands, whereas micro-evolutionary processes may have enough time and genetic variability to progressively ‘align’ with macro-evolutionary trends in large populations from big islands.
Résumé
esLes méthodes morphométriques permettent de quantifier des directions de changements phénotypiques et de les comparer statistiquement. Cette approche a été appliquée pour évaluer plusieurs facteurs explicatifs des différences de forme de la mandibule observées chez des populations de souris domestiques (Mus musculus domesticus) de Corse, de Sardaigne et d’un îlot voisin de la Corse. Le rôle de l’élargissement de la niche et du changement alimentaire concomitant a été testé en comparant le changement insulaire (échelle micro-évolutive) à celui observé entre mandibules de rongeurs omnivores et herbivores (échelle macro-évolutive). La plasticité phénotypique pourrait également contribuer à l’accélération des changements évolutifs sur les îles. Le changement mandibulaire associé a étéévalué en élevant des souris de laboratoire avec deux nourritures de consistance différente, en granulés ou gélifiée. La composante allométrique de la variation de forme a étéévaluée sur des populations continentales. Enfin, l’évolution phénotypique peut être facilitée le long des directions de plus grande variance. Cette hypothèse a été testée en évaluant ces directions dans les différentes populations sauvages et en les comparant aux directions des changements plastiques, allométriques, micro- et macro-évolutifs.
La congruence des directions micro- et macro-évolutives observée dans le cas de la différentiation continent – Corse, continent – Sardaigne et omnivores – herbivores, corrobore le rôle de l’adaptation dans le changement de forme de la mandibule chez les souris corses et sardes. La divergence de la population de l’îlot, en revanche, correspond aux directions de changement allométrique et de remodelage osseux en réponse à la consistance de nourriture. Ces résultats montrent l’intérêt de comparer quantitativement des directions de changement phénotypique pour analyser les causes complexes de différenciation des populations naturelles. Ils suggèrent en outre que différents processus agissent sur des îles de taille contrastée, possiblement à des pas de temps différents : plus long sur les grandes îles comme la Corse, et plus rapides, impliquant une réponse plastique, sur de petits îlots.
Introduction
Islands are often regarded as a ‘laboratory of evolution’, because processes of genetic and phenotypic evolution occur fast and may lead to pronounced intra-specific phenotypic differences (Berry 1964, 1996; Whittaker 1998; Millien 2006). The interpretation of the phenotypic changes is difficult because both adaptation and chance are invoked (Berry 1973, 1996). Adaptive interpretation of the observed phenotypic evolution is often invoked due to particular ecological conditions, such as a decrease in predation and interspecific competition and an increase in niche breadth. Random factors include founder effect and subsequent genetic drift due to reduced population size. Direct investigation of the underlying processes using experiments are rare and difficult to realize in the wild, as they require controlled introductions on islands devoid of the species of interest (e.g. Berry 1964; Losos et al. 1997), without risking to destabilize the local ecosystem. Experiments are obviously impossible on extinct species, although numerous taxa, from dwarf elephants to giant rodents, displayed extreme insular evolution in the fossil record (e.g. Sondaar 1977; Roth 1992; Palombo 2008). This lack of direct evidence may lead to a collection of ‘just-so’ stories (Berry 1996). Morphometric methods improved the quantification of such patterns of differentiation on islands, including shape modifications beyond size trends (Parra et al. 1999), and allowed the description of insular divergence in a morphospace including continental references (e.g. Davis 1983; Cardini et al. 2007; Renaud and Michaux 2007). We propose to apply such approaches to characterize the directions of morphological change associated with insular evolution, and compare them with controlled effects of candidate factors involved in insular divergence. Although still relying on indirect evidence, such methods may allow making a step further towards understanding the processes behind observed patterns.
We applied this approach to an investigation of the morphological differentiation of insular rodents. This group displays countless cases of insular evolution with more or less pronounced morphological differences (e.g. Michaux et al. 1996; Parra et al. 1999; Amori et al. 2008). Within this vast family, the house mouse Mus musculus domesticus Schwarz & Schwarz, 1943 offers a convenient model to investigate the processes involved in insular evolution as it repeatedly invaded both small islets and large islands, leading to numerous examples of insular divergence (e.g. Davis 1983; Michaux et al. 2007b). This aptitude to colonization is related to the commensal habit of the house mouse, making it prone to accompany human travels during prehistoric and historical times (Vigne 1999; Cucchi 2008). Together with providing numerous cases of insular divergence, this peculiarity of the house mouse may also make the colonization process more difficult to trace, by favouring introductions from remote areas (Cucchi 2008) and multiple colonizations (Navajas y Navarro and Britton-Davidian 1989), differently from non-commensal rodents for which a single introduction by chance is more likely.
We focused here on three cases exemplifying different insular conditions. Corsica and Sardinia are large oceanic islands that can sustain large populations of mice. Because of the early human colonization and subsequent intense commercial exchanges (Vigne 1999), multiple colonizations are likely, that would diminish random effects due to founder effect and drift. Adaptive processes may be involved because a change in diet and habitat is documented (Granjon and Cheylan 1988). While on the mainland M. m. domesticus is mostly a commensal species with rare outdoor populations in the Mediterranean zone, on Corsica the house mouse displays frequent outdoor populations because of the absence of its competitor Mus spretus. On islands such as Corsica, being successful in extending its niche, the house mouse even became a successful competitor of the wood mouse Apodemus sylvaticus in humid lowlands (Orsini 1982; Libois 1984; Granjon and Cheylan 1988).
Very different conditions characterized the population trapped on the small islet of Piana. On this islet, few hundred meters off Corsica, a very small population displaying a larger body size than continental and Corsican mice was documented in the mid-1980s (Navajas y Navarro 1986) but seemed to have gone extinct a few years afterwards (Granjon and Cheylan 1989). This islet has no permanent human settlement and is rather inhospitable, making a sustained gene flow from nearby Corsica unlikely. Hence, the size of the islet and the concomitant size of the population make genetic drift likely. Furthermore, strong selective pressures may have occurred because of the reduction in habitat diversity and types of food compared with Corsica, the islet vegetation being only composed of junipers, lentisk bushes and herbaceous halophytous plants (Granjon 1986; Navajas y Navarro 1986).
Many of the ecological changes invoked on these islands are related to food quality. Hence, focusing on a morphological character heavily involved in the food processing might shed light on the adaptive processes involved. The mandible was chosen, because it is involved in the mastication as zone of muscles insertion, pivot of jaw and bearing the incisor and jugal teeth, and because it can be a proxy of body size (Renaud 2005), hence relevant to an important aspect of insular evolution. The size and shape of the mandibular bone was quantified using an outline analysis (Renaud and Michaux 2003).
Morphometric methods allow the description of morphological changes as directions (vectors) in a morphological space in which they can be statistically compared. Directions of morphological changes related to insular differentiation were first quantified by comparing mice from Corsica and Sardinia with close mainland populations from southern France and northern Italy. Piana was probably colonized from the nearby Corsica. Hence, Piana mice were compared with Corsican ones.
These directions of change describe mandible shape differences between populations. Similarly shape changes associated with differences in diet and allometry were quantified and their role in the differentiation of the insular mice investigated. Although it cannot directly test causal relationships, congruence between directions of change would support the role of a candidate factor in the differentiation of populations on different islands.
