A longstanding entomological problem finally solved? Head morphology of Nannochorista (Mecoptera, Insecta) and possible phylogenetic implications
Ein hartnāckiges entomologisches Problem endlich gelōst? Die Kopfmorphologie von Nannochorista (Mecoptera, Insecta) und ihre phylogenetische Bedentung
Abstract
enExternal and internal head structures of Nannochorista species were examined and described in detail. The characters are discussed with regard to their functional and phylogenetic implications. The structure of the mouthparts indicates that adults of Nannochorista feed on fluids. The loss of the mandibular muscles and the precerebral pharyngeal dilators are presumptive autapomorphies of the genus. A possible clade comprising Nannomecoptera, Siphonaptera and Diptera is supported by the presence of a labral food channel, the absence of the galea, a sheath for the paired mouthparts formed by the labium, very strongly developed labial palp muscles and cibarial dilators, and the presence of a well-defined postcerebral pharyngeal pumping chamber. Closer affinities of Nannomecoptera with Diptera are suggested by the presence of a unique sensorial groove on the third maxillary palpomere. Further potential synapomorphies are the presence of a frontal apodeme and a primarily lamelliform mandible without teeth. The presence of a salivary channel on the laciniae and a subdivided labrum are shared derived features of Nannochorista and Siphonaptera. A derived condition present in Mecoptera including Boreidae but excluding Nannochoristidae is the secretion with a strongly developed intrinsic muscle of the salivary duct. The loss of the lateral labral retractor, the cranial muscle of the cardo, and of two of the three premental retractors, and the absence of transverse epipharyngeal muscles are potential autapomorphies of Antliophora. The formation of a maxillolabial complex is a possible synapomorphy of Hymenoptera and Mecopterida.
Zusammenfassung
deÄuβere und innere Kopfstrukturen von Nannochorista-Arten wurden untersucht und detailliert beschrieben. Die Merkmale werden bezüglich ihrer funktionellen und phylogenetischen Bedeutung bewertet. Die Struktur der Mundwerkzeuge zeigt, dass die Imagines sich von flüssigen Substraten ernähren. Der Verlust der Mandibelmuskeln und der praecerebralen pharyngealen Dilatatoren sind wahrscheinlich Autapomorphien der Gattung und Familie. Eine wahrscheinlich monophlyetische Gruppierung, die die Nannomecoptera, Siphonaptera und Diptera umfaβt, wird durch das Vorhandensein eines labralen Nahrungskanals, das Fehlen der Galea, das Vorhandensein einer labialen Scheide für die paarigen Mundwerkzeuge, sehr stark entwickelte Muskeln des Labialpalpus und des Cibarium und das Vorhandensein einer stark entwickelten postcerebralen Saugpumpe nahegelegt. Für ein Schwestergruppenverhältnis der Nannomecroptera mit den Diptera spricht das Vorhandensein einer einzigartigen Sinnesgrube auf dem dritten maxillaren Palpomer. Weitere potentielle Synapomorphien sind das Vorhandensein eines Frontalapodems und eine primär lamelliforme Mandibel ohne Zähne. Das Vorhandensein eines Speichelkanals auf den Laciniae und ein unterteiltes Labrum sind gemeinsame abgeleitete Merkmale der Nannomecoptera und Siphonaptera. Ein abgeleitetes Merkmal der Mecoptera inklusive Boreidae, aber exclusive Nannochoristidae ist der Sekretformer des Speichelgangs mit einem stark entwickelten intrinsischen Muskel. Der Verlust des seitlichen labralen Retraktors, des M. craniocardinalis, und von zwei von drei praementalen Retraktoren sowie das Fehlen von transversalen epipharyngealen Muskeln sind potentielle Autapomorphien der Antliophora. Die Bildung eines Maxillolabialkomplexes ist eine mögliche Synapomorphie der Hymenoptera und Mecopterida.
Introduction
Nannochoristidae (Tillyard, 1917) is a small group containing only one genus and eight extant species in southern South America (three species), in Australia (four species) and in New Zealand (one species) (Penny 1997). The group has a long fossil record. Definite nannochoristids appear in the Early Jurassic and were quite abundant in mid-Jurassic and Early Cretaceous in Siberia and China (Grimaldi and Engel 2005).
Traditionally, Nannochoristidae is assigned to Mecoptera (e.g. Penny 1975; Willmann 1987, 1989, 2005). However, its peculiarities, especially the strikingly divergent larval morphology and life style have been recognized in earlier studies (e.g. Pilgrim 1972). A status of a separate order Nannomecoptera was already suggested by Hinton (1981), a sistergroup relationship with Diptera by Wood and Borkent (1989), and a sistergroup relationship with a clade also comprising Boreidae and Siphonaptera by Whiting (2002) (see also e.g. Kristensen 1991, 1999; Grimaldi and Engel 2005; Beutel and Pohl 2006). It is evident that the systematic position of Nannochoristidae is not settled yet and that this question is crucial for the understanding of the evolution of Mecopterida as a whole. The status as a basal group of a monophyletic order Mecoptera (e.g. Willmann 2005: 754) cannot be ruled out at present. However, other options should be tested with a maximum amount of morphological and molecular data.
The external morphology and the habits of immature stages are comparatively well known (e.g. Williams 1968; Pilgrim 1972; Melzer et al. 1994). Different body parts and organ systems of adults have been investigated (e.g. Evans 1942; Imms 1944; Richards 1965; Richards and Richards 1969; Hepburn 1969a,b; Mickoleit 1975, 1976, 1978; Willmann 1981, 1989; Kristensen 1989; Krenn and Pass 1993; Simiczyjew 2002). However, the head anatomy and the thoracic skeletomuscular system are not well known yet. This lack of information on an obvious key taxon induced us to carry out this morphological study. The main aim was a detailed documentation of the head structures of Nannnochorista and the phylogenetic focus is on the inter-ordinal relationships. As it was not our intention to reconstruct the phylogenetic relationships of mecopteran subgroups, we examined only representatives of four families, Nannochoristidae, Boreidae, Panorpidae, and Bittacidae in this study. The morphological features are discussed in detail and presented as a matrix including representatives of all groups of Endopterygota. The phylogenetic hypotheses presented should be considered as preliminary as they are not based on a formal character analysis. In a future study, the data will be added to a comprehensive data set including DNA sequence data and morphological characters of different body parts and life stages. A reliable reconstruction of endopterygote and antliophoran relationships requires analyses of extensive data sets with a broad selection of taxa. This would be beyond the scope of the present contribution.
Materials and Methods
List of taxa examined (fixed in 70% ethanol unless otherwise noted)
Mecoptera, Nannochoristidae: Nannochorista neotropica (Navás, 1928; Bouin, ethanol), Nannochorista dipteroides (Tillyard, 1917), Nannochorista sp. (two heads, undetermined species from Tasmania).
Boreidae: Caurinus dectes (Russell, 1979), Boreus westwoodi (Hagen, 1866; fixed in FAE, formaldehyde–ethanol–acetic acid).
Bittacidae: Bittacus hageni (Brauer, 1860).
Panorpidae: Panorpa communis (Linnaeus, 1758).
Siphonaptera: Ceratopsyllus sp.
Diptera, Culicidae: Culex sp. (FAE).
Bibionidae: Bibio spp.
Tipulidae: Tipula maxima (Poda, 1761), Tipula spp.
Hymenoptera, Xyelidae: Macroxyela ferruginea (Say, 1824), Xyela julii (Brébisson, 1818).
Neuroptera, Chrysopidae: Chrysopa carnea (Stepehens, 1836).
Methods
One specimen of N. neotropica was dissected and two specimens were embedded in Araldite, cut at 1 μm (cross- and longitudinal sections) and stained with methylene-blue and acid fuchsine. The head capsule of one specimen used for dissection was macerated in KOH after examination of the musculature. Drawings were made using an ocular grid or a camera lucida (cross-sections) and processed and evaluated with Adobe Photoshop® and Adobe Illustrator CS® software. The serial sections were photographed with a PixelLINK PL-A622C digital camera on a Zeiss Axioskop. For SEM (Scanning Electron Microscopy) micrographs (FEI ESEM XL 30), specimens were cleaned ultrasonically, dried (critical point) and coated with gold.
Results
Head capsule (1, 10)
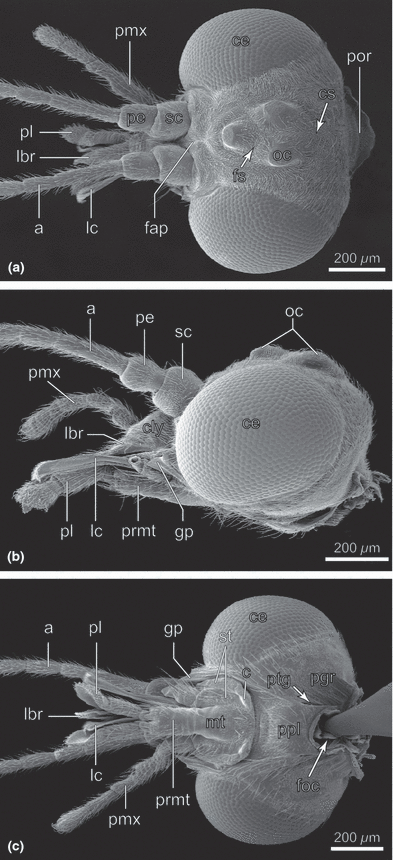
Nannochorista neotropica, head, SEM images. (a) frontal view; (b) lateral view; (c) posterior view. Abbreviations: a, antenna; c, cardo; ce, compound eye; cly, clypeus; cs, coronal suture; fap, frontal apodeme; foc, foramen occipitale; fs, frontal suture; gp, genal process; lbr, labrum; lc, lacinia; mt, mentum; oc, ocellus; pe, pedicellus; pgr, postgenal region; pl, palpus labialis; pmx, palpus maxillaris; por, postoccipital region; ppl, posterior plate; prmt, prementum; ptg, post. tentorial groove; sc, scapus; smr, submental region; st, stipes
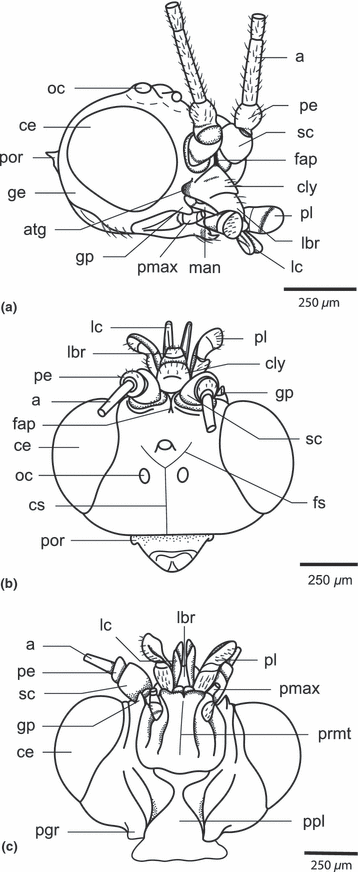
Nannochorista sp. (from Tasmania), head. (a) anterolateral view; (b) dorsal view; (c) ventral view. Abbreviations: a, antenna; atg, anterior tentorial groove; ce, compound eye; cly, clypeus; cs, coronal suture; fap, frontal apodeme; fs, frontal suture; ge, gena; gp, genal process; lbr, labrum; lc, lacinia; man, mandible; oc, ocellus; pe, pedicellus; pgr, postgenal region; pl, palpus labialis; pmx, palpus maxillaris; por, postoccipital region; ppl, posterior plate; prmt, prementum; sc, scapus
The head of Nannochorista is orthognathous. The posterior region is fully exposed and the foramen occipitale is strongly narrowed (foc; 1, 5). The head capsule is well sclerotised (hc; Fig. 2b). Most parts are densely covered with thin and very short setae, but the vestiture is sparse or almost absent in the area ventrad of the paired ocelli, the postgenal region (pgr), and the dorsal region of the posterior plate, which forms the ventral closure of the head capsule (1, 2). More widely spaced long setae are present on the latter two areas, vertex, clypeus (cly; 1, 2), and on the preocular part of the gena. In frontal view, the head capsule is broadly oval. The very large, nearly round, multifaceted compound eyes occupy approximately half of the entire surface of the head (ce; 1, 10). They are very slightly emarginated above the antennal bases and internally enclosed by a high circumocular ridge (cor; 2, 5). Three large ocelli with distinctly convex cuticular lenses are arranged as an equilateral triangle on the anterior head region (oc; Fig. 1a,b). The pigmentation between them is darker than on the other areas. The single ventral ocellus is slightly smaller than the others. The posterior ocelli are separated by a very indistinct coronal suture, which reaches the postocciput posteriorly (cs; Fig. 1a). The vestigial frontal sutures (fs) meet with each other and with the coronal suture within the ocellar triangle (Fig. 1a). They are obliterated ventrally. A well-developed internal median frontal apodeme reaches the frontoclypeal suture anteriorly (fap, 1, 4, 5, 7, 8). It is Y-shaped in cross-section ventrally but a simple ridge dorsad of the interantennal frontal constriction. The triangular frontal area ventrad of the antennal bases is very small. The large and strongly convex clypeus is posteriorly delimited by a deep inverse U-shaped furrow representing the frontoclypeal suture (fcs; Fig. 2a). Internally, the furrow corresponds with a high ridge. A short, transverse anteclypeus (acly) is separated from the postclypeus (pcly) by a medially interrupted transverse fold (Fig. 2a). The following trapezoid element is interpreted as a separate proximal part of the labrum (lbr; 1, 2, 3). It cannot be fully excluded that this is also a part of the anteclyeus. However, considering the sclerotization of the cuticle and the absence of a subdivided anteclypeus in other insects, this interpretation appears unlikely. The genal region posterad of the secondary mandibular articulation forms an apically pointed process (gp; 1, 2, 3). A subgenal suture is not recognizable. The subgenal region is likely represented by a small area between the anterior tentorial pit and the genal process (sgr; Fig. 3a,b). A postgenal bridge is probably not developed. An undivided posterior plate with unclear homology is adjacent with the foramen occipitale. It is distinctly separated from the postgenal region by lateral ridges and folds (ppl, 1, 2) and any traces of a median connecting line or zone of weakness are absent. The posterior tentorial grooves are fissure-shaped and very distinct (ptg; 1, 2). They enclose the caudal part of the posterior plate (ppl, 1, 2). The tentorial bridge divides the narrow foramen occipitale into an upper spindle-shaped alaforamen and a round lower neuroforamen. The postoccipital region forms a roof-like extension above the alaforamen (por, Fig. 1a). A separating line or suture between the occiput and postocciput is not present.
