Geography of morphological differentiation in Asellus aquaticus (Crustacea: Isopoda: Asellidae)
Geographisches Verbreitungsmuster der morphologischen Differenzierung beiAsellus aquaticus(Crustacea: Isopoda: Asellidae)
Abstract
enWe implemented a detailed morphometry and multivariate statistics to establish a general, large-scale racial differentiation in Asellus aquaticus (L.) sensu Racovitza. We ascertained that in surface populations a set of 11 morphometric characters might equivalently be represented by the pleopod respiratory area size alone. The analyses resulted in a distinct distribution pattern, with the large respiratory area populations disposed mainly along the Dinaric karst between southern Slovenia and western Macedonia and surrounded by the medium respiratory area morph, spatially irregularly substituted by the small area morph. This pattern is in contradiction with the distribution pattern of molecularly defined clades (as shown by Verovnik et al. 2005). We could find no ecological, hydrographical or paleogeographical explanations for such distribution pattern either. The only hypothetical explanation would be a preservation of the large respiratory area as a plesiomorphic character in the comparatively sheltered karst habitats, while throughout the easier accessible parts of the species range it was replaced by the ‘modern’ smaller area size. While a diminution of the respiratory area functionally means an increased sclerotization – hardening of pleopod IV–V exopodites, endopodites of pleopods III–V remain less sclerotized, probably respiratory and osmoregulatory functional.
Zusammenfassung
deDie globale Rassendifferenzierung von Asellus aquaticus (L.) sensu Racovitza wurde anhand eingehender Morphometrie und multivariater Statistik untersucht. Es stellte sich heraus, dass der gesamte Satz von 11 morphometrischen Merkmalen allein durch das Merkmal ‘Flächengröße der Pleopoden-Respirationsfläche’ ersetzt werden kann. Die Analysen ergaben ein deutliches Muster, in dem Populationen mit großen Respirationsflächen überwiegend im Dinarischen Karst zwischen Süd-Slowenien und West-Makedonien verbreitet sind, von Morphen mit mittelgroßen Respirationsflächen umgeben werden, welche wiederum räumlich zerstreut von Morphen mit kleinen Respirationsflächen ersetzt werden. Dieses Muster widerspricht der Verbreitung von molekular-systematisch ermittelten Gruppen (Verovnik et al. 2005). Wir konnten keine ökologische, hydrographische oder paläogeographische Erklärung dafür finden. Die einzige hypothetische Erklärung könnte eine Erhaltung der großen Respirationsflächen als eines plesiomorphen Merkmals in vergleichsweise isolierten Karstgebieten sein, während sie in leichter besiedelbaren Gebieten von den ‘modernen’ kleineren Respirationsflächen ersetzt wurden. Es muss betont werden, dass eine Verkleinerung der Respirations-Area mit der Sklerotisierung der Exopoditen an den Pleopoden IV-V verbunden ist, während die Endopoditen der Pleopoden III-V ihre geringe Sklerotisierung beibehalten und somit wahrscheinlich atmungs- und osmoregulatorisch aktiv bleiben.
Introduction
The range of Asellus aquaticus (Linné, 1758) sensu Racovitza 1919, reaches over almost all Europe except for Pyrenean Peninsula and some smaller Mediterranean areas (Birštejn 1951; Williams 1962; Argano 1979; Henry and Magniez 1983, 1995a,b; Sket 1994a; Henry et al. 1996). As A. aquaticus is an extremely eurytopic species, it inhabits all types of fresh to slightly brackish surface waters, as well as some types of subterranean fresh waters (Henry et al. 1996; Prevorčnik et al. 1998, Prevorc✓nik et al. 2004). We may find it in brackish water with salinity up to 15 ppt (Sket 1965; Pesce and Maggi 1983; Koehn and Gosselck 1989). It may even withstand high degree of the organic pollution, being an indicator and a successful member of the ‘α-mesosaprobic’ community (Liebmann 1962).
