New data from an enigmatic phylum: evidence from molecular sequence data supports a sister-group relationship between Loricifera and Nematomorpha
Nuevos datos sobre un filo enigmático: los estudios de secuencias moleculares apoyan una relación de grupos hermanos entre los Loricíferos y los Nematomorfos
Abstract
enLoricifera is one of the most recently discovered animal phyla. So far, the group has been considered closely related to Kinorhyncha and Priapulida, and assigned to the ecdysozoan clade Cycloneuralia. Using Bayesian inference, we present the first phylogeny that includes 18S rRNA and Histone 3 sequences from two species of Loricifera. Intriguingly, we find support for a sister-group relationship between Loricifera and Nematomorpha. Such relationship has not been suggested previously and the results imply that a revision of our conception of early ecdysozoan evolution is required. Additionally, the data suggest that evolution through progenesis (sexual maturation of larvae) may have played an important role among the ancestral cycloneuralians.
Resumen
frLos Loricíferos constituyen uno de los filos descubiertos más recientemente. Hasta hoy, este taxón se ha considerado estrechamente emparentado con los Kinorrincos y los Priapúlidos y se ha asignado al clado Cicloneuralios dentro de los Ecdisozoos. Presentamos aquí el primer estudio filogenético que, mediante la inferencia bayesiana, utiliza secuencias de ARNr 18S e histona 3 de dos especies de loricíferos. Sorprendentemente, nuestros resultados sugieren una relación de de grupos hermanos entre Loricíferos y Nematomorfos. Este parentesco no se ha propuesto con anterioridad, y estos resultados hacen necesaria una revisión de las ideas sobre la evolución de los primeros ecdisozoos. Además, los datos sugieren que la evolución mediante progénesis (maduración sexual de las larvas) puede haber tenido un importante papel entre los cicloneuralios ancestrales.
Introduction
Loricifera is among the most recently described metazoan phyla (Kristensen 1983). The group consists of marine, meiobenthic organisms with an adult size ranging from 80 to 500 μm, and are hence, among the smallest known animals. Their preferred habitats are in deep sea mud, on remote sea mounts or in small isolated shell gravel banks, which makes them hard to collect for studies (Kristensen 1983, 1991). In spite of their minute size loriciferan morphology is extraordinarily rich in details and they probably display some of the most complex life cycles (life histories) among all metazoans. The body of the adult specimens consists of a head, a neck, a thorax and an abdomen. The head is formed by a retractable introvert with a mouth cone surrounded by up to nine rings with appendages, the so-called scalids. Some species may have up to two hundred scalids and each of them is independently movable and used for locomotion and as sensory organs. The neck and thorax are accordion-like and the neck is equipped with rows of basal plates and small, flattened appendages, called trichoscalids. The abdomen is covered with either 6–10 lorica plates or 22–60 folds (plicae) (Kristensen 1983; Higgins and Kristensen 1986; Kristensen and Higgins 1991; Heiner 2004; Kristensen and Gad 2004). Their reproductive cycle differs between the genera and varies in complexity from regular gamogenetic mating between separate sexes and development through a series of moulting larval instars to much more complex cycles that include parthenogenesis, sexual maturity of larvae, paedogenesis, reduction and simplification of several life stages and the occurrence of hermaphroditic adults (Kristensen and Brooke 2002; Kristensen 2003; Gad 2004, 2005a,b; Heiner 2008).
Within the past century only four new animal phyla, Loricifera (Kristensen 1983), Gnathostomulida (Ax 1956), Cycliophora (Funch and Kristensen 1995) and Micrognathozoa (Kristensen and Funch 2000) were discovered and named. The latter three had great impact on our conception of animal evolution and metazoan phylogeny, and their phylogenetic positions are still highly debated (e.g. Littlewood et al. 1998; Giribet et al. 2000, 2004; Sørensen 2003; Halanych 2004; Jenner 2004; Funch et al. 2005; Passamaneck and Halanych 2006).
