Dynamic gastropods: stable shell polymorphism despite gene flow in the land snail Arianta arbustorumDynamische Schnecken: stabiler Schalenpolymorphismus trotz Genflusses in der Landschnecke Arianta arbustorum.
Abstract
enBased on a sequence fragment of mtDNA we analysed the genetic differentiation of the land snail Arianta arbustorum in the Alpine massif Gesäuse, where four morphotypes occur in close vicinity. We aimed at understanding actual and historical evolutionary processes among the morphotypes. With 135 haplotypes diverging up to 12.5%, genetic diversity was extremely high. Phylogenetic analyses indicated that A. arbustorum has colonized the Gesäuse several times in consecutive waves and that introgression of neutral markers across morphotypes and selection on shell shape have played an important role. Gene flow was more likely between locally close, different morphotypes than among distant populations of the same morphotype again indicating that cohesion of morphotypes was mainly due to selection. The actual distribution of morphotypes and haplotypes as well as the high genetic diversity also suggested that the area provided refugia during the Pleistocene glaciations.
Zusammenfassung
deWir analysierten die gentische Differenzierung der Landschnecke A. arbustorum in den Gesäusebergen der Alpen, wo vier Morphotypen in unmittelbarer Nachbarschaft vorkommen, basierend auf einem Fragment mitochondrieller DNS. Ziel war die Aufklärung der aktuellen und historischen evolutiven Prozesse zwischen den Morphotypen. Mit 135 Haplotypen, die um bis zu 12.5% divergierten, war die genetische Diversität extrem hoch. Phylogenetische Analysen zeigten, dass A. arbustorum das Gesäuse mehrfach in aufeinanderfolgenden Wellen besiedelt hat und dass Introgression neutraler Marker in andere Morphotypen und Selektion der Schalenform eine wichtige Rolle gespielt haben. Genfluss zwischen verschiedenen aber benachbarten Morphotypen war wahrscheinlicher als zwischen entfernten Populationen des selben Morphotyps, was ebenfalls auf die wichtige Rolle von Selektion für die Kohäsion der Morphotypen hinwies. Die aktuelle Verbreitung der Morphotypen und Haplotypen sowie die hohe genetische Diversität legten nahe, dass sich in dieser Region Eiszeitrefugien befunden haben.
Introduction
Polymorphisms in species of land snail including colour, shape and coiling direction have been the subject of numerous evolutionary investigations that have contributed importantly to our understanding of the roles of history and selection in diversification, adaptation and ultimately speciation (e.g. Cain and Sheppard 1954; Clarke 1978; Davison 2002; Schilthuizen and Davison 2005). In general, shell shape and colour have often been related to certain habitats or selective regimes (Cameron 1981; Solem and Climo 1985; Goodfriend 1986; Heller 1987; Chiba 2004). Shell shape shows a strong relation to the inclination of the preferred habitat (Cain and Cowie 1978; Cook and Jaffar 1984). In Cepaea nemoralis (Linnaeus, 1758), population specific patterns of shell colour and banding have been explained alternatively by local selection (Cain and Sheppard 1954) or historical factors causing ‘area effects’ (Cain and Currey 1963) including re-colonization from Pleistocene refugia, where diversity has been limited (Davison and Clarke 2000; Davison 2002). Land snails usually have a low potential for dispersal and are thus ideal objects for studying differentiation in space and time, because admixture of populations is restricted, often following a stepping stone model (Thomaz et al. 1996; Arnaud et al. 2001).However, both adaptive and historical explanations are not mutually exclusive (Gould and Woodruff 1990) and in many studies it has not been possible to find an unambiguous explanation for the morphological patterns encountered in and among various species of land snail (reviewed by Davison 2002). Most investigations have probably been conducted on a geographical scale too gross to allow for unambiguous conclusions (Davison 2002). Moreover, phylogenetic analyses based on mtDNA, have revealed that hybridization and introgression, even though difficult to show conclusively, may complicate the picture (e.g. Douris et al. 1998; Thacker and Hadfield 2000; Goodacre and Wade 2001; Haase et al. 2003). And thirdly, the methodology chosen may have been inappropriate to differentiate between alternative explanations. It needs to be stated, though, that often certain patterns such as incongruence between genetic and morphological data have unexpectedly been revealed (e.g. Thacker and Hadfield 2000; Haase et al. 2003). Explanations had to be sought ad hoc and could not be the result of rigorous hypothesis testing. To take our understanding of the evolutionary processes relating to morphological polymorphisms including adaptation, demographic factors, gene flow and hybridization a step further, it is necessary to investigate these processes on a fine spatial scale with a combination of morphological, ecological and genetic methods and to add the historical perspective.
In most alpine regions the globular nominate subspecies of Arianta arubstorum (Linnaeus, 1758), which has a wide distribution across Europe and occurs from the lowlands up to 3000 m asl. (Ehrmann 1933; Fechter and Falkner 1989), gets smaller in size and sometimes more conical with increasing altitude, which is attributable to phenotypic plasticity (Baur 1984; Burla 1984). In contrast, in certain, confined regions of the Alps, two or more clearly distinguishable morphotypes or subspecies of A. arbustorum occur in close vicinity, sometimes in parapatric situations. In the central Austrian Gesäuse mountains (Fig. 1), the nominate globular form inhabits lowland habitats such as meadows, bank slopes or the understorey of light, deciduous or mixed forests, whereas the flat-shelled A. a. styriaca (Frauenfeld, 1868), restricted to Northeast-Austrian massifs, possibly only the Gesäuse (but see Klemm 1974; Gittenberger 1991; Baminger 1997; Haase et al. 2003; Gittenberger et al. 2004), is largely dwelling on calcareous rock faces (Baminger 1997) (Fig. 2). At the lower ends of some of the steep trenches characteristic for the area, occasionally hybrid individuals between both forms can be found (Haase et al. 2003). These observations as well as breeding experiments between globular and depressed-shelled individuals resulting in intermediate offspring (Baumgartner 1997; unpublished data) indicate that shell shape has a genetic, heritable component. There are also intermediate-shelled populations occurring in the Gesäuse, which are probably not hybrids of globular and depressed ancestors. Their distribution is quite well defined (Fig. 1) and they are not couched between globular and depressed populations. In addition, if these populations were of hybrid origin, they should exhibit a considerable variance in shell shape. According to Baminger (1997), these populations may represent ecotypes, but the justification for this assumption is rather vague. Clear differences in shell shape are generally considered to be genetically controlled (see Gittenberger et al. 2004 for discussion and further references). These intermediate populations are found in a variety of habitats including shrubs, alpine pastures, scree and rocks. A fourth morph with a narrow, East-Alpine distribution, A. a. picea (Rossmässler 1837) sensu Klemm (1974) characterized by fragile shells and more inflated whorls (Fig. 2c), is separated from the Gesäuse populations to the South by the upper Johnsbachtal (Fig. 1).