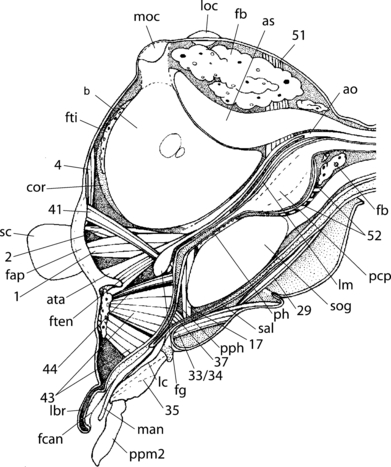
Nannochorista neotropica, head, sagittal section. Abbreviations: as, airsacs; ata, anterior tentorial arm; ao, cephalic aorta; b, brain; cor, circumocular ridge; fap, frontal apodeme; fb, fat body; fti, fat body tissue; fcan, food channel; fg, ganglion frontale; ften, frontotentorial muscle; lbr, labrum; lc, lacinia; lm, longitudinal muscle; loc, lateral ocellus; man, mandible; moc, median ocellus; pcp, postcerebral pharyngeral pumping apparatus; ph, pharynx; pph, prepharynx; ppm2, palpomere 2; sal, salivary duct; sc, scapus; sog, suboesophageal ganglion; 1, M. tentorioscapalis ant.; 2, M. tentorioscapalis post.; 4, M. tentorioscapalis med.; 17, M. tentoriocerdinalis; 29, M. tentoriopraementalis inferior; 33/34, Mm. praementopalpales; 35, M. palopopalpalis primus; 37, M. hypopharyngosalivaris; 41, M. frontohypopharyngalis; 43, M. clypeopalatalis; 44, M. clypeobuccalis; 51, M. verticopharyngalis; 52, M. tentoriopharyngalis
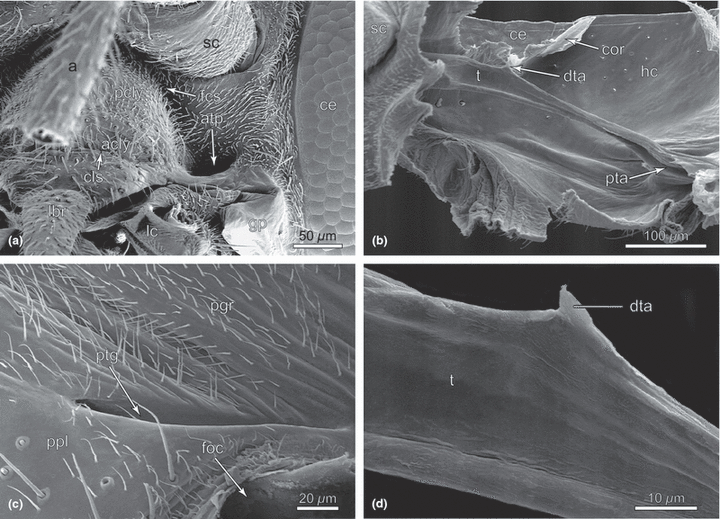
Nannochorista neotropica, head structures, SEM images. (a) anterolateral view, clypeus, and anterior tentorial groove; (b) tentorium, overview (macerated specimen); (c) posterior tentorial groove; (d) tentorium, detail (macerated specimen). Abbreviations: a, antenna; acly, anteclypeus; atp, anterior tentorial pit; ce, compound eye; cls, clypeolabral suture; cor, circumocular ridge; dta, dorsal tentorial arm; fcs, frontoclypeal suture; foc, foramen occipitale; gp, genal process; hc, head capsule; lbr, labrum; lc, lacinia; pcly, postclypeus; pgr, postgenal region; ppl, posterior plate; pta, posterior tentorial arm; ptg, posterior tentorial groove; sc, scapus; t, tentorium
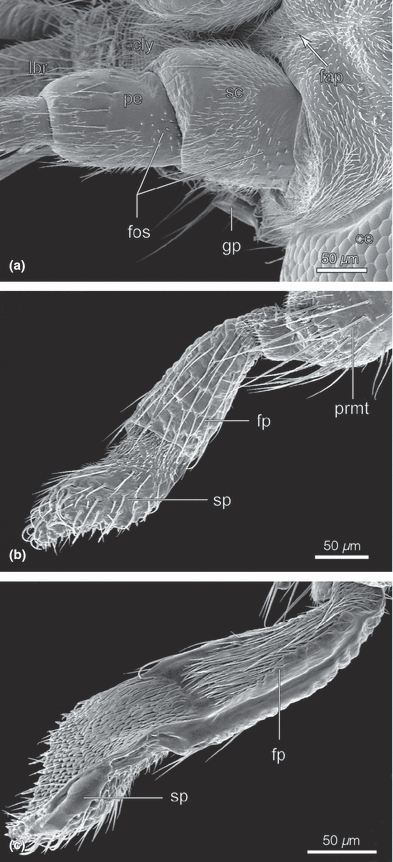
Nannochorista neotropica, head structures, SEM images. (a) antennal base, lateral view; (b) labial palp, lateral view; (c) labial palp, mesal view. Abbreviations: cly, clypeus; fap, frontal apodeme; fos, field of sensilla; fp, palpomere 1; gp, genal process; lbr, labrum; pe, pedicellus; prmt, prementum; sc, scapus; sp, palpomere 2
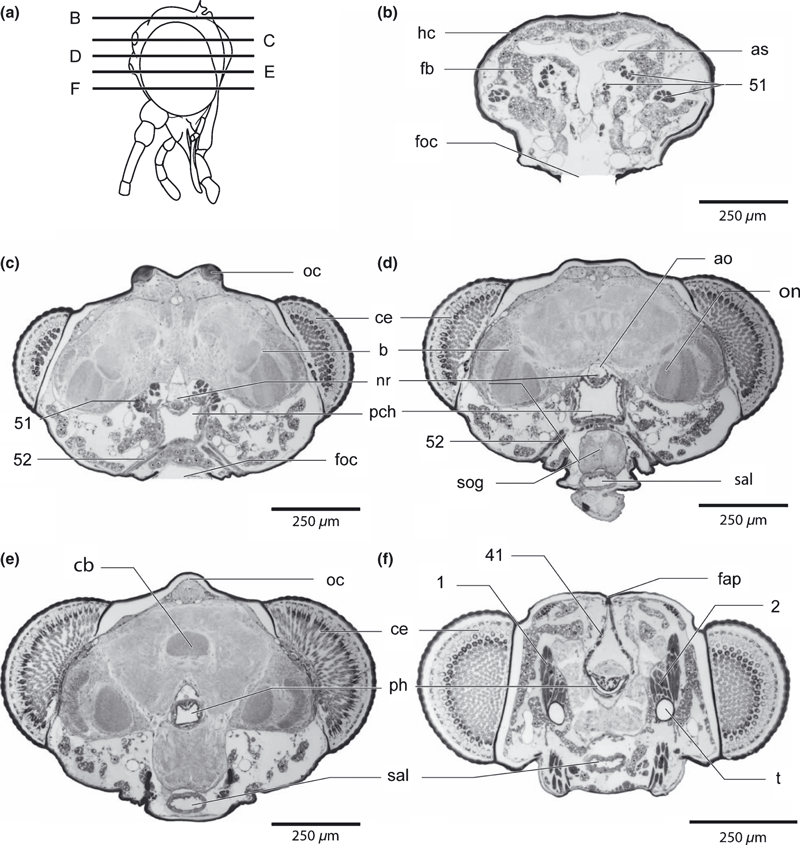
Nannochorista neotropica, head, cross-sections. (a) overview of the sections; (b–f) cross-sections of the upper head region. Abbreviations: ao, cephalic aorta; as, air sac; b, brain; cb, central body, ce, compound eye; fap, frontal apodeme; fb, fat body; foc, foramen occipitale; hc, head capsule; nr, nervus recurrens; oc, ocellus; on, optic neuropils, pch, postcerebral pumping chamber, ph, pharynx; sal, salivarium; sog, suboesophageal ganglion; t, tentorium; 1, M. tentorioscapalis anterior; 2, M. tentorioscapalis posterior; 41, M. frontohypopharyngalis; 51, M. verticopharyngalis; 52, M. tentoriopharyngalis posterior
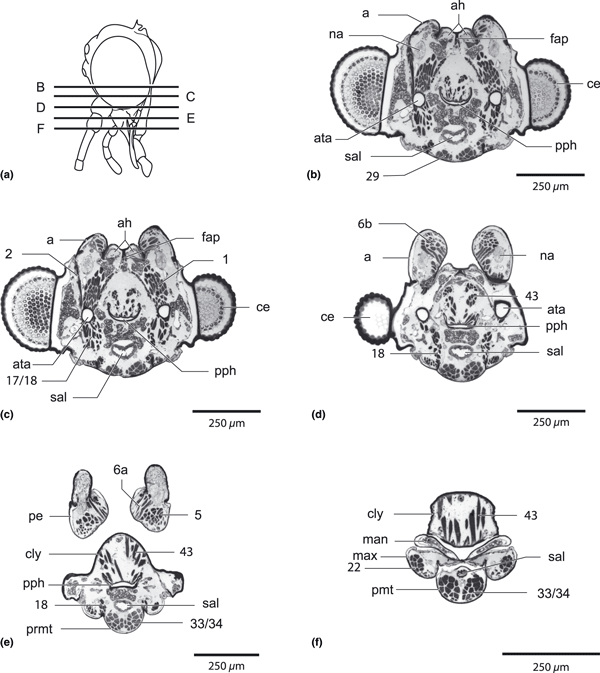
Nannochorista neotropica, head, cross-sections. (a) overview of the sections; (b–f) cross-sections of the lower head region. Abbreviations: a, antenna; ah, antenna-heart; ata, anterior tentorial groove; ce, compound eye; cly, clypeus; fap, frontal abodeme; man, mandible; max, maxilla; na, nervus antennalis; pe, pedicellus; ph, pharynx; pmt, prementum; pph, prepharynx, sal, salivarium; 1, M. tentorioscapalis anterior; 2, M. tentorioscapalis posterior; 5, M. scapopedicellaris lateralis; 6, M. scapopedicellaris medialis; 17/18, Mm. tentoriocardinalis and -stipitalis; 29, M. tentoriopraementalis inferior; 34/35, Mm. praementopalpales externus and internus
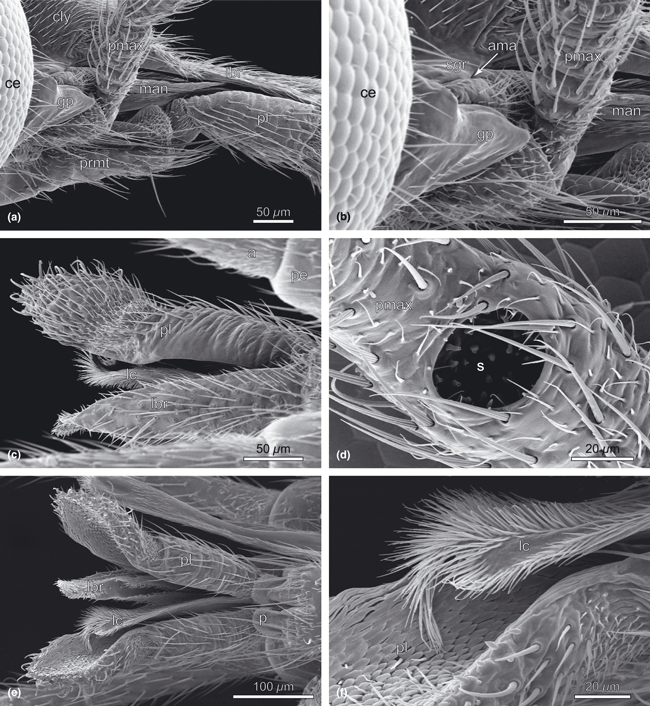
Nannochorista neotropica, head structures, SEM images. (a) genal region and bases of mouthparts; (b) bases of the mouthparts, details; (c) labrum, lacinia, and labial palp; (d) sensorium of maxillary palp; (e) mouthparts, ventral view; (f) lacinia and mesal view of labial palp. Abbreviations: a, antenna; ama, anterior mandibular articulation; ce, compound eye; cly, clypeus; gp, genal process; lbr, labrum; lc, lacinia; man, mandible; p, palpiger; pe, pedicellus; pl, palpus labialis; pmax, palpus maxillaris; prmt, prementum;, s, sensorium of maxillary palp; sgr, subgenal region
Tentorium (2, 7, 8)
The tentorium is formed by nearly straight, strongly developed rods connecting the large, round anterior pits (atp; Fig. 2a) with the fissure-shaped posterior grooves (ptg; 1, 2). The anterior part, i.e. the section comprising the anterior arms and the corpora tentorii is hollow and nearly round in cross-section (t; 7, 8). The lumen narrows posteriorly and the posterior arms are solid and flattened (pta; Fig. 2b). The dorsal arms are reduced, with a minute triangular process on the dorsal side of the middle section of the tentorial rods possibly representing a vestige (dta; Fig. 2b,d). The well-developed tentorial bridge separates the alaforamen from the neuroforamen. It is completely separated from the base of the well-developed posterior arms.
