Sexual size and shape evolution in European newts (Amphibia: Caudata: Salamandridae) on the Balkan Peninsula
Abstract
enWe used a phylogenetic perspective in an examination of the direction and extent of sexual dimorphism in body size and body shape in European newts from the Balkan Peninsula (alpine newts, Mesotriton alpestris; crested newts, Triturus cristatus superspecies; smooth newts, Lissotriton vulgaris). We found a strong, female-biased sexual size dimorphism (SSD) in the analysed clades of alpine newt, whereas within crested newts we found a less stringent female-biased SSD in Triturus carnifex, Triturus macedonicus and Triturus karelinii, and no significant SSD in T. cristatus or Triturus dobrogicus. Among the smooth newts, we found male-biased SSD in Lissotriton vulgaris vularis and Lissotriton vulgaris greacus and no SSD in Lissotriton vulgaris meridionalis. Most of these newts also exhibit a significant sexual dimorphism in body shape, which varied more randomly than body size, regardless of SSD level. Female and male body size as well as the degree of SSD displayed statistically significant phylogenetic signal, while sexual dimorphism in body shape was phylogenetically independent. The relationship between independent contrast data for female size and male size indicated that SSD in European newts could be driven by a disproportionate increase in female size as increase in female size was not accompanied by a proportional increase in male size.
Zusammenfassung
kaEvolution von sexueller Körpergröße und –form bei europäischen Wassermolchen (Amphibia: Caudata: Salamandridae) auf der Balkenhalbinsel
Richtung und Ausmaß des Geschlechtsdimorphismus bei Größe und Form der europäischen Wassermolche (Bergmolch, Mesotriton alpestris; Kammmolch, Triturus cristatus–ūSuperspezies; Teichmolch, Lissotriton vulgaris) wurden in Hinblick auf phylogenetische Fragestellungen untersucht. Wir fanden einen starken Geschlechtsdimorphismus in der Körpergröße (SSD) zu Gunsten der Weibchen in den analysierten Gruppen der Bergmolche; bei den Kammmolchen war dieser bei T. carnifex, T. macedonicus und T. karelinii weniger ausgeprägt und bei T. cristatus und T. dobrogicus dagegen nicht signifikant. Bei den Teichmolchen wurde Größendimorphismus zu Gunsten der Männchen bei L. v. vularis und L. v. greacus gefunden; bei L. v. meridionalis konnten keine Größenunterschiede zwischen den Geschlechtern festgestellt werden. Die meisten dieser Molche zeigen auch einen auffälligen Sexualdimorphismus in der Körperform, der allerdings zufälliger variiert als die Körpergröße, unabhängig vom Niveau des sexuellen Größendimorphismus (SSD). Körpergröße von Männchen und Weibchen sowie der Grad des Geschlechtsdimorphismus lassen ein stastistich signifikantes phylogenetisches Signal erkennen, während der Geschlechtsdimorphismus der Körperform phylogenetisch unabhängig ist. Das Verhältnis unabhängiger Kontrastdaten für weibliche und männliche Körpergröße deutet darauf hin, dass SSD in europäischen Molchen durch eine disproportionale Zunahme der weiblichen Körpergröße bewirkt wird, da Zunahme der Weibchengröße nicht durch eine proportionale Zunahme der Männchengröße begleitet wird.
Introduction
Long lasting issues of evolutionary biology addressing patterns of sexual size and shape differences among related taxa, and in particular mechanisms and processes driving these patterns, still remain controversial (e.g. Hedrick and Temeles 1989; Badyaev 2002; Delph 2005 and references therein). Thus, the statement that the sexual size dimorphism (SSD), together with other life-history traits, has a significant phylogenetic signal (the tendency for evolutionary related groups to resemble each other, Blomberg et al. (2003) has been confirmed in empirical surveys conducted on cross-species data sets (e.g. Cheverud et al. 1985; Butler et al. 2000; Huey et al. 2006). However, there is a real necessity for additional empirical justification for such a generalization. The same holds for the effect of body size on sexual dimorphism (allometry for SSD), well known as a Rensch’s rule, which states that in groups with male-biased SSDs, the sexual dimorphism increase in larger groups (species, races or populations) and opposite, in cases of female-biased SSD, the intersexual difference diminishes in larger groups (e.g. Abouheif and Fairbairn, 1997).
