A review of iguanian and anguimorph lizard genitalia (Squamata: Chamaeleonidae; Varanoidea, Shinisauridae, Xenosauridae, Anguidae) and their phylogenetic significance: comparisons with molecular data sets
Eine Revision der Genitalien der Iguania und Anguimorpha (Squamata: Chamaeleonidae; Varanoidea, Shinisauridae, Xenosauridae, Anguidae) und ihrer phylogenetischen Aussagekraft: Vergleiche mit molekularen Datensätzen
Abstract
enWe compare phylogenetic hypotheses for iguanian (chameleonids) and anguimorph lizard groups (varanoids, xenosauroids, anguids) which were generated from analyses of genital (hemipenial) morphology, with recent molecular phylogenetic approaches towards the same groups. Taxa with infraspecific communication by means of visible, sexually dimorphic epigamic characters usually have less diverse genital structures than taxa with less developed visible epigamic characters but with a more highly developed chemical intersexual communication. Generally, it turned out in the cases considered here, that phylogenetic hypotheses based on hemipenial characters coincide much better with molecular-genetic phylogenies than with earlier concepts based solely on external morphology. It seems that genital morphological characters are phylogenetically more informative – on both the species and the supraspecific level – than external morphological characters at least in these examples, because the former seem not to be affected by environmental selective pressures but seem to be only subject to sexual selection. Our data suggest that sexual selective pressure on genital structural diversity is higher the less sexually dimorphic, optical cues for infraspecific communication have evolved. They further suggest a correlation with the mating system (single versus multiple matings).
Zusammenfassung
deWir vergleichen auf genitalmorphologischen Analysen basierende phylogenetische Hypothesen für die Chamaeleonidae (Iguania) und die Varanoidea, Shinisauridae, Xenosauridae und Anguidae (Anguimorpha) mit aktuellen molekularen Phylogenien dieser Echsengruppen. Taxa, die über eine infraspezifische Kommunikation mittels sichtbarer, sexualdimorpher epigamischer Merkmale verfügen, zeigen gewöhnlich weniger diverse Genitalstrukturen als solche Taxa, die weniger ausgeprägte, sichtbare epigamische Merkmale aufweisen, dafür aber über eine besser entwickelte zwischengeschlechtliche chemische Kommunikation verfügen. Grundsätzlich stimmen die auf genitalmorphologischen Vergleichen beruhenden Hypothesen bei den von uns betrachteten Fällen sehr viel besser mit molekulargenetischen Verwandtschaftsanalysen überein als mit früheren, allein auf externmorphologischen Merkmalen beruhenden phylogenetischen Konzepten. Es scheint, dass genitalmorphologische Merkmale zumindest in diesen Fällen phylogenetisch aussagekräftiger sind – sowohl auf Artniveau als auch im supraspezifischen Bereich – als externmorphologische Merkmale, da erstere, so wie es scheint, lediglich durch die sexuelle Selektion, nicht aber durch Umweltselektion beeinflusst sind. Die hier vorgelegten Daten zeigen an, dass die sexuelle Selektion sich um so stärker auf die Diversität der Genitalstrukturen auswirkt, je geringer geschlechtsdimorphe, optische Merkmale in der innerartlichen Kommunikation entwickelt sind. Auch deuten unsere Daten eine weitere Korrelation hinsichtlich des Fortpflanzungssystems (einzelne versus multiple Kopulationen) an.
Introduction
When amniote tetrapods first definitely freed themselves from an aquatic environment, the main achievements making this success possible were (1) a cornified integument as a protective layer against desiccation and mechanical injuries, (2) a hard (parchment or calcareous) eggshell and (3) a new, additional embryonic envelope (amnion) to keep the embryo in a fluid environment within the water-independent egg. This key innovation gave the whole group (Reptilia, including mammals and birds) the name Amniota and serves phylogeneticists as a unique synapomorphy to define amniotes as monophyletic. However, there is another indispensable prerequisite for a successful conquest of the terrestrial habitat which has also to do with reproduction, viz. the possession of an intromittent organ (despite the fact that it has been lost again twice: viz. in Sphenodon and modern birds) to transport male gametes into the female because, in contrast to water, air is an unsuitable medium for sperm transport.
Male intromittent or copulatory organs have also been developed in a great variety by other terrestrial animal groups, e.g. in chelicerates and insects. In the former, these organs are not abdominal genital organs as in the appertaining females, but modified extremities (cheliceres in males of mites, pedipalps in those of spiders and daddy-long-legs) which collect the sperm at their genital opening to bring it into the female’s genital opening, while in insects, true genital structures have been developed at the end of the abdomina (see e.g. Steffan 1960; Kraus 1968; Scudder 1971; Dirsh 1973; Eberhard 1985, 1990; Shapiro and Porter 1989). In contrast to tetrapod vertebrates, it is therefore impossible to homologize the structural elements of the outer reproductive organs between the sexes in the Chelicerata with their modified extremities in males, and it is much more difficult in insects with their ‘true’ abdominal genitalia because these stem from different segments of the terminal part of the abdomen (Weber 1938; Clausnitzer 1996).
Chelicerae and pedipalps serving copulation might be termed ‘pseudogenitals’ (or ‘secondary genitals’ according to Eberhard 1985) because they are not true genitalia but are obviously nonetheless subjected to female sexual selection (Kraus 1968; Eberhard 1985, 1990). This makes them constant and species-specific in shape so that they also provide zoologists with indispensable diagnostic characters for species (or, in some groups, generic) identification. Even organs that do not serve for sperm transfer but for stimulation only, such as the love-arrows of landsnails have species-specific shapes that make taxonomic identifications on the species level possible (e.g. Kerney et al. 1983).
Pseudogenitals as intromittent organs in vertebrates have been developed by sharks (modified ventral fins: pterygopods) and some cyprinid teleosteans (live-bearing cyprinodonts with modified anal fins: gonopods) because internal fertilization is also in purely aquatic animals a prerequisite for giving birth to living young which is realized in these groups. In amphibians, only one primitive anuran, the famous Ascaphus truei, has a notable cloacal extension ‘or tail’ in males serving intromission and internal fertilization in rapid mountain torrents (Duellman and Trueb 1986). Male gymnophionans have also an evertible cloacal intromittent organ which is obviously infraspecifically constant but varies in shape between species and groups (Meisenheimer 1921; Gerhardt 1933; Duellman and Trueb 1986); however, it has not yet much been used for systematic purposes (Wake 1972; Himstedt 1996).