Musculature: A flat muscle band (M. frontotentorialis) originates between the mesal margin of the antennal foramen and the anterior margin of the frontal apodeme. It is inserted on the mesal side of the anterior tentorial arms. The homology of this muscle is unclear.
Labrum (1, 2, 3, 4, 5)
The labrum is divided into a trapezoid posterior part, which is connected with the anterior margin of the anteclypeus (acly; Fig. 2a) and a narrow, tongue-like elongated anterior part (lbr; 1, 3). The anterior part is distinctly higher than its width. On its ventral side a half-pipe structure forms the food channel (fch; 3, 4, 5, 9; see also epipharynx). Labral muscles are not present.
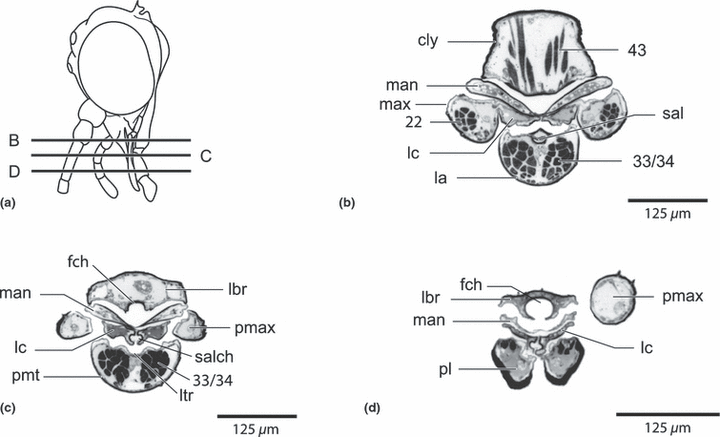
Nannochorista neotropica, head, cross-sections. (a) overview of the sections; (b,c,d) cross-sections of the mouth part region. Abbreviations: cly, clypeus; fch, food channel, la, labium; lbr, labrum; lc, lacinia; ltr, labial trough; man, mandible; max, maxilla; pl, palpus labialis; pmt, prementum; pmx, palpus maxillaris; sal, salivarium; salch, salivary channel; 22, M. stipitopalpalis externus, 33/34, Mm. praementopalpales externus and internus; 43, M. clypeopalatalis
Antenna (1, 2, 4)
The antenna (a; 1, 4) is composed of 32 segments with a moderately dense vestiture of short and longer setae. The insertion areas between the compound eye and the epistomal suture are almost contiguous (1, 4). Two inconspicuous protuberances are present on the base of the scapus dorsomesally and ventrolaterally. They form a loose articulation with the corresponding indistinct notches of the antennal ring. The connecting axis is oblique. A similar articulation is formed between the scapus and pedicellus, with projections on the lateral and mesal side of the pedicellus. Both basal antennal segments are about equally long, but the scapus is distinctly larger. Scapus and pedicellus show at the base a field of sensillary hairs on the lateral side. The first flagellomere is approximately one third longer than the cylindrical pedicellus, but only half as wide. The second flagellomere is half as long as the first. The following 22 antennomeres are of approximately equal size. The distal eight flagellomeres are slightly smaller than the preceeding segments.
Musculature (5, 7, 8): M. tentorioscapalis anterior (M. 1), well developed and fan-shaped, without tendon, O: on the dorsal side of the anterior tentorial arm, mesad of M. frontotentorialis, I: mesal margin of the scapal base; M. tentorioscapalis posterior (M. 2), well developed, with a short tendon, O: dorsolaterally on the anterior tentorial arm, I: dorsal margin of scapal base; M. tentorioscapalis lateralis (M. 3), absent; M. tentorioscapalis medialis (M. 4) (cranial rotator), short and flattened, O: on the frons mesad of the anterior margin of the compound eyes, I: on the tendon of M. 2; M. scapopedicellaris lateralis (M. 5), a compact, well-developed muscle, O: dorsal wall of scapus, I: laterally on the base of the pedicellus; M. scapopedicellaris medialis (M. 6), well developed, composed of a larger and a smaller subcomponent, M. 6a, O: mesally on the base of the scapus, I: mesally on the base of the pedicellus, M. 6b, O: dorsomesally on the base of the scapus, I: dorsomesally on the base of the pedicellus.
Mandible (3, 5, 8, 9)
The symmetrical mandibles are visible in the lateral view as a narrow triangle between the clypeus and the lacinia (man; 3, 9). A shallow concavity on the anterior side of the mandibular base is part of a modified secondary mandibular joint. It corresponds with a convexity on the ventral side of the anterior clypeus, close to its lateral margin and anterad to the anterior tentorial pits (Fig. 3b). The ventral primary mandibular joint is reduced. The basal part of the mandible is sclerotized. A mola is not developed. The distal parts cover the ventral opening of the epipharyngeal food channel (Fig. 8). They are strongly flattened and unsclerotized. Incisivi are absent.
Musculature: M. craniomandibularis internus (M. 11), M. craniomandibularis externus (M. 12), absent; M. hypopharyngo-mandibularis (M. 13), represented by two extremely thin fibres accompanied by a nerve, O: anterior tentorial arm, I: ventral surface of the mandibular base; M. zygomaticus mandibulae (M. 14), absent.
Maxilla (1, 3, 6, 9)
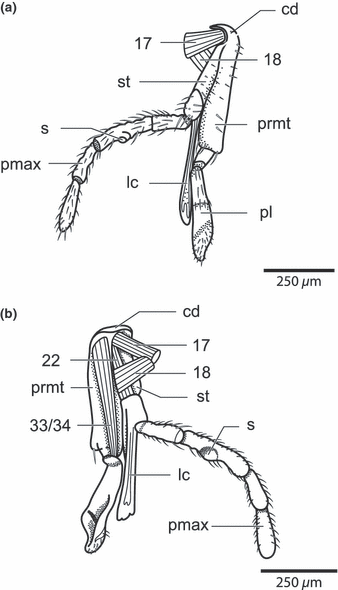
Nannochorista neotropica, head structures. (a) maxillolabial complex, lateral view; (b) maxillolabial complex, inner view. Abbreviations: cd, cardo; lc, lacinia; prmt, prementum; s, sensorium of maxillary palp; pl, palpus labialis; pmax, palpus maxillaris; st, stipes; 17, M. tentoriocerdinalis; 18, M. tentoriostipitalis; 22, stipitopalpalis externus; 33/34, Mm. praementopalpales
The maxillae are closely connected with the labium (la; 3, 6). Both elements together form a maxillolabial complex. The small cardo is recognizable as a convexity laterad of the base of the mentum (cd; Fig. 6a,b). Cardo and stipes are almost completely fused with each other (st; Fig. 6a,b). The stipes comprises a narrow and elongate lateral basistipes and a broader mesal mediostipes, which is covered with short hairs and longer setae, and mesally connected with the lacinia (lc; 1, 2, 3, 6). The well-developed five-segmented palp is inserted on the distal margin of the mediostipes (pmax; 1, 3, 6). It is densely covered with setae. The large proximal segment is approximately half as long as the second. Palpomere 1 is one-third longer than 2 and has an irregularly annulated surface structure. A deep cavity with sensilla and a ventrally directed round opening is present at about midlength of the segment (s; Fig. 3d). Palpomeres 4 and 5 are about as long as the second segment. The lacinia is mesally attached to the stipes. Its main part forms a flattened vertical lamella with a deep, narrow longitudinal furrow on the mesal site. The galea is absent.
Musculature (5, 6, 8, 9): M. cranioardinalis (M. 15), absent; M. tentoriocardinalis (M. 17), spindle-shaped and compact, O: anterior tentorial arm, on the opposite side of Mm. tentorioscapales anterior and posterior, I: apodeme of a sclerotized part of the mesal base of the cardo; M. tentoriostipitalis (M. 18), flattened and broader than M. 17, O: anterior tentorial arm, below M. 17, I: ridge between prementum and stipes; M. craniolacinialis (M. 19), long and very thin, composed of only three thin fibres, O: posterior genal region, I: membranous base of lacinia with a thin tendon; M. stipitolacinialis (M. 20), absent; M. stipitogalealis (M. 21), absent; M. stipitopalpalis externus (M. 22), strongly developed, O: external wall of the stipital base, below the insertion area of M. tentoriostipitalis, I: externaly on the basal margin of palpomere 1; M. stipitopalpalis internus (M. 23), absent; M. palpopalpalis maxillae primus (M. 24), composed of a larger and a very small subcomponent, O: laterally on the base of palpomere 1 and mesally at about midlength of the segment, respectively, I: externally on the base of palpomere 2 with a common tendon; M. palpopalpalis secundus (M. 25), absent; M. palpopalpalis tertius (M. 26), O: mesal wall of palpomere 3, close to the round sensory organ, I: mesally on the base of palpomere 4; M. palpopalpalis quartus (M. 27), O: mesal wall of palpomere 4, I: mesally on the base of palpomere 5.
Labium (1, 2, 3, 4)
Most parts of the labium are only weakly sclerotized. It cannot be fully excluded that the submentum is represented by the posterior plate (ppl; 1, 2), however, considering the articulation of the cardines, this interpretation appears rather unlikely. The submentum is probably not present as a separate structure. The strongly elongated narrow labial element between the maxillae contains the indistinctly separated mentum (or postmentum) and prementum (prmt; Fig. 1c). It is separated from the posterior plate by a very deep fold. The posterior part is closely connected with the basal parts of the maxilla (prmt; Fig 1c). The parallel-sided prementum is medially cleft at its apex. The inner surfaces of this divided apical region are membranous and hold the apical vertical parts of the lacinia in their position. Paraglossae and glossae are not developed. The two-segmented palp (pl; 3, 6) is inserted on a membranous bilobed palpiger (p; Fig. 3e). Palpomere 1 is about three times as long as wide and slightly widening distally. Its mesal side is unsclerotized (fp; Fig. 4b,c). Palpomere 2 is approximately three times as long as the basal segment and covered with hairs on the mesal side. Its proximal half is sclerotized whereas the distal part is membranous (sp; Fig 4b,c). In its distal third, palpomere 2 forms a spoon-like structure with a separate sclerite at its base and a medially oriented concavity (3, 4). The surface of this area is densely covered with minute scales (3, 4). The convex outer side is set with sort setae.
Musculature (5, 8, 9): M. submentopraementalis (M. 28), absent; M. tentoriopraementalis inferior (M. 29), a long and slender muscle, O: mesally on the posterior tentorial arms, I: apodeme of the posterolateral edge of the prementum; M. tentoriopraementalis superior (M. 30), absent or completely merged with M. 29; M. praementoparaglossalis (M. 31), M. praementoglossalis (M. 32), absent; Mm. praementopalpalis internus/externus (33/34), strongly developed, two subcomponents with common origin and only slightly separated points of insertion, O: surface of the labial element comprising the mentum and prementum, I: dorsomesally and dorsolaterally on the base of palpomere 1; M. palpopalpalis labii primus (M. 35), well developed, O: laterally on the base of palpomere 1, I: laterally on the base of palpomere 2; M. palpopalpalis labii secundus (M. 36), absent.
Epipharynx (8, 9)
The entire epipharynx is completely devoid of microtrichiae and most parts are sclerotized. Its distal part forms a cylindrical, ventrally open food channel on the ventral side of the narrow distal part of the labrum (8, 9). The opening of the channel widens towards the cibarium and it is divided by a low median ridge in its posterior section. It is continuous with the concave roof of the open preoral chamber. Like the anterior part, this intermediate section of the epipharynx is also sclerotized except for the membranous lateral margins. The thin but sclerotized posterior part is laterally connected with the posterior hypopharynx by a narrow semimembranous band. It forms the roof of the closed prepharyngeal tube. The walls of the tube are sclerotized. It is moderately long, U-shaped in cross-section, and the lumen is very narrow (Fig. 8b–f).
Musculature (5, 8, 9): M. clypeopalatalis (M. 43), strongly developed, composed of many bundles and two major subcomonents, M. 43a, O: anterior clypeus, from the anterior margin to the attachment area of M. 43b, I: roof of the anterior cibarium; M. 43b, composed of fewer individual bundles, but approximately three times as broad as M. 43a, O: posterior clypeus, dorsad of M. 43a, I: roof of the prepharyngeal tube; M. clypeobuccalis (M. 44), O: immediately dorsad of the last bundle of M. 43b, I: dorsal side of the bucca, below M. transversalis buccae and the frontal ganglion.
Hypopharynx (5, 8)
The hypopharynx is not present as a distinct individual structure, but consists of three subunits, which are more or less extensively fused with other structures. The distal part is fused with the anterior labium. The terminal section of the salivary duct lies within this combined structure. The intermediate part forms a flat lamella below the mandibles. Its anteromedian part is sclerotized (distal part of sitophore plate), trapezoid in cross-section, and fits with the sclerotized mesal edges of the basal part of the mandibles. The strongly sclerotized proximal part of the hypopharynx (proximal part of sitophore plate) is laterally fused with the posterior epipharynx. Both parts together form the closed prepharygeal tube. The tube is approximately U-shaped in cross-section and the walls are sclerotized. The sclerotized upper edges may be considered as a derivative of the hypopharyngeal suspensorium. However, a typical suspensorial sclerite is not developed.
Musculature (4, 7): M. frontohypopharyngalis (M. 41), well developed, O: frontal apodeme, I: sclerotized upper edge of the posterior prepharyngeal tube, anterad of the anatomical mouth; M. tentoriohyopharyngalis (M. 42), absent.
The muscle referred to as M. tentoriohyopharyngalis (M. 42) in studies on the head morphology of beetles and other insects (e.g. Beutel 1986; Beutel and Weide 2005; Beutel and Vilhelmsen 2007) functions as a tentorial retractor of the hypopharnyx, but is homologous to v. Kéler’s M. tentoriobuccalis anterior (M. 48). M. tentoriobuccalis anterior lies within the circumoesophageal connectives; M. tentoriohyopharyngalis lies laterad of them.