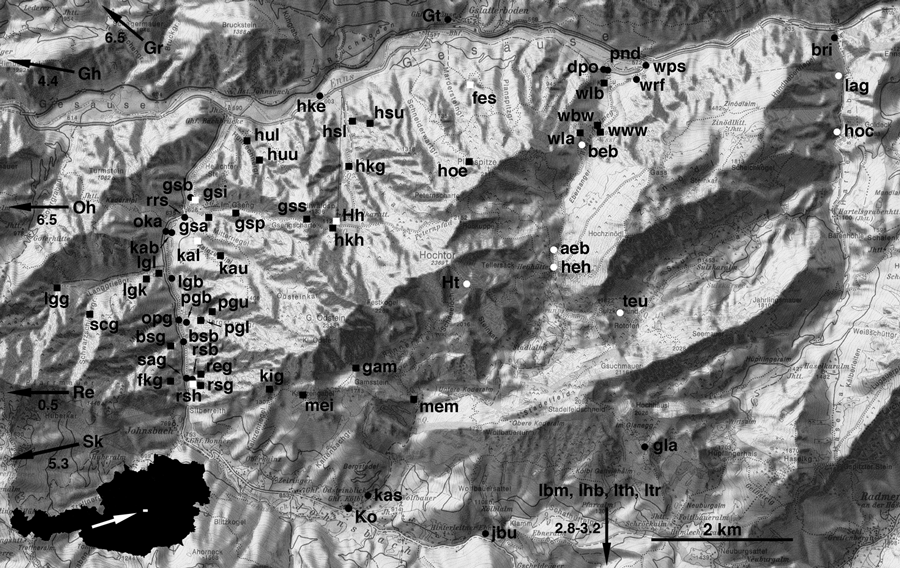
Map of the Gesäuse with sampling localities (for abbreviations see supporting information Table S1). Inset in lower left corner shows position within Austria. Black circles, populations with globular shells; white circles, populations with intermediate shells; white squares, localities where globular and flat-shelled specimens meet and shells with intermediate shape of apparently hybrid origin were found; black squares, populations with flat shells. Black arrows point at sites outside the map. These sites are in a distance from the arrowhead indicated by the accompanying number (in km)
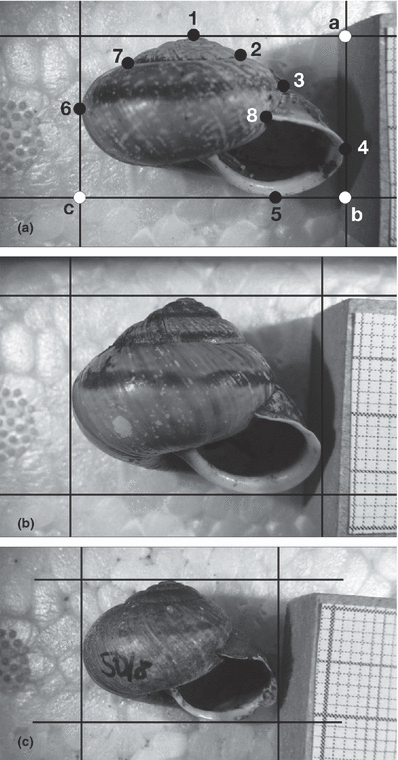
Shell shapes. A, flat shell from wlb with landmarks for morphometric analysis. Landmarks 1–8 describe shell shape, (a–c) were used to calculate the height–width ratio. (b) globular shell from pnd. (c) A. a. picea from ltr. For acronyms of localities see supporting information Table S1
The origin of these morphs, specifically, whether flat or globular shells are ancestral, is still under debate. By outgroup-comparison Gittenberger (1991) and Gittenberger et al. (2004) argued that the ancestor of A. arbustorum was flat-shelled and preferred high altitudes like all other members of the subfamily Ariantinae. The globular morph only evolved when the ancestral populations were driven into the lowlands by deteriorating conditions including glaciations during the Pleistocene. The few extant flat-shelled populations scattered throughout the Alps are considered relics which survived the unfavourable conditions in ice-free habitats such as nunataks. These authors further assumed that habitats, which were Arianta-free after the retreat of the glaciers, were re-colonized by the globular form. Alternatively, Baminger (1997) suggested that the flat shells are rather recent adaptations of a globular ancestor to the steep rock faces dominating the Gesäuse mountains. Phylogenetic analyses based on mtDNA did not unambiguously support either hypothesis. Nevertheless, Gittenberger et al. (2004) saw support for their hypothesis attributing the cases not fitting into the picture to incomplete lineage sorting or introgression, while Haase et al. (2003), remained cautiously undecided and did not suggest a direction of morphological evolution.