In this study, a phylogenetic framework was used to address general hypotheses about the evolution of sexual size and shape differences in European newts in the Balkans. These newts are a marked example of amphibians in which sexual differentiation includes dimorphism in body size, epigamic differences in colour and specific integument structures (i.e. dorsal crest in males), which are conspicuous during the breeding season only (e.g. Halliday 1977). Also these newts characterize with complex courtship behaviour which is particularly diversified (Griffiths 1996; Steinfartz et al., 2007). The Balkan Peninsula is especially an appropriate area for such investigations because of phylogenetic clade richness.
The Balkan’s newts include three groups: the group of small-sized smooth newts Lissotriton vulgaris (Linnaeus, 1758), the medium-bodied alpine newt taxa Mesotriton alpestris (Laurenti, 1768), and the large-bodied crested newts Triturus cristatus superspecies. All five species of crested newts occur on the Balkan Peninsula: T. cristatus (Laurenti, 1768), Triturus dobrogicus (Kiritzescu, 1903), Triturus carnifex (Laurenti, 1768), Triturus macedonicus (Karaman, 1922) and Triturus karelinii (Strauch, 1870) of which only T. cristatus appears to be without further taxonomic subdivision (e.g. Arntzen 2003; Arntzen et al. 2007).
Within the alpine newts (M. alpestris), a number of distinct phylogenetic clades have been recognized in the Balkans (Sotiropoulos et al. 2007), though they have yet to be taxonomically characterized. Within the genus Lissotriton, only the smooth newt (L. vulgaris), with its three conventionally recognized subspecies (vulgaris, meridionalis and graecus) (e.g. Raxworthy 1990; Babik et al. 2005), is present in the Balkans.
European newts have been the subject of many SSD studies, in which a number of conspecific and congeneric populations were examined (Kalezić et al. 1992; Malmgren 1999; Malmgren and Thollesson 1999). A female-biased pattern of SSD was found to prevail, but with marked interspecies differences in the degree of dimorphism. Since much of the size variation among species can be explained by shared ancestry, a comparative method that takes phylogeny into consideration is necessary (e.g. Felsenstein 1985; Harvey and Pagel 1991).
To date, the vast majority of comparative studies have focused only on size dimorphism, largely ignoring differences in body shape between sexes (but see Brãna 1996; Butler et al. 2000; and for European newts Malmgren and Thollesson 1999; Bovero et al. 2003), though there is no reason to believe that shape dimorphism is any less important than size dimorphism.
In this study, we explored the patterns of both sexual size and shape dimorphism in order to determine (1) whether the level and pattern of sexual size and shape dimorphism are phylogenetically constrained; (2) whether size-dependent shape changes (allometry) produce sexual shape dimorphism and (3) whether allometry for SSD in newts follows Rensch’s rule.
Materials and Methods
Phylogenetic analysis
The analysis of sexual dimorphism in European newts within a phylogenetic context poses several difficulties. First, species and samples used in the available molecular phylogenies only partially coincide with those analysed morphometrically in this study. Secondly, there is uncertainty in the phylogeny at various levels, as different tree topologies have been obtained (Zajc and Arntzen 1999; Steinfartz et al. 2002, 2007; Babik et al. 2005; Weisrock et al. 2006; Arntzen et al. 2007).
Therefore, for the purposes of this study, we produced a molecular phylogeny of all the morphologically analysed taxa (Fig. 1), using representative 16S rRNA sequences from the same or geographically related populations (see Appendix S1 for references and Genbank data).