Some attempts at presenting racial differentiation in the species across a large area have been made (e.g. Karaman 1952; Sket 1965), unfortunately not considering a sufficient number of samples, specimens and characters. Sket (1994a) summarized the taxonomy of the species. In addition to the list of 10 subspecies presumably representing the whole species’ range, he indicated that an extensive racial diversification seemed to be present especially in the S and SE parts of the range. His indications were confirmed after the primary patterns of racial differentiation in A. aquaticus were assessed. Prevorčnik et al. (2004) carried out extensive multivariate statistical analyses of geographic variation in 59 morphometric characters. A higher number of analysed specimens and samples provided the necessary insight into the variation within, as well as among samples. The pattern of geographic variation was distinctly clinal in samples comprising pigmented and eyed specimens; while samples from Slovenia (20) were arranged almost along the whole cline on the plot of the discriminant function analysis (DFA) scores (Prevorčnik et al. 2004; Fig. 1, p. 198) samples from other countries (18) were aggregated only in the smaller section of the cline. By the shape and size of the pleopod V respiratory area (RA, i.e. non-sclerotized part of the exopodite, limited by linea areae) three groups of samples were distinguished within the cline: the ‘type’, the ‘intermediate’ and the ‘Dinaric’ groups of samples. The authors argued that the roughly concentric distribution ranges of these groups could hardly be accidental. But while the explicit pattern of geographic distribution due to the great number of samples was easily recognizable in Slovenia, the pattern in the Dinaric region SE of Slovenia remained unknown. Two samples from Croatia and Macedonia used in the analysis, together with the high overall diversity known in other species, indicated the possibility of an extensive diversification in A. aquaticus also in the mentioned region.
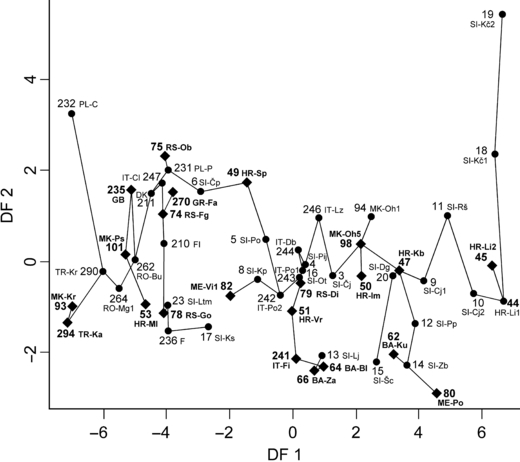
Minimum spanning tree based on Mahalanobis’√D2 distances, superimposed on the plot of 55 Asellus aquaticus sample centroids against first two discriminant functions (DF 1, DF 2), using 11 morphometric characters. Sample symbols are listed in Appendix S1. Dots (•) indicate 33 samples from previous studies (Prevorčnik et al. 2004); diamonds (♦) indicate 22 presently analysed samples
In the present study, we extend the diversification analysis of this ecologically important species to most of its European distribution area. As the material from as many as 24 countries is analysed (the Dinaric area of the former Yugoslavia remaining the most thoroughly sampled) we examine whether either a set of characters or even a single character are the best descriptors of a racial diversification among the surface populations. We also discuss the accordance between morphological variability and the anticipated phylogeographic situation (Verovnik et al. 2005) taking into consideration not only the much smaller number of samples used in the latter study, but also that samples from different sampling sites were mainly used in both studies.
Materials and Methods
Only males of A. aquaticus from 183 samples (Appendix S1, S8–S9, Fig. 2) were used in the analyses of the patterns of geographic distribution. Twenty-two samples mainly from the Dinaric region (termed ‘presently analysed samples’ further in the text) were included in the multivariate statistical analyses together with 38 samples from Prevorc✓nik et al. (2004; termed ‘samples from previous studies’ further in the text). Males from the additional 123 samples were included only in the analysis of size and shape of the pleopod respiratory area (RA). The detailed information on preparation of specimens is provided in the Appendix S2.