In the present account, we aimed at understanding actual and historical processes among populations of all morphotypes of A. arbustorum in the Gesäuse. Our inferences were based on phylogenetic and population genetic analyses of a fragment of mitochondrial DNA as well as a dense sampling design (Fig. 1, supporting information Table S1). We also intended to include information of microsatellites, however, we were only able to reliably amplify a single out of nine published loci (Armbruster et al. 2005) (see Materials and Methods). We focused on the extent and possible direction of gene flow within and across morphotypes. Morphotypes were defined based on the height–width ratio and using geometric morphometrics, which allows analysing shape independently of size (Bookstein 1991; Zelditch et al. 2004). We assumed that if morphological differentiation was a rather young phenomenon, genetic diversity of a derived morph should be a subset of the diversity of an ancestral morph. The spatial extent of introgression of morph-specific haplotypes across contact zones would be an indication of the importance of selection on shell shape. Our results are also discussed in the context of post-glacial re-conolonization and the still contentious issue of the existence of northern refugia (cf. Stewart and Lister 2001; Haase et al. 2003; Pinceel et al. 2005).
Materials and Methods
Collection
Snails were collected at 62 localities during two campaigns in May and September 2005, respectively. Locality data (supporting information Table S1) were recorded as GPS data or read out of the digital Austrian Map Fly 4.0 (BEV 2005). When ever possible, localities were selected along longitudinal transects through canyons including the adjacent low lands. Not more than 15 animals were taken at any locality in order to minimize damage to the populations. Animals were taken alive to the accommodation where they were decapitated with a razor blade and immediately dropped into absolute ethanol, which ensured both immediate death and high quality of DNA preservation. For morphometric analyses, also empty adult shells recognizable by an aperture with reflected lip were collected. A total of 462 snails were available for genetic analyses and 385 shells for morphometrics. For the phylogenetic analyses (Fig. 1, supporting information Table S1), we included also haplotypes from sites not visited in 2005 and haplotypes that were not collected in 2005 from our previous work (Haase et al. 2003). As outgroup we chose a congeneric species and one species each of two genera belonging to the same subfamily Ariantinae (supporting information Table S1). Sequences were deposited at GenBank (EF398129–EF398269).
Morphometrics
Shells of this species with determinate growth indicated by the formation of a reflected apertural lip were placed on a socket of styrofoam that had a hole accommodating the convex body-whorl so that pictures in apertural view could be taken. Digital photographs, all at the same scale, were made with a Panasonic DMC-FZ5EG camera fitted to a stand. Distortions controlled for with graph paper were corrected in Adobe Photoshop. These corrections were accurate in the central area of the picture containing the shell. Only at the borders slight distortions remained. Using auxiliary lines, two sets of landmarks were placed on the images. Set one comprised eight landmarks describing shell shape. Two of these, viz. numbers 1 and 8, were of type 1, the remainder, of type 3 (Bookstein 1991; Zelditch et al. 2004). The second set of landmarks comprised only three points placed on the intersections of the auxiliary lines (Fig. 2). These landmarks were used to calculate the height–width ratio. Images were transformed into the tps format using tpsUtil (Rohlf 2004a) and landmarks were defined in tpsDig2.0 (Rohlf 2004b; programmes available at http://life.bio.sunysb.edu/morph/). Analyses were made with the IMP suite of programmes by Sheets et al. available at http://www2.canisius.edu/~sheets/morphsoft.html and based on Partial Procrustes superimpositions generated in CoordGen6h. In A. arbustorum, assigning individual shells to a certain morphotype may be ambiguous, however, populations can well be characterized by simple descriptive statistics (Kothbauer et al. 1991; Bisenberger 1993; Baminger 1997). Thus, populations were assigned to morphotypes flat, intermediate and globular, respectively, which were defined based on mean height–width ratios calculated with TMorphGen6. In addition, A. a. picea was distinguished as separate morphotype although the height–width ratio was typical of globular shells. Specimens from hybrid populations were individually assigned to a morphotype. In these analyses, hybrid specimens were not distinguished from populations with intermediate shell shape, i.e. populations indicated by white circles in Fig. 1. All statistical analyses were based on individual shells. The height–width ratio of morphotypes flat, intermediate and globular was compared in a one-way anova. All further analyses were based on the shell landmarks 1–8 (Fig. 2). A principal component analysis (PCA) was performed with PCAGen6p in order to visualize the morphological variation across the four morphotypes. A canonical variance analysis (CVA) and jacknife-assignment tests were computed in CVAgen6n in order to discriminate the four morphotypes. TwoGroup6h was used for pair-wise comparisons of morphotypes including graphical visualization of differences between means as vectors of relative landmark displacement and thin-plate splines. With the same programme, we tested the repeatability by repeating the procedure with 25 randomly selected shells 4 months later. The measuring series could not be distinguished (Goodall’s F-test: F12,576 = 1.26, p = 0.237; resampling F-test, 4900 bootstrap replicates: p = 0.258) indicating good repeatability.
DNA preparation and amplification
DNA was isolated with Qiagen’s DNeasy Tissue Kit (Qiagen, Venlo, the Netherlands). For phylogenetic analyses a fragment of COI comprising 663 base pairs was amplified using the primers ACO 5′-CCTATTATAATTGGGGGTTTTGG-3′ and BCO 5′-GTATCGGCTGTAAAATAAGC-3′. 50 μl PCR mix contained 27.0 μl water, 8.0 μl 10 × buffer, 8.0 μl MgCl2 (25 mM), 1.5 μl of each primer (10 pmol), 2 μl BSA (10 mg ml−1), 0.8 μl dNTPs (10 mM), 0.2 μl Sigma Taq, and 1 μl genomic DNA. PCRs were run on GeneAmp 2700 or 2720 thermo cyclers under the following conditions: initial denaturation for 3 min at 95°C, 40 cycles of denaturation for 45 s at 95°C, annealing for 45 s at 48°C, and extension for 90 s at 72°C, final extension at 72°C for 7 min. The PCR-products had to be excised from 1.5% agarose gels using the MinElute Gel Extraction Kit from Qiagen to get rid of primer dimers. For direct sequencing on a Beckman Coulter CEQ 8000 modified primers ACOk 5′-ATTATAATTGGGGGTTTTGG-3′ and BCOk 5′-GTATCGGCTGTAAAATAA-3′ were used. 10 μl cycle sequencing reaction mix contained 40–60 ng PCR product, 4 μl Quick Start Master Mix, 1 μl primer and water. Reaction conditions were according to the manufacturer’s protocol (Beckman Coulter, Fullerton, CA, USA), but the number of cycles was increased to 40. The cycle sequencing product was cleaned using Clean Seq, the magnetic bead system of Agencourt. Sequences were edited in BioEdit 7.0.5 (Hall 2005) and the final alignment comprised 630 sites.