- 1
An ecological shift in diet will change the selective functional pressures related to the feeding apparatus. For instance, the consumption of food more resistant to grinding should select for more powerful masticatory muscles. It is difficult to evaluate the micro-evolutionary output of such a change, because within species variation in diet are difficult to measure, being subtle and affecting the relative abundance of the components rather than the qualitative composition of the food ingested (e.g. Butet 1990). By contrast, different species can be characterized by clear shifts in diet. We can hypothesize that functional requirements and selective pressures associated with mastication are comparable at the micro- and macro-evolutionary scales, and hence that differences among species may be regarded as the continued action of natural selection within populations, extended over considerably longer periods of time (Losos et al. 2001). To test this hypothesis, the difference between continental and insular mice was compared to the divergence between omnivorous and herbivorous clades of murine rodents (Michaux et al. 2007a). Congruence of these directions would provide support for the role of diet shift in mandibular differentiation of insular mice, and thus suggest a strong connection between micro- and macro-evolutionary processes.
- 2
Size changes are also often involved in insular differentiation as part of the ‘island rule’ (Lomolino 1985, 2005) including within-species variation in rodents (Angerbjörn 1986). Do size variations occur according to the island rule in the insular populations of mice? If yes, can the shape differences between continental and insular mice be explained by allometric changes only, related to the size increase typical of the island rule? We investigated patterns of size differences between continental and insular mice to test if our populations were congruent with the island rule.
- 3
Evolution on islands can be very fast (Mayr 1963) and evidence is available for an acceleration of morphological evolution in insular mammals (Millien 2006). A plastic component has been suggested to contribute in such rapid insular differentiation, as an efficient way to respond to short-term changes in a geographically isolated environment (Losos et al. 2001). We investigated how changes in food consistency may alter mandible shape by breeding laboratory mice with hard versus soft food and comparing their mandibular morphology. The shape change observed between the two experimental groups was compared with the shape differences characterising the insular populations.
- 4
Finally, rapid evolution can be facilitated along ‘lines of least resistance’ (Schluter 1996). These lines correspond to the directions of the greatest variance within a population: selection screens pre-existing variation and hence, response to selection is favoured along directions displaying an important variation. Directions of the greatest intra-population variation are usually estimated as the main direction of genetic variance (Steppan et al. 2002; McGuigan et al. 2005). The genetic variance–covariance (VCV) matrix (G), however, cannot be estimated for wild-trapped populations or fossil assemblages. Hence, in such cases, the major axes of within-population variation have to be estimated based on the VCV matrix of phenotypic characters (P). An intermediate to high heritability of the phenotypic characters (Cheverud 1988) makes the matrix of phenotypic variance a potentially good surrogate for the matrix of genetic variance G (Marroig and Cheverud 2001).
Methods
Breeding experiment
Forty 3-week-old female mice from the inbred strain C56BL/6J were obtained from the Charles River Laboratory, just after weaning. They were bred thereafter at the PBES (Ecole Normale Supérieure de Lyon, France) in four cages where they were provided with water and food ad libitum until they reached the age of 6 months (33 weeks) when they were killed by cervical dislocation. During this time, the mice were weighed several times in order to monitor their growth. Females were chosen for convenience, as they can be kept together in cages, and because no important sexual dimorphism is reported in murine feral populations (Davis 1983; Renaud 2005).
At the beginning of the experiment, the mice were randomly split into two groups. Half of the mice received the ordinary hard pellet diet (hard food group, HF). The other group (soft food group, SF) was fed a gelatinous food obtained by grinding the pellets to a powder which was then mixed with agar–agar, and hydrated when given to the mice. The final sample size was 20 mice for the SF group and 19 for the HF one. The protocol of breeding and killing has been validated as the regular procedure at the PBES. As the ingestion of food was not considered as harmful, no further validation by an ethics committee was required.
Wild-trapped populations
All wild-trapped populations consisted of a set of 132 domestic mice (M. m. domesticus) from the collection of the Institut des Sciences de l’Evolution (Montpellier, France). Only mature specimens with an erupted third molar were considered.
Mice considered in the present study included wild-trapped mice from Corsica, Sardinia, from the surrounding continent (France and Italy), and from Piana, an islet 0.06 km2 large and 300 m off the Corsica coast close to Bonifacio (Table 1). French mice included mice trapped in Montpellier and the surrounding region; Italian mice were trapped in Lombardy and in Reggiolo, a locality from the neighbouring region Emilia Romagna (northern Italy).
| Group | Locality | Md | |
|---|---|---|---|
| FR | Mainland France | Montpellier | 19 |
| Montpellier surroundings | 11 | ||
| IT | Mainland Italy | Lombardy | 20 |
| Reggiolo | 17 | ||
| CORS | Corsica | Bonifacio | 10 |
| Fango Valley | 38 | ||
| Piana | Piana islet | Piana | 6 |
| SARD | Sardinia | Colline Romana | 11 |
Macro-evolutionary differences
The effect of a shift in average diet was tested by a comparison with a direction of macro-evolutionary change, evaluated as difference between a subset of omnivorous versus herbivorous murine rodents (Murinae). The clades Mastomys and Apodemus are related to the clade of the house mouse, and all the species of these clades for which diet is known (Nowak 1999) share an omnivorous–granivorous diet. The Arvicanthini clade is the sister group of the Mus–Mastomys–Apodemus ensemble (Michaux et al. 2007a) and all the species documented in this clade are characterized by a shift towards an herbivorous diet (Nowak 1999).
An average mandible shape for omnivores was estimated as the mean shape obtained for the 18 taxa documenting the Mus, Apodemus and Mastomys groups, while an average herbivorous mandible was estimated based on 17 Arvicanthine taxa (Michaux et al. 2007a; see online supporting information Table S1). The mean shape of each taxon was based on ca 10 specimens whenever possible. The average ‘omnivorous’ and ‘herbivorous’ mandible shapes were thus estimated as an average of the mean shape per taxon.
Outline analysis
The shape of the mandible was estimated by its outline, corresponding to the 2D projection of the hemi-mandible placed flat on its lingual side. As the incisors may be mobile and some molars missing, only the outline of the mandibular bone was considered. This outline provides a good description of the processes involved in the insertion of the masticatory muscles, as well as of the alveolar region hosting the cheek teeth and incisors. This method has been used to describe fine-scale geographical variations in wood mice (Renaud and Michaux 2003, 2007; Renaud 2005).
A radial Fourier transform (RFT) was applied to the mandible outline (Renaud and Michaux 2003). From the x, y coordinates of the points along the outline, a set of radii (i.e. distance of each point to the centroid of the outline) was calculated. This set was analysed as a function of the cumulative distance along the outline using a Fourier method. The initial data set is thus described by a sum of trigonometric functions of decreasing wavelength, the harmonics. Each harmonic is weighted using two Fourier coefficients (FC). The zero harmonic (A0) is proportional to the size of each outline and was used to standardize all other FC in order to separate size and shape components of form differences. Previous studies on wood mice showed that considering the FC of the first seven harmonics offered a good compromise between measurement error, information content and number of variables to be considered (Renaud and Michaux 2003).