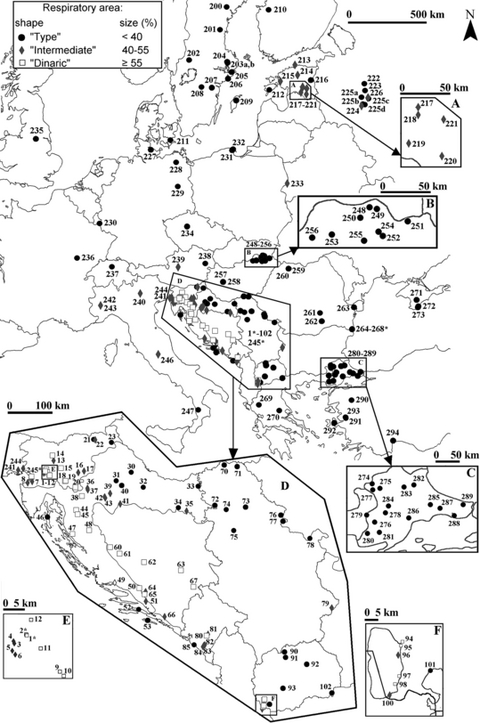
Concentric pattern of geographic variation in A. aquaticus according to the shape and size of pleopod V respiratory area (RA). Populations of the ‘large’ area (‘Dinaric’) group denoted by squares encircled by populations with the ‘medium’ and ‘small’ RA (‘intermediate’ and ‘type’) groups denoted by diamonds and circles respectively. Numbers identify samples as in Appendix S1
Statistical data analysis
In order to fill in the blank parts on the map of geographic variation in A. aquaticus, we used the same multivariate statistical methods as Prevorc✓nik et al. (2004; previous studies further in the text). Each sample of A. aquaticus was treated as an operational taxonomic unit. In order to diminish the time needed for the measurements simultaneously enabling the increase in number of the analysed samples, we first tried to provide an adequately reduced set of morphometric characters. The detailed information on all statistical methods used in the present study is provided in the Appendix S2 (Statistical data analysis).
Analysis of size and shape of the respiratory area on pleopod V
In previous studies, the size of the pleopod V exopodite respiratory area (PL5AS) turned out to be the main factor allowing discrimination of samples. Because of its major influence on the racial polymorphism in A. aquaticus, reflecting geography, we have anticipated that the analysis of respiratory areas may represent an adequate step towards a further elucidation of the geographic distribution of variation in the species. Therefore, in addition to males from 22 samples included in DFAs, five (mostly) adult males from 123 samples (Appendix S1) were prepared for the analysis of the size and shape of the respiratory area on pleopod V (Appendix S4) alone. The detailed information on methods of preparation and measuring as well as discussion about the results of previous studies is provided in the Appendix S2 (analysis of size and shape of the respiratory area on pleopod V).
Results
Statistically significant geographic variation (p < 0.001) was demonstrated in 11 chosen morphometric characters (Appendix S3). There was no information redundancy due to highly correlated characters; no character represented a pair or group of characters where |r| > 0.45.
In DFA 1 run on 22 non-troglomorphic (i.e. with pigmented body and/or eyes) presently analysed samples (Appendix S1), using 11 morphometric characters a continuous variation in character or frequency of character states along DF 1 is present (Appendix S5). The result allows no distinct grouping of samples along DF 1, along DF 2, however, sample from the Serbian Obedska bara (75 RS-Ob) is separated from all other samples. The first two axes (DF 1, DF 2) account for 69.2% of the total variance, DF 3 adding an additional 10.4%.
The contributions of the individual characters to the discrimination of the samples along the first three DFs, indicated by the discriminant function coefficients (DCs), are listed in Appendix S6. The rate of correctly classified specimens considering all 11 DFs, is 94.2%; six specimens from five samples are misclassified, while in the remaining 17 samples the observed classification equals the predicted. In an attempt to confirm the expected separation of the epigean from the hypogean samples also in the case of the presently analysed 22 samples, DFA 2 was run on the additional 38 samples from previous studies (Appendix S1), including five samples with depigmented specimens. The presently analysed samples are located within the group of the epigean samples from previous studies, distinctly separated from the hypogean ones.
The principal result of DFA 3 run on all 55 non-troglomorphic samples, the presently analysed and the ones from previous studies (Appendix S1) is as in DFA 1, a continuous disposition of samples along DF 1 (Fig. 1) with no evident transitions in the form of the sharp steps. But while the position of 33 samples remains consistent with their position in previous studies, the position of the presently analysed 22 samples changes slightly in comparison to their position in DFA 1. The first two axes (DF 1, DF 2) account for 71.6% of the total variance, DF 3 accounting for an additional 8.3%. Contributions of the characters to discrimination of the samples along the first three DFs (their DCs) are listed in Appendix S7.