Loricifera apparently shares several characters with kinorhynchs and priapulids, and these taxa are usually united in the clade Scalidophora (Lemburg 1995). Scalidophora is considered the sister group to the Nematoida, consisting of Nematoda and Nematomorpha, and the monophyly of these two clades has rarely been questioned, at least on morphological grounds (Kristensen and Higgins 1991; Neuhaus 1994; Schmidt-Rhaesa 1997/98; Schmidt-Rhaesa et al. 1998; Nielsen 2001; Neuhaus and Higgins 2002). Only Adrianov and Malakhov jeopardized this hypothesis and proposed in a series of papers the clade Cephalorhyncha, consisting of the scalidophoran taxa and Nematomorpha (Malakhov 1980; Adrianov and Malakhov 1995, 1996, 1999). More recently Park et al. (2006) presented the first phylogenetic study that included molecular sequence data from a species of Loricifera. The study supported the close relationship between kinorhynchs and priapulids, but the analyses did not find support for Scalidophora. Unfortunately, the study failed to establish the phylogenetic position of loriciferans, but it placed a great question mark on the prevalent hypothesis about their origin and closest allies.
One of the most important modern steps towards an understanding of metazoan evolution was the establishment of the clade Ecdysozoa (Aguinaldo et al. 1997) that contains all moulting animals, including arthropods, onychophorans, tardigrades, nematoids and scalidophorans. This hypothesis not only abandoned the former concept about evolution of articulation, namely an assumed homology between annelid and panarthropod articulation, but it also forced us to reset many ideas about the evolution of the most species rich group in the animal kingdom, namely the arthropods (e.g. Schmidt-Rhaesa et al. 1998; Scholtz 2002; Giribet 2003). According to the more traditional view, arthropods and annelids evolved from a common segmented ancestor, but an increasing amount of evidence supports that the panarthropods (= Tardigrada, Onychophora and Euarthropoda) are more closely related to Nematoida and Scalidophora. Hence, the establishment of the phylogenetic position of Loricifera can turn out to be highly significant; not only to clarify the phylogeny of these small, enigmatic critters, but also to understand the early evolution of the largest branch in the Tree of Life.
In the present study, we present a phylogenetic analysis of Loricifera based on Bayesian inference of molecular sequences. The data set includes 18S rRNA and Histone 3 sequences for representatives of all ecdysozoan main lineages (except for H3 of Nematoida), including two distantly related loriciferan species, and the most comprehensive taxon sampling of scalidophoran species presented in any molecular study up to now.
Materials and Methods
Analyzed sequences
The data set includes 53 ecdysozoan terminals and seven outgroup taxa, including five lophotrochozoans and two chordates. We choose not to include the taxa Meiopriapulus fijiensis (Priapulida) and Sagitta sp. (Chaetognatha) even though both were included in the study of Park et al. (2006). Meiopriapulus fijiensis was included in the initial analyses, but the taxon always formed a clade with the onychophorans most basally in the trees. A close relationship between these taxa seems very unlikely, and since grouping of apparently distantly related taxa near the root of the tree may indicate problems with long-branch attraction (see e.g. Bergsten 2005), we decided to exclude M. fijiensis from the analyses. Chaetognatha was not included in the outgroup because we preferred to use an outgroup composed of lophotrochozoans and chordates.
Molecular sequences of the nuclear locus 18S rRNA are included for all terminals, whereas the nuclear protein-coding gene Histone 3 (hereafter H3) were available for 21 taxa only. The non-scalidophoran sequences were downloaded from GenBank, whereas most of the scalidophoran sequences were obtained from new material (Table 1). We generally preferred GenBank sequences that had been used in previous studies by one of the authors. The new loriciferan sequences were obtained from an undescribed species of Nanaloricus. The specimens were collected in Trezen ar Skoden at Roscoff, France, in March 2005. The species was recorded for the first time at this locality in 1985.