An alternative approach to analyse outline data is the elliptic Fourier transform (EFT). This method is based on a separate Fourier decomposition of incremental changes along x and y as a function of the cumulative length along the outline (Kuhl and Giardina 1982). Each harmonic corresponds to four coefficients: two for x and two for y, defining an ellipse in the xy plane. This method offers an excellent reconstruction of the mandible outline using the inverse Fourier transform, useful for visual inspection. The RFT, however, has the advantage to provide a minimal number of variables. As both methods appear to provide highly correlated descriptors of shape variation (Michaux et al. 2007a), we decided to apply the most efficient method for each purpose: statistical analyses were therefore performed on the FCs of the RFT, whereas EFT was used to provide reconstructed outlines. When superimposed, reconstructed outlines were adjusted to be centred.
Statistical analyses
The size of the mandible was estimated using the zero harmonic of the RFT. Differences in size were tested using one-way analysis of variance (anova).
The shape of each outline was described by a set of 14 Fourier coefficients (seven harmonics per two FCs). The patterns of shape differentiation were first investigated using a principal components analysis (PCA) on the pooled VCV matrix, a method that provides synthetic multivariate axes summarizing the main directions of the total variance. Differences among groups were further tested using multivariate analyses of variance (manova). The overall influence of size was tested using a multivariate regression (Monteiro 1999) of the FCs against size, estimated using the zero harmonic of the mandible outline. To compare the relative importance of the geographic groups and size to explain shape differentiation, a mancova (multivariate analysis of covariance) was applied including geographic groups, size and their interaction as possible effects.
P matrices were computed as VCV matrices based on the 14 FCs per mandible. Simulations suggest that the principal components of variation of such VCV matrices can be robustly estimated when relying on more than 30 specimens (Polly 2005). These matrices can be compared using different methods that may provide complementary results (Ackermann and Cheverud 2000). The correlation of VCV matrices using Mantel t-tests provides a coefficient of correlation R and the probability that R between random matrices is less than the observed R. This approach evaluates similarities between matrices and tends to support correlations between them. Alternatively, common principal components analysis (CPCA) tests whether matrices share complex relationships (Flury 1988). Two matrices may be equal, proportional but not equal or might share principal eigenvectors. A CPCA tests each of these hypotheses. By looking at complex relationships shared between matrices, this approach tends to emphasize differences rather than similarities (Ackermann and Cheverud 2000; Steppan et al. 2002).
Comparison between P matrices and evolutionary directions
Eigenvectors were extracted from the VCV matrix of a group and normalized to unit length. These eigenvectors were compared among groups as well as with directions of differences among groups (evolutionary directions). These directions were also compared with each other. The shape change Δz between groups G1 and G2 was expressed as the difference between the mean values of the FCs for each group: 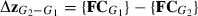 .
.
The direction of shape change related to allometry was estimated using a multivariate regression of the FCs against size within the pooled mainland sample to increase accuracy by using the largest available sample (Cardini and Elton 2007). Prior to pooling, using mancova the existence of differences in the allometric relationship between the different mainland localities was tested. The vector describing allometric change corresponds to the series of 14 partial regression coefficients provided by the multivariate regression of the FCs on size.
The angle between two vectors is the arc cosine of the inner product of the two vector elements. Simulations of angles between random vectors were used to assess the statistical significance of this correlation (Klingenberg 1996; Renaud et al. 2006). Fifty thousand simulations of the correlation between two random vectors of 14 elements were performed. They provided the following significance thresholds for the absolute value of the inner product R, probability that the observed R is higher than random: p > 0.95, R = 0.517; p > 0.99, R = 0.651; p > 0.999, R = 0.770; p > 0.9999, R = 0.860.
Bootstrap procedures
The sampling of the initial population may affect the evaluation of shape parameters such as mean shape and variance, especially when sample size decreases to less than 10 specimens (Cardini and Elton 2007). This is the case of the Piana sample where the reduced size of the islet did not allow for a more extensive trapping. Furthermore, estimation of allometry and major directions of variance are even more sensitive to sample size and seem to require at least 30 specimens for a robust estimation (Polly 2005; Cardini and Elton 2007). We are close to this threshold given the sampling of the largest populations. Hence, the robustness of the directions of morphological change had to be investigated with regard to initial sampling.
A bootstrap procedure was used to estimate the precision of each vector. For the shape change Δz between groups G1 and G2, each group G1 and G2 was bootstrapped 100 times, providing 100Δzi that were compared with the shape change Δz based on the original sample. The number of independent bootstrap samples is given by (2N − 1)!/N!(N − 1) (Cardini et al. 2007), i.e. 11 088 for a sample with N = 6. The bootstrap procedure can thus be applied to the reduced sample of Piana.
The direction of within-population allometry was tested by calculating multivariate regressions between FCs and size on 100 bootstrapped samples of the mainland group.
Finally, the robustness in estimating the eigenvectors of the P matrix was evaluated in bootstrapping each group 100 times. The corresponding VCV matrix and eigenvectors of the bootstrapped samples were compared with the original vectors.
To evaluate the impact of sampling on the stability of all these vectors, the distribution of the bootstrapped directions around the original one was described by providing the mean and median coefficients of correlation and angles, as well as their values including 75% and 95% of the bootstrapped vectors.
We thereafter performed a series of comparisons between these directions of change. To assess their robustness regarding initial sampling, we correlated the 100 bootstrapped estimates of each initial vector, thus providing 100 × 100 = 10 000 bootstrapped correlations for one initial correlation. We provided the average and median coefficients of correlation obtained, as well as the percentage of significant correlations among these 10 000 values. This may be regarded as an estimate of the sensitivity of the observed correlations to initial sampling of the populations.
Results
Breeding experiment
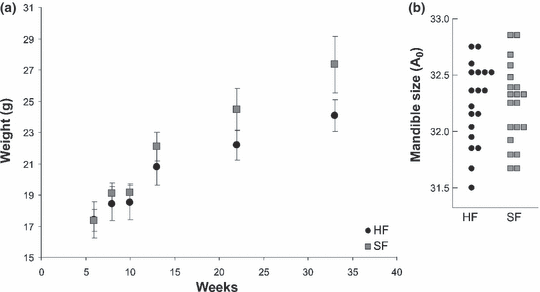
The mice fed on soft food reached a larger weight at the end of the experiment (anova on weight: p < 0.001; Fig. 1a). This difference in body size did not translate into a significant difference in mandible size (anova on A0: p = 0.937; Fig. 1b). The mandibles of the two groups showed a marked shape difference (manova: p < 0.0001) that emerged as the main signal on the first principal plane of a PCA on the Fourier coefficients (Fig. 2; PC1 = 45.3% of variance, PC2 = 23.3%). The reconstructed outlines evidence that the shape difference of SF versus HF mice corresponds to a less robust incisor alveolus, an uplift of the molar alveolar region, and a less robust angular process. The latter is the main zone of insertion of the masseter muscles which are heavily involved in mastication.