Considering all 11 DFs, 77% of specimens are correctly classified; 12 specimens from eight presently analysed samples and 166 specimens from 29 samples from previous studies are misclassified, while in the remaining samples the observed classification equals the predicted.
Analysis of size and shape of the pleopod respiratory area (RA)
Simultaneous use of nine RA size intervals together with three RA shape intervals on the map of geographic variation in A. aquaticus (Appendix S8–S9) shows a fairly well correspondence between shapes and sizes of the male pleopod V respiratory areas. The only exception is the Estonian sample from the pond on the eastern shore of Lake Võrtsjärv (217) where the large RA size is recorded in pleopods of indubitably ‘intermediate’ RA shape. In addition, the use of nine size intervals supports our previous choice of only three size intervals while the concentric pattern of geographic variation (Fig. 2) is not existent at a lower scale, i.e. within three main shape/size groups.
In the Dinaric region, data obtained from the analysis of 145 samples (22 used in present DFAs and 123 used only for the respiratory area analysis, Appendix S1) supporting information previously observed concentric pattern of geographic variation. Outside the Dinaric region, however, some changes in the mentioned pattern may be observed.
Populations with a small respiratory area, i.e. the ‘type’ group of samples, inhabit most of the species range. The mentioned samples encircle the Dinarides at the edge of the Pannonian basin from the north, in Serbia and Macedonia from the east, in Greece from the south and in the Adriatic isles (and partly in its coast) and south Italy from the west (Fig. 2, Appendix S1, S8–S9). Surface populations with an extremely small size respiratory area (PL5AS <27%) are found in Romanian Mangalia (265), in Swedish Mörkö (206), Arkö (207) and island Gotland (209), in Turkish Kazikli (294) in Anatolia and in lake Dojran in Macedonia (102), as well as in a Polish brackish peat-bog ponds (232). The only hypogean A. aquaticus population with the small respiratory area is A. a. infernusTurk-Prevorc✓nik and Blejec, 1998, in the Romanian Dobrogea (267*, 268*).
The range of populations with a large respiratory area, i.e. the ‘Dinaric’ group of samples is bound to a considerable area along the Dinaric karst. The present study supplemented the previously outlined range by adding 15 samples from Slovenia, Croatia, Bosnia and Herzegovina, Montenegro and the Ohrid area in Macedonia. The gap between Montenegro and Macedonia is due to the total lack of samples from the intermediate Albania. One has to note that also all Dinaric hypogean populations share the mentioned type of the respiratory area.
Between the belt of populations with the large and the surrounding populations with the small size respiratory area, a narrow belt of populations with an intermediate size respiratory area is inserted within the Dinaric region. Outside the Dinarides, however, the ‘medium area’ type is spread in North and Central Italy, Estonia and close by in western Russia, whilst in the scarcely sampled Central Europe (Central Austria, West and Northeast Germany, East Poland) the ‘medium’ respiratory area samples are isolated.
Considering the size of the respiratory area seven out of 145 presently analysed samples include one outlier. All mentioned outliers, however, fit into their sample group according to the shape of their area. The assigned types of the respiratory areas for the outliers are listed in Appendix S1 (under G/O).
In three presently analysed locations contacts of two different types of pleopod respiratory area sizes and shapes may be observed; along the shores of Lake Ohrid in Macedonia (the co-existence of the ‘medium’ and the ‘large’ area types), as well as in the Slovenian Drava River and the Russian Tver region (the co-existence of the ‘small’ and the ‘medium’ respiratory area types).
Discussion
Prevorčnik et al. (2004) demonstrated the significant geographic variation in 58 out of 59 morphometric characters used in the studies of racial differentiation in adult A. aquaticus males. Using multivariate discriminant analysis, they managed to recognize seven characters sufficient for the equivalent separation of samples comprising completely depigmented animals from those samples with pigmented ones. As the actual analysis ran exclusively on the morphologically much more homogeneous samples of pigmented animals, a somewhat enlarged set of required characters (11) is explicable.
Discordances in the position of A. aquaticus samples can be observed in DFA 1 in comparison to DFA 3; main displacements are present in the Serbian sample from Obedska bara (75), as well as samples from the Croatian Karlobag and Imotski (47, 50) and the Macedonian Ohrid lake (98). Obviously the lack of samples (as in DFA 1), especially from the anticipated area of the greatest racial differentiation, may result in at least inadequate if not completely false conclusions.