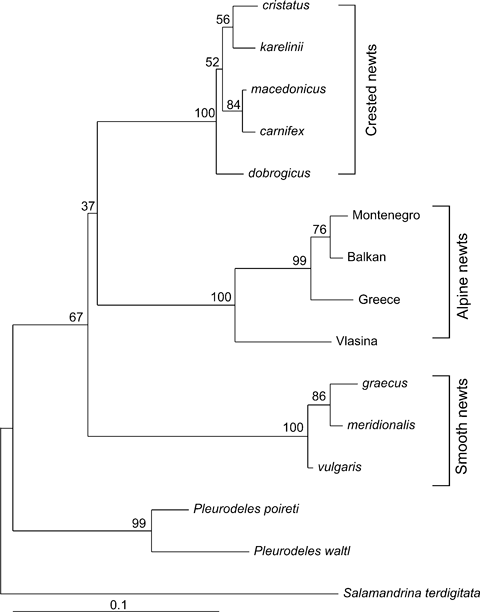
Phylogenetic relationships among newt clades in the Balkans based on a maximum-likelihood analysis of 16S rRNA sequences under the GTR + I + G model of DNA substitution. Bootstrap values for the major nodes are shown on branches
The 16S rRNA sequences were aligned with clustal x (Thompson et al. 1997) and manually corrected. Although some alignment gaps were inserted to resolve length differences between sequences, all positions could be unambiguously aligned and were therefore included in the analyses. Phylogenetic inference was analysed using the maximum-likelihood (ML) method (Felsenstein 1981). Nucleotides were used as discrete, unordered characters. The best-fit model of DNA substitution and the parameter estimates employed for tree construction were chosen using the Akaike Information Criterion (Akaike 1974) in modeltest (version 3.7, Posada and Crandall 1998). The general time reversible (GTR) model with rate heterogeneity and a non-zero proportion of invariable sites had the highest likelihood score (−lnL = 1895.4391) and showed a significantly better fit than the other less complicated models (model parameters: GTR + I + G, I = 0.6205, G = 1.0150; base frequencies A = 0.3664, C = 0.2023, G = 0.1740, T = 0.2573). A ML tree with modeltest-derived parameters was constructed with the phyml version 2.4.4 program using the method of Guindon and Gascuel (2003). We tested the robustness of the topology with 1000 bootstrap replicates.
Morphometric analyses
Morphometric data from 98 populations, including those studied in Kalezić et al. (1992), were collected and analysed. The complete list of examined populations, along with their associated attributes and sample sizes, is given in the Appendix S2.
Seven body measures were scored: snout–vent length (SVL), distance between forelimb and hindlimb axillas (D), head width (LTC), head length (LC), tail length (LCD), forelimb length (PA) and hind limb length (PP).
A principal component analysis (PCA) was performed to investigate the levels and patterns of sexual size and shape variation, together with the relationship between the variation patterns within and between European newt clades. This analysis was performed using the correlation matrix for a pooled data set. PC1 was interpreted as an axis of variance of body size (Bookstein et al. 1985). PC1 scores for each individual were used to calculate body size index.
There are a variety of ways to quantify SSD (e.g. Lovich and Gibbons 1992; Badyaev and Hill 2000). For the purposes of this study, the multivariate SSD index (IPC1 = PC1 female scores − PC1 male scores) was calculated as a measure of dimorphism in overall body size. To investigate the level of inter-population variation in SSD, two-way anovas were performed using PC1 scores as dependent variable and with population, sex and their interaction as factors. To analyse sexual dimorphism in body shape, regression of all the extracted shape variables on the PC1 score was carried out for each taxa and sex separately. A significant multivariate regression indicates significant allometry. Secondly, the pattern and the difference in allometric relation of size-dependent shape changes between sexes were examined by comparing regression vectors between sexes. Angles between regression vectors were calculated. The statistical significance of differences between vectors was obtained by bootstrapping (400 iterations), using the veccompare program in the integrated morphometrics programs series (Sheets 2000). When the angles between two vectors are not significantly different from each other, the sexes exhibit similar size-related shape changes.