(a) Growth curve of the laboratory mice. Symbols correspond to average value ± standard error. (b) Mandible size at 6 months (each symbol corresponds to an individual measure)
(a) Variation in mandible shape of C57BL/6J mice represented on the first principal plane of a PCA on the FCs (RFT7). Reconstructed outlines visualize average shape for mice fed on hard food (HF) and gelatinous food (SF). (b) Fivefold magnified difference between outlines of the mice fed hard and soft food
Patterns of differentiation of the island mice populations
Corsican mice were not larger than continental ones (p = 0.255; Fig. 3). Mice on Sardinia might be slightly larger than their continental (p = 0.051) and Corsican (p = 0.057) counterparts, but these differences do not reach the significance threshold. These reduced differences are not due to the confounding effect to pool several localities to document each region, because localities within a region displayed similar size (France: Montpellier versus surroundings, p = 0.904; Italy: Lombardy versus Reggiolo, p = 0.334; Corsica: Fango versus Bonifacio, p = 0.095). Hence, these localities were thereafter pooled for increasing sample size.
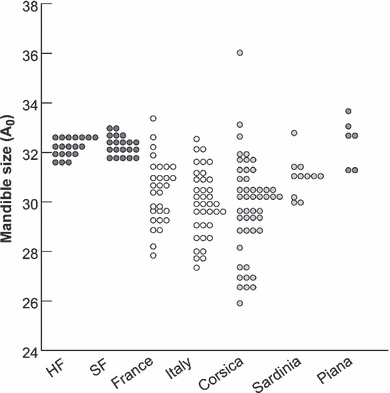
Mandible size in laboratory and wild mice. Each symbol corresponds to an individual measure
By contrast, Piana’s mice are significantly larger than all other groups despite the small number of specimens (comparison with mainland: p < 0.001; with Corsica p = 0.002; with Sardinia p = 0.003).
The mandibles of the different wild populations also differed significantly in shape (manova: p < 0.0001). Differences among localities of a same region evidenced some differentiation (France: Montpellier versus surroundings, p = 0.023; Italy: Lombardy versus Reggiolo, p = 0.016; Corsica: Fango versus Bonifacio, p = 0.0004). A correlation of the FCs on size evidenced an overall significant relationship (p < 0.0001). A mancova including both size and localities nevertheless suggested that the later was a more important factor of differentiation than size among all wild populations (group, p = 0.008; size, p = 0.297; interaction, p = 0.011). A similar result was obtained when considering larger geographic grouping by pooling mainland specimens according to nationality (large geographic group, p = 0.009; size, p = 0.476; interaction, p = 0.011).
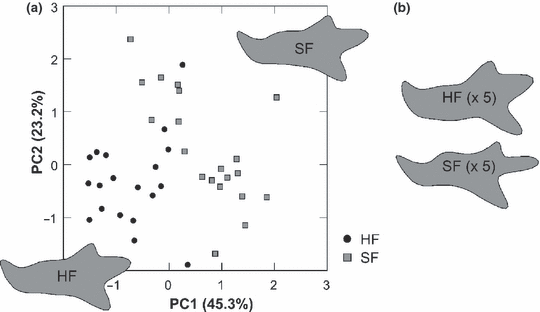
The pattern of differentiation between mainland and island populations was visualized using a PCA (PC1 = 39.7% of variance, PC2 = 25.7%, PC3 = 16.2%) including the mice from the breeding experiment (Fig. 4). The C57BL/6J mice differed largely from their wild relatives by having a relatively longer condylar process, and more robust alveolar regions. HF and SF were separated from each other along PC1. Regarding the wild animals, mainland populations from France and Italy were very close and their confidence ellipses largely overlapped, showing a low differentiation of both groups (manova, p = 0.040). The populations from the two large islands Corsica and Sardinia diverged from their mainland relatives mostly along PC2. Their confidence ellipses did not overlap with those of the mainland samples, a differentiation confirmed using two-by-two comparisons (Corsica and Sardinia versus Mainland: p < 0.0001). The ellipse of Sardinia included the one of Corsica, despite a significant differentiation (p = 0.0006). Mandibles from Corsica and Sardinia shared shortened coronoid and angular processes. The limited sample sizes for both Sardinia and Piana led to their larger confidence ellipse and a non-significant differentiation of both samples (p = 0.127). However, despite this limited sampling, the mandibles from Piana were well differentiated along PC1, as confirmed by significant differences with other samples (Piana versus Mainland and Corsica, p < 0.0001) and were characterized using an elongated condylar process, and an uplift of the molar alveolar region.
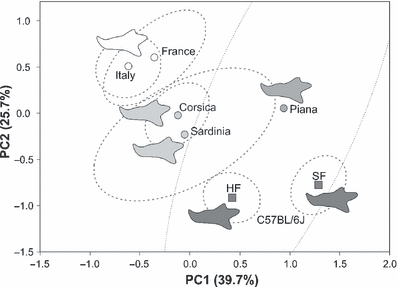
Mandible shape variation among laboratory and wild mice visualized on the first principal plane of a PCA on the FCs (RFT7). Each symbol corresponds to a group mean. In dotted grey, confidence ellipses (95%) around the centroid (light dotted grey, ellipse of the Piana sample; note the large ellipse due to small sample size). Reconstructed outlines were obtained using the EFT7. They correspond to average outlines of the wild populations and laboratory groups
Eigenvectors of the P matrices
A first step was to investigate whether the pattern of phenotypic variation was comparable among geographic groups. Only France (N = 30 specimens), Italy (N = 37) and Corsica (N = 48) presented enough specimens to provide a robust evaluation of VCV matrices and associated eigenvectors. Mantel comparisons between P matrices (Table 2) showed highly congruent patterns of variation among France, Italy and Corsica. The strongest correlation associated the P matrices of France and Corsica, and mainland (estimated by pooling samples from Southern France and Northern Italy) and Corsica. This pattern had to be moderated by considering the results of CPCA. VCV matrices were never found equal, but the hypothesis of CPCs could not be rejected at the 1% threshold except in the comparison of France and Corsica. This suggested that despite not being equal, the VCV matrices of the different geographic groups shared a relatively high degree of similarity between the main axes of variation.
| Mantel | CPCA | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | R Mantel | 1 − PMantel | Equal | CPC |
| Mainland France | Mainland Italy | 0.760 | 0.000 | 0.0006 | 0.0102 |
| Corsica | 0.913 | 0.000 | 0.0004 | 0.0019 | |
| Mainland Italy | Corsica | 0.851 | 0.000 | 0.0043 | 0.0399 |
| Mainland (France + Italy) | Corsica | 0.944 | 0.000 | 0.0032 | 0.0223 |
- Two methods are applied. Matrix comparison using Mantel tests of the VCV matrices based on the Fourier analysis (RFT7) of the mandible outline (R: coefficient of correlation; 1 − P: 1 − probability that random R < observed R). Common principal components analyses (CPCA): probability that matrices are equal or share CPCs). Values in bold are significant at the 1% level.