Pharynx and oesophagus (5, 7, 8)
The anterior pharynx is strongly narrowed between the brain and the suboesophageal complex (Fig. 5) and approximately quadrangular in cross-section, without distinct folds which usually serve for muscle attachment. The inner surface is densely covered with minute tubercles bearing fine spines. The pharynx strongly widens in the posterior head region, where it forms a well-defined, thick-walled postcerebral pumping apparatus, which is also quadrangular in cross-section and densely covered with spiniferous tubercles. Distinct dorsolateral and ventrolateral folds serve as attachment areas for postcerebral dilators. The anteriormost oesophagus, which enters the head capsule is U-shaped in cross-section.
Musculature of the precerebral pharynx (5, 7, 8): M. frontobuccalis anterior (M. 45), absent; M. frontobuccalis posterior (M. 46), absent; M. frontobuccalis lateralis (M. 47), absent.
Musculature of the postcerebral pharynx (5, 7): M. verticopharyngalis (M. 51), a strongly developed muscle complex composed of three subcomponents, M. 51a, three well-developed bundles, O: vertex, I: dorsolateral fold of the postcerebral pharynx, M. 51b, strongly developed, O: posterior genal region, I: dorsolateral fold of the postercerebral pharynx, together with one bundle of M. 51a; M. 51c, a slender muscle, O: postoccipital region, dorsad of the alaforamen, I: dorsolateral fold of the posteriormost pharynx; M. tentoriopharyngalis (M. 52), strongly developed, composed of four subcomonents; M. 52a, two slender bundles, O: laterally on the postoccipital ridge, close to the origin of the posterior tentorial arm, I: ventrally on the postcerebral pharynx; M. 52b, very strongly developed, composed of a series of parallel bundles, O: postgenal region, laterad of the neuroforamen, I: ventrolateral fold of the postcerebral pharyngeal pumping apparatus, below the common insertion of two bundles of M. 51a and M. 51b; M. 52c, two well-developed bundles, O: laterad of the alaforamen, close to the origin of the tentorial bridge, I: ventrolateral fold of the postcerebral pharyngeal pumping apparatus, below the insertion of the posterior bundle of M. 51a and of M. 51c, respectively.
Intrinsic musculature of the pharynx: M. transversalis buccae (67), a thin transverse muscle band extends over the dorsal wall of the anterior pharynx, immediately posterad of the anatomical mouth opening, above the insertion of M. 44; M. anularis stomodaei (68), a typical ring musculature is not developed; very thin muscle bands attached to the upper edges of the anterior pharynx enclose only the lateral and ventral sides; the intrinsic musculature of the postcerebral pumping apparatus is similar in its attachment to the pharyngeal wall, but strongly developed; M. longitudinalis stomodaei (69), a median longitudinal muscle is present on the dorsal side of the anterior pharynx; well-developed dorsal, ventral and lateral longitudinal muscle bands are present below the ring musculature of the postcerebral pumping apparatus.
Salivarium (5, 7, 8, 9)
The anterior part of the salivarium is represented by a narrow, moderately flattened tube with a sclerotized ventral wall. It opens on the unsclerotized dorsal surface of the complex formed by the anterior hypopharynx and labium, below the posterior opening of the salivary channels formed by the mesal edges of the laciniae. At the level of the mandibular articulation the salivary tube widens. The posterior part is oval in cross-section and nearly as broad as the prepharynx. The wall is thick and its inner surface is covered with minute tubercles carrying very fine spines. In the cervical region, the salivary tube splits into paired ducts, which form the connection with the salivary glands in the prothorax.
Musculature (3, 4): M. hypopharyngosalivarialis (M. 37), well developed, O: dorsal wall of the anterior labiohypopharyngeal complex, I: unsclerotized dorsal wall of the anterior salivary duct; M. praementosalivarialis anterior (M. 38), absent; M. praementosalivarialis posterior (M. 39), absent; M. annularis salivarii (M. 40), absent.
Cerebrum, suboesophageal complex and stomatogastric nervous system (5, 7, 8)
The brain is large in relation to the head capsule. It is located in the anterordorsal area of the head capsule and fills out a large part of this region. The protocerebrum is not distinctly separated from the other parts of the brain. The corpora pedunculata, the central body, and the optic neuropils are well developed. The antennal nerves originating from the deutocerebrum are unusually large. The tritocerebral commissure is not present as a separate structure. It is fused with the suboesophageal complex, which nearly fills out the entire space between the posterior salivarium, the posterior tentorial arms, and the pharynx. The circumoesophageal connectives are very broad and short. The brain and the suboesophageal complex form a compact structure around the pharynx. The posterior part of the suboesophageal complex reaches the cervical region posteriorly. The frontal ganglion is large. The nervus recurrens is enclosed by the cephalic aorta. The frontal connectives are very short and thick.
Glands (5, 7, 8)
Glands are not present within the head capsule. The well-developed paired salivary glands are located in the prothorax.
Circulatory system (5, 7, 8)
The cephalic aorta is wide and approximately quadrangular in cross-section in the occipital region, but more rounded and narrower below the brain (ao; Fig. 7c,d). The ventral wall is extremely delicate and appears attached to the musculature of the dorsal side of the pharynx. Well-developed antenna-hearts are present between the antennal insertion area and the frontal apodeme (ah; Fig 8b,c).
Tracheal system (7, 8)
A large trachea enters the head capsule medially and two pairs of larger and one pair of smaller tracheae laterally. The median trachea divides into two large air-sacs posterad of the brain (as; Fig. 7b). Two tracheae are present below the median fat body layer of the anterior surface of the brain, close to the median line. They unite below the paired ocelli and are posteriorly connected with the air sacs.
Fat body (5, 7, 8)
Smaller lobes of fat body are present between the basal part of the mandible, between the salivarium and the prepharynx, in the frontal region, anterad of the brain, in the anteroventral and posteroventral head regions, and in the vicinity of the posterior tentorial arms (fb; 5, 7). The anteromedian and the anterolateral surface of the protocerebrum are covered by a layer of fat body. The fat body lobes are massively developed posterad of the brain.
Interspecific differences (10)
The labrum and the labial palps are distinctly shorter in the undetermined species from Tasmania.
Phylogenetically relevant characters (Tables 1, S2)
| Taxa/characters | 1fap | 2tes | 3pgb | 4cls | 5acl | 6rst | 7ais | 8dta | 9ata | 10ftm | 11lbr | 12fch | 137 | 148 | 159 | 16cra |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nannochorista | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| Boreus | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2? | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 |
| Caurinus | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 |
| Panorpa | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 |
| Bittacus | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 |
| Culicidae | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 0? | 1 | 1 | 1 | 1 | 0 |
| Tipulidae | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 2 | – | 0 | 0 | ? | ? | ? | 1 | 2 |
| Bibionidae | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 0? | 1 | ? | 1 | 1 | ? |
| Ceratopsyllidae | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 2 |
| Micropterigidae | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Rhyacophilidae | 0 | 1 | 0 | 1 | 1? | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 |
| Xyelidae | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Tenthredinidae | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cupedidae | 0 | 0 | 0 | 0 | 1 | 0 | 0&2 | 0 | – | 0 | 0 | 0 | 0 | 2 | 1 | 0 |
| Leiodidae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 |
| Sialidae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Raphidiidae | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chrysopidae | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mengenillidae | 0 | 0 | 0 | 0&– | – | 0 | 0 | 2 | – | 0 | 0 | 0 | 1 | 2 | 1 | 2 |
| Taxa/characters | 17pmj | 18smj | 19inc | 20shm | 2111 | 2212 | 2313 | 24mlc | 25fsp | 26gal | 27sch | 28sen | 2915 | 30o17 | 31cal | 32npm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nannochorista | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 |
| Boreus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Caurinus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Panorpa | 0 | 0 | 0 | 0 | 0 | 0 | 1? | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Bittacus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Culicidae | 0 | 1 | 1 | 1 | 0&1 | 0&1 | 0 | 2 | – | 1 | 0 | 1 | 1 | 0 | 1 | 2 |
| Tipulidae | – | – | – | – | – | – | – | 2 | – | 1 | 0 | ? | 1 | 0 | 1 | 2 |
| Bibionidae | – | – | – | – | – | – | – | 2 | – | 1 | 0 | ? | 1 | 0 | 1 | 2 |
| Ceratopsyllidae | – | – | – | – | – | – | – | 1 | 0 | 1 | 1 | 0 | 1 | – | 1 | 1 |
| Micropterigidae | 0 | 0 | 0 | 0 | 0 | 0 | 1? | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0&2 |
| Rhyacophilidae | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xyelidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tenthredinidae | 0 | 0 | 0 | 0 | 0 | 0 | 1? | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cupedidae | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiodidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sialidae | 0 | 0 | 0 | 0 | 0 | 0 | 1? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Raphidiidae | 0 | 0 | 0 | 0 | 0 | 0 | 1? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chrysopidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mengenillidae | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | – |
| Taxa/characters | 33lbp | 34sca | 35glo | 36pgl | 37ret | 3834 | 3937/8 | 40sit | 4141 | 4242 | 4343 | 44tme | 4545/6 | 4648 | 47ims | 48pum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nannochorista | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Boreus | 0 | 0 | 1 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 0 |
| Caurinus | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Panorpa | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 0 |
| Bittacus | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 0 |
| Culicidae | 0 | 1? | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Tipulidae | 0 | 0 | 1 | 1 | 2 | 1 | ? | 1 | ? | ? | 1 | 1 | ? | 0 | 0 | 1 |
| Bibionidae | 0 | 0 | 1 | 1 | 2 | 1 | ? | 1 | 1 | ? | 1 | 1 | 0 | 0 | 0 | 1 |
| Ceratopsyllidae | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 0? | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Micropterigidae | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhyacophilidae | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0? | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Xyelidae | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tenthredinidae | 0 | 0 | 0 | 0 | ? | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cupedidae | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | – | 0 |
| Leiodidae | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0 |
| Sialidae | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 |
| Raphidiidae | 0 | 0 | 1 | 1 | 1 | 0 | 1? | 0 | 0? | 0 | 0 | ? | ? | 0 | ? | 0 |
| Chrysopidae | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mengenillidae | – | – | 1 | 1 | 3 | – | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | – | 0 |
The characters are listed in a morphology-based sequence (see Results). Presumably plesiomorphic character states are coded as (0), although this convention is inconsequential in cladistic analyses. Table S2 with head muscles of representatives of endopterygote orders and Zorotypos– see Supporting Information.
1. Frontal apodeme (fap): (0) absent; (1) present. A well-developed median internal frontal apodeme is present in Nannochorista (1, 5, 7, 8) and also in Culicidae and some other members of Diptera (e.g. Schiemenz 1957: Eristalis [Syrphidae]; Wenk 1962: Wilhelmia [Simuliidae]: fig. 35; Hennig 1973). It is absent in Bibio (Bibionidae) and Tipulomorpha (Rees and Ferris 1939; Hennig 1973: fig. 72), and also in Mecoptera excluding Nannochoristidae (e.g. Caurinus, Boreus, Bittacus; Heddergott 1938; Hepburn 1969a; Beutel et al., 2008) and other groups of Endopterygota (e.g. Crampton 1921; Röber 1942; Wenk 1953; Hannemann 1956; Klemm 1966; Anton and Beutel 2004; Beutel and Pohl 2006; Beutel and Vilhelmsen 2007). It is conceivable that the presence is a derived groundplan feature of a clade comprising Nannomecoptera and Diptera.
2. Temporal suture (occipital sulcus) (tes): (0) absent; (1) present. A temporal suture or occipital sulcus is usually present in Mecoptera (e.g. Hepburn 1969a: figs 30, 41–43), but is absent in Nannochorista (1, 10), Boreus, Caurinus, and Bittacus (Hepburn 1969a; Russell 1979; Beutel et al., 2008). Similar structures are also present in some groups of Diptera (e.g. Bibio, personal observation, Beutel; Eristalis, Schiemenz 1957), in symphytan Hymenoptera (with exceptions; Beutel and Vilhelmsen 2007), and in Rhyacophila (Trichoptera). Whether they are homologous to the complete occipital suture of Micropterix (Lepidoptera; Hannemann 1956: fig. 3) is uncertain. The suture is absent in Siphonaptera (Snodgrass 1946), many groups of Diptera (e.g. Rees and Ferris 1939: Tipula; Snodgrass 1959: Culicidae; Wenk 1962: Wilhelmia), Coleoptera (e.g. Beutel 1997), Strepsiptera (Beutel and Pohl 2006), and in Neuropterida (Crampton 1921; Maki 1936; Röber 1942; Aspöck and Aspöck 1971). Whether the very widely separated lines in Neuroptera (Ferris 1940: furrow-like depression) are homologous with the occipital sulcus of Hymenoptera and members of other groups is an open question.
3. Postgenal bridge (pgb): (0) absent; (1) present. In contrast to Hepburn (1969a; see also Evans 1942), a postgenal bridge is not developed in Nannochorista (1, 2, 10). This is likely plesiomorphic compared with the condition in other mecopterans. The bridge is present in Caurinus (Beutel et al., 2008), Boreus, Panorpa (Heddergott 1938), Panorpodes, Merope (Otanes 1922: figs 17, 25), and Bittacus (Hepburn 1969a: fig. 30), and also in Siphonaptera (Snodgrass 1946; Wenk 1953: fig. 3). A postgenal bridge does also occur in Hymenoptera, but is not present in the groundplan (Beutel and Vilhelmsen 2007). It is absent in most groups of Endopterygota (e.g. Crampton 1921; Hannemann 1956; Maki 1936; Röber 1942; Hörnschemeyer et al. 2002). The condition in Diptera is somewhat unclear due to homology problems (see Hennig 1973). The bridge is absent in Theobaldia (Schiemenz 1957: fig. 4) but present in Tipula (Rees and Ferris 1939) and Wilhelmia (incompletely closed; Wenk 1962: fig. 4).