Our study confirmed the previously observed main contribution of PL5AS to the discrimination of samples. A strong connection between the results of DFAs and the analysis of the pleopod respiratory area shape/size is a logic consequence of the fact that PL5AS is the sole character ‘responsible’ for discrimination of samples along DF1, accounting for the majority of the total variance. The contribution of all other characters is almost negligible. Therefore, we believe that the results of both mentioned analyses may be treated as comparable and consequently, that the analysis of RA size/shape is adequate for the present and all further morphological studies of geographic variation in A. aquaticus.
Our supplemented map of the pattern of the geographic distribution of the three respiratory area size (RA) groups of samples shows that in spite of the existence of a small intra-population variation, the geographic dependence of the character remains well expressed in the Dinaric region, as shown by Prevorčnik et al. (2004). A range of the ‘large’ RA group along the mentioned region up to Macedonia forms a core of a distribution pattern, representing its most prominent feature. The ‘medium’ RA group is surrounding the core as a narrow and irregularly shaped belt, which is extending westwards along the Southern Calcareous Alps. The ‘small’ RA populations generally inhabit the rest of Europe, though some regions with ‘medium’ RA populations are present as well. Unfortunately, some parts of Europe remain scarcely sampled despite our sampling effort.
We see three possible explanations for such a conspicuous geographic pattern.
- 1
It could be hydrographically defined i.e. congruent with the actual drainage areas.
- 2
It could be historically defined in which case it should be congruent either with the distribution patterns of some other taxa, or with its own phylogenetic lineages.
- 3
It could be ecologically defined and also congruent with the distributions of any environmental parameters; the latter might act either directly through a phenotypic definition or indirectly, by positive selection of characters (causing convergent phenotypes).
Ad. 1) The distribution of RA morphs is definitely not hydrographically defined; samples of the ‘large’ RA group in Dinarides are shared by both the Adriatic and the Black Sea drainages, as well as a number of lower-order drainages within these (Sket 1970, Sket 2002). The ‘small’ RA morph is present nearly in all drainages including those inhabited by the ‘medium’ and the ‘large’ RA morphs.
Ad. 2) While the distribution of the ‘large’ RA morph is at least a bit congruent with the ‘holodinaric distribution pattern’sensuSket (1994b), it has almost nothing in common with the mended definition of the later (Sket and Zagmajster 2006).
As the holodinaric distribution pattern is exhibited by some troglobiotic species or genera, e.g. the clam Congeria kusceri Bole, 1962, the tubeworm Marifugia cavatica Absolon in Hrabe, 1930, the copepods Acanthocyclops troglophilus (Kiefer, 1932) and Troglodiaptomus sketi Petkovski, 1978 and the amphibian Proteus anguinus Laurenti, 1768, a low level of congruence with the eurytopic, eurythermal and expansive species as A. aquaticus may seem less noteworthy. Ranges of the former taxa and the ‘large’ RA morph are similar in their NW parts while in SE the latter is highly exceeding the holodinaric range.
Results of phylogeographic analysis of the colonization history of A. aquaticus (Verovnik et al. 2005) are in overall incongruent with morphological results. Unfortunately, the exact rate of the (in)congruence cannot be assessed due to incompatibility in the number of samples and sampling sites in both analyses. Nevertheless, one striking resemblance exists; samples from two morphologically defined groups (the ‘large’ and the ‘medium’ RA groups) (see Fig. 2, Appendix S9) inhabit the Slovenian Drava River, where also two out of six main A. aquaticus haplotype groups (the ‘Central European’ and the ‘Trans Alpine’ groups) overlap and, possibly, the oldest refugium and origin of dispersion is situated. Also some other conclusions of the mentioned authors seem to back our findings, e.g. about ‘Individuals all over Europe sharing the same 28S rRNA gene, while only populations from hydrographically isolated karst water systems having distinct 28S sequences’. The homology can, however, only be observed in the NW Dinaric Karst, where the ‘NW Dinaric Karst’ and the ‘Trebiciano cave’ haplotypes coincide with the ‘large’ area morph and the ‘Trans Alpine’ haplotype covers the range of the ‘medium’ area morph. According to the hypothesized colonization scheme (Verovnik et al. 2005) all main haplotype groups on the territory of the ‘large’ RA morph dispersed separately from the west Pannonian refugium in pre-Pleistocene, but only in the NW Dinaric karst was isolation strong enough to prevent homogenization of the rRNA gene family. Among recent phylogeographic studies of freshwater fauna, Bunje (2005) hypothesized the similar ancestral range (the Ponto-Pannonian region) and time of the dispersion for the freshwater snail Theodoxus fluviatilis (Linné, 1758). Unfortunately, total lack of samples from the area of former Yugoslavia does not allow a proper comparison of the analyses.