| Phylum | Species | 18S rRNA | Histone 3 |
|---|---|---|---|
| Chordata | Branchiostoma lanceolatum | AY428817 | AY428831 |
| Ciona intestinalis | AB013017 | – | |
| Entoprocta | Loxosomella murmanica | AY218100 | AY218150 |
| Cycliophora | Symbion pandora | EF142083 | AY218153 |
| Rotifera | Encentrum astridae | DQ297695 | DQ297800 |
| Branchiopoda | Neocrania anomala | DQ279934 | DQ279997 |
| Polychaeta | Lumbriculus variegatus | AY040693 | – |
| Euarthropoda | Limulus polyphemus | U91490 | AF370813 |
| Siro rubens | AY428818 | AY428834 | |
| Opilioacarus texanus | AF124935 | – | |
| Nesticus cellulanus | AF005447 | – | |
| Damon gracilis | AY829919 | AY829981 | |
| Scutigerina weberi | AY288689 | DQ222182 | |
| Cormocephalus monteithi | AF173249 | – | |
| Hypogastrura sp. | AY338691 | AY338616 | |
| Anaspides tasmaniae | L81948 | – | |
| Alvinocaris longirostris | AB231688 | – | |
| Libinia emarginata | AY743953 | – | |
| Homarus americanus | AY743645 | AF370819 | |
| Onychophora | Peripatopsis capensis | AF119087 | – |
| Peripatoides novaezealandiae | AF342794 | – | |
| Euperipatoides leuckarti | U49910 | AF110849 | |
| Tardigrada | Richtersius coronifer | AY582123 | – |
| Pseudechiniscus islandicus | AY582119 | – | |
| Echiniscus viridissimus | AF056024 | – | |
| Milnesium tardigradum | AY582120 | – | |
| Ramazzottius oberhauseri | AY582122 | – | |
| Thulinius stephaniae | AF056023 | – | |
| Macrobiotus sp. | U49912 | – | |
| Nematoda | Ascaris lumbricoides | U94366 | – |
| Trichinella spiralis | AY497012 | – | |
| Alaimus sp. | AJ966514 | – | |
| Tylolaimophorus minor | AJ966512 | – | |
| Contracaecum microcephalum | AY702702 | – | |
| Howardula aoronymphium | AY589304 | – | |
| Bursaphelenchus xylophilus | AY508034 | – | |
| Nematomorpha | Gordius paranensis | AF421766 | – |
| Paragordius sp. | AY428819 | – | |
| Paragordius tricuspidatus | AF421771 | – | |
| Euchordodes nigromaculatus | AF421764 | – | |
| Spinochordodes tellinii | AF421773 | – | |
| Chordodes morgani | AF036639 | – | |
| Neochordodes occidentalis | AF421768 | – | |
| Nectonema agile | AF421767 | – | |
| Kinorhyncha | Centroderes n. sp. | EU669452 | EU669445 |
| Echinoderes horni | EU669453 | – | |
| Echinoderes collinae | EU669454 | EU669446 | |
| Echinoderes spinifurca | EU669455 | EU669447 | |
| Echinoderes truncatus | EU669456 | – | |
| Pycnophyes kielensis | U67997 | – | |
| Pycnophyes greenlandicus | AY428820 | AY428836 | |
| Pycnophyes beaufortensis | EU669457 | EU669448 | |
| Priapulida | Priapulopsis bicaudatus | EU669458 | EU669449 |
| Priapulus caudatus | AF025927 | – | |
| Halicryptus spinulosus | AF342790 | AY428837 | |
| Tubiluchus corallicola | AF119086 | – | |
| Tubiluchus troglodytes | EU669459 | – | |
| Tubiluchus sp. | EU669460 | EU669450 | |
| Loricifera | Nanaloricus n. sp. | EU669461 | EU669451 |
| Pliciloricus sp. | AY746986 | – |
- Boldfaced taxa were sequenced by the authors for the present study.
New DNA sequences were mostly extracted from fresh tissues using the Qiagen DNeasy® Tissue Kit (Qiagen AB, Ballerup, Denmark). We attempted to amplify four different molecular loci, but only 18S rRNA and histone H3 amplified for Nanaloricus n. sp. The complete 18S rRNA loci were amplified into three overlapping fragments using the following primer pairs: 1F – 4R, 3F – 18sbi, and 18Sa2.0 – 9R (Giribet et al. 1996; Whiting et al. 1997). H3 were amplified with H3aF – H3aR (Colgan et al. 1998). The amplified samples were purified using QIAquick® PCR Purification Kit (Qiagen AB, Ballerup, Denmark). The purified fragments were labelled using BigDye® Terminator v3.0 sequence reaction, and sequenced with an ABI 3730 genetic analyzer. Chromatograms obtained from the automatic sequencer were read and ‘contig sequences’ (assembled sequences) were assembled using the sequence editing software Sequencher™ 4.0. All new sequences have been deposited in GenBank under accession numbers EU669445–EU669461.
The sequences were aligned with a Clustal W (Thompson et al. 1994) implementation in BioEdit Sequence Alignment Editor ver. 7.0.4.1 (Hall 1999). Gap opening and extension costs were set to 10 and 0.1, respectively. The aligned sequences were carefully examined and non-alignable or hypervariable regions were discarded manually.