4. Clypeolabral suture (cls): (0) absent; (1) present. In contrast to Evans (1942: fig. 1), Hepburn (1969a), and Willmann (2005) the clypeolabral suture is distinctly developed in Nannochorista (cls; 2, 10) and this is also the case in Caurinus (Beutel et al., 2008) and apparently also in Panorpodes and Apterobittacus (Otanes 1922: figs 1, 5). It is absent in other mecopteran genera (including Boreus) according to Otanes (1922), Setty (1940), Heddergott (1938), and Hepburn (1969a). Transverse sutures anteriorly delimiting the clypeus (or postclyeus; see following character) are depicted for Brachypanorpa, Apteropanorpa, and Chorista (very distinct) in Hepburn (1969a), but not discussed in that study. It is likely, that the presence of the sutures belongs to the groundplan of Mecoptera (excluding or including Nannochoristidae and Boreidae) and was reduced several times independently. The clypeolabral suture is also absent in Siphonaptera (Snodgrass 1946; Wenk 1953: fig. 2) and the labrum is almost always reduced in Strepsiptera (Beutel and Pohl 2006). The suture is present in the groundplan of Diptera (Hennig 1973), and in contrast to Weber (1933) also present in Tipulidae (Rees and Ferris 1939). It is also usually present in other groups of Endopterygota (e.g. Crampton 1921; Maki 1936; Röber 1942; Hannemann 1956; Hörnschemeyer et al. 2002; Beutel and Vilhelmsen 2007).
5. Clypeus (acl): (0) subdivided into postclypeus and anteclypeus; (1) not subdivided. The clypeus is divided into a posterior postclypeus and an anterior weakly sclerotized anteclypeus in Nannochorista (Fig. 2a) and this is also the case in Caurinus (Russell 1979; Beutel et al., 2008). The subdivision is lacking in Panorpa (Heddergott 1938) and other members of Mecoptera (Otanes 1922; Hepburn 1969a). The division into a postclypeus and anteclypeus does also occur in Raphidioptera (Crampton 1921) and is a common feature of Diptera according to Crampton (1942). However, this is not the case in Culicidae and several other groups (e.g. Tipula, Wilhelmia; Eristalis; Rees and Ferris 1939; Schiemenz 1957; Wenk 1962). The clypeal area is undivided in Siphonaptera (Snodgrass 1946; Wenk 1953: fig. 2) and other groups of Endopterygota (e.g. Crampton 1921; Maki 1936; Hannemann 1956; Hörnschemeyer et al. 2002).
6. Rostrum (rst): (0) absent or extremely short; (1) rostrum formed by elongation of clypeus and genae; (2) rostrum composed of elongated clypeus and genae and parts of the ventral mouthparts. A rostrum formed by elongation of the clypeus and genae is usually more or less well developed in Mecoptera (e.g. Panorpa, Bittacus, Boreus; Otanes 1922: very short in Apterobittacus; Hepburn 1969a: very short in Brachypanorpa). It is absent in Nannochorista (1, 10) and in Caurinus (Russell 1979; Beutel et al., 2008). It is likely that the typical elongate rostrum as it is found in Boreus and Panorpa has evolved several times independently. A rostrum is absent in Siphonaptera (Snodgrass 1946; Wenk 1953) and other groups of Endopterygota including most groups of Diptera (e.g. Wilhelmia; Schiemenz 1957; Wenk 1962). However, a distinctly elongated anterior head region, which also includes elements of the maxillae and labium (Weber 1933: fig. 95) is present in Tipulomorpha (Rees and Ferris 1939; Hennig 1973).
7. Antennal insertion: (0) dorsally and nearly adjacent; (1) dorsally but distinctly separated; (2) laterally and widely separated. The antennal insertions lie closely together on the anterior side of the head capsule between the compound eyes in Nannochorista (1, 10) and other mecopterans with the notable exception of Caurinus (Russell 1979). More or less closely adjacent antennal sockets are also present in Diptera (e.g. Rees and Ferris 1939; Hennig 1973), Hymenoptera (Beutel and Vilhelmsen 2007), Trichoptera (Klemm 1966), Lepidoptera (Kristensen 2003), and in some groups of Coleoptera (e.g. Cupedidae excl. Priacma; Hörnschemeyer et al. 2002). The antennae insert laterally in Siphonaptera (Snodgrass 1946) and anterolaterally in front of the compound eyes in most groups of Coleoptera and in Sialis (e.g. Röber 1942; Hörnschemeyer et al. 2002). An intermediate condition is found in Chauliodes (Maki 1936), Raphidioptera (Crampton 1921), and Micropterix (Hannemann 1956).
8. Dorsal tentorial arm (dta): (0) well developed; (1) present as very thin thread; (2) absent or vestigial. Typical dorsal tentorial arms are not present in Mecoptera according to Hepburn (1969a: ‘…antero-dorsal process, …, which extends to the base of the antenna’). Very thin threads are present in Caurinus (Beutel et al., 2008; coded as 1) and also in Boreus according to V. Michelsen (personal communication). However, the dorsal arms are not identifiable on several 1-μm microtome series of B. westwoodi available to us (coded as 2?). The dorsal arms are vestigial in Nannochorista (Fig. 2b,d) and absent in Panorpa (Heddergott 1938) and Bittacus. They are also absent in Bibio (personal observation, Beutel), Culicidae (Schiemenz 1957) and the tentorium is entirely reduced in Tipula (Rees and Ferris 1939). The dorsal arms occur in some groups of Diptera according to Hennig (1973), but whether the extensions of the distal anterior arms described by Wenk (1962: fig. 2, DT) are indeed dorsal arms is questionable. In agreement with Wagner (1927) and in contrast to Snodgrass (1946), we assume that the dorsal arm is always absent in Siphonaptera (see also Wenk 1953). The anterior point of attachment anterad of the eyes and antennal fossae in species with developed tentorial rods suggests that this structure is the anterior arm. The dorsal arms are vestigial in Trichoptera (Klemm 1966) and absent in Micropterix (Hannemann 1956). The tentorium is entirely reduced in Strepsiptera (Beutel and Pohl 2006). All parts of the tentorium are present in most beetles (e.g. Beutel 1986; Belkaceme 1991; Anton and Beutel 2004), Megaloptera (Maki 1936; Röber 1942), and in Chrysopa. The tentorium is weakly developed, but all parts are present in the groundplan of Raphidioptera (Aspöck and Aspöck 1971: Raphidia flavipes).
9. Shape of anterior tentorial arms (ata): (0) not developed as thick hollow rods; (1) thick, nearly round in cross-section and hollow. The anterior arm is a thick hollow rod in Nannochorista (2, 7, 8), Bittacus, Bibio (personal observation, Beutel) and Culicidae (Schiemenz 1957: fig. 5, Tentoriumrohr; Wenk 1961: fig. 2), and a similar condition is found in Chrysopa. It is also hollow but thin in Wilhelmia (Wenk 1962), whereas the lumen is absent in Eristalis (Schiemenz 1957). The anterior arms are absent or thin in Siphonaptera (Snodgrass 1946: pl. 3H) and flattened in Boreus. They are very massive anteriorly, without lumen, and flattened posteriorly in Caurinus (Beutel et al., 2008). Enlarged and hollow anterior arms are not described for non-antliophoran groups of Endopterygota (Maki 1936; Röber 1942; Hannemann 1956; Aspöck and Aspöck 1971; Anton and Beutel 2004; Beutel and Vilhelmsen 2007).
10. Frontotentorial muscle band (ftm): (0) absent; (1) present. M. frontotentorialis, a muscle which likely stabilizes the anterior tentorium, is present in Nannochorista (ften, Fig. 5) and also in Syrphidae (Schiemenz 1957: stabilisator tentorii). It is not described for any other group of endopterygote insects (e.g. Miller 1933; Maki 1936; Röber 1942; Hannemann 1956; Anton and Beutel 2004; Beutel and Vilhelmsen 2007).
11. Division of labrum (lbr): (0) absent; (1) divided into proximal fulcrum and narrow distal part. The characteristic divison of the labrum into a basal fulcrum and a narrow distal portion is found in Nannochorista (Fig. 2a) and interestingly also in Siphonaptera (Michelsen 1996/97). It was pointed out by Michelsen (1996/97) that the slender and elongate structure referred to as epipharynx by Snodgrass (1944, 1946) and Wenk (1953: fig. 20a,b) is indeed the distal part of the labrum. The labrum differs widely in Diptera, and a division like in Nannochorista and fleas is not explicitly described (Rees and Ferris 1939; Schiemenz 1957; Wenk 1962; Hennig 1973). However, it cannot be excluded that the small proximal part of the labrum in Culicidae (Schiemenz 1957) is equivalent to the proximal element in Nannochorista. The labrum is undivided in all other groups of Endopterygota (e.g. Maki 1936; Röber 1942; Hannemann 1956; Anton and Beutel 2004; Beutel and Vilhelmsen 2007).
12. Labro-epipharyngeal food canal (fch): (0) absent; (1) present. A narrow food canal is present on the ventral side of the labrum of Nannochorista (5, 9) and this is usually also the case in Diptera (e.g. Vogel 1920; ventrally closed in Culicidae: Weber 1933; Snodgrass 1959: fig. 20G,H; Schiemenz 1957: figs 8, 28 [Theobaldia], 56 [Eristalis]; Hennig 1973: figs 80, 81; Krenn et al. 2005; shallow in Bibio, personal observation, Beutel) and in Siphonaptera (Snodgrass 1946; Wenk 1953: fig. 21). It was pointed out by Michelsen (1996/97) that the structure bearing the food canal in fleas is not only formed by the epipharynx but also by the labrum (see previous character). Its presence is considered as a potential synapomorphy of Nannomecoptera, Siphonaptera, and Diptera, with loss or secondary modification in many representatives of the latter group.
13. M. labroepipharyngalis (7): (0) present; (1) absent. M. labroepipharyngalis is absent in Nannochorista (5, 9) and Caurinus (Beutel et al., 2008), but is present in Panorpa, Bittacus, Boreus, and in other groups of Mecoptera (Heddergott 1938; Hepburn 1969a: e.g. Nothiothauma). It is usually also present in Diptera (Bibio, personal observation, Beutel; Schiemenz 1957: Eristalis; Wenk 1962; Hennig 1973), but is apparently absent in Culicidae (Schiemenz 1957: fig. 28; Hennig 1973: fig. 81). All labral muscles are absent in Siphonaptera (Wenk 1953) and Strepsiptera (Beutel and Pohl 2006). M. labroepipharyngalis is present in Micropterix and occurs in different groups of beetles (e.g. Hörnschemeyer et al. 2002; Anton and Beutel 2004). A full set of labral muscles is present in Neuropterida (Miller 1933; Maki 1936; Röber 1942; Matsuda 1956).
14. M. frontolabralis (8): (0) present, origin on frons; (1) origin on clyepus; (2) absent. M. frontolabralis is absent in Nannochorista (Fig. 5), Panorpa, Bittacus, Boreus, Caurinus (Beutel et al., 2008) and other groups of Mecoptera (Heddergott 1938; Hepburn 1969a) and is also missing in Siphonaptera (Wenk 1962). It occurs in Diptera (Schiemenz 1957; Wenk 1962) but originates on the clypeus and not on the frons. This shifted origin is a potential autapomorphy of Diptera. The muscle is present in several groups of endopterygote insects, but absent in Macroxyela (Beutel and Vilhelmsen 2007), Rhyacophila, Micropterix (Hannemann 1956), Coleoptera (e.g. Hörnschemeyer et al. 2002; Anton and Beutel 2004), and Strepsiptera (Beutel and Pohl 2006).
15. M. frontoepipharyngalis (9): (0) present; (1) absent. The muscle is absent in Nannochorista (Fig. 5) and other mecopterans (e.g. Panorpa, Bittacus, Boreus, Caurinus; Beutel et al., 2008), and also in Diptera (Bibio, personal observation, Beutel; Schiemenz 1957; Wenk 1962; Hennig 1973) and Siphonaptera. It is also missing in Rhyacophila (Klemm 1966) and some groups of Coleoptera (e.g. Hörnschemeyer et al. 2002; Anton and Beutel 2004). The loss is a potential autapomorphy of Antliophora.
16. Cranial rotator of the antenna (cra): (0) absent; (1) present, origin on frons; (2) all antennal muscles originate from the head capsule. A cranial rotator of the antenna with an origin posterad of the antennal insertion is present in Nannochorista (Fig. 5), Boreus and Panorpa (Hepburn 1969a), but is absent in Caurinus (Beutel et al., 2008) and Bittacus (Setty 1940; Hepburn 1969a: fig. 19). The muscle is absent in Rhyacophila (Klemm 1966), Micropterix (Hannemann 1956), Coleoptera (e.g. Beutel 1986; Belkaceme 1991; Hörnschemeyer et al. 2002; Anton and Beutel 2004), Raphidioptera (Matsuda 1956), and Megaloptera (Maki 1936; Röber 1942). All antennal muscles originate on the head capsule in Tipula, Ctenocephalus (Wenk 1953) and Strepsiptera (Beutel and Pohl 2006). This is obviously related with the partial or complete reduction of the tentorium.
17. Primary mandibular joint (pmj): (0) present; (1) absent. The primary mandibular joint is absent in Nannochorista. It is present in Caurinus (Russell 1979; Beutel et al., 2008) and other mecopterans (Heddergott 1938; Hepburn 1969a), and also in the groundplan of Diptera (Schiemenz 1957: fig. 17 [Theobaldia]; Hennig 1973). Both points of articulation are well developed in most other lineages of Endopterygota (e.g. Maki 1936; Röber 1942; Hannemann 1956; Anton and Beutel 2004; Beutel and Pohl 2006; Beutel and Vilhelmsen 2007). The mandibles and associated structures are absent in Siphonaptera (Snodgrass 1946; Wenk 1953; Michelsen 1996/97), in the vast majority of Lepidoptera (Kristensen 2003), and in large subgroups of Diptera (e.g. Tipulomorpha, Bibionomorpha, Cyclorrapha; Hennig 1973). The mandibles are also distinctly reduced in Trichoptera and the primary joint is absent (Kaltenbach 1978).