To compare these results further, the P matrices were decomposed into their successive eigenvectors. In order to evaluate the robustness of the evaluation of these eigenvectors, the initial populations were bootstrapped 100 times, eigenvectors extracted from these bootstrapped replicates and compared with the original eigenvectors (Table 3). In all populations except Italy, the first eigenvector pmax appeared to be reliably estimated (average angle ≤15°). V2 and V3 appear less robustly estimated (angle up to 36°, except Italy once again more variable). It is likely that permutations occur between the estimate of V2 and V3 depending on the group, an effect that may easily decrease the stability of eigenvectors when the structure of the variation is closer to a sphere rather than to an ellipse (Zelditch et al. 2006), suggesting that comparisons between eigenvectors except the first one should be considered cautiously. This has been further investigated by comparing the eigenvectors among groups.
| PC1 | PC2 | PC3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | |R| | Angle | % | |R| | Angle | % | |R| | Angle | |
| Corsica | 45.3 | 0.949 | 15 | 26.8 | 0.908 | 21 | 12.5 | 0.890 | 23 |
| Mainland France | 59.7 | 0.982 | 10 | 16.5 | 0.914 | 21 | 8.4 | 0.848 | 30 |
| Mainland Italy | 33.5 | 0.781 | 33 | 24.3 | 0.659 | 45 | 20.4 | 0.717 | 41 |
| Mainland (France + Italy) | 45.5 | 0.978 | 11 | 18.2 | 0.749 | 36 | 15.5 | 0.743 | 36 |
- The stability of each eigenvector has been estimated by bootstrapping the initial group, extracting eigenvectors and comparing them with the initial vectors. Average correlation (|R| = inner product of the vectors) and average angle (degrees) between bootstrapped and initial eigenvector are given.
The first eigenvectors V1 are highly correlated between the three samples of France, Italy and Corsica (0.917 ≤ R ≤ 0.988). By contrast, the second eigenvectors were correlated only between Corsica and France (R = 0.960). The third eigenvector of Italy (20% of variance) correlated with V2 of France (16.5% of variance, R = 0.860) and Corsica (26.8% of variance, R = 0.924). In turn, the second eigenvector for Italy (24% of variance) correlated with the third eigenvector of French (R = 0.827) and Corsican (R = 0.827) populations.
These results evidenced a good stability of the first eigenvector across populations but a possible permutation of V2 and V3. In order to increase the size of the population on which the evaluation of the eigenvectors is based, and because of their limited differentiation, the two mainland populations were pooled. V1mainland correlates with V1France (R = 0.995) and V1Italy (R = 0.950), V2mainland with V2France (R = 0.982) and V3Italy (R = 0.973). Reconstructions (Fig. 5) show that V1 opposes mandibles with a broad alveolar region and a thin and elongated coronoid process, versus mandibles with a slender alveolar region, accompanied by an posterior shift of the angular process and a shorter coronoid process. V2 is characterized by more pronounced coronoid and angular processes, and a bending of the incisor alveolar region.
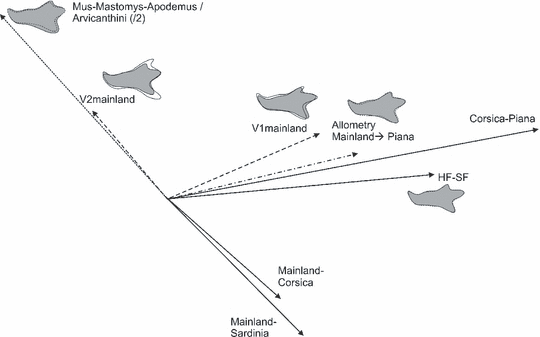
Comparison of directions of micro- and macro-evolutionary differences, allometry and plasticity, and major directions of variance. All the vectors were projected onto the first principal plane represented in Fig. 4. Micro-evolutionary differences are vector relating mean shape of two samples (e.g. Corsica–Piana). The effect of plasticity is shown by the difference between mice fed hard versus soft food (HF–SF). The macro-evolutionary direction (MMAp–Arvicanthini) corresponds to average difference between omnivorous and herbivorous murine rodents; its length has been reduced twofold. The dashed outline corresponds to the omnivore murines belonging to Mus, Mastomys and Apodemus clades, the grey outline to Arvicanthini. The direction of allometric change has been evaluated on the mainland sample; its length corresponds to a change from the average mainland size (A0 = 30.2) to the average Piana size (A0 = 32.4). Reconstructed outlines represent magnified differences (dashed outline A0 = 26; grey outline A0 = 34). The direction of intrapopulation variance (V1mainland and V2mainland) have been represented, the length of the vectors being arbitrary. The superimposed outlines visualize the direction of change along V1mainland and V2mainland. Light grey outlines correspond to the mean mainland shape. Reconstructed outlines were based on the EFT7 in order to provide accurate reconstructions. They either correspond to average outlines, or were obtained by projecting the coefficients of the RFT7 on the EFT7 coefficients using a multivariate multiple regression (allometric direction and changes along V1 and V2)
Directions of variation and direction of evolution
Several directions of morphological change were determined. Micro-evolutionary changes were evaluated among wild mouse populations from mainland to Corsica, from mainland to Sardinia, and from Corsica to Piana. The direction of macro-evolutionary change between representatives of Mus, Mastomys and Apodemus on the one hand, and of the Arvicanthini on the other hand, exemplified a shape change from omnivorous to herbivorous species, and corresponded to a transition from slender to robust mandibles with enlarged coronoid and angular processes (Fig. 5). The direction of plastic change of the mandible was evaluated as the difference between C57BL/6J mice fed hard food versus soft food.
The allometric direction was evaluated using a multiple regression of the FCs on size (estimated using A0). This was performed on the mainland sample which presented the best sample size, because estimation of allometric directions seems particularly unstable in reduced samples (Cardini and Elton 2007). Pooling appeared as appropriate as a mancova between mainland groups evidenced an effect of size but no effect of groups or interaction between size and groups, suggesting that the different mainland groups shared similar allometric relationships (group, p = 0.058; size, p = 0.023; interaction, p = 0.055).The multiple regression was significant (N = 66, Wilks’ lambda, p = 0.0012) and the coefficients of the regression provided a vector expression of the allometric change. The major directions of variance were estimated using the V1 and V2 vectors of the mainland sample.
The robustness of these directions was assessed by bootstrapping 100 times the original populations, calculating 100 vectors of differences, multiple regressions or eigenvectors and comparing the resulting bootstrapped vectors to the original estimation. The distribution of these bootstrapped vectors around the original one was described using mean, median and values including 75% and 95% of the distribution for the correlation (R = inner product) and angle (arc cosine of R) between each bootstrapped and original vector (Table 4). Not surprisingly, the amount of variation around the original vector depended on the number of specimens available when differences between two samples were considered. The Mainland – Sardinia direction was, however, less reliable than Corsica – Piana, despite Sardinia being slightly better sampled (N = 11) than Piana (N = 6). The vector describing the effect of food consistency on laboratory mice (HF–SF) appeared robustly estimated (mean angle <15°), as well as the macro-evolutionary direction between omnivorous and herbivorous rodents. This is reassuring because such a direction might have appeared as highly dependent on the taxa chosen to document each diet. The multiple regression did provide a moderately robust estimate (mean angle 21°) despite being based on a large group (N = 66), corroborating the observation that estimating allometric directions requires large samples (Cardini and Elton 2007). Finally, the major direction of variance within the mainland sample appeared as the most stable regarding initial sampling (mean angle 10.5°). By contrast, the second direction V2 appeared as the least stable (mean angle 35°).