A true, unpaired penis, together with its homologous, miniaturized female equivalent (clitoris), is originally present in all extant (and certainly also fossil) amniotes and has been retained by all mammals, chelonians, crocodiles and primitive (ratite and anseriform) birds (Gadow 1887; Boas 1891; Wiedersheim 1909; King 1981a,b; Lake 1981). However, in recent amniotes it has been lost twice: in the lepidosaurian clade (Rhynchocephalia and Squamata) and in modern birds. But while the only extant rhynchocephalian (Sphenodon) has to carry out sperm transfer as also modern birds must, viz. by adpressing the cloacas of both sexes against each other (Robb 1977; Moffat 1985), male squamates have developed a new system of paired intromittent organs (hemipenes) which are, as miniaturized equivalents, also present in females (hemiclitoris: Böhme 1995; Ziegler and Böhme 1997). In snakes, the structure and interspecific variation of hemipenes has since long been used for taxonomic and phylogenetic purposes (e.g. Cope 1895; Camp 1923; Bogert 1940; Dowling and Savage 1960;Domergue 1962; Myers and Cadle 1994; Zaher 1999). Comparative studies of lizard hemipenes have, with the exception of Cope (1896) and Camp (1923), started much later (Böhme 1971, 1988; Arnold 1973, 1983; Presch 1978; Branch 1982; Klaver and Böhme 1986; Card and Kluge 1995; Ziegler and Böhme 1997; Rösler 1998, 2000a,b; Rösler and Böhme 2006; and some more studies dealing with particular species groups only). Ziegler and Böhme (1997) showed that even a hemiclitoris alone may serve as a substitute in a comparative hemipenis study when the respective male organ is lacking in the sample because relevant structures are present in miniaturized form (see also Ziegler and Böhme 1999; Ziegler et al. 2005).
Our own studies of lizard taxonomy and phylogeny based on genital morphology (Böhme 1971, 1988, 1995; Klaver and Böhme 1986; Ziegler and Böhme 1997) have always laid emphasis on functional constraints which may influence shape and structure of lizard hemipenes and thus weaken their phylogenetic value. In an ophidian example, Böhme and Sieling (1993) were able to demonstrate that even the knowledge of mating behaviour combined with female cloacal anatomy can be necessary to understand form and function of hemipenial morphology (see also Ziegler and Böhme 1997). A major, general correlation was noted between low hemipenial diversity and marked, sexually dimorphic, epigamic characters on the one hand (e.g. iguanian lizards), versus high hemipenial diversity and nearly lacking sexual dimorphism (e.g. varanoids) on the other (Ziegler and Böhme 1997) (Fig. 1). Similar correlations have been found in other animal groups, for instance saltatorians where taxa with diversified acoustic (and optic) signalling in males tend to have uniform genitals whereas in mute forms it is the reverse (Alexander and Otte 1967; Rentz 1972).
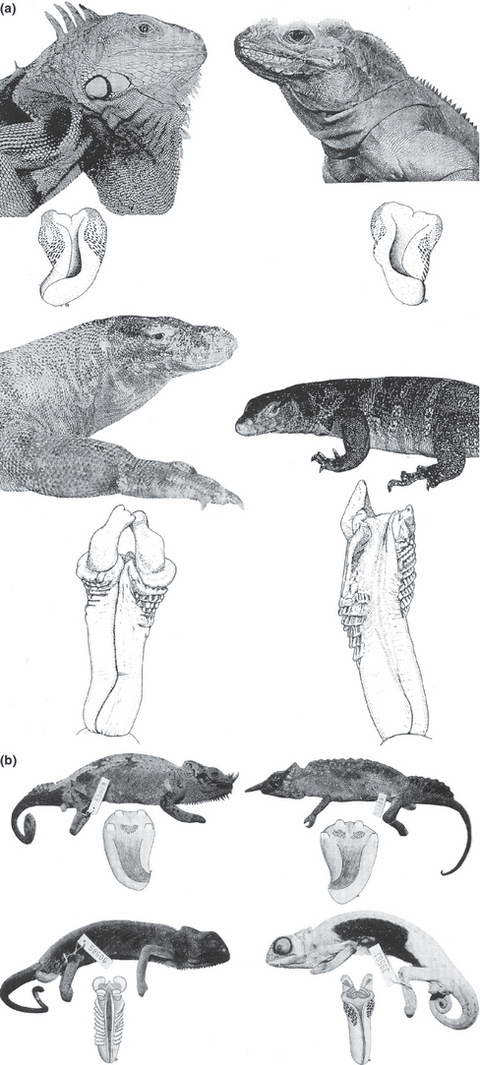
(a) Correlation between genital structures and external morphology respectively, premating isolating mechanisms. Above: The externally-morphologically highly diversified iguanines [left: Iguana iguana (Linnaeus, 1758), right: Cyclura cornuta (Bonnaterre, 1789)] show a rather uniform genital morphology even between genera. Below: The phenetically much more similar monitor lizards [left: Varanus komodoensis (Ouwens, 1912], right: V. salvator [Laurenti, 1768)] have highly diversified genital structures (after Ziegler and Böhme 1997). (b) Even within the Chamaeleonidae, which are mostly diverse in external and genital morphology, a similar correlation is demonstrable. Above: the sympatric, externally contrasted males of Chamaeleo quadricornis Tornier, 1899 (left) and C. montium Buchholz, 1874 (right) are similar in their hemipenial structures. Below: Chamaeleo gracilis Hallowell, 1844 (left) and C. senegalensis Daudin, 1802 (right) are externally most similar, but have each markedly differentiated hemipenial structures (after Ziegler and Böhme 1997)
Despite these functional constraints of genital morphological characters, there remain numerous peculiarities of shape and structure which do not seem to be functionally correlated and which are therefore considered to contain valuable phylogenetic signals so that we had constructed phylogenies on this basis, in particular of the iguanian family Chamaeleonidae (Klaver and Böhme 1986) and the anguimorph family Varanidae (Böhme 1988; Ziegler and Böhme 1997). We compare these two families because:
(1) Chameleons are a highly specialized, arboreal group of sit-and-wait predators with an aberrant, uniform bauplan. As a result of this uniformity, they had traditionally, for many decades, been classified in only two (or three) genera, the tree-dwelling genus Chamaeleo, and the ground-dwelling genus Brookesia (or Brookesia and Rhampholeon, respectively) (Werner 1902, 1911; Mertens 1966). In contrast, other iguanian families used to be phenetically much more diverse and to occupy a much wider range of niches. For example, the true iguanas (Iguanidae s.str.) offer examples of ground (sand and rock) dwellers (Conolophus, Ctenosaura part., Cyclura, Dipsosaurus, Sauromalus) tree dwellers (Brachylophus, Ctenosaura part., Iguana) and even one marine species (Amblyrhynchus cristatus). Despite this phenetic diversity, not only interspecific (Ctenosaura: Köhler and Blinn 2000), but also intergeneric hybrids are known (Amblyrhynchus × Conolophus; Ctenosaura ×Iguana: Snell et al. 1984; Rassmann et al. 1997; Lücker and Feiler 2002; Dirksen 2004) even from the wild, demonstrating genotypic relatedness and suggesting that genus rank here might overestimate (phylo-) genetic distance (cf. Dubois 1988).
Within iguanians, chameleons are unique because of their greatly varying sexual dimorphism; some groups have males much larger than females, some have equal-sized sexes, and some have females much larger than males. Only species belonging to the first category have males with epigamic attributes such as rostral and/or orbital appendages, elevated helmets, tarsal spurs etc., and their interspecific hemipenial differences are usually less expressed than in species with smaller and non-adorned males, where, conversely, interspecific hemipenial differences are much better developed (Klaver and Böhme 1986; Ziegler and Böhme 1997).
(2) Monitor lizards, in contrast, are generalized, active foragers on the ground, in fresh and brackish water as well as on trees and are characterized by a weakly expressed sexual dimorphism. Males tend to be larger than females and fight for them in strongly ritualized combats but in similar combats, they may compete also with females for food resources (Horn et al. 1994). They have highly diversified genitals which seem to provide much more reliable information on their phylogenetic relationships (Böhme 1988; Ziegler and Böhme 1997) than earlier classifications based on external morphology (e.g. Mertens 1942, 1959, 1962).