Analyses
Bayesian analyses were conducted using MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003). MrModeltest v 2.2 (Nylander 2004) was used to select the best-fit model of nucleotide substitution for the data sets. All models were tested independently for the two loci, and in both cases the best fit was reported for the GTR + I + Γ model (hereafter GTR model). Analytical parameters were set according to the model of sequence evolution selected by MrModeltest. MrBayes were run for 4 million generations with four chains starting from a random tree. Samples were taken at every 400 generations giving a total sample of 10 001 trees. All parameters were checked for stationarity using the program Tracer 1.4 (Rambaut and Drummond 2004) and the first 1000 trees were discarded as burnin (the number of generations until parameters reach stationarity). A 50% majority-rule consensus tree was constructed from the remaining 9001 trees. The number of gamma categories was set to eight. A Maximum likelihood analysis were also conducted using PAUP* 4.0 (Swofford 2002). A heuristic search using 100 random-addition-sequence replications with TBR (tree bisection-reconnection) branch-swapping using the GTR + I + Γ model. Nodal support for all topologies was furthermore assessed by parsimony jackknifing (Farris et al. 1996; Farris 1997). The jackknife analysis was done with PAUP* 4.0 (Swofford 2002) using heuristic search method, a deletion percentage of 50 and 1000 replicates.
To test the robustness of the data set’s dependence on the rate distribution, we furthermore analyzed the data set using autocorrelated rates across sites (GTR-A model). This was done using the ‘lset = adgamma’ option in MrBayes which sets the marginal rate of substitution to gamma, but assign correlated rates to the adjacent sites. The standard ‘lset = invgamma’ option sets a proportion of the sites to be invariable while the rate for the remaining sites are drawn from a gamma distribution independently of each other. These two different types of gamma rates were further analyzed with the ‘covarion = yes’ option (GTR-COV and GTR-A-COV models) which forces the use of a covarion-like model of substitution (Tuffley and Steel 1998). The covarion model (GTR-COV) allows the rate at a site to change over its evolutionary history. Specifically, the site is either on or off. When it is off, no substitutions are possible. When the process is on, substitutions occur according to the specified substitution model.
A Bayes factor test, as implemented in Tracer 1.4 (Rambaut and Drummond 2004) was used to test our result obtained by Bayesian inference against an alternative topology that included monophyletic Scalidophora [Loricifera + (Priapulida + Kinorhyncha)].
Results