| Mean | Median | 75% | 95% | |||||
|---|---|---|---|---|---|---|---|---|
| R | Angle | R | Angle | R | Angle | R | Angle | |
| Mainland–Corsica | 0.958 | 15.9 | 0.970 | 14.1 | 0.946 | 18.9 | 0.902 | 25.5 |
| Mainland–Sardinia | 0.883 | 26.2 | 0.907 | 24.9 | 0.839 | 33.0 | 0.688 | 46.6 |
| Corsica–Piana | 0.916 | 21.4 | 0.945 | 13.2 | 0.891 | 27.0 | 0.767 | 39.9 |
| HF–SF | 0.972 | 12.8 | 0.978 | 12.1 | 0.967 | 14.8 | 0.924 | 24.2 |
| Allometry | 0.920 | 20.7 | 0.951 | 18.0 | 0.909 | 24.6 | 0.679 | 47.2 |
| MMAp–Arv | 0.976 | 11.6 | 0.983 | 10.5 | 0.968 | 14.5 | 0.935 | 20.8 |
| V1mainland | 0.978 | 10.5 | 0.986 | 9.6 | 0.972 | 13.5 | 0.932 | 21.2 |
| V2mainland | 0.749 | 35.5 | 0.895 | 26.5 | 0.621 | 51.6 | 0.126 | 82.8 |
- Mean and median of the distribution are provided as well as the values including 75% and 95% of the distribution of the 100 correlations. Angles are given in degrees.
Each of these directions of change can be regarded as vectors in the space defined by the 14 FCs. If various factors (e.g. diet and allometry) would lead to similar shape change of the mandible, the vector describing their morphological effect should be correlated in the morphospace of the 14 FCs. Hence, the different directions have been compared with each other and with the eigenvectors of the P matrix (Table 5). A visualization can be obtained by projecting all the vectors on the first principal plane of the PCA displaying the intergroup differences (Fig. 5). The robustness of these correlations was further estimated by considering correlations among the 100 bootstrapped vectors (hence 100 × 100 = 10 000 correlations), and in how many cases a significant correlation still emerged (Table 5).
| Bootstrapped vectors | ||||||
|---|---|---|---|---|---|---|
| First vector | Second vector | R | Angle (degrees) | |R| median | % |R| p** | % |R| p* |
| Mainland–Corsica | Mainland–Sardinia | 0.789 | 37.9 | 0.711 | 64.3 | 84.8 |
| Corsica–Piana | 0.268 | 74.6 | 0.363 | 5.4 | 22.6 | |
| HF–SF | 0.364 | 68.7 | 0.389 | 5.7 | 22.9 | |
| Allometry | 0.012 | 89.3 | 0.172 | 0.5 | 4.1 | |
| MMAp–Arv | 0.707 | 45.0 | 0.657 | 53.0 | 92.9 | |
| V1mainland | 0.079 | 85.5 | 0.178 | 0.2 | 3.3 | |
| V2mainland | −0.835 | 33.4 | 0.710 | 60.8 | 73.3 | |
| Mainland–Sardinia | Corsica–Piana | 0.374 | 68.0 | 0.457 | 17.9 | 40.0 |
| HF–SF | 0.317 | 71.5 | 0.325 | 8.6 | 23.9 | |
| Allometry | 0.131 | 82.5 | 0.245 | 3.2 | 12.5 | |
| MMAp–Arv | 0.568 | 55.4 | 0.505 | 4.8 | 46.0 | |
| V1mainland | 0.046 | 87.4 | 0.249 | 3.5 | 11.9 | |
| V2mainland | −0.665 | 48.3 | 0.554 | 33.6 | 55.7 | |
| Corsica–Piana | HF–SF | 0.754 | 41.1 | 0.698 | 61.1 | 84.7 |
| Allometry | 0.823 | 34.6 | 0.677 | 58.9 | 89.0 | |
| MMAp–Arv | 0.486 | 60.9 | 0.312 | 1.7 | 11.7 | |
| V1mainland | 0.806 | 36.3 | 0.670 | 53.7 | 74.6 | |
| V2mainland | −0.015 | 89.1 | 0.253 | 3.7 | 11.9 | |
| HF–SF | Allometry | 0.799 | 37.0 | 0.749 | 79.1 | 91.7 |
| MMAp–Arv | 0.364 | 68.7 | 0.371 | 0.2 | 10.5 | |
| V1mainland | 0.754 | 41.1 | 0.719 | 73.1 | 94.2 | |
| V2mainland | −0.224 | 77.1 | 0.238 | 1.8 | 7.9 | |
| Allometry | MMAp–Arv | −0.069 | 86.0 | 0.158 | 0.0 | 1.0 |
| V1mainland | 0.900 | 25.8 | 0.830 | 83.3 | 92.1 | |
| V2mainland | 0.007 | 89.6 | 0.212 | 3.1 | 10.0 | |
| MMAp–Arv | V1mainland | −0.028 | 91.6 | 0.159 | 0.0 | 0.1 |
| V2mainland | 0.726 | 43.4 | 0.549 | 37.2 | 67.1 | |
- Significant probabilities in bold (p > 0.99, R = 0.651) and in italics (p > 0.95, R = 0.517). HF–SF: mice bred hard versus soft food. MMAp–Arv: direction of change from omnivores (MMAp: Mus–Mastomys–Apodemus groups) to herbivores (Arv: Arvicanthini). Allometry: direction obtained using the multivariate regression of FCs on size within the mainland group. For each correlation between the first and second vectors, R (=inner product of both vectors) and angle between both vectors (arc cosine of |R|) are indicated first, followed by results regarding correlations between 100 bootstrapped vectors (BV) for each original vector, i.e. 10 000 correlations. Median of the distribution of R (|R| Median) is given, as well as percentages of correlations above p > 0.99 (%|R|p**), in bold more than 50% of significant correlations at this threshold; and percentage of correlations above p > 0.95 (%|R|p*), in bold more than 75% of significant correlations.
Some sets of vectors highly correlated with each other (Table 5). Considering the amount of change involved (length of the vector; Fig. 5) provided a clue of how much a process may be sufficient to explain the differences of insular populations. The ‘plasticity vector’ (HF–SF) paralleled the direction of change between Corsican and Piana mandibles. The amount of shape difference due to plasticity was probably exaggerated due to the extreme differences in food consistency the laboratory mice were exposed to. Even thus, the difference was smaller than the one between Corsican and Piana mice.
The possible effect of size increase in Piana mice was evaluated based on the allometric direction estimated on the mainland sample. The direction of allometric change paralleled the Corsica–Piana direction as well (Table 5). As an attempt to assess whether this allometric change may account for the observed differentiation on Piana, an extrapolation was performed along the mainland allometric direction, by estimating the difference in shape corresponding to a size change from mainland to Piana values. The length of the vector corresponding to this extrapolated allometric change was only about half the difference observed between mainland and Piana mice (Fig. 5). Hence, neither plasticity nor allometry could account for the whole divergence of Piana mice. Both combined, however, were of the order of magnitude of the observed difference characterising the population of this islet.