The aim of this study is to confront these genital morphology data and phylogenetic hypotheses with new molecular genetic approaches to the phylogeny of these two families (Ast 2001; Townsend and Larson 2002; Matthee et al. 2004; Fitch et al. 2006; Mariaux and Tilbury 2006; Tilbury et al. 2006; Ziegler et al. 2007). In addition, we shall also compare genital morphological (Böhme 1988) and molecular genetic data (Macey et al. 1999) in a few other anguimorph families or sections of families, viz. xenosauroids and anguine anguids because they also belong to those groups with weakly developed sexual dimorphism. We do not add respective paragraphs on iguanian groups other than chameleons because, despite their obvious suitability for such comparisons, either hemipenial or molecular genetic data are insufficient or lacking, or the latter are merged with external morphology which in turn is not separated from hemipenial morphology in the available phylogenetic analyses (see e.g. Titus and Frost 1996; Wiens and Hollingsworth 2000; Frost et al. 2001; Schulte et al. 2003; Torres-Carvajal et al. 2006).
Materials and Methods
For the genital morphology methods applied, the reader is referred to the extensive descriptions of hemipenial/hemiclitorial preparation/eversion in both fresh and fixed specimens given in Böhme (1988, 1995), Pesantes (1994) and Ziegler and Böhme (1997). Character polarity was assessed by outgroup comparisons (see Klaver and Böhme 1986; Ziegler and Böhme 1997). A comprehensive terminology of the single structures on lizard intromittent organs has been set up for chameleons by Klaver and Böhme (1986), and for other lizard groups, in particular monitor lizards, by Böhme (1988, 1995) and Ziegler and Böhme (1997), including the female homologous structures.
Some of the most important terms in chamaeleonid and anguimorph copulatory organs are as follows (in alphabetical order): apex – distal portion of the copulatory organ; asulcal – portion of the copulatory organ opposite to the sperm groove; auriculae – curved denticulate ridges (singular: auricula) at the asulcal side of the pedunculi; calyces – shallow pockets between retiform ridges, which may vary in depth, size and shape, the ridges surrounding them may either be smooth, fringed, serrated or denticulated at the outer margin: hence the surface is said to be calyculate (=‘reticulately honeycomb-like pits’: McCann 1949; ‘flounced’, ‘ridged’, ‘plicated’: Cope 1896; McCann 1949; ‘franges cellulaires’, ‘collerettes superposes à bord frangé’: Brygoo and Domergue 1969); hemibacula – terminal supportive structures (singular: hemibaculum) in the hemipenis as insertion of the retractor muscle (=‘Knorpelplatten’, ‘ligamentöse Faserknorpel’: Müller 1838; ‘cartilaginous bodies’: Günther 1861; ‘os penis’: Smith 1935; ‘horns’: Branch 1982; ‘hemipenial bones’: Shea and Reddacliff 1986; ‘cornua’: Savage 1997); hemibaubella – terminal supportive structures (singular: hemibaubellum) in the hemiclitoris as insertions of the retractor muscle; papillae – fleshy and pliable projections varying in size and shape, which may either be single, grouped in pairs or rows, evenly scattered over the apex, or concentrated on various locations on the apex in papillary fields; paryphasmata – transversal, mostly roof tile-shaped supportive fringes not only in the varanoid but also in other anguimorph copulatory organs (=‘flounces’: Cope 1896; ‘cartilaginous transverse lamellae’: Günther 1861; ‘frills’: Branch 1982); pedicel – proximal portion of the copulatory organ; pedunculi – in their basic form (singular: pedunculus) thick stalks, that protrude over the distal end of the sulcus spermaticus, and which may have numerous pointed papillae that are often arranged in rows on the sulcal surface; rotulae – an apical ornament (singular: rotula) with semicircular discs with a denticulated or serrated outer margin (=‘papillae’: Cope 1896; ‘erect cresentic plates’: McCann 1949; ‘apical discs’, ‘wings’, ‘cogwheels’: Broadley 1971; Böhme and Klaver 1980; Klaver 1981b; ‘inward curving denticulate apical structures’: Raw 1976; ‘halbkreisförmige Strukturen’: Klaver 1981a); sulcal – portion of the copulatory organ bearing the sperm groove; sulcus spermaticus – sperm groove; truncus – median portion of the copulatory organ.
For methodological details concerning the molecular phylogenies based on DNA sequences we refer the reader to the reviews by Macey et al. (1999), Ast (2001), Townsend and Larson (2002), Matthee et al. (2004), Tilbury et al. (2006), Mariaux and Tilbury (2006) and Ziegler et al. (2007).
Results and Discussion
Iguania: Chamaeleonidae
As mentioned above, chameleons have traditionally been classified in only two (or three) genera, viz. Chamaeleo and Brookesia (e.g. Loveridge 1957; Mertens 1966) (plus Rhampholeon as a third genus: Werner 1902, 1911). Both genera have meanwhile been partitioned (or, in case of Brookesia, re-partitioned). In a first phylogenetic approach, based on lung and – primarily – hemipenial characters, Chamaeleo was subdivided into two African and two Malagasy genera by Klaver and Böhme (1986):
In Africa, a species group of Chamaeleo with few large lung septa was definable by an apomorphic hemipenis character, viz. augmentation of the originally four rotulae to up to 10. It left a plesiomorphic ‘remainder’ with always four rotulae only for which the name Trioceros was used (definable only by the unique occurrence of annulated horns). Trioceros was proposed as a subgenus of Chamaeleo. The primitive (i.e. without large lung septa) African tree chameleons were lumped in a second genus Bradypodion accomodating a South African monophyletic radiation together with an East African clade of still uncertain relationships. The SW African ground-dwelling desert chameleon Chamaeleo namaquensis Smith, 1831 displayed the plesiomorphic four-rotulae condition and was tentatively retained as a basal member in the subgenus Chamaeleo.
In Madagascar, also two different clades could be detected by means of hemipenial characters, viz. a plesiomorphic group with four hemipenial rotulae, and a derived group bearing one pair of auriculae and pedunculi each on the apex of their hemipenes, a type of ornamentation exclusive to Madagascar. The latter group, thereby well defined as a monophyletic group, was placed in its own genus Furcifer (Fig. 2), while the former plesiomorphic ‘remainder’ was lumped under Calumma. Within Calumma, however, species groups could be defined as monophyletic on the basis of unique hemipenial structures. This was true for e.g. the Calumma brevicornis (Günther, 1879) and Calumma gastrotaenia (Boulenger, 1888) groups which possess long bifid papillae not found in any other chameleon. Similar papillae are also present in Calumma tigris (Kuhl, 1820), a Seychellean endemic which was formerly considered to be related with the South African Bradypodion clade because of the common possession of scaly skin flaps under the chin (Hillenius 1959), but was now interpreted to belong to a Madagascan radiation (Klaver and Böhme 1986).