At the same time, we failed in finding any references on either phyllogenetic or morphological distribution patterns in other freshwater fauna corresponding to such characteristic distribution or differentiation pattern as it was found in Asellus.
Ad. 3) The spread of the RA size and shape genes (alleles) between the haplotypes without the accompanying reflection in the sequenced genes (the case of historically defined distribution pattern) is highly improbable. A complementary explanation would be a convergent evolution of the RA size. It is possible that the ‘large’ RA has an adaptive advantage in the karst area although we are not able to point out the ecological parameter responsible for it (see below). In an attempt to exclude or confirm the latter, we have first checked possible similarities between regions inhabited by the A. aquaticus of the ‘large’ and the ‘medium’ respiration areas.
Populations with the ‘medium’ RAs distributed within the Dinaric karst and the Italian Southern Calcareous Alps, as well as all populations with the ‘large’ RAs, seem to be highly limestone-dependent. The limestone-dependence of the ‘medium’ RA populations in the central and northeastern parts of Europe, however, is disputable.
The carbonate hardness of the water within the karst region inhabited by the ‘large’ RA morph may vary from 104 to 878 mg l−1 CaCO3 (Ivković et al. 1983), so we are dealing with ‘hard’, as well as with ‘soft’ waters in the limestone area.
We failed to find a correlation between the presence of some other chemical elements and ions (Ca, Fe, Mg, K, Na, Cl−, HCO3, SiO2) checked up in the Geochemical Atlas (Salminen 2007) and the distribution area of both morphs.
Also the recognized diversity of the habitats within the range of the ‘large’ RA morph is providing no proper ‘environmental’ explanation for its existence, as the diversity of the habitats outside the mentioned range is approximately the same.
Unfortunately, our set of samples is still too poor and the information about the nature of localities too incomplete to allow the statistical elaboration of the possible relations. In addition, no compilation or atlas of petrographical structure in European measures is available.
We reached possible, yet indirect explanation on the development of the ‘large’ areas during our findings in the distribution of the populations with the ‘small’ RAs. Our study showed that all troglomorphic populations share the ‘large’ RAs except for the hypogean populations from Rumania where water is ‘thermal sulfidic’ with 0.24‰ chlorinity (Sarbu 2000). Respiratory areas in these populations are extremely small (14–24% of exopodite surface), even smaller than in their epigean ancestors with quite small RAs (25–37% of exopodite surface). Populations with the ‘small’ RAs consistently inhabit also waters along the seacoasts, the RAs getting particularly small (c. 30% of exopodite surface or less) in waters with higher chlorinity (brackish peat-bog ponds in Poland with RAs 22–32% of exopodite surface). These observations led us to the supposition that RA size may somehow be linked to the osmoregulation in Asellus, the ‘small’ areas representing the mechanism for diminishing water efflux from the body fluids (Prevorčnik et al. 2004).
While many authors emphasized respiratory function of pleopods in isopods (Tschetwerikoff 1911, Remy 1925, Dejdar 1930, Franzl 1940), less is known about their function in the processes involved in salt and water balance. Hrabě (1949) first pointed out that the method of silver staining is not a reaction specific for the respiratory structure (Dejdar 1930, Franzl 1940) but the indirect indication of the importance of the structure in ion transport; it allows identifying areas with a high permeability to chloride ion. Several authors have used it since to indicate that isopod pleopods may be the site of ion transport (Babula 1979, Babula and Bielawski 1981, Bubel and Jones 1974, Holliday 1988). The indications were certified by the studies of the possible role of the branchial Na+/K+-ATPase in ion transport in the isopod crustacean Sphaeroma serratum (Fabricius, 1787) (Thuet et al. 1969, Philippot et al. 1972). The Na+/K+-ATPase activity in the gills (pleopods) of Sphaeroma proved highest in the endopodites of pleopods 4 and 5 and the acclimation in dilute media increased the enzyme activity in these appendages. Holliday (1988) reported similar results for the intertidal Idotea wosnesenskii Brandt, 1851 except that also the endopodites of pleopods 3 were active during osmoregulation.