To estimate the phylogenetic signal, we used a method of phylogenetically independent contrasts (Felsenstein 1985) to calculate independent contrast values with the software cactus version 1.13 (Schwilk 2000; Schwilk and Ackerly 2001). Based on the obtained values, the quantitative convergence indices (QVI) of Ackerly and Donoghue (1998) were calculated. A randomization method was used to provide null distributions for testing the statistical significance of QVI (Ackerly and Reich 1999; Blomberg et al. 2003). If the variance of the data in their position on the phylogenetic tree was smaller than 5% of the variances of the randomized data sets, the null hypothesis of no phylogenetic signal was rejected.
The relation between body size and SSD (allometry for SSD; Fairbairn 1997) was estimated from a log/log plot of the independent contrast values for male size on independent contrast values for female size. Significant departure from isometry (the slope of a model II regression of log male size on log female size significantly different from 1) indicates an association between the level of SSD and body size for one sex.
Results
Phylogenetic relations among newts in the Balkans
Of the 502 16S rRNA sites examined, 103 sites were variable, 83 of which were parsimony-informative (130 and 101, respectively, including the outgroups). ML analysis under the GTR + I + G model resulted in a topology with ln L = −1897.4467 (G-shape parameter with four discrete rate categories = 1.015; proportion of invariable sites = 0.621; nucleotide frequencies: A = 0.348, C = 0.206, G = 0.191, T = 0.255) (Fig. 2).
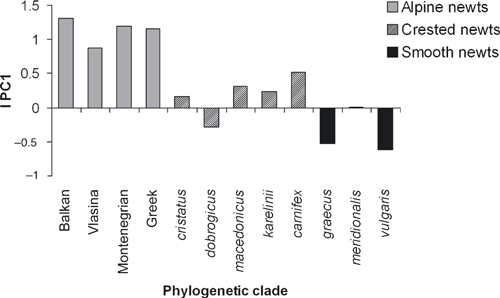
The multivariate SSD indices IPC1, calculated as mean female PC1 scores – mean male PC1 scores
The 16S rRNA sequences, each representing a single representative haplotype, fell into three distinct clades (crested newts, alpine newts and smooth newts) (Fig. 2). The crested newt clade comprised five haplotypes, each one corresponding to one of the analysed Triturus taxa (T. cristatus, T. dobrogicus, T. karelinii, T. carnifex and T. macedonicus). The respective haplotype clades are further referred to as the cristatus, dobrogicus, karelinii, carnifex and macedonicus clades. Since the taxonomic status of the four alpine newt haplotype clades is still under consideration (Sotiropoulos et al. 2007), they were named according to their geographic origin: the Balkan clade, which is endemic and widespread on the Balkans; the Montenegrin clade, confined mostly to Montenegro; the Vlasina clade, confined strictly to the Vlasina region in South-East Serbia; and the Greek clade, the southern-most clade of alpine newts. Finally, the three smooth newt haplotype clades were named the vulgaris, meridionalis and graecus clades.
Variation in the patterns of sexual size and shape dimorphism
PC1 explained the largest proportion in overall variation (88.1%), with proportionally positive loadings of all analysed traits. The largest proportion of shape variation (5.6%) was explained by PC2, while the remaining five PC, PC3–PC7, explained 6.3% of the variation in shape (Table 1).