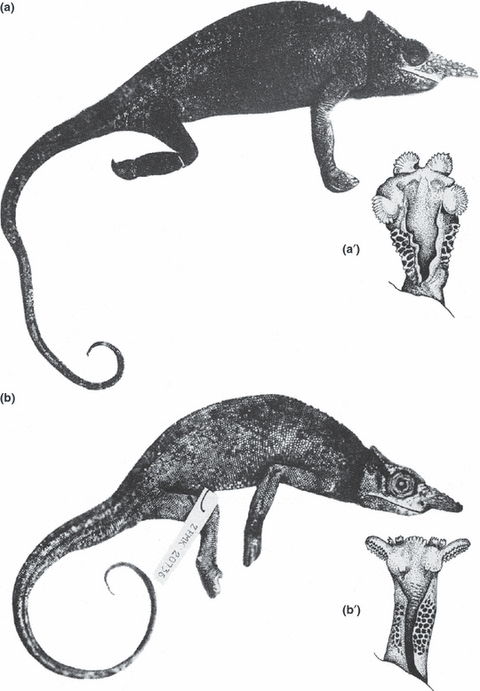
Body contours and hemipenis sketches of Kinyongia fischeri (Reichenow, 1887) (above) from East Africa, and Furcifer bifidus (Brongniart, 1800) (below) from Madagascar, to show the convergently similar body shape versus the strikingly different hemipenial structure (after Böhme 1988)
The analysis by Raxworthy et al. (2002) combined external morphological and molecular data in one phylogenetic tree. Monophyly was suggested for two basal clades of Brookesia (one for B. nasus Boulenger, 1887 and B. lolontany Raxworthy & Nussbaum, 1995 and one for the remaining species) followed by a clade for two Rhampholeon species studied [R. brevicaudatus (Matschie, 1892) and R. spectrum (Buchholz, 1874)]. Low bootstrap support was received for a monophyletic Calumma clade with four better supported subgroups, but without the Ca. gastrotaenia (Boulenger, 1888) group. High support was received for the genus Chamaeleo with its two subgenera Chamaeleo s.str. and Trioceros, and finally, also the Ca. gastrotaenia (Boulenger, 1888) species group and the genus Furcifer came out as monophyletic. Within Calumma next to the gastrotaenia group, the Ca. parsonii (Cuvier, 1824), Ca. brevicornis (Günther, 1879) and Ca. nasuta (Mertens, 1933) groups formed clades as well.
The exclusively molecular genetic analysis by Townsend and Larson (2002) revealed nine distinct clades: (1) Brookesia (Madagascar), (2) Chamaeleo subgenus Chamaeleo (but without C. namaquensis; whole of Africa and SW Asia), (3) Chamaeleo subgenus Trioceros (Central and E Africa), (4) viviparous Bradypodion (S. Africa), (5) oviparous Bradypodion (East Africa), (6) Furcifer [Madagascar; but without F. balteatus (Duméril & Bibron, 1851)] and (7–9) three clades of Calumma (Madagascar). Chamaeleo namaquensis Smith, 1831 seems to be the African sister taxon of one of three Calumma subgroups (parsonii species group), while B. nasus Boulenger, 1887 comes out as a sister taxon of all other Brookesia. The African ecological equivalents, R. brevicaudatus (Matschie, 1892) and R. spectrum (Buchholz, 1874), form distinct lineages of their own, further congeners not being included in the study.
The position of the SW African C. namaquensis Smith, 1831 as the sister taxon to a Madagascan clade would offer a serious zoogeographic problem if this relationship will be further substantiated.
The hemipenis of ‘Furcifer’balteatus (Duméril & Bibron, 1851) has been described by Brygoo and Domergue (1969) and indeed provides data for doubting its generic assignment: the very slim organ does have two asulcal auriculae but the pedunculi are differently shaped as compared with true Furcifer species, consisting of irregular denticulations which are not arranged in one plane. Moreover, there are no long papillae or tufts of superimposed papillae at the base of the pedunculi thus giving additional support to not place balteatus in Furcifer. These data support the view of placing ‘F.’balteatus (Duméril & Bibron, 1851) basal to all other (true) Furcifer, which are otherwise definable as a monophyletic unit on the basis of their hemipenial structure.
Tilbury et al. (2006) analysed the East African fraction of Bradypodion sensu Klaver and Böhme (1986), Klaver and Böhme (1997) and were able to demonstrate that they are not only generically distinct from the South African viviparous clade (Bradypodion s.str.) but contained two distinct genera which they described as Kinyongia (i.e. the B. fischeri (Reichenow, 1887) group, see Fig. 2) and Nadzikambia [monotypic for B. mlanjense (Broadley, 1965)]. As noted by the authors, a uniquely derived hemipenis (Fig. 10 in Klaver and Böhme 1986) also argues strongly for an independent generic status of this species.
Due to the recent study by Matthee et al. (2004) which refines molecular phylogenetics of Rhampholeon, this genus proves to provide more good examples for congruence between hemipenial and molecular data. Within Rhampholeon, the East African savanna species R. kerstenii (Peters, 1868) holds the plesiomorphic chameleonid condition with its hemipenial ornamentation of four rotulae (see above). A total reduction (loss) of this ornamentation is seen in R. brevicaudatus (Mariaux and Tilbury, 2006). Strongly differing and highly derived ornaments are found in the hemipenes of R. temporalis (Matschie, 1892) and R. spectrum (Buchholz, 1874) (Fig. 3). A third type of ornamentation is seen in R. boulengeri Steindachner, 1911, R. brachyurus Günther, 1893, R. chapmanorum Tilbury, 1992, R. marshalli Boulenger, 1906, R. moyeri Menegon, Salvidio & Tilbury, 2001, R. nchisiensis Loveridge, 1953, R. platyceps Günther, 1893 and R. uluguruensis Tilbury & Emmrich, 1996, viz. two apical, diverging horn-like structures which are adorned with an interspecifically varying number and arrangement of papillae (Klaver and Böhme 1986; Tilbury 1992). Rampholeon marshalli Boulenger, 1906 takes a special position in that the horns in this species show a basal, asymmetrical, asulcally directed bifurcation (Klaver and Böhme 1986: Fig. 16b). These observations fit well with the new molecular analysis by Matthee et al. (2004) who split Rhampholeon into two genera, viz. Rieppeleon and Rhampholeon, while they subdivide the latter into the three subgenera Rhampholeon, Bicuspis und Rhinodigitum. Here, Rieppeleon (which branches off first) corresponds with the hemipenially plesiomorphic kerstenii/brevicaudatus group, followed by Bicuspis with the autapomorphic bifurcated apical horn and by Rhinodigitum with the diverging papillose apical horns (Fig. 3).