In order to incorporate nuclear information we intended to analyse the nine microsatellite loci developed for a Swiss population of A. arbustorum by Armbruster et al. (2005). However, only two of the published primer sets, those for loci H8 and H9, amplified alleles in the Austrian populations, but still with high frequencies of null alleles. For these initial tests G. Armbruster kindly provided two Swiss specimens as positive control, which gave the published results (Armbruster et al. 2005). We then tried to develop new primers based on the sequences deposited in GenBank, but were successful in only one case, viz. for locus A9: A9F153 5′-CTGTCCACGCGTCCGTATGTGT-3′ (JOEE labelled for detection on the sequencer) and A9R287 5′-TGTTGGGGACGGGGTAGAAGATT-3′. The PCR mix contained 5.6 μl water, 1 μl buffer, 1 μl MgCl2, 0.5 μl BSA, 0.5 μl of each primer, 0.2 μl dNTPs, 0.075 μl Taq, and 0.75 μl genomic DNA. PCRs were run over 35 cycles with denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec, and extension at 72°C for 30 sec followed by a final extension step lasting 8 min. Alleles were separated on an ABI Prism 377 DNA Sequencer. Because this microsatellite was surprisingly variable, it was amplified across the whole set of samples and analysed as well. In general, these data fully support the results based on the COI fragment. However, we report only some general findings and refrain from presenting the detailed analyses, because a single microsatellite locus is most likely not representative for the entire nuclear genome.
Phylogenetic and population genetic analyses
The sequence data contained several deeply coalescing, hardly variable clades. Therefore, we conducted both tree and network reconstructions. Because of the data structure we performed two tree reconstructing approaches, a Bayesian and a distance analysis. The Bayesian analysis (BA), conducted with MrBayes 3.1.1. (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003), probably allows the most comprehensive and specific incorporation of assumptions on sequence evolution including the distinction of separate partitions and delivers node support values in reasonable computation time. However, BA tends to be over-parameterized for young lineage splits. These young evolutionary events may be better represented by maximum parsimony (MP) and distance analyses. We refrained from MP, because the innumerable alternative equally parsimonious topologies within clades of low divergence resulted in prohibitive computation times. Trading time against the potential drawbacks of distance methods, viz. not distinguishing different causes and types of similarity, we reconstructed a neighbour joining tree based on a HKY + G substitution model inferred as best fitting model by Modeltest 3.7 (Posada and Crandall 1998) using paup* 4.0b10 (Swofford 1998). For the BA, substitution models were fitted to combined first and second codon positions and to third positions, respectively, using a Bayes factor test (Kass and Raftery 1995; see also Nylander et al. 2004). Both partitions had homogeneous base frequencies, one of the assumptions of model based methods. First and second positions were combined because there were too few substitution events in either position to meaningfully fit separate models. The best fitting models were HKY and GTR, respectively. MrBayes runs two parallel analyses with each one cold and three heated chains by default. Our analysis had the default flat priors and ran over 15 Mio generations of which every 100th was sampled. The first 500 000 generations were discarded as burnin. Likelihood scores had converged after about 300 000 generations.
Since intraspecific phylogenies are often better represented by networks rather than trees, relationships were also reconstructed with haplotype networks using statistical parsimony and the programme TCS (Templeton et al. 1992; Clement et al. 2000; Posada and Crandall 2001).
For the analyses of population differentiation we only used the specimens collected in 2005, because the sampling regime of our previous study (Haase et al. 2003) was different. In the current investigations, some populations had to be merged or excluded because of small sample sizes (Table 1 and supporting information Table S1). Using Arlequin 3.01 (Excoffier et al. 2005), we conducted analyses of molecular variance (amova) based on the unstructured set as well as several subdivided sets. For the latter, populations were attributed to four to six groups according to geography, morphology or a combination of both (Table 1). Since hybrid populations could not be attributed to a single morphotype, another four populations had to be excluded. Significance of variance components was tested with 20 022 permutations. We also computed group specific FST values. Their significance was tested with 5040 permutations. Along transects through trenches, the direction of gene flow was inferred based on presence/absence of data of haplotypes. In an ideal case, a longitudinal series of nested haplotype compositions might indicate gene flow from the most to the least diverse population. The following transects were investigated (Fig. 1, supporting information Table S1): Gseng (gss-gsp-gsa-gsi-gsb), Haindlkar (gss-hkh-hkg-hks-hke), Langgriesgraben (lgg-scg-lng-lgb), Rotenedergraben (rsg-rsh-rsb-reg), and Wasserfallweg (wla-www-wbw-wlb-pnd-wrf-wps).