All these vectors also correlated with pmax (V1mainland), the direction of main variance within the mainland group. On the other hand, the change from mainland to Corsica and Sardinia correlated with the macro-evolutionary direction corresponding to a change from herbivory to omnivory (negative correlation with the difference between Mus–Mastomys–Apodemus and Arvicanthini), the amount of change being by far less than the macro-evolutionary difference. Both correlated with the second major axis of variance within the mainland population, V2mainland.
The stability of the correlation between vectors representing various patterns of differentiation, regarding the initial sampling, was evaluated by correlating bootstrapped estimates of the initial vectors (Table 5). Results were coherent with those obtained for each vector (Table 4), robust correlations being observed between robustly estimated vectors. However, even vectors that displayed a relatively high variability in their bootstrapped estimates provided robust correlations. It was the case of the direction involving Piana (Corsica–Piana) as well as the allometric direction. The only vector leading to overall poor correlations was the difference Mainland–Sardinia.
Discussion
This study aimed at investigating processes involved in phenotypic differentiation on islands, using morphometric quantification and comparing the morphological output of different controlled factors, focusing on a few exemplary cases in the house mouse. The preliminary condition for this study was to evidence a significant differentiation in size and shape on islands, which indeed has been confirmed using our results, shape differentiation occurring in Corsica, Sardinia and Piana, whereas size increase characterized only the mice from Piana. Patterns, however, were contrasted between the large islands Corsica and Sardinia, and the small islet Piana. This discrepancy points to different processes acting on different spatial and temporal scales.
Adaptation to a niche shift: a process over many generations on large islands
House mice on Corsica (Libois 1984; Granjon and Cheylan 1988) and Sardinia (Orsini 1982) occupy a broader range of habitats than on mainland, displaying successful outdoor populations that are rare on mainland even under favourable climatic conditions prevailing in the Mediterranean area, due to the competition with M. spretus. Such a shift in the ecological niche should involve a change in the average diet of the population, and an adaptive interpretation of the mandible shape evolution in Corsica and Sardinia is tempting. Such an interpretation finds support in the congruence between these vectors of change (mainland–Corsica and mainland–Sardinia) with the macro-evolutionary signal corresponding to the difference between omnivorous and herbivorous murine rodents (Fig. 5). Grass being very resistant to abrasion and mastication, the consumption of such food requires more powerful masticatory muscles. In agreement, herbivorous rodents display a more robust mandible with larger posterior processes serving as the zone of attachment to these muscles (Michaux et al. 2007a). Evolution of mice on Corsica and Sardinia correlated with this macro-evolutionary direction of change, although corresponding to a change towards a relatively more generalized, omnivorous diet. Possibly house mice in these outdoor populations increased the consumption of seeds, a food that crumbles during the mastication process and require relatively less force during mastication.
Noteworthy, mice on Corsica and Sardinia displayed very similar mandible changes compared with mainland ones, despite rather different environments on both islands. This may be due to niche widening occurring on both islands and leading to parallel evolution, a process that might explain recurrent patterns of differentiation observed in insular rodents (Pergams and Ashley 2001). This may also arise from genetic closeness, as mice from both islands share common origins of colonization, and a subsequent evolution in relative isolation from the mainland but with gene flow among both islands thereafter (Navajas y Navarro and Britton-Davidian 1989). Both processes are not mutually exclusive and can both contribute to the morphological closeness of Corsican and Sardinian mandibles.
At what time did such an evolution occur? The house mouse started to spread and colonize the entire western Mediterranean basin since the first millennium BC, taking advantage of the extensive commercial exchanges of the Phoenicians and of foundation of Greek colonies (Cucchi and Vigne 2006). In agreement, the mouse appears in a Corsican archaeological sequence just under the layer dated 9th–5th centuries BC (Vigne and Valladas 1996). Hence, the process of morphological evolution on Corsica and Sardinia probably acted upon many generations of mice in well-established populations that did not suffer dramatic bottlenecks after their implantation (Navajas y Navarro and Britton-Davidian 1989).
Allometry and insular evolution
The pattern of morphological divergence of Piana mice completely differs from the one characterising Corsica and Sardinia. Piana mice display an increased body size, a trait that is a large part of the ‘insular syndrome’ in small mammals (Lomolino 1985). Extinct endemic rodents displayed cases of gigantism (e.g. Canariomys on the Canary islands; Michaux et al. 1996), but cases of relative gigantism can also be displayed within extant species. Larger body size is documented on several insular wood mice (Angerbjörn 1986), although this trend is not systematic (Renaud and Michaux 2007); it is also displayed in some insular house mice (e.g. on Canary Islands, Michaux et al. 2007b). Can the morphological divergence of the Piana mice be the mere product of an allometric trend?
The existence of an allometric trend may be expected due to functional reasons. Increasing body size leads to a relative increase in mandible weight that has consequences on the functioning of masticatory muscles. The force required to move the mandible with a constant acceleration is proportional to its weight (Satoh 1997); hence the strength exerted by the muscles in the forward movement of the mandible should increase with size. Accordingly, an allometric component has been identified in the mandible shape variations at the scale of the murine family (Michaux et al. 2007a). The shape associated with larger size corresponds to a mandible with more massive zone of insertion of the masticatory muscles.
At the intra-population level within our sample of continental house mice, an allometric influence on mandible shape was indeed evidenced using the significant multiple regression of outline parameters against mandible size. The shape changes involved, however, were limited (Fig. 5) and regarded not only the posterior processes but also the alveolar region. The amount of allometric change caused by the size increase from mainland and Corsican populations to Piana was only approximately half of the observed divergence of Piana mice. Hence, allometry alone cannot account for the divergence of the population on the islet.
Phenotypic plasticity: a component of rapid insular evolution?
The direction of shape change from Corsican to Piana mice further matched, however, the direction of change observed between laboratory mice bred on food of different consistency (hard regular rodent pellets versus same food as jelly), suggesting a possible role of phenotypic plasticity in the differentiation on Piana. The laboratory mice fed soft food displayed a less robust incisor-bearing part, a dorsally shifted molar alveolar region and less robust angular processes. These observations were in agreement with similar experiments on growing rats that were characterized using a thinner condylar process and a vertically reduced angular process (Mavropoulos et al. 2004), together with a decrease in bone density (Mavropoulos et al. 2004). These changes are due to bone remodelling of the mandible, a process that occurs along the entire animal’s life, depending on the conditions of growth (Katsaros et al. 2001; Mavropoulos et al. 2004, 2005).
Hence, the mandible shape change on Piana may be, at least partly, the result of the food available on this small, uninhabited islet. Its vegetation is composed of herbs and halophytous vegetation. Mice, confronted to an intense competition with the black rat Rattus rattus (Navajas y Navarro 1986), possibly switched to a diet largely composed of tender halophytous plants, or even invertebrates as documented in some islands with extreme environments (Smith et al. 2002), in turn causing a plastic change of the mandible similar to that of mice fed soft versus hard diet.