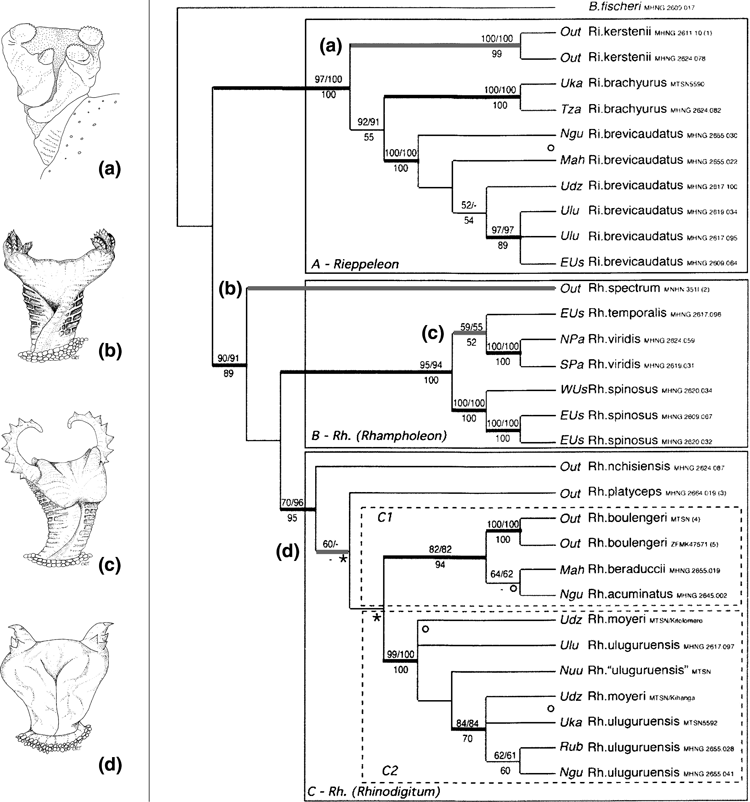
Molecular tree after Mariaux and Tilbury (2006). Parsimony analysis, strict consensus of four shortest trees (numbers above branches are bootstrap values over 50% for heuristic parsimony searches, 1000 repeats, and below branches bootstrap of ML searches 100 repeats). Hemipenis of Rieppeleon kerstenii (Peters, 1868) by courtesy of Roman Kernchen; hemipenis drawings of Rhampholeon (Rh.) spectrum (Buchholz, 1874), of Rhampholeon (Rh.) temporalis (Matschie, 1892) (for the temporalis group) and of Rhampholeon (Rhinodigitum) acuminatusMariaux and Tilbury, 2006 (for the platyceps group) after Mariaux and Tilbury (2006)
Anguimorpha: Varanidae
Varanid lizards include dwarf and giant species, among the latter are the biggest lizards in the world. Traditionally, they are all placed in one single genus Varanus which sheds some light on their morphological uniformity which is determined by their lifestyle as active foragers (Böhme 1988). In his reviews of the Varanidae, Mertens (1942, 1959) divided the species known at that time into nine subgenera, of which the following were monotypic: Psammosaurus, Polydaedalus, Dendrovaranus, Indovaranus, Tectovaranus and Philippinosaurus. According to Mertens (1942), the subgenus Empagusia comprised only two species whereas his typical subgenus Varanus and the subgenus Odatria were relatively species-rich. In 1962, Mertens added a seventh monotypic subgenus Papusaurus. The concept on which these infrageneric taxa were based was typological, i.e. the characters defining them were adaptive morphological structures linked with habitat and life habits (e.g. compressed versus round tails, position of nostrils etc., head and skull shape, dentition) (Böhme 1988). Subsequent studies using karyological (King and King 1975), electrophoretic (Holmes et al. 1975), osteological (Estes et al. 1988) and cytogenetic approaches (King 1990) reached different conclusions, as did the microcomplement fixation analysis of serum albumines by Baverstock et al. (1993). A first hemipenial approach was made by Branch (1982), followed by more detailed reviews by Böhme (1988) and Ziegler and Böhme (1997) which resulted in a first genital morphologically-based phylogenetic hypothesis. Card and Kluge (1995) concentrated on the hemipenial skeleton alone to construct a phylogenetic hypothesis; however, the X-ray method applied turned out to be insufficient to analyse the internal supportive structures (Ziegler and Böhme 1997; Ziegler et al. 2007).
All these studies provided evidence that conflicted with Mertens’ typological concept. For instance, his typologically uniform subgenus Varanus turned out to be very heterogenous whereas some of the monotypic subgenera recognized by Mertens (1942, 1959, 1962) proved to be homogenous to each other with regard to their karyotypes, immunogenetics and hemipenial structure. Therefore, Böhme (1988) considered the subgeneric rank of Dendrovaranus, Indovaranus and Tectovaranus as overestimated. He recognized only Empagusia, Odatria, Papusaurus, Philippinosaurus, Polydaedalus, Psammosaurus and Varanus; however, in some cases with different species compositions (see also Ziegler and Böhme 1997). Because this was overlooked by Ast (2001), we will discuss the details below.
As already mentioned, not only the works by Holmes et al. (1975), King and King (1975), Becker et al. (1989), King (1990) and Baverstock et al. (1993) but also our hemipenial findings clearly demonstrated that the subgenus Varanus sensu Mertens (1942) is a paraphyletic assemblage of morphologically similar species. It still accommodated V. indicus (Daudin, 1802) together with V. komodoensis Ouwens, 1912, the large Australian species, such as V. gouldii (Gray, 1838) or V. varius (Shaw, 1790), and with V. salvator (Laurenti, 1768) and V. salvadorii (Peters & Doria, 1878), which later proved to belong to distinct evolutionary lineages (see below). The sister taxon of the V. indicus (Daudin, 1802) group, however, viz. the V. prasinus (Schlegel, 1839) group, was placed by Mertens (1942) together with the Australian dwarf monitors of the subgenus Odatria based on the round cross section of the tail. In turn, the semi-aquatic Australian dwarf monitor species V. mitchelliMertens, 1958 was placed into the typical subgenus Varanus (Mertens, 1958) because it has, because of its adaptation, a laterally compressed tail. These examples may suffice to demonstrate the Mertensian typological approach however it must be taken in mind that his work was pre-Hennigian.
Our studies of hemipenial morphology provided results that were in strong conflict with Mertens (1942, 1959) concept. The most unorthodox finding was that the mangrove-dweller V. indicus (Daudin, 1802) (and its close relatives) was closely linked to the arboreal, prehensile-tailed V. prasinus (Schlegel, 1839) (and its close relatives) by two unique hemipenial synapomorphies, viz. (1) a highly asymmetrical sperm groove which does not end medially between the apical lobes but stretches to one of them only and (2) a strong left/right asymmetry of the shape of their hemibacula (contra Card and Kluge 1995). We therefore regarded both species groups as collectively forming a monophyletic group and placed them in a joint subgenus for which the name Euprepiosaurus was available (Böhme 1988; Ziegler and Böhme 1997) (Fig. 4). The latter reference was able to demonstrate that even the miniaturized hemiclitores showed just the same picture. The monophyly of this Euprepiosaurus clade was clearly supported by several subsequently published molecular phylogenies (Ast 2001; Fitch et al. 2006; Ziegler et al. 2007; see Fig. 5).