| No structure | 46 pops | % var | FST | 42 pops | % var | FST | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| among pops | 29.84 | 0.2984 | among pops | 29.55 | 0.2955 | |||||||
| within pops | 70.16 | within pops | 70.45 | |||||||||
| Structure | GEO | MORPH | GEO-MORPH | |||||||||
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 1 | Group 2 | Group 3 | Group 3 | Group 4 | Group 5 | Group 6 | |
| Track 665 | Waterfall | Gseng | Uval | Lval | Flat | Glob | Inter | Flat/Gseng | Flat/Val | Glob/LVal | Glob/Uval | |
| bsg | ||||||||||||
| fkg | ||||||||||||
| gam | ||||||||||||
| gsp | ||||||||||||
| gss | ||||||||||||
| bsb | hkg | bri | ||||||||||
| bsg | hkh | bsb | ||||||||||
| fkg | hoe | gla | ||||||||||
| kau | hsg | gsb | ||||||||||
| lgb | hum | jbu | ||||||||||
| lgg | kau | kas | bsg | |||||||||
| aeb | lng | lgg | lgb | fkg | ||||||||
| beb | gsb | oka | lng | oka | gam | bsb | ||||||
| heh | gsp | opg | mei | opg | gsp | kau | gsb | |||||
| pnd | gss | peg | peg | pgb | aeb | gss | lgg | lgb | ||||
| Bri | wla | hkg | pgb | reg | pnd | beb | hoe | lng | oka | |||
| Gla | wlb | hkh | gam | reg | rsg | rsb | heh | hkg | mei | opg | ||
| Hoc | wps | hoe | jbu | rsb | wla | sag | hoc | hkh | peg | pgb | ||
| Lag | wrf | hsg | kas | rsg | wlb | wps | lag | hsg | reg | rsb | jbu | |
| Teu | www | hum | mei | sag | www | wrf | teu | hum | rsg | sag | kas | |
| Within groups | % var | % var | % var | % var | % var | % var | % var | % var | % var | % var | % var | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Among pops | 0.00 | 43.26 | 30.25 | 15.06 | 13.27 | 36.75 | 16.54 | 10.72 | 30.57 | 18.05 | 0.00 | 4.49 |
| Within pops | 100.00 | 56.74 | 69.75 | 84.94 | 86.73 | 63.25 | 83.46 | 89.28 | 69.43 | 81.95 | 100.00 | 95.51 |
| FST | 0.0000 | 0.4326 | 0.3025 | 0.1506 | 0.1327 | 0.3675 | 0.1654 | 0.1072 | 0.3057 | 0.1805 | 0.0000 | 0.0449 |
| Across Groups | % var | Fixation Indices | % var | Fixation Indices | % var | Fixation Indices | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Among groups | 5.44 | FCT | 0.0544 | 6.33 | FCT | 0.0633 | 12,35 | FCT | 0.1235 | |||
| Am pops wi gr | 24.91 | FSC | 0.2635 | 24.80 | FSC | 0.3114 | 12,98 | FSC | 0.1480 | |||
| within pops | 69.64 | FST | 0.3036 | 68.86 | FST | 0.2648 | 74.67 | FST | 0.2533 | |||
Results
Morphometric analysis
Mean height–width ratios ranged from 0.52 to 0.62 for flat-shelled populations, 0.64 to 0.68 for populations with intermediate shells and from 0.71 to 0.83 for globular-shelled samples. The division of this continuum may appear to be somewhat arbitrary. However, an anova based on individual snails was highly significant (F2379 = 708.2, p < 0.001) and Tukey’s pairwise post-hoc comparisons distinguished these three morphotypes with high significance as well (p < 0.001 in all three cases). Data had to be log-transformed to meet the assumptions of normality (Shapiro–Wilk test: p > 0.101 in all three cases) and homogeneity of variance (Levene’s test: p = 0.119).
The more comprehensive landmark-based analyses of individual shells including A. a. picea confirmed that our distinction of four morphotypes probably has more than only heuristic value. The continuous variation is illustrated by the PCA (Fig. 3a), which aimed at finding linear combinations maximizing the total variance and had six distinct eigen-values. The first three principal components explained 86.08% of the total variance. Only A. a. picea did not overlap with the morphologically nearest type with globular shells. The CVA, which maximized differentiation among morphotypes and had three distinct canonical variates, showed a much clearer separation (Fig. 3b). CVA-distance based groupings were 86% (globular)–100% (A. a. picea) correct. In a jack-knife assignment test with 1000 replicates using 20 individuals as ‘unknowns’ 96.7% of individuals were correctly classified, 88.7% significantly. All pairwise comparisons of morphotypes were highly significant (resampling F-test with 4900 bootstrap replicates: p < 0.001 in all six cases).
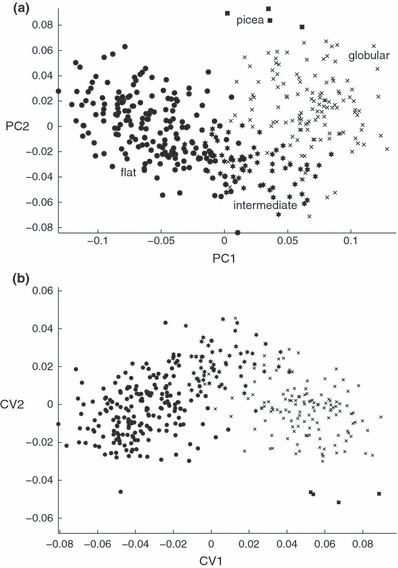
Ordinations based on landmark data. (a) Principal component analysis; (b) canonical variates analysis. CV, canonical variate; PC, principal component
The graphical comparisons of morphotypes (supporting information Fig. S1) indicate that in terms of shape shells with intermediate height–width ratio are not simply intermediate in transformations from flat to globular shells. This becomes especially clear comparing relative lengths, angles and directions of the vectors of landmarks 3, 4, 5 and 8 describing aperture and last whorl. A. a. picea, which cannot be distinguished from the nominate, globular form based on the simple height–width ratio, is less conical than the latter and its last whorls are relatively higher thus appearing more inflated (Fig. 2).