Though no analysis of Na+/K+-ATPase activity exists in A. aquaticus, experiments on the reduction of silver nitrate (AgNO3), as well as potassium permanganate (KMnO4) have been carried out by Hrabě (1949). He discovered that the reduction abilities of both substances differ in Asellus; silver from AgNO3 is deposited not only on the endopodites of pleopods 3–5 (as observed by Franzl 1940) but also on the RAs of pleopods 4 and 5 and on the heart shaped area on the ventral side of the pleotelson, while KMnO4 is reduced on the endopodites of pleopods 3–5 exclusively. He assigned the observed differences to two unknown factors that may be components of the same or of two various vital processes.
C. Lagoutte Université de Dijon, unpublished results confirmed Hrabě’s findings but used also some vital staining techniques to investigate the respiratory function of pleopods in the epigean A. aquaticus, as well as in five hypogean species of Proasellus. She established that all structures quoted by Hrabě represent sites of ion exchange, as well as respiration, in all mentioned species. She also discovered the enlarged exopodite exchange surface in Proasellus species in comparison to Asellus, describing it as a possible compensation for the lower ventilation rate (slower pleopod beating) of the hypogean species.
In his study of structure of the respiratory organs in A. aquaticus, however, Babula (1979) reported about the ultrastructural details typical of crustacean branchial ion transport epithelia only in the endopodites of pleopods 3–5 while much thinner epithelia, probably involved in respiration, were found in the exopodites of the same appendages. Such topographic difference in ultrastructure between the anterior (exopodites) and posterior (endopodites) gills has also been observed in the crab Eriocheir sinensis H. Milne-Edwards, 1853 (Barra et al. 1983) and in the crayfish Astacus leptodactylus Eschscholtz, 1823 and Austropotamobius pallipes (Dalman, 1820) (Dunel-Erb et al., 1982).
In view of the staining experiments and results of the analyses in Na+/K+-ATPase activity in isopods (described above) the presence of the differentiated epithelium also within RAs would be expected. As the increase in the surface area available for ion transport has been observed in the intertidal isopod Idotea in comparison to the marine Sphaeroma, further enlargement (on account of the RAs and the heart shaped area) would be logical in the freshwater Asellus. As Babula (1979) examined just parts of the endo- and exopodites, a possible explanation for his report about the absence of the differentiated epithelium on exopods would be that he did not examine epithelium from the RA as well.
To confirm the proposed connection between the RA shape/size and its osmoregulation function, i.e. to confirm the existence of the environmental parameter phenotypically defining the area size, another histological analysis and possibly, also the analysis of Na+/K+-ATPase activity in A. aquaticus would be necessary. Simultaneously, water chemistry analyses at least in the area of the greatest diversification (in Slovenia) would be needed.
In addition to the mentioned parameter phenotypically defining the area size, the similarity of the ‘large’ RA populations might as well be explained as an evolutionary convergence caused by selection.
Though paedomorphies are very common in the cave fauna, Sket (1965) could not find such ontogenetic changes on A. aquaticus pleopods that could reveal a large respiratory area as a paedomorphy or as caused by changes in allometric growth.