Synapomorphic genital morphology (i.e. the asymmetrical sulcus spermaticus) reveals the mangrove-dweller Varanus indicus (Daudin, 1802) (V. indicus species group: above) to be sister to the arboreal, prehensile tailed V. prasinus (Schlegel, 1839) (V. prasinus species group: below); figures after Ziegler and Böhme (1997) and from Dryden and Ziegler (2004)
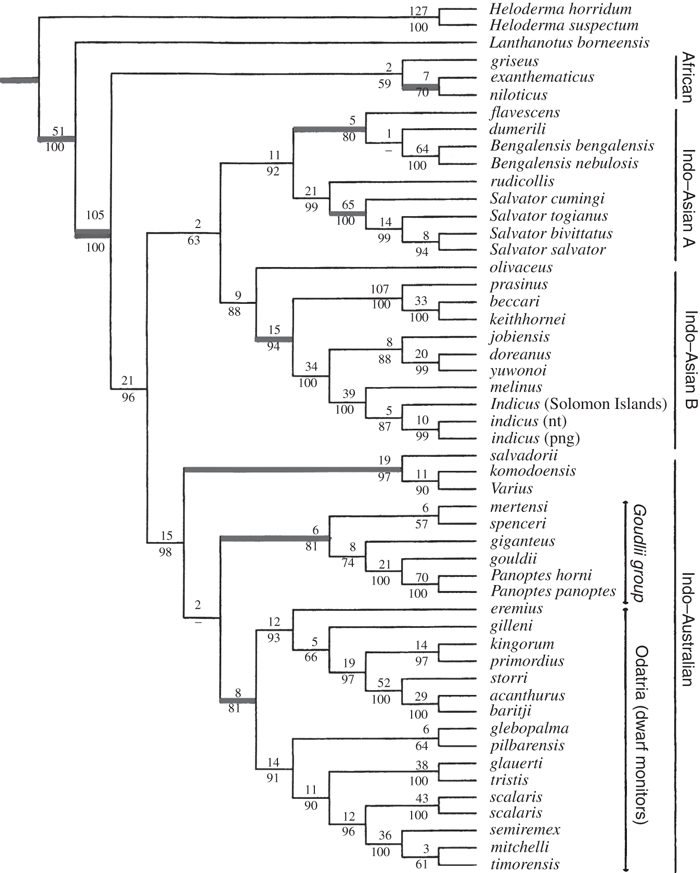
Ast’s (2001) most parsimonious hypothesis based on simultaneous analysis of 1474 informative characters (length = 11 274, CI = 0.246, RI = 0.487), with species ranges noted (NT, Northern Territory (Australia); PNG, Papua New Guinea), without outgroup taxa. Nodes which were supported by our genital morphological findings (Böhme 1988; Ziegler and Böhme 1997) were marked with bold lines
Another unexpected result was that V. salvator (Laurenti, 1768), phenetically very similar to V. komodoensis (Ouwens, 1912), differed from this species and also from all large Australian monitor lizards by a strong asymmetry of its copulatory organ: The hemibacula were strongly asymmetrically structured and the paryphasmata were not continuous around the organ, but separated into two asymmetrical halves. As a result of this unique apomorphic character state, which is shared with taxa in the Philippine-Sulawesi realm and considered today in part as distinct species within the salvator group (Koch et al. 2007), a separate clade was suggested (Böhme 1988) and a separate subgenus Soterosaurus subsequently was erected for this clade (Ziegler and Böhme 1997). Genetically, the V. salvator (Laurenti, 1768) group proved in fact to be very distant from the V. komodoensis (Ouwens, 1912), V. varius (Shaw, 1790) and V. salvadorii (Peters & Doria, 1878) clade, and forms in fact a monophyletic group (Ast 2001) but in Ast’s cladogram, V. rudicollis (Gray, 1845) appears as its sister taxon. Because V. rudicollis (Gray, 1845) has a more primitive, symmetrical hemipenis, this species was considered to be closer to Empagusia (sensu Böhme 1988), i.e. to V. bengalensis (Daudin, 1802) (including the taxon nebulosus, see Böhme and Ziegler 1997), V. dumerili (Schlegel, 1839) and V. flavescens (Hardwicke & Gray, 1827) which share derived hemipenial features (Ziegler and Böhme 1997) and do form a monophyletic clade also in Ast’s (2001) analysis. The remaining monotypic Mertensian (1942) subgenera Dendrovaranus, Indovaranus and Tectovaranus, synonymized with Empagusia by Böhme (1988), might become partly valid names again, should the genus Varanus be partitioned into several distinct genera.
As for Empagusia sensu Mertens (1942), the placement of the African savannah monitor V. exanthematicus (Bosc, 1792) together with the phenetically superficially similar Indian V. flavescens (Hardwicke & Gray, 1827) was easily falsified by their hemipenial characters. V. exanthematicus (Bosc, 1792) shares apomorphic character states in the hemibacular structure with V. niloticus (Linnaeus, 1766), formerly (Mertens 1942) the sole member of the monotypic subgenus Polydaedalus, and was therefore grouped with the latter by Böhme (1988). Their monophyly has also been confirmed by Ast’s (2001) analysis. Polydaedalus comprises the species V. albigularis (Daudin, 1802), V. exanthematicus (Bosc, 1792), V. niloticus (Linnaeus, 1766) and V. yemenensis Böhme, Joger & Schätti, 1989 (Ziegler and Böhme 1997; Böhme 2003). Sister taxon to the Polydaedalus clade is according to Ast (2001) the monotypic subgenus Psammosaurus [V. griseus (Daudin, 1803) group] which contains the desert monitors of the Saharo-Sindian realm and represents a distinct lineage according to the various character sets used by Holmes et al. (1975), King and King (1975), Becker et al. (1989), King (1990), Sprackland (1991a,b), Baverstock et al. (1993), Card and Kluge (1995) and – finally – also by our hemipenial studies (Böhme 1988; Ziegler and Böhme 1997).
From a genital morphological point of view, separate subgeneric status (Philippinosaurus, see Mertens 1959) for the uniquely frugivorous V. olivaceus Hallowell, 1856 (and its recently discovered, likewise frugivorous sister species V. mabitang Gaulke & Curio, 2001) seems justified (Ziegler and Böhme 1997; Ziegler et al. 2005) and has also been demonstrated genetically by Ast (2001).
In Australia’s monitor lizard fauna, the subgenus Odatria contains the small-bodied species. Its monophyly can be supported by their fully mineralized hemibacula which are basically round (rather than plate-like) in cross section (Ziegler and Böhme 1997). The only species (or species complex) with terminally flattened, triangular hemibacula, which are serrated on their inner margins, is V. acanthurus Boulenger, 1885 (Branch 1982; Böhme 1988; Ziegler and Böhme 1997). According to Ast (2001) and Fitch et al. (2006), Odatria is indeed monophyletic, V. acanthurus Boulenger, 1885 and V. baritji King & Horner, 1987 being nested in one of two clades including also V. eremius Lucas & Frost, 1895, V. gilleni Lucas & Frost, 1895, V. kingorum Storr, 1980 and V. storriMertens 1966.
Varanus komodoensis and the large Australian species exhibit rather plesiomorphic hemipenes (Branch 1982; Böhme 1988; Ziegler and Böhme 1997) but are linked by synapomorphic hemibacula. This is partly supported by the findings of Ast (2001) but it is remarkable that in the phylogenies by her and also by Fuller et al. (1998), V. komodoensis (Ouwens, 1912) and V. varius (Shaw, 1790) (type species of the genus Varanus) are separated from the V. giganteus (Gray, 1845)/gouldii (Gray, 1838) group (for which the subgeneric name Pantherosaurus is available; see Böhme 2003). The sister taxon of the two former species is according to Ast (2001) and to Fitch et al. (2006)V. salvadorii (Peters & Doria, 1878) from New Guinea. This giant species, placed by Mertens (1962) in a monotypic subgenus Papusaurus, gets only weak support from its hemipenial characters (Ziegler and Böhme 1997), and Ast (2001) suggests reconsidering the use of this supraspecific taxon.