Phylogenetic analyses
We identified 119 unique haplotypes among the 450 sequenced individuals of A. arbustorum. For the phylogenetic analyses another 16 haplotypes were added from our foregoing study (Haase et al. 2003). The maximum pairwise distance (uncorrected p) was 12.5%. In the BA, in contrast to the model parameters, the diagnostics for convergence of both runs had not reached the critical value after 15 Mio generations. The potential scale reduction factor did not approach 1 and the average standard deviation of split frequencies had a final value of 0.0119. This was probably due to the large number of similarly likely topologies within clade A (Fig. 4). Since the interrelationships within this large haplotype group were not of primary importance and considering that they would be better represented by a network anyway, we stopped the analysis at this point. Topologies of the large clades of BA and distance analysis were practically identical. The only difference was the paraphyly of clade A relative to clade B in BA (Fig. 4), which were sister clades in the neighbour joining tree (not shown). All except three of the well differentiated, large clades were supported by posterior probabilities of ≥99%. Clade A united individuals from all four morphotypes from all over the investigated area. Only few subclades were consistent with morphology or geography. In contrast, clade B′ consisted of entirely flat-shelled specimens from trenches directed north towards the river Enns. The remaining clades, subdivided into two groups, were sister clade to A and B. The first group, clades C–F, where E and F consisted each of a single specimen (see haplotype network, supporting information Fig. S2), contained three flat-shelled specimens, one hybrid snail and otherwise exclusively globular individuals. Members of these clades were restricted to the low lands of the Ennstal and lower Johnsbachtal (here defined as north-directed stretch of the valley of the Johnsbach, Fig. 1). One specimen, ‘clade’ F, was collected at Schloss Kaiserau, SW of the Gesäuse. The flat-shelled specimens stemmed from localities very close to low land habitats, and the hybrid animal was collected at the Haindlkarhütte, which has a little garden with a compost heap, where a number of gastropod species normally not occurring in alpine habitats including globular A. arbustorum were found. Among the remaining clades, only G exhibited some geographic cohesion with snails coming from the Ennstal, the lower Johnsbachtal, as well as the Gsengscharte and Haindlkarhütte (1, 4).
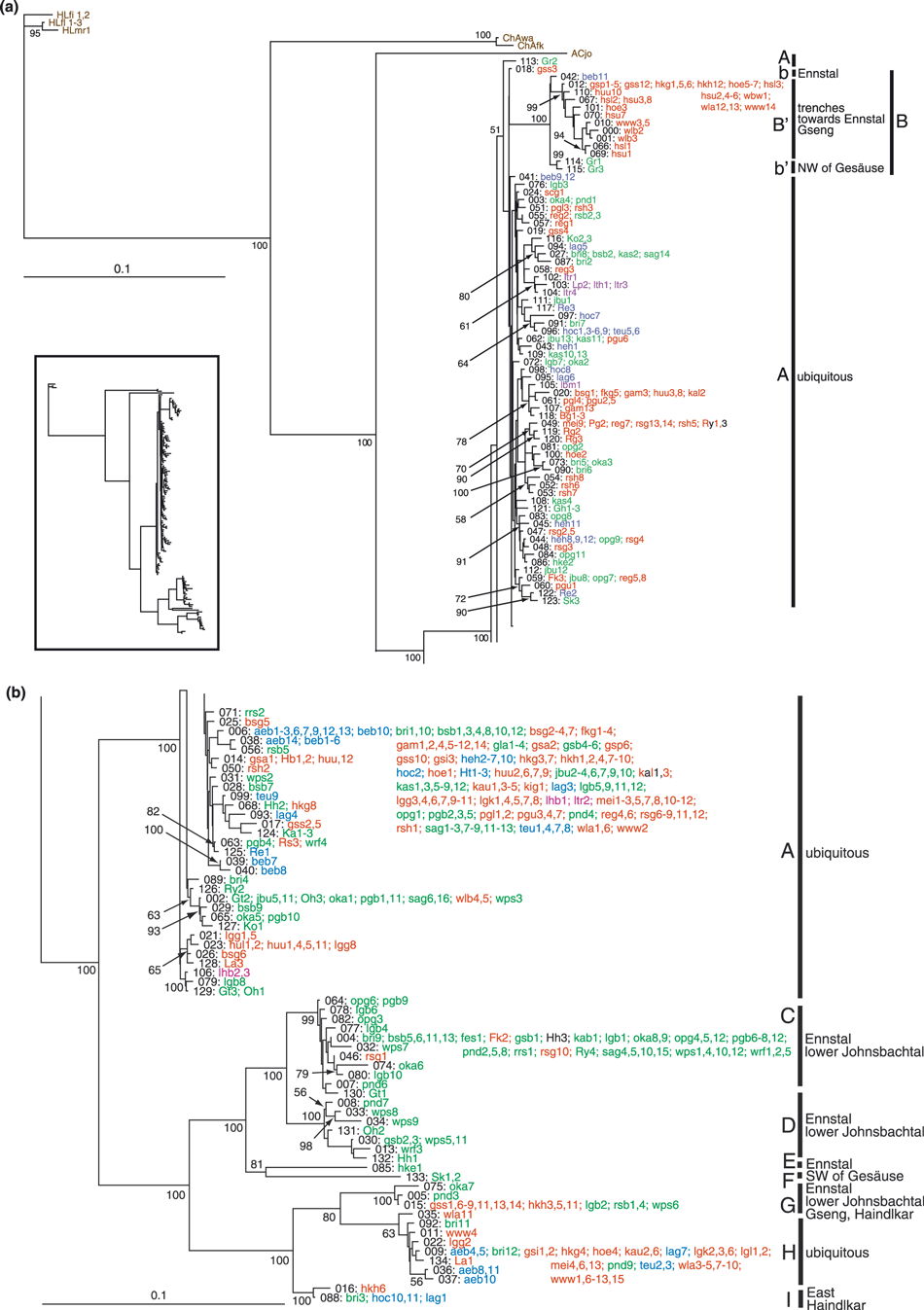
Tree with maximum likelihood (ln L = −4411.449) among a total of 290 002 sampled trees from Bayesian analysis. Numbers at nodes represent posterior probabilities from a 50% majority rule consensus tree. Haplotypes are identified by numbers, for population acronyms see supporting information Table S1. Red, flat shells; blue, intermediate shells; green, globular shells; violet, A. a. picea; black, hybrid individuals; brown, outgroup. Inset shows entire tree topology
The network reconstruction using statistical parsimony was based on the entire data set including 500 specimens. Under the 95% connection limit, the set collapsed into nine separate networks, two of which consisting each of a single specimen, one containing two and one three haplotypes (supporting information Fig. S2). In order to join these networks, the connection limit had to be set to 52 steps (not shown). Thus, the network reconstruction confirmed the deep splits between as well as the low variation within clades already recovered in the trees and revealed substantial homoplasy shared among the clades. Haplotypes identified as ancestral were largely those with most connections. They did not occupy basal positions in the trees, though. Clades A–D exhibit numerous alternative connections to certain haplotypes, which, in case both paths to each or most of these nodes had been historically realized, indicates a considerable degree of homoplasy also within clades.