| Trait | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 |
|---|---|---|---|---|---|---|---|
| SVL | 0.396 | −0.138 | 0.284 | −0.116 | −0.027 | −0.191 | −0.832 |
| D | 0.377 | −0.313 | 0.622 | −0.123 | −0.378 | 0.155 | 0.437 |
| LTC | 0.379 | 0.357 | 0.236 | −0.199 | 0.762 | −0.066 | 0.219 |
| LC | 0.355 | 0.651 | 0.023 | 0.581 | −0.335 | 0.015 | −0.004 |
| PA | 0.389 | 0.048 | −0.443 | −0.338 | −0.078 | 0.722 | −0.091 |
| PP | 0.389 | −0.016 | −0.462 | −0.327 | −0.250 | −0.643 | 0.231 |
| LCD | 0.360 | −0.574 | −0.258 | 0.612 | 0.308 | 0.015 | 0.079 |
| Eigenvalue | 6.167 | 0.388 | 0.172 | 0.148 | 0.085 | 0.024 | 0.015 |
| Proportion | 0.881 | 0.056 | 0.025 | 0.021 | 0.012 | 0.003 | 0.002 |
To test for sexual size and shape dimorphism, the PC1 and PC2 scores were subjected to a two-way anova using sex, population and their interaction as factors. The significant interaction between sex and population indicated the presence of variation in sexual size or sexual shape dimorphism among populations (Table 2).
| Clade | PC1 | PC2 | |||||
|---|---|---|---|---|---|---|---|
| Population | Sex | Population × sex | Population | Sex | Population × sex | ||
| Alpine newts | Balkan | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0075 |
| Greece | 0.0001 | 0.0001 | 0.1276 | 0.0001 | 0.0001 | 0.5552 | |
| Montenegro | 0.0001 | 0.0001 | 0.1510 | 0.0001 | 0.0001 | 0.0001 | |
| Vlasina | 0.0001 | 0.0001 | 0.1778 | 0.0001 | 0.0044 | 0.2396 | |
| Crested newts | cristatus | 0.0001 | 0.1950 | 0.0173 | 0.8249 | 0.2443 | 0.0116 |
| dobrogicus | 0.0001 | 0.0154 | 0.2026 | 0.0001 | 0.0547 | 0.0261 | |
| macedonicus | 0.0001 | 0.0015 | 0.0169 | 0.0001 | 0.0001 | 0.0004 | |
| carnifex | 0.0001 | 0.0001 | 0.1132 | 0.0001 | 0.0001 | 0.0160 | |
| karelinii | 0.0001 | 0.0004 | 0.2642 | 0.0001 | 0.0001 | 0.2052 | |
| Smooth newts | vulgaris | 0.0001 | 0.0001 | 0.0033 | 0.0001 | 0.0001 | 0.0353 |
| graecus | 0.0001 | 0.0022 | 0.5418 | 0.0001 | 0.0022 | 0.5782 | |
| meridionalis | 0.0001 | 0.3575 | 0.0001 | 0.0001 | 0.3374 | 0.0001 | |
- Significant values (after Bonferroni corrections) are given in bold.
Statistically significant sexual dimorphism in both size and shape was found for all alpine newt clades. Significant variation among populations was found for the Balkan clade (for size) and for the Montenegrin clade (for shape). The analysis of PC1 and PC2 individual scores revealed differences in the magnitude and pattern of sexual dimorphism among the crested newt clades. While no sexual dimorphism in size and shape was evident for the cristatus and dobrogicus clades, the remaining crested newt clades showed much more pronounced and statistically significant female-biased SSD as well as significant dimorphism in shape. Significant inter-population variation was found for body shape in the macedonicus clade only. Among smooth newts, non-significant sexual size and shape dimorphism was found in the meridionalis clade, with high inter-population variation in both parameters. The other two smooth newt clades showed significant male-biased SSD (Table 2, Fig. 2) and statistically significant sexual dimorphism in body shape. Previous analyses (Table 2) and SSD indices calculated upon PC1 scores (Fig. 2) showed that: (1) among analysed newts, female-biased SSD predominated; (2) a reversal in the pattern of SSD is occasionally seen, as significant male-biased SSD occurred in two smooth newt clades (vulgaris and graecus).