In the above paragraphs, we dealt mostly with the nodes of species groups (including several monotypic ones) rather than of single species. However, also within groups of closely related species, hemipenial morphology can contribute valuable data and taxonomic insights which are in a broad consensus with new molecular data. It is beyond the scope of this review to discuss all existing instances here (see e.g. Ziegler et al. 2007 for hemipenial and molecular relationships of species within the V. indicus (Daudin, 1802) and V. prasinus (Schlegel, 1839) groups), but we want to refer, as an example, to the recent paper by Aplin et al. (2006) who state: ‘Ziegler and Böhme (1997) considered hemipeneal similarities between V. caudolineatus Boulenger, 1885 and V. gilleni Lucas & Frost, 1895 to indicate a close relationship between these species. Their view is vindicated by our molecular and extended morphological studies’.
When viewed from genital morphology, the more than 60 species of monitor lizards currently known (Böhme 2003; Böhme and Ziegler 2005; Eidenmüller and Wicker 2005; Aplin et al. 2006; Ziegler et al. 2007) can be classified in the nine subgenera Varanus, Empagusia, Euprepiosaurus, Odatria, Papusaurus, Philippinosaurus, Polydaedalus, Psammosaurus and Soterosaurus (Böhme 1988; Ziegler and Böhme 1997). The respective genital structures discussed above largely agree, in contrast to the morphological concept by Mertens (1942, 1959, 1962), with the molecular approaches by Fuller et al. (1998), Ast (2001) and Fitch et al. (2006).
Differences include the placement of V. indicus (Daudin, 1802) by Fuller et al. (1998) outside the Indo-Asian B group by Ast (2001) and the placement of V. acanthurus Boulenger, 1885 distinct from other pygmy monitor lizards by Fuller et al. (1998) and instead as sister clade to the V. gouldii (Gray, 1838) group. However, Fitch et al. (2006) replaced V. acanthurus Boulenger, 1885 with the remaining pygmy monitors and V. indicus (Daudin, 1802) as the sister taxon to the V. prasinus (Schlegel, 1839) group, thus corroborating the results by Ast (2001).
However, before the phylogeny of varanid lizards will be fully understood, further studies (using additional genes including nuclear ones) on additional species are necessary. A good example is V. spinulosusMertens, 1941 from the Solomon Islands. It had been described as a subspecies of V. indicus (Daudin, 1802) (Mertens 1941), later elevated to species rank (Sprackland 1994) and stayed in Euprepiosaurus for convenience only, as neither hemipenial nor genetic data were available. Recently, when a specimen with everted hemipenis became available, V. spinulosusMertens, 1941 had to be removed from Euprepiosaurus because it lacked the unique synapomorphies of this clade, but there was no evidence to assign it to any other clade (Böhme and Ziegler 2007). Future studies have to show whether V. spinulosusMertens, 1941, currently incertae sedis, will be assignable to any of the subgenera or whether it will even represent an own distinct lineage meriting subgeneric rank.
Additional comments on anguimorph lizards: lanthanotids, helodermatids, xenosauroids and anguids
Lanthanotidae and Helodermatidae
Also the remaining two varanoid families are diagnosable by means of genital morphological characters. Based on plesiomorphic character states such as a simple, unforked sulcus spermaticus, an apically undivided retractor muscle and the lack of hemibacula (or hemibaubella respectively) the two species that form the family Helodermatidae have a basal position within the Varanoidea. Their main apomorphy is here the asymmetrical truncal cover with paryphasmata with a separate paryphasma field at the basis of the pedicel (Ziegler and Böhme 1997). Lanthonotus borneensis Steindachner, 1878, in contrast, as the sole representative of the Lanthanotidae, shares a derived, forked retractor muscle with the Varanidae but has no hemibacula at the two terminal ends (contra Branch 1982). An autapomorphy of this species is a longitudinal tissue well separating the paryphasmata into two separate areas. The Varanidae, at last, are clearly defined by their possession of hemibacula and hemibaubella, respectively, at the end of their forked retractor muscles. This hierarchical pattern fits the molecular phylogeny published by Ast (2001) in that she postulates the Varanidae and Lanthanotidae to be sister clades while the Helodermatidae form the sister group of both (see also Fuller et al. 1998) (Fig. 6).
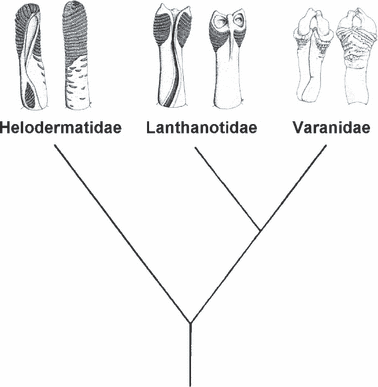
The general varanoid bauplan based on hemipenial characters is identical with Ast’s (2001) molecular topology; from left to right: hemipenis drawings of Heloderma horridum (Wiegmann, 1829), Lanthanotus borneensis Steindachner, 1878 and Varanus (Varanus) komodoensis (Ouwens, 1912) (after Böhme 1988 and Ziegler and Böhme 1997)
Xenosauridae and Shinisauridae
Because of the classic monograph on anguimorph lizards by McDowell and Bogert (1954), the famous Chinese crocodile lizard Shinisaurus crocodilurusAhl, 1930 was believed to be the closest relative of the morphologically similar Mexican Xenosaurus species. Both were placed in a joint family Xenosauridae, a concept widely accepted until the end of the 1990s. However, based on osteological evidence, Rieppel (1980) and Estes (1983) doubted the monophyly of this pair of genera, and Hu et al. (1984) added further osteological evidence for a distinct family Shinisauridae which they also regarded to have many more plesiomorphic character states than the Xenosauridae. This view was substantially supported by the study of hemipenis morphology (Fig. 7): In Shinisaurus, the organ is tripartite (pedicel, truncus, apex: a plesiomorphic character state), the truncus being covered by distinct, papillate paryphasmata, and the apex adorned with two long, diverging tips or ‘horns’ (Böhme 1988: Fig. 31; Zhang 1986: Fig. 1E; Ziegler et al. 2008). Xenosaurus, in contrast, has an apomorphic, smooth, spherical organ without any trace of paryphasmata but with denticulate sulcal lips and a likewise denticulate, downward-directed median apical process not observed in any other lizard (Böhme 1988: Fig. 32). It was concluded that both genera cannot be placed in one family. The molecular approach to this problem by Macey et al. (1999) clearly rejected xenosaurid monophyly, and the authors also recommended placing Shinisaurus in its own family Shinisauridae. This is not only congruent with the hemipenial data but also reflects the first tentative familial assignment of the discoverer (Ahl 1930) of the only extant species S. crocodilurus for which he had postulated a monotypic family.