18. Secondary mandibular joint (smj): (0) present; (1) absent. The secondary mandibular joint is modified but still present in Nannochorista (ama; Fig. 3b). It is well developed in Caurinus (Russell 1979) and still recognizable in other members of Mecoptera (Hepburn 1969a; Hennig 1973). It is always absent in Diptera (Hennig 1973) and Siphonaptera, and also in Strepsiptera excluding Mengenillidae (Beutel and Pohl 2006). In contrast to Willmann (2005) (see also Krenn 2007), the reduction of the secondary (anterior) mandibular articulation cannot be considered as an autapomorphy of Antliophora.
19. Sclerotized mandibular incisivi (inc): (0) present; (1) absent. Sclerotized mandibular incisivi are absent in Nannochorista (3, 8, 9) and are also lacking on the blade-like mandibles belonging to the dipteran groundplan (Schiemenz 1957; Hennig 1973). They are also completely reduced or vestigial (Stenopsyche) in Trichoptera (Klemm 1966; Malicky 1973). Sclerotized incisivi are present in Panorpidae (Heddergott 1938) and other members of Mecoptera (e.g. Caurinus; Matsuda 1965: figs 92A–D; Hepburn 1969a: figs 30, 32, 38) and the other lineages of Endopterygota with preserved mandibles.
20. Shape and size of mandible (shm): (0) not elongated and blade-like or lamelliform; (1) elongated, flattened and lamelliform or blade-like. The mandibles are elongated, strongly flattened distally and lamelliform in Nannochorista (3, 4). This is similar to the blade-like condition found in members of Diptera with preserved mandibles (e.g. Schiemenz 1957; Hennig 1973), although the nannochoristid mandibles are not suitable for piercing. The mandibles of Nannochorista cover the labro-epipharyngeal food channel like in Culicidae (Schiemenz 1957). We consider these conditions as shared derived groundplan features of Nannomecoptera and Diptera, or a more inclusive clade which includes also Siphonaptera, with secondary loss of the mandibles in the latter group and in many dipterans. Blade-like mandibles are not found in other lineages of Endopterygota. The mandibles of Caurinus are well developed and broad at their base (Beutel et al., 2008). They are equipped with a strong, acuminate apical tooth and additional teeth on the mesal side. They are moderately flattened and elongated in Bittacus and small and shifted to the anterior part of the rostrum in Boreus and Panorpa. In contrast to Willmann (2005) and Krenn (2007) slender mandibles should not be considered as a derived groundplan feature of Antliophora.
21. M. craniomandibularis internus (11): (0) present; (1) absent. The muscle is absent in Nannochorista (5, 7), but is present in other groups of Mecoptera (e.g. Caurinus; Beutel et al., 2008; Heddergott 1938; Hepburn 1969a) and also in the groundplan of Diptera (Wenk 1961: e.g. Anopheles;Wenk 1962: Wilhelmia). The muscle is reduced in many groups of Diptera (e.g. Tipula, Bibio, Schiemenz 1957; 1961; Wenk 1962; Hennig 1973; personal observation, Beutel). All mandibular muscles are absent in Siphonaptera (Wenk 1953). They are also lacking in Mengenilla but occur in other groups of Strepsiptera (Beutel and Pohl 2006).
22. M. craniomandibularis externus (12): (0) present; (1) absent. The abductor of the mandible is absent in Nannochorista (5, 7) but present in other groups of Mecoptera (e.g. Caurinus, Boreus; Beutel et al., 2008; Heddergott 1938; Hepburn 1969a). Like M. craniomandibularis internus it is preserved in the groundplan of Diptera (Wenk 1961: e.g. Anopheles;Wenk 1962: Wilhelmia ♀♀), but is absent in many groups (Schiemenz 1957: Theobaldia; Wenk 1961; Hennig 1973; personal observation, Beutel: Bibio).
23. M. tentoriomandibularis (13): (0) present; (1) absent. The muscle is present in Nannochorista, Caurinus (very strongly developed; Beutel et al., 2008), Boreus (extremely thin, atypical origin on lateral side of clypeus) and Bittacus, and also in the groundplan of Diptera (Schiemenz 1957; Wenk 1961, 1962), Macroxyela (Beutel and Vilhelmsen 2007), Rhyacophila (Klemm 1966), and in Chauliodes (Maki 1936). It is absent (or has been overlooked due to its very small size) in Coleoptera (e.g. Beutel 1986; Belkaceme 1991; Hörnschemeyer et al. 2002), Strepsiptera (Beutel and Pohl 2006), Myrmeleon (Wundt 1961), and Agulla (Matsuda 1965). M. tentoriomandibularis is also well developed in Chrysopa (Beutel, personal observation). In contrast to Miller (1933), it originates from the anterior tentorial arm in the typical manner.
24. Labio-maxillary complex (lmc): (0) absent; (1) present, proximal parts of maxilla without lateral movability; (2) proximal parts of maxilla strongly modified or reduced, without lateral movability. The maxilla and labium form a structural and functional complex in Nannochorista (1, 6) and other groups of Mecopterida (Hannemann 1956: fig. 3; Klemm 1966; Hepburn 1969a; Hennig 1973: fig. 82). The articulatory membrane is absent and the tentoriostipital muscle usually inserts on the ridge connecting the mesal stipital margin with the prementum. A very similar condition is found in Hymenoptera (Beutel and Vilhelmsen 2007). The maxillolabial complex of adults is probably not an autapomorphy of Hymenoptera (see e.g. Beutel and Vilhelmsen 2007), but of a more inclusive lineage also comprising Amphiesmenoptera and Antliophora. The condition in Diptera is obviously strongly modified (e.g. Rees and Ferris 1939; Schiemenz 1957; Hennig 1972). However, maxillae with exposed proximal parts with lateral movability as they are present in Neuropterida (e.g. Röber 1942) or Coleoptera are never retained (coded as 2). The loss of the postlabium was proposed as an antliophoran autapomorphy by Willmann (2003) and Krenn (2007). We consider this character as problematic, as a posterior element representing the mentum (and possibly a submentum) is present in Nannochorista and other mecopterans (Heddergott 1938; see also Hennig 1973: fig. 82, postmentum).
25. Fusion of the mesal edge of the maxillary base and the labium (fsp): (0) absent; (1) present. The mesal edges of the main body of the maxilla (cardo and stipes) are fused with the posterior part of the labium in Caurinus (Russell 1979; Beutel et al., 2008) and Boreus. The resulting structural unit is medially divided by a ridge. The fusion is a potential autapomorphy of Boreidae. It does not occur in Nannochorista (Fig. 2) and other lineages of Endopterygota, although the labium and maxillae may form a closely connected functional complex (coded as ‘–’ for Diptera).
26. Galea (gal): (0) distinctly developed; (1) vestigial or absent. The galea is lacking in Nannochorista, Diptera (Hennig 1973), and Siphonaptera (Snodgrass 1946; Wenk 1953; Michelsen 1996/97), and is also absent or vestigial in Trichoptera (e.g. Klemm 1966; Malicky 1973). In agreement with Michelsen (1996/97), we consider this feature as a potential synapomorphy of the three groups. The galea is also absent in extant Strepsiptera (Beutel and Pohl 2006) and in some groups of Coleoptera (Beutel 1997), but usually present in endopterygote insects (e.g. Maki 1936; Röber 1942; Hannemann 1956; Hörnschemeyer et al. 2002).
27. Salivary channel formed by laciniae (sch): (0) absent; (1) present. Narrow salivary channels are present on the mesal side of the lacinia in Nannochorista (salch; Fig. 9c,d). They are also present in Siphonaptera (Wenk 1953: fig. 2). In contrast to Nannochorista, the salivary channels of the laciniae are closed in fleas. The presence is a shared derived feature of Nannomecoptera and Siphonaptera. It is conceivable that the channel is secondarily absent in Diptera in correlation with modifications of the hypopharynx.
28. Sensorium of third maxillary palpomere (sen): (0) absent; (1) present. A round and deeply excavated pit with characteristic club-shaped sensilla on the mesal side of palpomere 3 is present in Nannochorista (s; Fig. 3d) and in the groundplan of Diptera (Hennig 1973). The ovoid sensillum of basal lineages of Strespiptera (Beutel and Pohl 2006) is almost certainly not homologous with this structure. It lies on the lateral side of the unsegmented palp and is only slightly concave. The sensorium is absent in Siphonaptera (Snodgrass 1946; Wenk 1953; Michelsen 1996/97) and in other groups of endopterygote insects.
29. M. craniocardinalis (15): (0) absent; (1) present. The muscle is absent in Nannochorista, Caurinus (Beutel et al., 2008), Boreus, Bittacus, and Panorpa, and also in Diptera (personal observation, Beutel; Schiemenz 1957; Wenk 1962) and Siphonaptera (Snodgrass 1946; Wenk 1953; Michelsen 1996/97). Extrinsic maxillary muscles are absent in Mengenilla and other members of Strepsiptera (Beutel and Pohl 2006). M. craniocardinalis is present in Xyelidae (Beutel and Vilhelmsen 2007), Rhyacophila (Klemm 1966), Micropterix (Hannemann 1956), Neuropterida (Miller 1933; Maki 1936; Röber 1942; Matsuda 1956), and in Coleoptera (e.g. Hörnschemeyer et al. 2002; Anton and Beutel 2004). The absence of the muscle is a potential autapomorphy of Antliophora.
30. Origin of M. tentoriocardinalis and M. tentoriostipitalis (17/18): (0) tentorium; (1) head capsule. M. tentoriocardinalis and a part of M. tentoriostipitalis originate on the clypeofrontal region in Boreus (Hepburn 1969a: figs 8, 10), and both muscles arise on the clypeus in Panorpa (Heddergott 1938: fig. 8). This highly unusual condition is not found in Nannochorista and other groups of Mecoptera (e.g. Nothiothauma; Hepburn 1969a; Caurinus; Beutel et al., 2008). M. tentoriocardinalis and M. tentoriostipitalis originate from the tentorium in Nannochorista like in representatives of the other groups of Endopterygota (e.g. Maki 1936; Röber 1942; Wenk 1953, 1962; Hannemann 1956; Schiemenz 1957). M. tentoriocardinalis is absent in Siphonaptera (Wenk 1953; Michelsen 1996/97) and in Eristalis (Schiemenz 1957), and also in other groups of Diptera (Hennig 1973).
31. Dorsal concavity of the anterior labium for reception of elements of the paired mouthparts (cal): (0) absent; (1) present. The proximal part of the basal palpomere and the unsclerotized dorsal side of the prementum form a trough or sheath, which encloses the laciniae in Nannochorista (Figs 3e,f, ltr, 9c) and in Siphonaptera (Wenk 1953: fig. 28). This is also often the case in Diptera and likely a groundplan feature of the order (Hennig 1973: figs 80, 81: ‘…Dorsalfläche ist rinnenartig vertieft, und in dieser Rinne liegen [falls vorhanden] die Stechborsten...’). This condition is considered as a potential synapomorphy of Nannomecoptera, Siphonaptera, and Diptera, with secondary modification within the latter group. It is not found in other representatives of Mecoptera examined (e.g. Caurinus, Boreus, Bittacus; Beutel et al., 2008: Heddergott 1938) and in other groups of Endopterygota. The identity of the labial parts of fleas is still unclear. In contrast to the interpretation of Snodgrass (1946) and Wenk (1953), Michelsen (1996/97) considers the basal rods as prementum and the anterior unpaired part as fused basal palpomeres.
32. Number of labial palpomeres: (0) three; (1) five; (2) two or less. Two palpomeres are present in Mecoptera (Fig. 2; Hepburn 1969a) and in the groundplan of Diptera (Rees and Ferris 1939; Snodgrass 1959; Hennig 1973; Krenn 2007). Three are present in Trichoptera (Neboiss 1991), in the groundplan of Lepidoptera (e.g. Agathipgagidae, Eriocraniidae, 3-1 in Micropterigidae; Kristensen 2003) and Hymenoptera (apical palpomere subdivided; Beutel and Vilhelmsen 2007), and in Coleoptera and Neuropterida (with some exceptions; Aspöck and Aspöck 2005). The distal parts of the labium are completely reduced in Strepsiptera (Beutel and Pohl 2006). The palps are five-segmented in Siphonaptera (Michelsen 1996/97).
33. Shape of apical labial palpomere (lbp): (0) not spoon-shaped; (1) spoon-shaped. The apical labial palpomere is medially concave and only sclerotized on its lateral side in Nannochorista (Fig. 3e,f). This is also the case in Siphonaptera (Wenk 1953). A spoon-shaped apical palpomere is unknown in non-antliophoran groups of Endopterygota (e.g. Maki 1936; Röber 1942; Hörnschemeyer et al. 2002; Beutel and Pohl 2006).
34. Scales on apical labial palpomere (sca): (0) absent; (1) present. The mesal concavity of the apical labial palpomere is densely covered with scales in Nannochorista (3, 4). Interestingly, scales do also occur on the apical parts of the labium in Culicidae (Harbach and Kitching 1998: fig. 9). However, the homology is questionable as the position of the scales is not identical (coded as 1? for Culicidae).
35. Paired glossae (glo): (0) present; (1) absent or vestigial (gl). The glossae are absent in Nannochorista as in the other groups of Mecopterida (e.g. Hepburn 1969a; Malicky 1973; Hennig 1973). A small unpaired structure is present in Micropterix (Hannemann 1956: fig. 16; coded as 1). The glossae are usually well developed in Hymenoptera (Vilhelmsen 1996; Beutel and Vilhelmsen 2007). The glossa is also absent or vestigial in Coleoptera (probably fused with the paraglossae in Priacma; Hörnschemeyer et al. 2002), Megaloptera (Röber 1942) and Raphidioptera (Aspöck and Aspöck 1971: recognizable as small membranous lobes). The ligula of Neuroptera (Miller 1933; Ferris 1940: fig. 7D) is probably not homologous with the glossa as no muscles are associated with this structure (Miller 1933; coded as 1).