Do clades differ in the direction and magnitude of sex-related allometry?
Statistically significant multivariate regressions of shape variables (PC2–PC7) on size (PC1) were found within all analysed phylogenetic clades for both sexes (Wilks’ Lambda, p < 0.01 in all comparisons). To estimate the difference in the pattern of size-dependent shape changes between sexes, the angles between sex-specific allometric vectors were calculated. This analysis revealed significantly different patterns in allometric relations between females and males for the Vlasina clade of alpine newts, for the cristatus, dobrogicus, carnifex and macedonicus clades of crested newts and for the graecus and meridionalis clades of smooth newts (Table 3).
| Clade | Angle | F | M | Difference | |
|---|---|---|---|---|---|
| Alpine newts | Balkan | 6.9° | 12.6° | 16.2° | ns |
| Greece | 22.8° | 45.1° | 49.7° | ns | |
| Montenegro | 63.1° | 66.6° | 66.2° | ns | |
| Vlasina | 117.4° | 75.0° | 52.2° | * | |
| Crested newts | cristatus | 106.8° | 74.1° | 38.8° | * |
| dobrogicus | 71.6° | 45.3° | 46.9° | * | |
| macedonicus | 57.1° | 35.4° | 44.5° | * | |
| carnifex | 57.6° | 29.0° | 35.1° | * | |
| karelinii | 60.5° | 82.3° | 65.7° | ns | |
| Smooth newts | vulgaris | 33.5° | 26.8° | 36.6° | ns |
| graecus | 88.9° | 74.5° | 53.6° | * | |
| meridionalis | 28.2° | 33.9° | 42.9° | ns |
- F, females; M, males; ns, non-significant; *p < 0.05.
Is the level and pattern of sexual size and shape dimorphism phylogenetically constrained?
In order to estimate the phylogenetic effect on body size and on the degree of sexual size and shape dimorphism among European newts, the QVI of Ackerly and Donoghue (1998) was calculated (Table 4). With the exception of the difference between sex-specific allometric vectors as expressed by the angle between the regression lines, all traits displayed a statistically significant phylogenetic signal.
| Trait | Observed QVI | Expected QVI | p for phylogenetic signal |
|---|---|---|---|
| PC1 females | 0.149 | 0.825 | 0.001 |
| PC1 males | 0.083 | 0.801 | 0.001 |
| IPC1 | 0.316 | 0.832 | 0.002 |
| Angle | 0.985 | 0.857 | 0.150 |
- Randomization methods were used to provide null distributions for testing the statistical significance of the quantitative convergence index (QVI; Ackerly and Reich 1999).
Analyses of calculated independent contrast values showed that the correlation between female size (PC1 females) and SSD (IPC1) significantly increased after phylogenetic correction (Table 5). The statistically significant coefficient of correspondence between the independent contrast values for female size and the degree of SSD indicated a correlated evolutionary change between female size and SSD (Table 5). A statistically significant coefficient of correspondence was also found between the independent contrast values for male size and SSD. However, the regression slope obtained for the independent contrast values of male size on female size (y = 0.819, CI = 0.713−0.942), indicated that SSD in European newts is driven by a disproportionate increase in female size. The increase in female size was not accompanied by a proportional increase in male size, for a statistically significant departure from isometry was found (F = 10.502, p < 0.01).
| Var1 | Var2 | AC | CC |
|---|---|---|---|
| PC1f | PC1m | 0.968* | 0.959* |
| PC1f | IPC1 | 0.289 | 0.687** |
| PC1m | IPC1 | 0.038 | 0.455*** |
| IPC1 | Angle | −0.219 | −0.337 |
- The values before (AC) and after (CC) correction for phylogeny are given. Coefficients of correspondence (CC) represent the pairwise correlation among selected variables based on the calculated independent contrast values.
