Geometric morphometrics applied to viscerocranial bones in three populations of the Lake Tanganyika cichlid fish Tropheus moorii
Abstract
Lake Tanganyika comprises the oldest and most diverse species flock of cichlid fishes. Many species are subdivided into numerous populations, often classified as geographical races, colour morphs or sister species. Unlike younger species flocks, in which speciation is accompanied by eco-morphological diversification that of Lake Tanganyika is at a mature stage with little further morphological change, most probably caused by stabilizing selection. This study addresses body shape differences among three genetically distinct but morphologically similar populations of Tropheus moorii, by focusing on bony structures of the cichlid head. We test by means of geometric morphological methods whether shape changes in the cichlid head are based on particular osteological differences. Specimens were disarticulated enzymatically, and standardized digital images of the disarticulated bony elements were taken. A landmark system was established for the dentary, articular, premaxilla, quadrate and the preopercle. Only the dentary shows significant differentiation among the three populations. Since all three populations live in similar cobble habitats and occupy the same trophic niche, the observed difference in the shape of the dentary can either be explained by different directional selection due to subtle habitat differences, or by neutral drift constrained by borders enforced through stabilizing selection. Lack of difference might indicate stabilizing selection on bone shape.
Introduction
Lake Tanganyika, with its estimated age from 9 to 12 millions years, an extension of 650 km and a maximum depth of 1470 meters, is the oldest, longest and deepest of the three East African Lakes (Coulter 1991; Cohen et al. 1993, 1997; see Fig. 1). It is well known for its large numbers of endemic species of cichlid fishes and their outstanding eco-morphological and behavioural diversity (Kawanabe et al. 1997 and references therein). The radiation process in Lake Tanganyika is already at a mature stage with a multitude of eco-morphologically as well as behaviourally diverse cichlid species, but little further morphological change, most probably caused by the action of stabilizing selection on the community level (Sturmbauer 1998). Such a mature species flock is well suited for reconstructing pathways of explosive speciation and adaptive radiation, and for the study of evolutionary change in mature communities. According to the structuring of the lake shore in rocky and sandy habitats, several species are subdivided into geographically separated and genetically isolated populations (Fryer and Iles 1972; Kornfield and Smith 2000). These were often classified as geographical races, colour morphs or sister species. The astonishing number of genetically and phenotypically distinct populations was attributed to their high degree of specialization to particular trophic niches and consequently low dispersal ability (Sturmbauer and Meyer 1992; Sturmbauer 1998). Population differentiation was repeatedly influenced by fluctuations of the lake level causing cycles of isolation and admixis of populations (Rossiter 1995; Sturmbauer 1998; Sturmbauer et al. 2001).
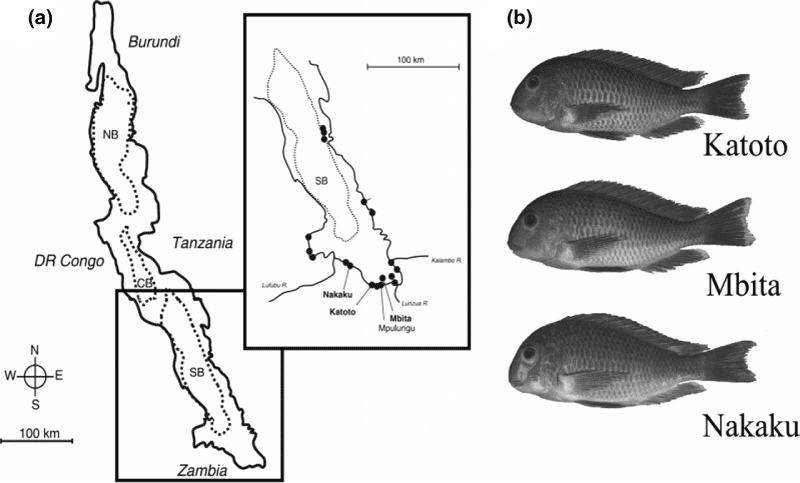
(a) Map of Lake Tanganyika with sampling locations (bold) on the southern shore. (b) Typical specimens of the three analysed populations Katoto, Mbita and Nakaku
The best-studied example for this phenomenon is the genus Tropheus (Sturmbauer and Meyer 1992; Sturmbauer et al. 1997, 2005; Baric et al. 2003; Egger et al. 2007). Currently there are six nominal species (Poll 1986) and about 120 distinctly coloured local variants described within Tropheus Boulenger, 1948 (Konings 1998; Schupke 2003). In general, populations of Tropheus moorii Boulenger, 1898 are found in the shallow zone of rock or cobble shores where the water is well illuminated for rich algal growth. All Tropheus are strictly herbivorous and feed by scraping algae from rocks. Also, they all show the same social system and breeding behaviour (Yanagisawa and Nishida 1991; Sturmbauer and Dallinger 1995). While colouration may differ markedly among populations, their external morphology remained highly similar (Snoeks et al. 1994). Morphology seems to be constrained to some extent by stabilizing selection, given that all Tropheus occupy the same ecological niche, with the exception of Tropheus duboisi Marlier, 1959 which was placed as paraphyletic to the remaining congeners (Sturmbauer et al. 2003).
This study analyses three selected populations of T. moorii (see Fig. 1) in the very south of the lake, to elucidate the degree of morphological differentiation in allopatric populations. Certain bony elements of the oral jaw apparatus, which were shown to be relevant for ecological specialization (Liem 1973, 1980; Yamaoka 1983, 1997; Smits et al. 1996; Albertson and Kocher 2001; Albertson et al. 2003a,b), were chosen for a landmark-based geometric morphometrics analysis (Rohlf and Slice 1990; Bookstein 1991; Marcus et al. 1996), in order to find out if these populations can also be discriminated via osteological structures. This study complements a sister paper focusing on general shape analysis by means of traditional and geometric morphometrics (Maderbacher et al. 2007).