MrModeltest suggested the general time reversible model of nucleotide substitution (GTR; Tavaré 1986; Rodríguez et al. 1990) with gamma-distributed rates among sites and a correction for invariable sites (GTR + Γ + I = GTR model) as the best-fit model of substitution for the data set when evaluating the 24 models available in MrBayes.
The three independent analyses based on the GTR model produced majority rule consensus trees with identical topologies. One of the resulting majority rule consensus trees based on the GTR model is presented to the left in Fig. 1, whereas the tree to the right summarizes the trees analyzed under the GTR, GTR-AUTO and GTR-COV models. Fig. 2 shows a phylogram indicating the actual branch lengths in the tree obtained under the GTR model. Analyses under the three different models produced trees with almost identical topologies, which imply that the result is stable to choice of model. Trees analyzed under the GTR-A-COV model never reached stability and are not considered any further in the following.
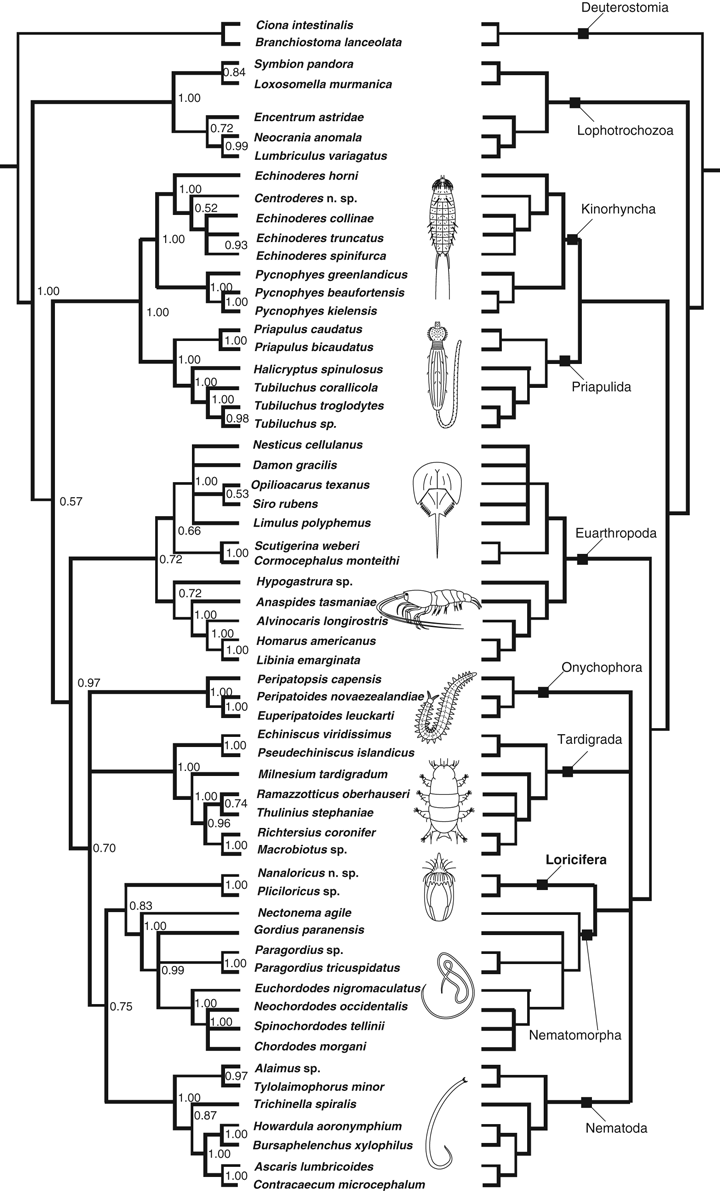
Resulting trees from Bayesian analyses of two molecular markers: 18S rRNA and Histone 3. Left: the phylogeny under Bayesian inference using the GTR + I + Γ model of nucleotide substitution. The values on the braches indicate the posterior probabilities for the data set. Right: a strict consensus of trees produced under all three tested models (see the Method section)
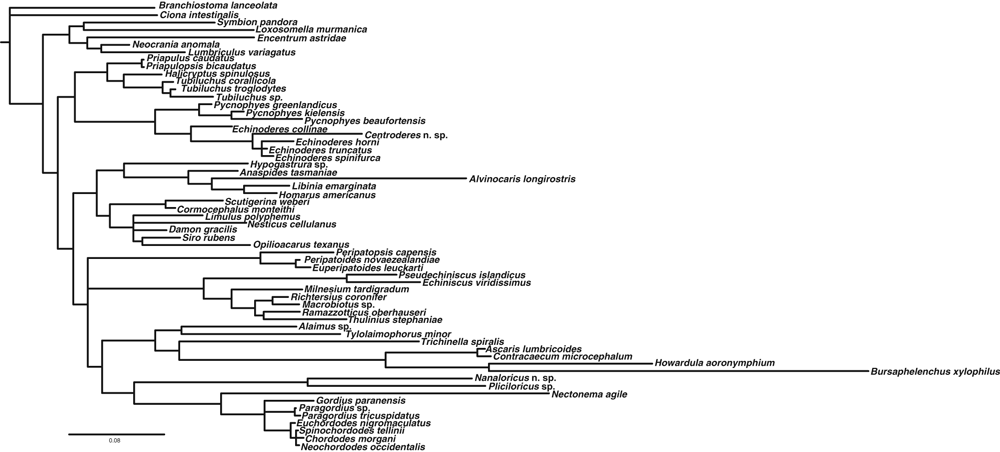
Phylogram indicating branch lengths in the tree obtained under the GTR + I + Γ model of nucleotide substitution
The obtained trees support monophyly for all included phyla (Fig. 1). The root is set at the two chordate outgroup taxa Ciona and Branchiostoma, and the first clade to branch off consist of the remaining outgroups that all are sampled from the Lophotrochozoa. Within the ingroup, Priapulida and Kinorhyncha form the most basal clade (pp = 1.00), which is the sister group to a clade with the remaining ecdysozoans (pp = 0.97 under the GTR model). The next clade to branch off is the euarthropods. Euarthropod monophyly is supported in all analyses, but the posterior probability for the clade ranges between 0.72 and 1.00. The highest pp (= 1.00) is found with the GTR-COV model. However, support by parsimony jackknifing is below 50 (Fig. 3). The euarthropod sister group is a weakly supported clade (pp = 0.70 under the GTR model) with the remaining panarthropods and cycloneuralians. The relationships between the phyla within this clade differ slightly, depending on choice of model. Trees analyzed under the GTR and GTR-A models form a trichotomy with Onychophora, Tardigrada and a clade with Nematoda, Nematomorpha and Loricifera, whereas trees analyzed under the GTR-COV model place Onychophora as sister group to a clade with Tardigrada, Nematoda and Nematomorpha + Loricifera. However, in spite of these incongruent topologies, all trees find a sister-group relationship between Loricifera and Nematomorpha (pp = 0.83 under the GTR model).