Plasticity has been advocated as a component of insular differentiation, allowing a rapid adjustment to local conditions (Losos et al. 2001). Enhanced plasticity may have even been selected for on islands, as a way to match the variety of environments (Aubret et al. 2004). In the long run, it may lead to a heritable change due to the process of genetic assimilation (Badyaev 2005; Parsons and Robinson 2006). What is, however, the timing of evolution on Piana? The mouse population was estimated at 12 individuals at the time of trapping and apparently did not recover from this campaign (Navajas y Navarro and Britton-Davidian 1989). The sharing of rare alleles between mice from Piana and Corsica (Navajas y Navarro 1986; Navajas y Navarro and Britton-Davidian 1989) suggests that the genetic divergence of the Piana population was limited, possibly because the population was too small to survive in the long term, and rather functioned as a sink for recurrent immigrants from the nearby Corsica. Hence, morphological differentiation may have occurred on a short timescale on this islet, compared with the processes involved on the large islands such as Corsica and Sardinia.
Congruence of changes involving remodelling during late growth
A striking feature was the similarity between shape changes caused by plasticity and allometry, these directions being further correlated with the major direction of intrapopulation variance. Plasticity and allometry are two processes that involved bone remodelling in response to food consistency or to growth.
In our experimental design, mice were bred on different foods from 3 weeks onwards. In the natural population on which allometry was estimated, young mice were excluded by only considering animals with the third molar erupted. This happens at around 3 weeks of age, a time corresponding to weaning.
Ontogenetic trajectories of the skull appear to be dynamic and to change through the post-natal developmental time (Zelditch et al. 2003). In M. m. domesticus, allometric trajectories stabilize at around 25–30 days, the skull shape having then reached 69% and skull size 95% of maturity (Zelditch et al. 2003). Extending these results to mandible ontogeny, our results documented only the late growth, after stabilization of the allometric trajectories. It seems that irrespective of the processes causing bone remodelling during this period of life (either plastic changes related to food consistency or late allometric growth), the morphological effects are similar. Moreover, these processes may deeply mould the intrapopulation shape variation, as suggested by the correlation of the allometric and plastic directions with the major axis of variance.
Some parts of the mandible should be more prone to changes during late ontogeny, corresponding to zones where remodelling occurs in response to biomechanical stimulation associated with mastication activities (Atchley et al. 1985). The processes related to the bone–muscle interactions would lead to shape changes distributed all over the mandible, blurring developmental modularity and/or localized genetic effects and emerging as major patterns of variation (Zelditch et al. 2008).
Noteworthy, different results might be obtained if considering early (pre-weaning) ontogeny. Hence, depending on life-history traits causing a size increase on islands, one might then expect different morphological signatures, if size increase is triggered early or later on during life.
Directions of greatest variance, lines of least resistance to phenotypic change
Main directions of within-population variation are more and more investigated for their role as ‘lines of least resistance to evolution’ (Schluter 1996). They may provide a conceptual bridge between micro- and macro-evolution, as they can be integrated as the channel to evolution towards or away from peaks in the adaptive landscape (Arnold et al. 2001). Can we bring some support to their role in the cases of insular evolution we documented? A prerequisite for considering directions of the greatest variance as lines of least evolutionary resistance is their stability over time. Conflicting evidences were provided by results pointing to conserved patterns of phenotypic variance over more than 10 Myr (Ackermann and Cheverud 2000; Marroig and Cheverud 2001; Renaud et al. 2006; Cardini and Elton 2008) and short-term fluctuations (Agrawal et al. 2001; Steppan et al.2002; Polly 2005; Guillaume and Whitlock 2007). Our results also provided conflicting results, depending on the method testing either similarities or differences between matrices, and depending on the consideration either of the P matrix or of its major axis only. P matrices appear to differ among populations, regarding the magnitude of the components and also the successive order of the components, this instability among the vectors being due to an approximately equal variance on the main axes (Zelditch et al. 2006). Yet, the major axis of variance based on the whole mainland sample appeared as robust regarding resampling, confirming that such an axis is satisfyingly estimated when based on a large population (Polly 2005; Cardini and Elton 2007).
Both random drift and adaptive response can be facilitated along these ‘lines of least resistance’ (Roff 2000; Ackermann and Cheverud 2004). In the case of the insular populations, observed morphological changes indeed occurred parallel to the first vectors of the P matrix, possibly due to a mixed effect of random and adaptive processes. The first axis paralleled the differentiation of Piana mice, but also the direction related to bone remodelling during late growth such as plasticity and allometry. Hence, several processes may contribute to the matching of the micro-evolutionary direction and the major axis of variation. (1) Size as line of least evolutionary resistance was already evidenced in monkeys (Marroig and Cheverud 2005; Cardini and Elton 2008), suggesting that allometric changes may be important in driving macro- as well as micro-evolutionary changes. (2) The same direction, however, corresponded to plastic variation due to food consistency, and possibly an effect of late growth driving the intra-population allometry. Therefore, the major axis of mandible shape variation in the wild mice populations may include a part of non-heritable variation, and may be influenced by local life-history traits including age structure of the population and diet. This may contribute to fluctuations observed in the P matrices between populations or closely related species. (3) The population of Piana was particularly reduced, being estimated at a dozen of individuals at the time of trapping (Navajas y Navarro and Britton-Davidian 1989) and hence, might have been prone to drift, more likely to occur along the major direction of variance of the source population. These hypotheses are mutually non-exclusive but would cause congruence between the variance in Piana and its direction of differentiation. Such congruence may help explain why, despite the reduced sample and its large variability, the shape change involving Piana robustly correlated with the major axis of variance and the directions of plastic and allometric change.
The second axis (V2) coincides with the direction of differentiation of Corsican and Sardinian mice, and with the macro-evolutionary change from herbivorous to omnivorous murines. It concerns particularly more or less pronounced coronoid and angular processes, both zones of attachment of important masticatory muscles (Satoh 1997). These zones are also those involved in a latitudinal gradient in mandible shape observed in different wood mice species (Renaud and Michaux 2003) interpreted as a response to a clinal change in average diet. Hence, these features on the mandibles appear as variable within a population and evolvable within or among species. This propensity to vary might be underlain by a complex genetic architecture in which several genes apparently cause mandibles changes localized in these zones (Klingenberg et al. 2001), favouring parallel evolution in different species and at different timescales.
Shape changes observed in wild populations are difficult to relate directly to the underlying processes involved. Comparing directions of morphological changes attributable to different candidate factors to explain divergence among populations remains speculative but can bring a stronger support to some hypotheses. We particularly investigated hypotheses involving different aspects related to diet as the mandible had been selected as the morphological character of interest, because of its importance in the feeding process. Studies including more insular populations documenting various ecological contexts would be required to further investigate the complex interactions underlying insular divergence, including the relative role of adaptation and drift. Island as ‘laboratory of evolution’ is still fertile in case studies for understanding processes of phenotypic divergence.
Acknowledgements
We thank Maria Navajas, Laurent Granjon and Janice Britton-Davidian for very useful information on the trapping campaigns that delivered most of the material included in this study. The manuscript greatly benefited from the comments of Janice Britton-Davidian, and from the very constructive reviews of Andrea Cardini and one anonymous reviewer. The breeding was managed at the PBES of the Ecole Normale Supérieure de Lyon and benefited from the skills of M. Texeira and J.-L. Thoumas. This work was supported by the GDR 2474 CNRS ‘Morphométrie et évolution des formes’. Contribution ISEM 2009-009.