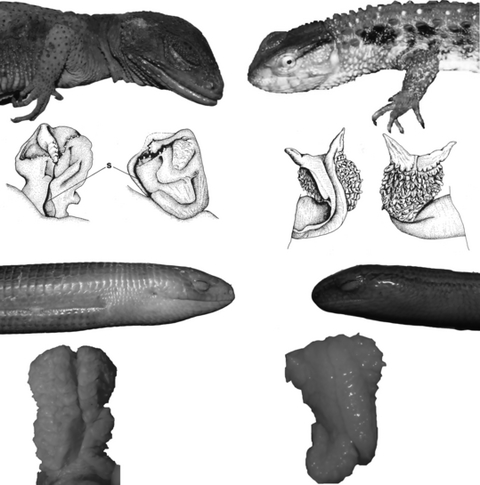
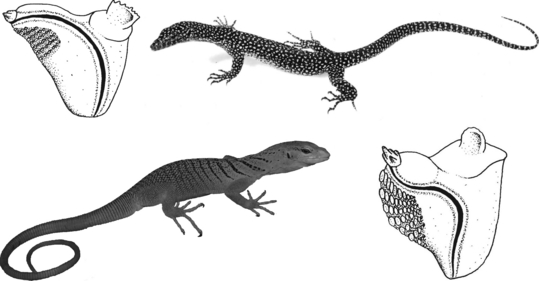
(above) Distinctly differing genital morphology characters reveal Xenosaurus grandis (Gray, 1856) (left) and Shinisaurus crocodilurusAhl, 1930 (right) to be members of different families as was recently confirmed by molecular findings (Macey et al. 1999); after Böhme (1988) and Ziegler et al. (2008); s = position of sulcus spermaticus. (below) Differences in the genital morphology (i.e. paryphasman ornamentation) of Pseudopus apodus (Pallas, 1775) (left) and Anguis fragilis (Linnaeus, 1758) (right) argue against lumping all anguines under one single genus Anguis (Macey et al. 1999)
Anguidae: Anguinae
Here, we restrict ourselves to the subfamily Anguinae which comprises the western Palearctic slow worms (Anguis) and the cosmopolitan glass lizards (Ophisaurus, Pseudopus), a group of large anguids with representatives in Europe, West Asia, North Africa, East and Southeast Asia and North America (Macey et al. 1999). Hemipenial data were available for Anguis fragilis (Linnaeus, 1758), the western Palearctic giant species Ophisaurus apodus (Pallas, 1775), the North African O. koellikeri and two North American species (O. attenuatus Cope 1880, O. compressus Cope, 1900).
Paraphyly of Ophisaurus was already suspected by Klembara (1979) and Estes (1983) and was substantiated by hemipenial data (Böhme 1988): The North American species (Ophisaurus s.str.) share the derived condition of an asymmetrical, smooth longitudinal rim parallel to their sperm groove; they differ, however, in the possession of spiny versus smooth paryphasmata making a close relationship or monophyly equivocal. The western Palearctic O. koellikeri (Moroccan endemic) and the SE European/West Asian giant species O. apodus (Pallas, 1775) agree largely in their hemipenial structure, but the former lacks supporting spines on the paraphysmata. These are moreover relatively larger and thus less numerous than in the latter. The slow worm [A. fragilis (Linnaeus, 1758)] has also a hemipenis which resembles that of O. apodus (Pallas, 1775); therefore, a monotypic subfamilial status for Anguis as suggested by Dowling and Duellman (1978) is not reflected by its genital morphology. The molecular analysis by Macey et al. (1999) reveals the Anguinae to be monophyletic and to contain the genera Anguis and Ophisaurus, but the genus Ophisaurus proved to be a paraphylum, the Old World species being closer to Anguis than to their North American congeners. The monophyly of the North American section of the genus (Ophisaurus s.str.) is statistically neither supported nor rejected. The Old World species O. koellikeri, O. apodus (Pallas, 1775) and A. fragilis (Linnaeus, 1758) form a monophyletic group with a first split of O. koellikeri at least 10 myr b.p., and a separation between of O. apodus (Pallas, 1775) and Anguis at about 9 myr. These results mirror the situation found in the hemipenial data, and a consequence would be to accomodate the former Moroccan Ophisaurus in an own genus Hyalosaurus, and the former O. apodus (Pallas, 1775) in the genus Pseudopus which is already widely used in current literature (e.g. Crochet and Dubois (2004). We do not agree with the alternative view proposed by Macey et al. (1999), viz. to lump all anguines under one single genus Anguis (Fig. 7). This would not only hide the deeply divergent diversity within this subfamily but would also seriously threaten nomenclatural stability.
Unfortunately, the East Asian species O. buettikoferi Lidth de Jeude, 1905, O. gracilis (Gray, 1845), O. sokolovi Darevsky & Nguyen, 1983 and O. wegneriMertens, 1959 have been studied neither in terms of genital structures nor genetically. Only in the Chinese/Vietnamese O. harti Boulenger, 1899 a drawing of the hemipenis has been published (Zhang 1986), but no details are discernible from this figure (Böhme 1988).
Conclusions
When evaluating phylogenetic hypotheses for selected iguanian (chameleonids) and anguimorph lizard groups (varanoids, xenosauroids, anguids) based on genital morphology, with recent molecular phylogenetic approaches towards the same groups, it turns out that the former coincide much better with the latter than with earlier concepts based on external morphological characters. We suggest that the better agreement in regard to phylogenetic signals between genital structures and genetic data is due to the fact that squamate genital organs are ‘hidden’ in the tail root and thus not affected by environmental selective pressures, in contrast to ecologically dependent, for example peripheral structures of the animals: namely that convergence because of natural selection is less likely to arise in genital morphology.
The infraspecific constancy and interspecific evolutionary diversification of genital structures – not only in squamates – is driven by sexual selection. Here, lizard groups with mating systems of dominant, territorial males using visible sexually dimorphic cues (iguanid, agamid lizards and many chameleons: for the latter see also Stuart-Fox and Moussalli 2008) have less diversified genital structures than groups with weakly expressed sexual dimorphism and territoriality, as it is the case in anguimorph lizards. Arnquist (1998) studied the mating system of several holometabolous insects (orders Ephemeroptera, Lepidoptera, Diptera, Coleoptera) and was able to demonstrate that in insects genital diversification depends on the mating system: if females copulate multiply with several males, the rate of genital diversification is twice as high in the respective group as compared with females that mate only once. For squamates, few data are yet available, but for monitor lizards it has been proven that at least some species belong to Arnquist’s group I [e.g. V. varius (Shaw, 1790): Carter 1991; V. exanthematicus (Bosc, 1792): Ziegler and Böhme 1997; V. glauerti Mertens, 1957, V. tristis (Schlegel, 1839): Sweet 1999, 2007) while the specific, typical pregnancy colouration of most iguanian lizards typifies them as belonging to Arnquist’s group II: many agamid females including those that show pregnancy colouration live in harem groups under one dominant male, and many chameleons (which are generally solitary) have their females with pregnancy colouration guarded by the males – two strategies effectively reducing genetic input from multiple mating and – consequently – multiple paternity. The correlation with more diversified genital structures in the group I (Anguimorpha) and with less diverse genital structures in group II is obvious, but leaves a lot of work to test Arnquist’s hypothesis in a vertebrate group which seems particularly suitable for such a test, viz. the squamate reptiles.
Acknowledgements
Professor Dr Aaron Bauer (Department of Biology, Villanova University), Professor Dr Samuel Sweet (Department of Ecology, Evolution and Marine Biology, University of California) and Dr Jakob Hallermann (Zoologisches Institut und Zoologisches Museum, Universität Hamburg) provided helpful comments on the manuscript. Furthermore, we are indebted to Philipp Wagner (Zoologisches Forschungsmuseum Alexander Koenig, Bonn) for his support with the composing of the figures.