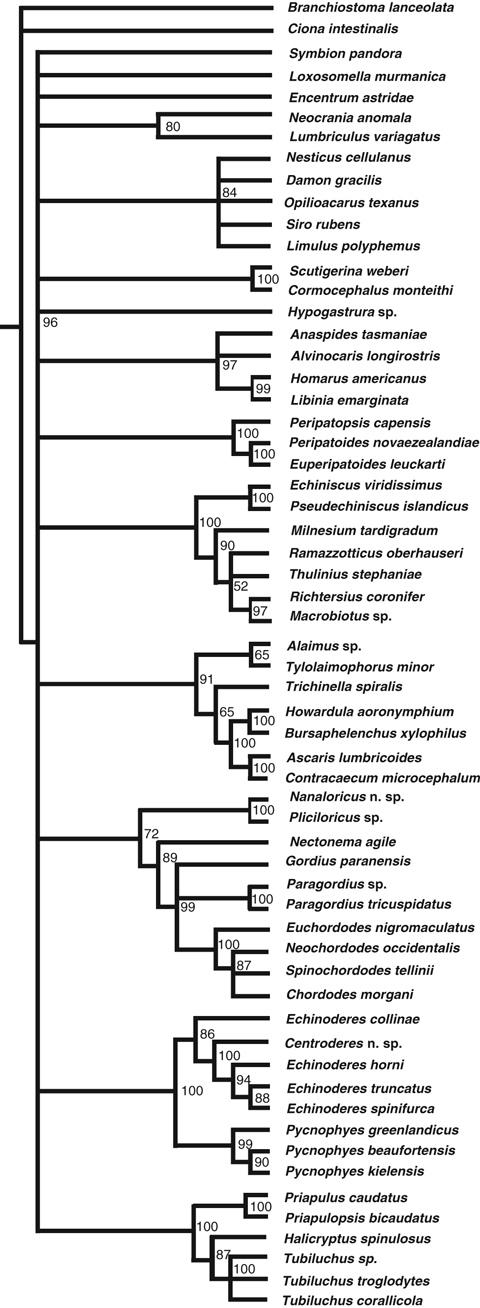
Jackknife 50% majority-rule consensus tree. Numbers at nodes indicate jackknife proportions above 50%
The Maximum likelihood analysis (tree not shown) also recovered a Nematomorpha–Loricifera sister-group relationship, with Nematoda as their closest relative. Jackknife proportion for the Nematomorpha–Loricifera clade was 72 (Fig. 3). The Bayes factor test rejected the alternative topology with scalidophoran monophyly – we find substantial support (K = 7.165) for the hypothesis found using Bayesian analysis.
Discussion
The monophyly of Scalidophora (Priapulida, Kinorhyncha and Loricifera) and Nematoida (Nematoda and Nematomorpha) have rarely been questioned based on morphology, and both clades are apparently supported by several unique apomorphies (Schmidt-Rhaesa 1997/98; Schmidt-Rhaesa et al. 1998; Kristensen 2003). Bayesian inference of the present sequence data indicates, however, that these interrelationships may need to be reconsidered. Our analyses supported a sister-group relationship between Loricifera and Nematomorpha under all tested models (Fig. 1) and this relationship was recovered by the maximum likelihood analysis and jackknifing analysis (Fig. 3) as well, hence we consider this relationship to be consistent and well supported. As noted previously, the position of some taxa in the analysis may be sensitive to long-branch problems, and in some of the initial analyses especially the onychophorans tented to form a basal clade in the obtained trees together with Loricifera (or Meiopriapulus as mentioned above). However, this problem got minimized and finally disappeared as more terminals were added, and it appears that the extensive taxon sampling has minimized the effect of eventual long-branch artifacts.