- *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Patterns of sexual size and shape dimorphism in the Balkan newts
A remarkable diversity of patterns in sexual size and shape dimorphism was found among the analysed clades of newts from the Balkan Peninsula. Although female-biased SSD prevailed, a variable relationship between sexes, with respect to size and shape, was found within the smooth newts, ranging from an insignificant female-biased SSD (meridionalis), to a significant male-biased dimorphism (vulgaris and graecus). Within crested newts, this relationship extended from an insignificant male-biased SSD (dobrogicus) to a statistically significant female-biased SSD (carnifex, macedonicus, karelinii).
Female-biased SSD is usually explained as result from asymmetric selective pressures favouring either larger females or smaller males, with selection for large females followed by selection for small males as a prerequisite for stable female-biased SSD (e.g. Zamudio 1998; Blanckenhorn 2000, 2005; Cox et al. 2003). Key factors in the evolution of female size seem to be primarily correlated with strong selective pressures on female fecundity, as the relative size and shape of the female’s abdomen may be tightly associated with fecundity (e.g. Shine 1989). However, no direct evidence for such a correlation was found in newts (Verrell et al. 1986). Clearly, we have a limited understanding of the interplay between selective pressures related to sex, viability and fecundity, which are, according to SSD theory, important ultimate factors in shaping differences between sexes (e.g. Blanckenhorn et al. 2007). Among potential proximate factors and processes that affect sexual dimorphism from a life-history perspective, it seems that differences in growth rates between sexes are important in mediating SSD in European newts. A study of the ontogeny of sexual size and shape differences in crested newt (T. macedonicus) found that juvenile females have a much higher growth rate than males, especially in the period between the first and the second hibernation (Cvetković et al. 1997). Other life history traits that have been studied (age at sexual maturity, longevity, annual survival rates and growth rates during adult phase) do not differ between the sexes (Kalezić and Djorović 1998).
The causes of sexual dimorphism in shape are the same as those that could produce SSD, including an allometric response to selection for sexual dimorphism (Reiss 1989; Andersson 1994). In most species and/or phylogenetic clades within species, changes in shape are concordant with those of size (see Butler and Losos 2002 and references therein). Our results showed that a relatively small amount of the total morphometric variation accounts for shape differences among the analysed newts. However, the shape changes were significantly related to size within each clade and sex. These results indicate that allometric scaling could be responsible for the observed differences in shape between sexes.
The effects of phylogeny on sexual size and shape variation
We found that female and male body sizes and degree of SSD displayed a statistically significant phylogenetic signal. This result may have been due to the inclusion of multiple sets of closely related groups that represent the traditionally recognized species (e.g. four clades for M. alpestris) or subspecies (three clades of L. vulgaris), and their particular positions within the phylogeny. In that case, the detected phylogenetic effect could have arisen from the fact that these groups invariably cluster at the tips of the phylogenies, and not from the branching patterns within or among these groups (Guill et al. 2003). However, the same analyses found no phylogenetic signal for sexual shape dimorphism, despite the use of the same phylogenetic tree.
The relationship between phylogenetically-corrected female and male size indicated that SSD in newts from the Balkan Peninsula is driven by a disproportionate increase in female size. This result indicates that the level of SSD increases with larger female body size, contrary to the expectations derived from Rensch’s rule. While numerous data and hypotheses have been generated that support and explain this rule (e.g. Abouheif and Fairbairn 1997), our results should be added to the growing amount of evidence that questions the generality of the Rensch’s rule as a scaling phenomenon, especially in the groups with female-biased SSD (Tubaro and Bertelli 2003;Blanckenhorn et al. 2007; Webb and Freckleton 2007).
Acknowledgements
We gratefully acknowledge the constructive reviews provided by Tim Halliday and Martin Fischer. Their efforts greatly improved the presentation and interpretation of our data. This study was partially funded by the Serbian Ministry of Science (project no. 143052).