Genetic differentiation of populations
Population differentiation was analysed based on 46 samples. Because of a small number of individuals, some samples had to be merged, and those, which could not be meaningfully merged, excluded (Table 1, supporting information Table S1). 32 populations (71%) had private haplotypes. The single microsatellite locus had 18 alleles. Five populations had each a private allele. Interestingly, the alleles sorted into two size classes, 138–144 and 187–206 base pairs. Some alleles differed only by a consistently and repeatably scorable single position, which was probably due to an indel in the flanking region of the microsatellite.
amovas based on haplotypes are summarized in Table 1. The analyses of both data types yielded similar results, although those based on haplotypes were clearer probably because of the higher diversity (microsatellite data not shown). The overall FST value of 0.298 was quite high indicating substantial population differentiation. The hierarchical structure defined combining geography and morphology, GEO-MORPH, had very low group specific FST values, three of them even non-significant. All fixation indices resulting from amovas were significant. The amovas of GEO-MORPH had the highest among group variance and FCT value, whereas the variance among populations within groups with the corresponding component of differentiation, FSC, was lowest. This suggests that genetic cohesion is highest among geographically close populations of a certain morphotype. This also means that gene flow between distant populations belonging to the same morphotype is less likely than between close populations of different morphology.
On a smaller scale, a general direction of gene flow along transects through trenches and adjacent low land sites could not be inferred based on presence/absence data of haplotypes. We found no nesting pattern. The haplotype composition of one population was never a subset of a neighbouring, let alone of a more distant population. In almost all populations the number of specific haplotypes was larger than that of haplotypes shared with other populations of a transect. Shared haplotypes were usually ubiquitous, thus uninformative. There was only one case suggesting top down gene flow in the transect Gseng with populations gss, hkh, hkg and hsg. Numbers of haplotypes shared between adjacent populations were 3, 2 and 1 respectively. But all sites had up to four specific haplotypes.
Discussion
Northern refugia
Much of our discussion is based on the following conclusions from the genetic diversity and distribution of haplotypes. Wide-spread haplotypes with local derivatives spread quickly and differentiated locally after having been established in a population. If spread and subsequent differentiation was a slow process we would expect a ladderized tree shape and a more linearized topology in the network analysis allowing to identify a centre of origin, which were clearly not given. Nevertheless, the distribution of haplotypes is certainly also a function of time. In addition, we assume that differentiation within a small area such as the Gesäuse would be reflected by relatively short branches whereas long branches would indicate immigration from an outside place of origin. Since the molecular clock could not be rejected (data not shown), branch lengths were proportional to divergence time. Thus, the phylogenetic analyses indicated that A. arbustorum has colonized the Gesäuse several times and that introgression of neutral or near neutral markers and selection on shell shape have played an important role. The clades diverged deeply and the maximum genetic differentiation between haplotypes of 12.5% in this small area was almost as large as that found across large parts of Europe (Haase et al. 2003; Gittenberger et al. 2004). The most basal and thus oldest clade A had the widest distribution in the Gesäuse and the surrounding areas and was found in all four morphotypes. Since the main clades of Fig. 4 were well differentiated, younger ones probably had an origin outside the Gesäuse. After their arrival, they spread and differentiated within the Gesäuse. Some remained rather local and more or less restricted to a single morphotype such as clades B′ or C (Fig. 4). Other haplotypes and their immediate derivatives became ubiquitous and introgressed into other morphotypes, e.g. clade H. The Pleistocene glaciations certainly extinguished local populations. Colonization and spread of new haplotypes and alleles was probably most dynamic immediately after the retreat of glaciers when large areas of new habitat became available. Once habitats were occupied, local mutations contributed importantly to population differentiation.
The actual distribution of morphotypes and haplotypes as well as the high genetic diversity leading to the scenario of consecutive waves of colonization followed by local differentiation outlined above also suggested that the area provided refugia during climatically adverse periods, i.e. during the Pleistocene glaciations. Mountaintops were covered by glaciers but did not reach the Ennstal glacier leaving the steep trenches ice-free. The Johnsbachtal as well remained ice-free during the last cold period (van Husen 1987). Thus, especially the trenches may have been effective refugia for the flat-shelled A. a. styriaca during the Pleistocene glaciations suggesting an origin preceding this period. Also Nunataks within the Alpine ice cover (van Husen 1987) could have served as refugia. Globular populations may well have survived in the low lands of the Johnsbachtal.
The existence of northern ice age refugia is still debated, but there is increasing evidence that central and northern Europe were not a tabula rasa during cold periods as implicitly suggested by Hewitt (1996, 2000) or Taberlet et al. (1998). A number of animal and plant taxa apparently survived north of the Mediterranean peninsulas (e.g. Stewart and Lister 2001; Brunhoff et al. 2003, 2006; Haase et al. 2003; Pinceel et al. 2005; Schönswetter et al. 2005; and literature cited in these papers). For several species of land snail including A. arbustorum there is a continuous fossil record in the pleniglacial (7 0000–3 4000 year BP) Loess deposits of the last glaciation in Western Germany (Moine et al. 2005). Eastern Alpine areas including the Gesäuse were identified as potential peripheral Alpine refugia of plants. Interestingly, evidence for plant survival on calcareous bedrock dominating in the Gesäuse massif is lacking, whereas siliceous bedrock did provide refugia for several species (Schönswetter et al. 2005). Since A. arbustorum apparently survived the glacial periods in the calcareous Gesäuse this may also be taken as evidence for plant survival in this region.