Several recent studies have discussed the cycloneuralian interrelationships and in summary following characters have been suggested as synapomorphic for Scalidophora: (1) introvert with inner and outer retractor muscles, (2) introvert with sensorial scalids, (3) introvert used for locomotion, (4) division of body into proboscis and abdomen, (5) one pair of lateral cuspidate spines (= basally swollen spines) mesially on trunk, (6) specialized sensory organs (papillar sensory spots and flosculi), and (7) compound filter of protonephridia built by two or more terminal cells (Schmidt-Rhaesa 1997/98; Lemburg 1999; Neuhaus and Higgins 2002). The list of characters definitely seems impressive, but after a critical revision one might question whether some of them should be considered plesiomorphic, at least for the scalidophoran taxa. It should furthermore be kept in mind that the nematomorph larva must have undergone several modifications during adaptation to their endoparasitic life strategy, and that some character traits might have been reduced or lost in this process. This means that, e.g. characters related to locomotion and sensorial functions might have been lost, which would explain the absence of movable and sensorial scalids and sensory spots in the nematomorph larva. The absence of protonephridia in nematomorphs (and nematodes) is also usually interpreted as a secondary reduction (Sørensen et al. 2000; Schmidt-Rhaesa 1997/98). The presence of lateral cuspidate spines mesially on the trunk is a rather dubious synapomorphy as such spines are not present in any loriciferan species. The Higgins larva may truly be equipped with ventrolateral appendages, but these are segmented and movable, and structurally very different from cuspidate spines (e.g. see Heiner 2004; Kristensen and Gad 2004). A division of the body into proboscis and abdomen is present in larvae of Nematomorpha as well as the scalidophoran taxa. Hence, most of the suggested scalidophoran synapomorphies can be questioned.
Nematoida is, on the other hand, apparently supported by several strong synapomorphies, such as presence of (mostly) unpaired dorsal and ventral epidermal nerve cords, reduction of circular muscles, reduction of protonephridia, reduction of spermatozoan flagellum and the presence of cloaca in both sexes (Schmidt-Rhaesa 1997/98). Most of these characters appear rather convincing but it should be noted that all of them relate to the adult morphology, whereas most nematomorph similarities with the Loricifera are displayed in the larvae. The incongruence between larval and adult morphology impedes a straightforward comparison of the cycloneuralian taxa, but it also suggests an interesting hypothesis about their evolution. It has often been suggested that progenesis, viz. sexual maturation of larval stages, has played a significant role in the evolution of various meiofaunal groups (Westheide 1987; Worsaae and Kristensen 2005), and Loricifera has been suggested as being a progenetic priapulid (Warwick 2000). One might speculate whether some of the cycloneuralian phyla evolved from a free living or burrowing ancestor with a vermiform adult stage and a larval stage that resembled the nematomorph larva. In such case, it would be likely that certain groups, i.e. loriciferans and the kinorhynch ancestor evolved by progenesis from a larval stage, whereas the nematodes retained the adult stage but lost the larvae, and the nematomorphs retained both stages (Fig. 4). This hypothesis would explain the similarities between on one hand adult nematode and nematomorph stages, and on the other larval nematomorphs and loriciferans. It is furthermore supported by specific traits in the cycloneuralian cuticular ultrastructure: Priapulids, kinorhynchs and loriciferans possess chitin in their body cuticle, whereas cuticular chitin apparently is missing in adult nematomorphs (Neuhaus et al. 1997; Lemburg 1998). However, Neuhaus et al. (1996) demonstrated the presence of chitin in the basal fibrillar cuticle layer in juveniles of the marine nematomorph Nectonema munidae. The juvenile cuticle in Nectonema is the same as carried in the larvae, and the position of chitin corresponds exactly to the location of cuticular chitin in Loricifera and Priapulida. If the presence of cuticular chitin is considered a larval trait, as demonstrated in Nematomorpha, this would support the progenetic origin of loriciferans and probably other scalidophorans as well.
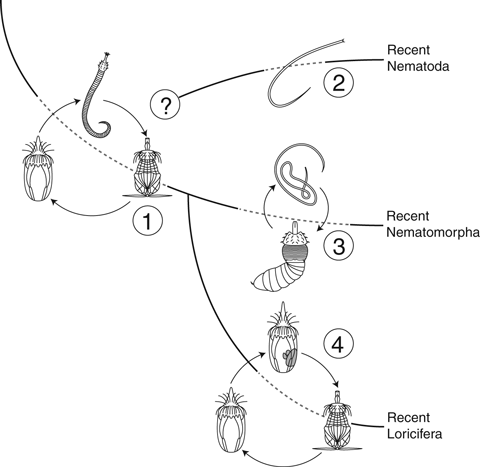
Hypothetical evolution of life cycles within the Loricifera-Nematomorpha-Nematoda clade showing (1) the ancestral life cycle including loriciferan-like larvae and postlarvae and vermiform adults. (2) The nematode lineage branches off and the larval stages are reduced and lost, producing recent nematodes with direct development. As indicated with the question mark, it still remains uncertain whether the nematodes branched from the common Loricifera–Nematomorpha ancestor or shortly after the two lineages had diverged. (3) Recent nematomorph life cycle evolve, resulting in endoparasitic larval stages. The morphology of the larvae changes slightly, e.g. due to adaptations to endoparasitism, but the general biphasic life cycle with introvert bearing larvae and vermiform adults is retained. (4) Loriciferans evolve through progenesis. The sexual maturation of the ancestral (post)larva affects that the adult, vermiform stage is lost
The Loricifera–Nematomorpha sister-group relationship
A sister-group relationship between Loricifera and Nematomorpha has not been suggested previously. Only in the molecular study of Park et al. (2006), some analyses suggested a sister group relationship of loriciferans to the nectonematoid nematomorphs, but never to the entire clade Nematomorpha, which is monophyletic in another molecular analysis (Bleidorn et al. 2002). The position of Loricifera never becomes well-established in the study of Park et al. (2006), and the included loriciferan terminal either branches out most basally in the tree or with unlikely sister taxa, such as the crustacean Cephalocarida or with onychophorans. The much more stable position of loriciferans in our analyses is probably mostly due to an improved taxon sampling, especially among the non-arthropod taxa, whereas the different analytic approach may play a role as well.