36. Paraglossae (pgl): (0) present; (1) absent or vestigial. The paraglossae are also absent in Nannochorista and in the other groups of Mecopterida (e.g. Hepburn 1969a; Hennig 1973; Malicky 1973). They are usually present in Hymenoptera (Vilhelmsen 1996; Beutel and Vilhelmsen 2007) and also in Micropterix (Hannemann 1956: fig. 16). Paraglossae are also vestigial or absent in Coleoptera (see previous character), Strepsiptera (Beutel and Pohl 2006), and in Neuropterida (Miller 1933; Ferris 1940: fig. 7D; Röber 1942: fig. 15; Aspöck and Aspöck 1971).
37. Premental retractors (28–30): (0) three muscles; (1) two muscles; (2) one muscle; (3) absent. Due to more or less far-reaching modifications of the labium in Antliophora it is difficult to assess the homology of the extrinsic labial muscles. Consequently, we do not code these muscles (M. submentopraementalis [M. 28], Mm. tentoriopraementales inferior [M. 29] and superior [M. 30]) separately. We assume that the single muscle preserved in antliophoran adults is M. 29 or a product of fusion of both tentorial muscles. Only one muscle is present in Nannochorista (5, 7) and other mecopteran genera (e.g. Caurinus; Beutel et al., 2008), and this is also the case in Diptera (e.g. Bibio, personal observation, Beutel; Graham-Smith 1930, 1934; Schiemenz 1957: fig. 14; Wenk 1962: fig. 12; Hennig 1973). Extrinsic labial muscles are absent in Siphonaptera according to Michelsen (1996/97) and a typical premental retractor is also missing in Boreus. The homology of a muscle arising on the surface of the stipital region and attached to the anterior (upper) membrane of the maxillolabial complex with a thin tendon is very uncertain (coded as 3). The interpretation of the single muscle in Siphonaptera is problematic (Snodgrass 1946; Wenk 1953; Michelsen 1996/97). Two extrinsic muscles are present in Raphidioptera (Matsuda 1956), and three in Micropterix (Hannemann 1956), Xyelidae, Tenthredinidae (Taylor 1931), Neuroptera (Miller 1933; Korn 1943), Megaloptera (Maki 1936; Röber 1942), and in most groups of Coleoptera (e.g. Hörnschemeyer et al. 2002; Anton and Beutel 2004). All labial muscles are absent in Strepsiptera (Beutel and Pohl 2006).
38. Mm. praementopalpales (34/5): (0) not enlarged; (1) enlarged. The prementopalpal muscles are unusually large in Nannochorista (5, 8), and are also strongly developed in Bittacus, Diptera (e.g. Tipula, Bibio, personal observation, Beutel; Schiemenz 1957: figs 22, 56) and Siphonaptera (Michelsen 1996/97). The single prementopalpal muscle of Panorpa is elongate but slender (Heddergott 1938: fig. 9). It is also moderately sized in Boreus and Caurinus (Beutel et al., 2008). Both prementopalpal muscles are absent in Strepsiptera (Beutel and Pohl 2006), and they are not enlarged in other endopterygote insects (e.g. Maki 1936; Röber 1942; Hannemann 1956; Hörnschemeyer et al. 2002; Anton and Beutel 2004).
39. Mm. praementosalivaris (37/38): (0) present; (1) absent. Both muscles are absent in Nannochorista, Caurinus (Beutel et al., 2008), Boreus, Bittacus, and Panorpa (Heddergott 1938), and also in Theobaldia, Eristalis (Schiemenz 1957), and Wilhelmia (Wenk 1962: fig. 12). At least one bundle is present in basal hymenopteran groups (Taylor 1931; Beutel and Vilhelmsen 2007), Micropterix (Hannemann 1956), Chrysopa (Miller 1933), and in Megalptera (Maki 1936; Röber 1942). The salivarium and its muscles are lacking in Coleoptera and Strepsiptera (e.g. Beutel 1986; Belkaceme 1991; Hörnschemeyer et al. 2002; Anton and Beutel 2004; Beutel and Pohl 2006). The prementosalivary muscles are not recorded for Agulla by Matsuda (1956).
40. Sclerotised sitophore plate (sit): (0) absent; (1) present. This structure is present in Mecopterida and Hymenoptera (Heddergott 1938; Hannemann 1956; Schiemenz 1957: Fulcralplatte; Klemm 1966; Vilhelmsen 1996; Kristensen 1999). It is weakly sclerotized in Boreus compared with Nannochorista. A sclerotized prepharyngeal floor does also occur in Coleoptera, but is absent in Priacma (Hörnschemeyer et al. 2002) and other beetles (e.g. Belkaceme 1991; Anton and Beutel 2004).
41. M. frontohypopharyngalis (41): (0) present; (1) absent. M. frontohypopharyngalis is well developed in Nannochorista (Fig. 5), Boreus, Caurinus (very short; Beutel et al., 2008) and Bittacus. It is also present in Theobaldia, Eristalis (Schiemenz 1957: m. fronto-fulcralis) and Wilhelmia (Wenk 1962: rao), but is absent in Bibio. The homology of a muscle dorsolaterally attached to the posterior roof of the prepharynx is unclear. The muscle referred to as m. retractor hypopharyngis (6) by Wenk (1953) is probably a frontohypopharyngeal muscle with the origin shifted to the gena. M. frontohypopharyngalis is usually well developed in Endopterygota (e.g. Miller 1933; Röber 1942; Anton and Beutel 2004; Beutel and Pohl 2006) but is apparently missing in Micropterix (Hannemann 1956). The muscle is probably present in Agulla (origin from the clypeus; Matsuda 1956: pl. 1C [5]) and Rhyacophila (Klemm 1966: fig. 10, 3dlphy), but the homology in both cases is not entirely clear.
42. M. tentoriohypopharyngalis (42): (0) present; (1) absent. The lateral retractor of the hypopharynx is absent in Nannochorista (Fig. 3) and in other mecopterans examined (Heddergott 1938), and is also missing in Theobaldia (e.g. Schiemenz 1957) and probably also in Wilhelmia (Wenk 1962) and Siphonaptera (see previous character; Wenk 1953). M. tentoriohypopharyngalis is possibly represented by a strong muscle originating on the ventrolateral wall of the head capsule in Tipula (tentorium reduced). It is present in Hymenoptera (Beutel and Vilhelmsen 2007: M. 30) and Neuropterida (Miller 1933; Maki 1936; Röber 1942; Matsuda 1956), but absent in Strepsiptera (Beutel and Pohl 2006) and Coleoptera. The muscle designated as M. tentoriophypopharyngalis in Beutel (1986), Belkaceme (1991), Anton and Beutel (2004) and other studies is in fact M. tentoriobuccalis anterior (see char. 46).
43. Size of M. clypeopalatalis (43): (0) not enlarged; (1) two major subcomponents and multiple bundles; (2) arranged as a long series of bundles. M. clypeoopalatalis is composed of multiple bundles and two major subcomponents in Nannochorista (43, 5, 8), and a similar condition is found in Diptera (e.g. Tipula, Bibio, personal observation, Beutel; Graham-Smith 1930, 1934; Schiemenz 1957: fig. 14; Hennig 1973), Siphonaptera (Snodgrass 1946: pl. 6; Wenk 1953: fig. 38), and in Rhyacophila (Klemm 1966: figs 9, 10). The muscle is also comparatively large and complex in Xyela (Beutel and Vilhelmsen 2007), but arranged in a different manner (coded as 0). It is moderately sized in Tenthredinidae (Taylor 1931). M. 43 is composed of a long series of bundles in Boreus, Caurinus (Beutel et al., 2008) and Panorpa (Heddergott 1938: fig. 7), but not in Caurinus (Beutel et al. 2008). The muscle is not distinctly enlarged in the other groups of endopterygote insects (Röber 1942; Korn 1943; Hannemann 1956; Hörnschemeyer et al. 2002; Beutel and Pohl 2006; Beutel and Vilhelmsen 2007).
44. Transverse muscles of the epipharynx (tme): (0) absent; (1) present. The transverse musculature of the epipharynx is absent in Nannochorista (Fig. 5), Caurinus (Beutel et al., 2008), Boreus, Bittacus Panorpa (Heddergott 1938: fig. 7; Grell 1938: figs 13–17), Diptera (e.g. Tipula, Bibio, personal observation, Beutel; Graham-Smith 1930, 1934; Schiemenz 1957: fig. 14; Wenk 1962: fig. 12; Hennig 1973), and in Siphonaptera (Wenk 1953: fig. 38). This is considered as a potential autapomorphy of Antliophora. The muscles group is very strongly developed in Xyelidae (Beutel and Vilhelmsen 2007) and Strepsiptera (Beutel and Pohl 2006), and is also present in Rhyacophila (Klemm 1966: fig. 10), Coleoptera (e.g. Beutel 1986; Belkaceme 1991), and Micropterix (Hannemann 1956).
45. Precerebral pharyngeal dilators (M. 45/6): (0) at least one bundle; (1) absent. M. frontopharyngalis anterior and posterior are absent in Nannochorista (Fig. 5) and also in Eristalis (Schiemenz 1957). One undivided precerebral dilator is present in Panorpa (Grell 1938: Musc. frontopharyngalis, fig. 2), Bittacus, and Boreus, and both muscles are very strongly developed in Caurinus (Beutel et al., 2008). Both muscles are also strongly developed in Siphonaptera (Wenk 1953: fig. 38 [2, 3]), and they are also present in Wilhelmia (Wenk 1962). One muscle is described for Theobaldia (Schiemenz 1957: M. frontopharyngalis) and a group of several slender bundles is present directly above the frontal ganglion in Bibio. Mm. frontopharyngales anterior and posterior are usually well developed in other groups of Endopterygota (e.g. Heddergott 1938; Röber 1942), but they are not clearly separated in Strepsiptera (Mengenilla; Beutel and Pohl 2006).
46. M. tentoriobuccalis anterior (48): (0) present; (1) absent. The muscle which functions as median tentorial retractor of the hypopharynx is absent in Nannochorista (Fig. 5), Bittacus, and Panorpa (Grell 1938), and also in Siphonaptera (Wenk 1953), Strepsiptera (Beutel and Pohl 2006), and in some groups of Coleoptera (Hörnschemeyer et al. 2002). It is present in Boreus, Theobaldia (Schiemenz 1957: M. tentoriofulcralis), Micropterix (Hannemann 1956), Neuropterida (Miller 1933; Maki 1936; Röber 1942; Matsuda 1956), and in the majority of beetles (e.g. Beutel 1986; Belkaceme 1991; Anton and Beutel 2004; referred to as M. tentoriohypopharngalis [M. 42]). The homology of the muscle designated as pao by Wenk (1962) is unclear, but it is conceivable that it is derived from a median tentorial retractor (M. 48).
47. Strongly developed instrinsic muscle of salivarium (Sekretformer) (ims): (0) absent; (1) present. The muscle, which is likely a detached M. hypopharyngosalivaris, is present, strongly developed and composed of numerous very fine fibres in Boreus, Caurinus (Beutel et al., 2008), Bittacus, and Panorpa (Heddergott 1938). M. hypopharyngosalivaris with a typical hypopharyngeal origin is present in Nannochorista (37, Fig. 4) and other endopterygote insects (orgin from the sitophore plate in Siphonaptera; Wenk 1953: figs 34, 38). The peculiar muscle of Mecoptera excluding Nannochoristidae is part of the apparatus designated as ‘Sekretformer’ (secretion former) by Grell (1938: fig. 37). The presence is a potential autapomorphy of Mecoptera excluding Nannochoristidae.
48. Postcerebral pharyngeal pumping apparatus (pum): (0) absent; (1) present. A well-defined postcerebral pharyngeal pumping chamber is present in Nannochorista (pcp, 5, 7). It is distinctly wider than the anterior pharynx, approximately quadrangular in cross-section, and equipped with a strongly developed intrinsic musculature and with very strong dorsal, lateral and ventral dilators. The postcerebral pharyngeal pumping apparatus is also very strongly developed in Siphonaptera (Wenk 1953: fig. 37), Tipula, Culicidae (Schiemenz 1957; Snodgrass 1959), and Wilhelmia (Wenk 1962: fig. 2), and less strongly in Bibio. It is likely that it belongs to the groundplan of Diptera. It is absent in Eristalis (Schiemenz 1957) and other representatives of Brachycera (Graham-Smith 1934; Gouin 1949; Hennig 1973). Its presence is considered as a potential synapomorphy of Nannomecoptera, Siphonaptera, and Diptera, with secondary modification or reduction in the latter group. A well-defined pumping chamber is not present in Panorpa (Grell 1938: figs 15, 23; Heddergott 1938: fig. 7), Caurinus (Beutel et al., 2008), Boreus, Bittacus, and Nothiothauma (Hepburn 1969a), although the ventral postpharyngeal dilators may be strongly developed. The dorsal dilators are slender and serve mainly for the fixation of the posterior pharynx (Heddergott 1938). Well-developed postcerebral dilators are also present in Rhyacophila (Klemm 1966; fig. 9, see also pp. 23–24). However, this is clearly different from the pumping chamber found in Nannochorista, Siphonaptera, and in representatives of Diptera. A pumping chamber is also absent in Hymenoptera (Beutel and Vilhelmsen 2007), Micropterix (Hannemann 1956), Neuropteridae (e.g. Maki 1936; Röber 1942; Matsuda 1956), Coleoptera (e.g. Beutel 1986; Hörnschemeyer et al. 2002), and Strepsiptera (Beutel and Pohl 2006).