The supposition that the ‘large’ area is a plesiomorphy, still characterizing the whole species when it had spread over the Europe and divided into three haplotypes, seems to be the most probable explanation of the described situation. The apomorphic and for some unknown reason more successful smaller RAs that occurred anywhere within the distribution area, could have driven the plesiomorphy out in most exposed ranges while the latter got protected in the hydrologically isolated, from the invasions comparatively well protected karst. One has to note that also in the related species, as much as this could be checked, the RAs are small: in A. hilgendorfii Bovallius, 1886 (own data), A. primoryensisHenry and Magniez 1993 (Henry and Magniez 1993) and A. monticola Birštejn, 1932 (Henry and Magniez 1996). Simultaneously, it has to be noted that a diminution of the RAs on the exopodites of pleopods IV and V does not necessarily mean a serious handicap for the respiratory and osmoregulatory function; the reduction of the pleopod V RA from c. 61% to 33% of the exopodite surface (i.e. from the ‘large’ to the ‘small’ RA type), accounts only for the diminution from c. 54% to 49% considering all less sclerotized surfaces (exopodites RAs and endopodites) on pleopods III–V. Finally, all data about the respiratory and/or the osmoregulatory function of the RAs (Tschetwerikoff 1911, Remy 1925, Dejdar 1930, Franzl 1940, Hrabě 1949, C. Lagoutte (Université de Dijon, unpublished results); Babula 1979) are of an indirect nature. So, we may only present the stated geographical situation as a fact, while any inferences about the reasons would be highly speculative.
Taxonomical implications
Since we do not know the nature of the morphs established here, we will abstain from further taxonomical evaluation. We are only forced to state the relations of the already named taxa. Asellus a. aquaticus, for which we propose the neotype population in the Linné’s Uppsala, Sweden (will be described in another paper), is morphologically definitely the ‘small’ RA morph. Similar are (according to the RA size) A. aquaticus fribergensis Schneider, 1887, A. a. bercziki Ponyi, 1956, A. arthrobranchialis Dudich, 1925, A. a. arthrobranchialis f. balcanicaKaraman 1952 and f. cresanaKaraman 1952; A. a. var. abyssalis Odenwall, 1927. The ‘large’ RA morph is correspondent to A. a. carsicusKaraman 1952 (=A. a. cavernicolus f. carsica). Within this pleopod morph some subspecies have been defined, some of them troglomorphic, differing in some other characters (Sket 1965, 1994a; Verovnik et al. 1996). We will not discuss their validity and feasibility here.
Conclusions
With use of uni- and multivariate statistics we managed to confirm high rate of morphological differentiation in A. aquaticus particularly in the western Balkans, including southern Slovenia in NW direction and western Macedonia in SE direction. The reduction of character number from 59 (Prevorčnik et al. 2004) to 11 did not influence the discriminatory quality of multivariate statistical analyses.
Pleopod V respiratory area size proved to be the most significant in discrimination of samples in Prevorčnik et al. (2004), as well as in present DAs, having the major influence on the racial polymorphism in A. aquaticus reflecting geography. Therefore, we found the mentioned character to be adequate for additional analyses of the geographical variability in this species.
The result of our analyses was the separation of groups of samples, which revealed a very distinct, ‘concentric’ geographic pattern (as in Prevorčnik et al. 2004). The range of A. aquaticus with the ‘large’ RA (pleopod V respiratory area) along the Dinaric karst (called the ‘Dinaric’ group) is surrounded by the range of the ‘medium’ RA (or the ‘intermediate’) group which is further irregularly replaced by the range of the ‘small’ RA (the ‘type’) group.
The distribution of the three RA morphs is largely discordant with respect to the phylogeographic patterns established by the molecular analyses. It is also discordant with a hydrographic and with any paleohydrographic situation. We could detect no actual ecological or geological parameters with a geographical distribution pattern, which could cause the actual morph distribution pattern either phenotypically, or support it by selection. An exception is the particular diminution of RA in the cases of elevated chlorinity. It remains a possibility that the ‘large’ RA is a plesiomorphy, once present in the whole species, which persisted only in comparatively sheltered habitats in karst ranges.
Footnotes
Acknowledgements
We thank T. Timm (Tartu, Estonia), K. Sindemark Kronestedt (Stockholm, Sweden), C. O. Coleman (Berlin, Germany), P. C. Dworschak (Wien, Austria), Laszlo Forro (Budapest, Hungary), B. Camur-Elipek (Edirne, Turkey), M. Ozbek (Izmir, Turkey), U. Wüest (Basel, Switzerland) and I. B. Muskó (Tihany, Hungary) for sending us samples from their institutions. We also thank all the collectors mentioned in the list (see Appendix S1, under Leg.). We are especially thankful to M Zagmajster for providing the maps for illustrating the geographic distribution of A. aquaticus using GIS software. We are grateful to P. Trontelj for his translating abstract to German language. This study was financially supported by the Slovenian Research Agency.