Loricifera and the nematomorph larva may share some rather interesting similarities in their mouth cones. Contrary to kinorhynchs and priapulids, that display pentaradial symmetry in their introvert appendages as well as the internal armature (Brown 1989; Adrianov and Malakhov 2001), both loriciferans and nematomorphs have hexaradial symmetry in their internal armature as well as musculature (Kristensen 1991, 2003; Müller et al. 2004). This feature could very well be synapomorphic for loriciferans and nematomorphs. Hexagonal symmetry is also present in the arrangement of some cephalic structures in nematodes. If this symmetry pattern is homologous with that in loriciferans and nematomorphs, the trait could be considered synapomorphic for all three groups, which would support a close relationship as suggested by analyses under the GTR (Fig. 1) and GTR-A models (the third tested model, GTR-COV cannot resolve the relationships between Tardigrada, Nematoda and Nematomorpha + Loricifera and places the three clades in a trichotomy).
In the original description of the phylum Loricifera, Kristensen (1983) considered them as a ‘link’ between Nematomorpha and the two scalidophoran phyla, Priapulida and Kinorhyncha. In this context he pointed out several loriciferan and larval nematomorph traits that could be considered homologous, including a curved and twisted buccal canal, and a diaphragm separating the thorax and abdomen. Kristensen (1983) considered these traits as symplesiomorphies, but in light of our new findings, they could be reinterpreted as loriciferan–nematomorph synapomorphies.
Based on these potential synapomorphies we propose that Loricifera and Nematomorpha are sister groups that evolved from a common ancestor with a Loricifera-like larva that developed through several molts until it reached maturity and metamorphosed into a vermiform, nematomorph-like adult stage (Fig. 4). After the split between the two groups, one of the loriciferan larval stages developed sexual maturity, which prompted the loss of the ancestral adult stage. Contrarily, nematomorphs maintained both larval and adult stages, and their parasitic life cycle evolved subsequently.
Conclusions
Bayesian inference of molecular sequences from two loci supports a sister-group relationship between Loricifera and Nematomorpha. Loricifera has previously been assigned to the morphologically well-supported clade Scalidophora, but a re-evaluation of the scalidophoran characters reveals that most of them could be considered symplesiomorphies. Morphologically, a loriciferan–nematomorph relationship is mostly supported by characters related to the larval nematomorph morphology. The suggested synapomorphies include hexaradial symmetry of internal armature and musculature in the introvert, a curved and twisted buccal canal, and the presence of a diaphragm separating the thorax and abdomen. The obvious similarities between loriciferans and nematomorph larvae, suggest that the Loricifera evolved by progenesis. Nematodes, a likely sister taxon to Nematomorpha–Loricifera, have on the other hand lost their larval stages, which explains the complete dissimilarity between adult nematodes and loriciferans.
Acknowledgements
We thank Professor Claude Jouin-Toulmond and the staff on the Biological Station at Roscoff, France, for providing excellent research facilities, Dr Antonio Todaro for sending us specimens of Tubiluchus troglodytes, Gonzalo Giribet for valuable suggestions to the manuscript, and Fernando Pardos for providing the Spanish translation of the summary. The study was based upon work supported by the US National Science Foundation under grant no. EF #0531757 to MVS and RMK, by the Danish Natural Science Research Council under grant no. 21-04-0331 to MVS, and grant no. 21-04-0047 to MVS and RMK. EW and MBH were supported by the Carlsberg Foundation and the Danish Natural Science Research Council, and HG by the Velux Foundation.