Discussion
Function of the feeding apparatus (3, 5, 7, 8, 9)
The following functional interpretations are deduced from the observed structural features and not based on direct observations or experiments. Although no information is available on the feeding habits of adults of Nannchorista and the diet is unknown, the structure of the mouthparts strongly suggests that they feed on liquid substances. It is possible that nectar is the main food source but other liquids such as juices of rotten fruits may also play a role. Nectar is frequently used as an alternative or supplementary food source by nematoceran and brachyceran Diptera, especially the males (Krenn et al. 2005; Grimaldi and Engel 2005). It was pointed out by Kingsolver and Daniel (1995) and Krenn et al. (2005) that two principal mechanisms are responsible for the uptake of surface liquids, adhesion (e.g. capillarity) and suction. Both mechanisms are combined in Nannochorista as it is also the case in many groups of Diptera (Krenn et al. 2005). Scales on the concave mesal side of the distal palpomere and fringes of hairs on the apical part of the lamelliform mandibles create capillary forces supporting the initial uptake of the fluid. It is then transported towards the opening of the closed prepharyngeal tube (functional mouth) in the food canal on the ventral side of the narrow distal part of the labrum. The canal (fch; Fig. 9d) is ventrally open but sealed by the flattened and unsclerotized distal part of the mandibles. Salivary fluid is likely transmitted in the opposite direction in mesal channels of the laciniae (salch; Fig. 9c). Like in Siphonaptera (Wenk 1953), the salivary duct opens on the dorsal surface of the structural unit formed by the distal labial and hypopharyngeal parts, exactly at the rear end of the narrow half-pipes on the mesal side of the lamelliform or stylet-like inner lobes of the maxilla. A closed salivary channel is formed in Nannochorista when the mesal sides of the laciniae of either side are laid together. They are held in this position by the unsclerotized dorsomesal parts of the distal prementum and the basal palpomeres. The concave dorsal side of the prementum forms a trough and the palps a sheath for the paired mouthparts. After the initial phase of the uptake, the fluid is sucked into the preharynx (pph; Fig. 5) and anterior pharynx by the contraction of the very strongly developed, bipartite M. clypeopalatalis (43; Fig. 5). The anatomical mouth is dilated by the contraction of M. frontopalatalis. Extensions and contractions of the strongly developed postcerebral sucking pump (pcp; Fig. 5) transport the food in the oesophageal region of the digestive tract.
Phylogeny (11)
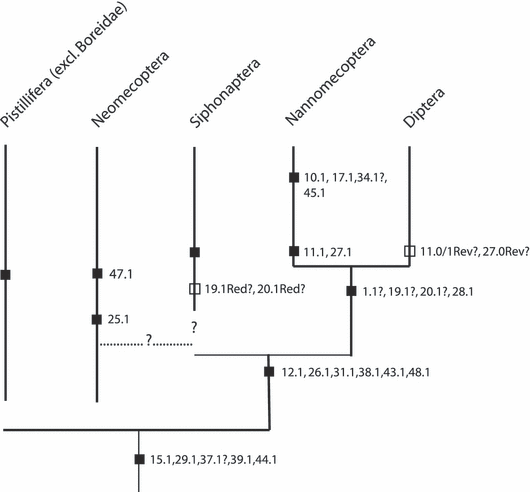
Tree based on informal character evaluation showing hypothesized placement of Nannochorista. Potential apomorphies mapped on tree (see text for detailed explanation). Red? refers to the reduction of the mandible in Siphonaptera; Rev? refers to the possible reversal in Diptera, i.e the secondary absence of a separate proximal labral piece and the secondary absence of salivary ducts on the laciniae
Our study shows that the characters of the adult head proposed as potential autapomorphies of Mecoptera (e.g. Willmann 1989, 2005) are invalid. A hypopharyngeal muscle, M. frontohypopharyngalis, is present in Nannochorista, Bittacus, and Boreus. The labrum is not fused with the clypeus in Nannochorista, and this is also the case in Caurinus (Beutel et al., 2008; Russell 1979), a little known genus assigned to Boreidae (e.g. Byers 1987). The absence of M. tentoriomandibularis (Mickoleit 1971) is not verified for most genera of Mecoptera, and the muscle is very strong in Caurinus (Beutel et al., 2008). It is extremely thin but also present in Nannochorista, Bittacus, and Boreus. The muscle was obviously reduced several times independently (e.g. Sialis, Röber 1942; Micropterix, Hannemann 1956; Coleoptera [partim], Beutel 1986, 1989; Belkaceme 1991), and it is easily overlooked if very good microtome section series are not available.
A potential autapomorphy of the order is the presence of a cranial rotator of the scapus. However, it is absent in Bittacus (Hepburn 1969a) and Caurinus (Beutel et al., 2008), and may also be missing in other genera. The remaining arguments for the monophyly of the traditional Mecoptera are related to the male genital segments or larval features. The median fusion of the basistyli (genital capsule) was suggested as an autapomorphy of Mecoptera, but this feature is absent in Boreidae. The basistyli are widely separated in Caurinus (Russell 1979: figs 81–83; Beutel et al., 2008). The genital capsule is present in Nannochorista, but differs distinctly from what is found in other mecopteran genera (Willmann 1981). The stylar organ of the dististylus is missing in three families and the homology in Bittacidae is doubtful (Willmann 1981). A detailed description of the structure is not available (not recognizable in fig. 15 [Nannochorista] in Willmann 1981) and the function is unknown (Willmann 1981). Despite the detailed contributions made by Mickoleit (1975, 1976, 1978) and Willmann (1981), a documentation of external and internal features of the genitalia of both sexes using modern techniques (e.g. SEM, three-dimensional computer reconstruction, μ-computer tomography) appears desirable.
The character state polarity of one of the two potential larval autapomorphies is very uncertain. The assumption that modified compound eyes (see e.g. Paulus 1986; Melzer et al. 1994), which occur not only in Mecoptera but also in Hymenoptera, are derived is not well supported. Besides this, stemmata seem to be present in Caurinus (Russell 1982: figs 2, 3). The other presumptive autapomorphy, the non-functionality of the metathoracic spiracles (peripneustic condition; Hinton 1958; Kristensen 1981; Willmann 2005), is not well documented, unknown for several genera, and not precisely defined. The meso- and metathoracic spiracles appear to be absent rather than non-functional in the larvae depicted in Byers (1987), and an amphipneustic spiracular condition is present in Caurinus (Russell 1982).
Our results suggest that Nannochorista is probably not part of a conventional order Mecoptera. This and a close relationship between Bittacidae and Panorpidae is in agreement with recent molecular investigations (Whiting 2002), and also with investigations of characters of the ovaries (Simiczyjew 2002; see also Beutel and Pohl 2006). A character linking Mecoptera excluding Nannochoristidae but including Boreidae is the presence of the Sekretformer (Grell 1938), with its characteristic intrinsic muscle of the salivary duct. M. hypopharyngosalivaris is present in Siphonaptera (Wenk 1953) and Nannochorista but absent in all other mecopterans examined.
Our findings support a monophyletic lineage comprising Nannomecoptera, Siphonaptera, and Diptera, but not Boreidae and other mecopteran families. The three lineages share a number of apomorphic features of the head such as the presence of a labro-epipharyngeal food canal, a cibarial pumping apparatus with a very strongly developed bipartite M. clypeopalatalis, a well-defined postcerebral pumping chamber with very strongly developed dilators, a trough or sheath formed by the anterior parts of the labium, exceptionally strong labial palp muscles, and the absence of the galea. The presence of salivary channels on the laciniae is only shared by Nannochorista and fleas. However, it is conceivable that they are secondarily absent in Diptera in correlation with the formation of an elongated hypopharyngeal stylet (absent or very short in Tipulidae and Tephritidae; Gouin 1949; Vijaysegaran et al. 1997), which transmits the salivary fluid. Within the siphonapteran–nannomecopteran–dipteran clade, a sistergroup relationship is favoured between the latter two taxa. The presence of a deep sensory groove on the mesal side of the third maxillary palpomere is a unique feature not occurring in any other group of insects. This character is not generally present in Diptera, but it is plausible to assume that it belongs to the groundplan. Lamelliform mandibles without teeth are also a potential synapomorphy of Nannochorista and Diptera. However, it cannot be excluded that similar mandibles belong to the groundplan of the more inclusive clade. The mandibles are completely absent in Siphonaptera. Considering the function, i.e. the creation of capillary forces, it is conceivable that the scales on the apical labial palpomeres of Nannochorista are a preceeding stage of the typical dipteran pseudotracheae. However, this interpretation is uncertain, and as already pointed out by Hennig (1973), a detailed comparative study of these structures in basal dipterans is wanting. Nannomecoptera and Diptera share the tendency to reduce the cranial mandibular muscles. However, they are also absent in Siphonaptera, whereas they are very obviously present in the dipteran groundplan (e.g. Wenk 1961; Hennig 1973).
The phylogenetic concept suggested here is in agreement with larval features (Beutel, Pohl, Kristensen unpublished results). The head of larvae of Nannochorista, Siphonaptera, and Diptera is distinctly prognathous and flattened, in contrast to all pistilliferan larvae (incl. Boreidae; Byers 1987). The prelabio-hypopharygneal lobe, which bears the opening of the salivary duct in other endopterygote lineages, is transformed into a prominent sclerotized process (Beutel, Pohl, Kristensen unpublished results).
Species of the extinct family †Pseudopolycentropidae share several features with Nannochorista, which may represent groundplan character states of the clade comprising Nannomecoptera, Siphonaptera, and Diptera. The external shape of the labrum is similar and elements of the paired mouthparts are elongated and stylet-like. Similarities of the female terminalia with those of Nannochorista were pointed out by Grimaldi and Engel (2005) and also affinities with Diptera. The strong reduction of the hind wings to small knob-like structures (Grimaldi and Engel 2005) suggest that this taxon branched off after Nannomecoptera, and belongs to the stem group of Diptera.
The rejection of the sperm pump as an autapomorphy of Antliophora (Hünefeld and Beutel 2005) distinctly weakened the case of the monophyly of this lineage. The present study revealed new potential synapomorphies shared by the groups traditionally assigned to Mecoptera and by Siphonaptera and Diptera. The partial (Boreidae) or complete reduction of the dorsal tentorial arm, the loss of the cranial extensor of the cardo, and the absence of transverse cibarial muscles are shared derived features of these groups. The loss of M. craniocardinalis is likely related with the formation of a maxillolabial complex, which is probably a synapomorphy of Hymenoptera and Mecopteridae. The loss of two of three extrinsic premental muscles, and the complete loss of glossae and paraglossae and their musculature are also potential antliophoran autapomorphies. However, the latter structures are also missing in Trichoptera, and also in Neuropterida, Coleoptera, and Strepsiptera. Another presumably derived character state occurring in different representatives of Antliophora is the wide and hollow anterior tentorial arm. However, as this feature is not consistently present and does also occur in other groups (e.g. Chrysopa), a phylogenetic evaluation is problematic at present.
Nannochorista is also characterized by some presumptive autapomorphies. The complete absence of the cranial mandibular muscles is likely autapomorphic, with parallel evolution in Siphonaptera and Diptera. Another potential autapomorphy is the complete reduction of the precerebral pharyngeal dilators (Mm. frontopharyngales anterior and posterior), which are rarely missing in other insect lineages. The presence of scales on the distal labial palpomere is also a possible autapmorphy. However, as pointed out before this may also be an apomorphy of a clade comprising Nannomecoptera and Diptera, with secondary modification in the latter group.
The placement of Nannochoristidae and Siphonaptera proposed in this contribution differs distinctly from the results of other recent studies (see Beutel and Pohl 2006), especially with respect to Boreidae. The molecular data suggest a clade including Nannochoristidae, Boreidae, and Siphonaptera (Whiting 2002), and this pattern is also supported by similarities of the ovarioles (Biliński et al. 1998; Simiczyjew 2002). However, the results on Nannochorista in Simiczyjew (2002) were considered as preliminary by the authors. A closer relationship between the latter groups is also supported by similarities of the process of resilin secretion (Schlein 1980) and by the presence of multiple sex chromosomes (Bayreuther and Brauning 1971). However, more data for potentially related groups appear desirable. Specific similarities of the proventriculus of boreids and fleas were not pointed out in Richards and Richards (1969). The characteristic acanthae are also present in Panorpidae and other mecopterans.
The monophyly of a lineage comprising Nannochoristidae, Boreidae, and Siphonaptera would imply that labral food channels have evolved independently in Siphonaptera, Nannochorista, and Diptera, and also the labial sheath, the postcerebral sucking chamber, and other shared characteristics of these groups (or several parallel losses). It would also imply parallel acquisition (or several losses) of the sensorial grooves on the third maxillary palpomere and also of other features occurring in Nannochoristidae and Diptera. Such a scenario cannot be excluded and it is evident that a solution of the Nannomecoptera enigma is still pending. A final settlement clearly requires an analysis of an extensive molecular and morphological data set as pointed out before.
A possible evolutionary scenario
Assuming that Siphonaptera, Diptera, Nannomecoptera, and †Pseudopolycentropidae form a clade, it appears likely that the last common ancestor of this lineage ingested fluids. They may have used a labral food channel ventrally covered by blade-like or lamelliform mandibles for the uptake of the liquid substrate, and a salivary channel formed by the laciniae. The paired mouthparts were partly enclosed in a sheath formed by the anterior labium, and the food uptake was facilitated by specific surface structures, possibly scales, on the distal part of the labial palp. All these structures are preserved in Nannochorista. The mandibles and their muscles were secondarily reduced in fleas and independently in several dipteran lineages. The salivary channel on the mesal sides of the laciniae was replaced by a novel structure formed by the hypopharynx in Diptera (not in Tipulidae and Mycetophilidae; Weber 1933), and the food uptake was optimized by the formation of pseudotracheae, which evolved within this order.
Acknowledgements
The authors are very grateful to Prof. Dr M.F. Whiting (Brigham Young University, Provo, Utah) and Prof. Dr N. Kristensen (Zoologisk Museum Copenhagen) for the gift of specimens of Nannochorista and Caurinus. Specimens of Caurinus were also provided by Prof. Dr Paul Johnson (South Dakota State University) and specimens of Boreus by Ronald Bellstedt (Museum der Natur, Gotha). This is gratefully acknowledged. We are also very grateful to Dr V. Michelsen (ZMUC) for numerous helpful comments and criticisms, which helped greatly in improving this manuscript.