Or cryptic speciation?
It has been suggested that phylogenetic structure as reflected by several statistical parsimony networks separated by more than the parsimony connection limit would be indicative of cryptic speciation (Hart and Sunday 2007). This, however, would imply that the considerable shell polymorphism encountered in the Gesäuse was not related to species differentiation but instead showed multiple homoplasies between species diagnosed by their mitochondrial haplotypes. It would further imply, that sympatric, morphologically indistinguishable snails were assemblages of several species rather than populations. To give just a few examples, the Gsengscharte (gss), the bridge across the Hartlsbach (bri) and the bank of the pond in Kummer (pnd) would harbour three, four and five morphologically identical species, respectively. We concede that the deep phylogenetic structure is in accordance with speciation scenarios. However, it is primarily evident in neutral or near neutral third codon positions and the sequence variation was largely due to synonymous substitutions. The divergence of functional sequence regions may be much more constrained promoting cohesion through gene flow. An allozyme study found only week differentiation among 15 Austrian populations (Haase and Bisenberger 2003). In this light and especially given the above cited evidence for reproductive compatibility of distantly related populations and different morphotypes as well as for the genetic control of shell shape we reject the possibility of cryptic speciation.
The deep phylogenetic structure is also well in accordance with findings in other species of land snail (e.g. Thomaz et al. 1996; Chiba 1999; Guiller et al. 2001). Apart from the existence of cryptic species, Thomaz et al. (1996) discussed four mutually non exclusive reasons for this extreme diversity, viz. selection, rapid mitochondrial evolution, ancient isolation and divergence of populations, and unusually structured or exceptionally large populations. There is no evidence for selection or rapid evolution in A. arbustorum, but ancient and probably recurrent isolation during the Pleistocene as well as the population structure have certainly played a major role in shaping the genetic diversity (Haase et al. 2003). In addition to these reasons, Haase et al. (2003) also invoked the hermaphroditic nature and thus higher effective mitochondrial population size (Hoelzer 1997) of these predominately outcrossing snails (Chen 1993, 1994) as important factor contributing to the preservation of a higher diversity of mitochondrial lineages.
Thus, the genetic diversity and phylogenetic structure of A. arbustorum in the Gesäuse bears the signature of demographic factors holding for hermaphroditic land snails in general and the particular historical factors of the Gesäuse.
Gene flow and shell shape
Genetic differentiation among populations indicated that gene flow is in the first place a local, i.e. geographical phenomenon. The morphological cohesion especially of the flat-shelled A. a. styriaca and the globular nominate form is apparently determined by selection. Only a single population of A. a. styriaca, from the Meierweg, did not occur on steep rock faces but on scree, while all globular populations were collected in more or less moist low land habitats. Habitats of populations with intermediate shell were somewhat more heterogeneous including rock, scree and alpine meadows. Most of these populations occur in the east of the Gesäuse. This suggests that for this morphotype gene flow has a more important role. The shell shape and structure of A. a. picea is probably an adaptation to its substrate, bedrock consisting at the Leobener of siliceous porphyroid and paleolithic lime stone (Flügel and Neubauer 1984), from which calcium carbonate may be less readily available. Hatchlings raised on a lime-rich diet in the lab still developed the characteristically fragile shell (Bisenberger, pers. comm.) indicating genetic control.
Hybrids between flat and globular specimens were rare in this as well as the previous study (Haase et al. 2003). In the first place this is certainly because populations of these morphotypes are only rarely contiguous such as in the hybrid zone of the Rotenederseitengraben. Secondly, there is only rarely a wider transition zone between steep, rocky and low land habitats so that areas where intermediate-shelled hybrids might have an advantage are small. Nevertheless, mtDNA and microsatellite alleles, both in general presumably selectively neutral or near neutral appear to cross morphotype boundaries readily. However, some haplotypes or haplotype groups were restricted to certain morphotypes in defined areas. In mating experiments between Swedish and Swiss populations (Baur and Baur 1992) as well as a population of A. a. styriaca from the Gesäuse and a globular population from Klosterneuburg near Vienna (Baumgartner 1997), formation of homotypic pairs was more likely than matings of heterotypic snails. If assortative mating does play a role in the distribution of haplotypes among morphotypes, we would expect that new haplotypes spread and diversify within the original morphotype first before being established in a different one. This may be the case, e.g. in the younger clades B′and C.
Diversity of mtDNA and microsatellite alleles, phylogenetic relationships as well as population differentiation indicated that both genetic and morphological diversity are old. In no case and in no locality was the genetic diversity of one morph a subset of the diversity of another, ancestral morph. Therefore a direction of morphological differentiation can still (cf. Haase et al. 2003) not be inferred. Our analyses revealed that dispersal was at least at times surprisingly dynamic extending beyond the boundaries of the study area. In order to improve our understanding of processes of genetic and morphological differentiation among Alpine populations analyses with a similar sampling design need to be geographically extended to other areas inhabited by flat-shelled populations and areas in between these rather isolated mountain massifs.
We are aware that sampling across populations was not even, which we partly compensated for by pooling or exclusion of smaller samples. This certainly did not completely equilibrate the analysis design. However, based on many years of experience with A. arbustorum in the Gesäuse of the first author and several persons acknowledged for their field assistance, we believe that it is hardly possible to optimize the sampling regime. The imbalance is probably reflecting the true patchy and uneven distribution of the snails rather than uneven collecting effort.
Acknowledgements
This paper would not have been possible without the invaluable help of Helmut Baminger, Elisabeth Haring, Doris Kleewein, Hans and Renate Kothbauer, Martin Kraushofer, Luise Kruckenhauser, Helmut Sattmann, Elisabeth Singer and Ilse Wenger in the field. We thank Daniel Kreiner from the Nationalpark Gesäuse for issuing the collecting permit. Financial support was received from the German Science Foundation (MI 649/5-1). Michael Hart and two anonymous referees provided helpful comments on an earlier version of the manuscript.