Materials and Methods
Preparation of specimens
The specimens of T. moorii (n = 120; standard length (SL) 57.8–87.8 mm) used in this study were adult fish collected in May 2005 from three different locations in the southern part of Lake Tanganyika (Katoto n = 38; Nakaku n = 40; Mbita Island n = 42; see Fig. 1b). The fish were sent to our laboratory (Graz, Austria) alive via air freight. After scanning the clove-oil anaesthetized fish with a flatbed scanner (Herler et al. 2007) individuals were killed by an overdose of clove oil, preserved in 10% formalin and ascending concentrations of ethanol, to be finally stored in 70% EtOH (for detailed information on the specimens see Table A1 in the Appendix).
The heads were cut off behind the fifth dorsal fin ray and the eyes and scales were removed. Sixty of the 120 specimens were cleared and stained with alcyan blue and alizarin red using a modification of the procedure described by Potthoff (1984). After rinsing the heads overnight they were stained with alcyan blue for 48 h (70 ml ethanol 70%, 30 ml glacial acetic acid and 20 mg of alcyan blue for each head). Specimens then were neutralized in sodium-tetraborate for 2 h (55 g l−1 aqua dest. dissolved at 100°C). Next the skin was scarified prior to bleaching with H2O2 and KOH (3–5 ml H2O2 per 100 ml 0.178 M KOH). The bleaching time depended on specimen size and varied between 4 and 5 h. Before starting the maceration heads were cleared for about 1 week in 0.178 M KOH at room temperature. Then they were transferred into trypsine solution (35 g trypsine in 1000 ml sodium-tetraborate plus 2.33 l aqua dest.) to be digested at 30 to 35°C for 3 days. To support the process and to avoid decay the solution was changed every day. Subsequently, specimens were macerated further in 0.178 M KOH as long as necessary, but never exceeding a total processing time of 14 days. The heads were then carefully stained with alizarin red (100 mg alizarin red in 1000 ml 0.178 M KOH) for up to 30 min maximum and washed out for about 60 min in 0.178 M KOH. Finally, heads were transferred to pure glycerine for 24 h as follows: The first rinse overnight in 0.089 M KOH and glycerine in a ratio of 2 : 1, followed by 0.089 M KOH and glycerine in a ratio of 1 : 2 for 24 h, and finally two changes of pure glycerine.
For 60 individuals the head was disarticulated by digestion with KOH and trypsine for 21 days (0.178 M KOH, before weekend 0.089 M KOH; trypsine-solution: 35 g trypsine in 1000 ml sodium-tetraborate plus 2.33 l aqua dest.). The other 60 specimens were disarticulated with Enzyrim® (Bauer Handels GmbH, Adetswil, Switzerland) (Grundmann and Roetzscher 2000) following the standard recipe with an incubation overnight at 55°C (20 g Enzyrim® l−1 water, 10 g l−1sodium chloride and 2.5 g l−1 Supralan, a detergent, adjusted at pH 8.5 with sodium carbonate).
Geometric morphometrics and statistical analyses
Following Albertson and Kocher (2001), the following skeletal elements of the viscerocranium were analysed: dentary, articular, premaxilla, quadrate and preopercle. All elements were taken from the left body side unless they were damaged. Digital images were obtained in standardized position with an Olympus digital camera (Olympus E-1 – Zuiko Digital 14-54 mm f 2.8–3.5; Olympus Optical Co., GmbH, Hamburg, Germany) mounted on an Olympus SZX-ILLB2-200 binocular eyepiece (Olympus Life and Material Science Europa GmbH, Hamburg, Germany). The images were imported into the software tpsDig 2.10 (TPS Software Series, Stony Brook, NY, USA) (Rohlf 2006) and digitized. Details about the positioning of the landmarks are given in Table 1 and Fig. 2. The positioning of these landmarks was standardized to represent all structural features in the two-dimensional image and to capture the overall shape of each bone.
| Element | Landmark number | Descriptions (after Barel et al. 1976) |
|---|---|---|
| Dentary (caudal) | 1 | Medial tip of the ventral process |
| 2 | Lateral tip of the ventral process | |
| 3 | Lateral tip of the dentigerous part | |
| 4 | Rostral tip of the dentigerous part | |
| 5 | Ventral-most point of the dentigerous part | |
| Dentary (lateral) | 1 | Dorsal tip of the dentary process |
| 2 | Ventral-most endpoint of the mandibular sensory canal (located on the last and ventral-opened pore) on the ventral process | |
| 3 | Rostral tip of the dentary | |
| Articular (lateral) | 1 | Retroarticular process |
| 2 | Tip of the rostral articular process | |
| 3 | Dorsal tip of the articular process | |
| 4 | Dorsal process of the suspensoriad articulation facet | |
| 5 | Postarticulation process (of suspensoriad articulation facet) | |
| Premaxilla (lateral) | 1 | Dorsal process of the ascending spine |
| 2 | Dorsal process of the maxillad spine | |
| 3 | Ventral-most point of the interprocess edge | |
| 4 | Rostral-most point of the dentigerous arm | |
| Quadrate (lateral) | 1 | Outside-most dorsal point of the quadrate |
| 2 | Caudal-most point of upper plane part of the quadrate | |
| 3 | Caudal-most tip of lower arm of the quadrate | |
| 4 | Rostroventral-most point of the articulation process | |
| Preopercle (lateral) | 1 | Dorsal-most tip of the preopercle |
| 2 | Rostral-most point of lower part of the preopercle | |
| 3 | Outer-most point of curvature of lower part of preopercle |
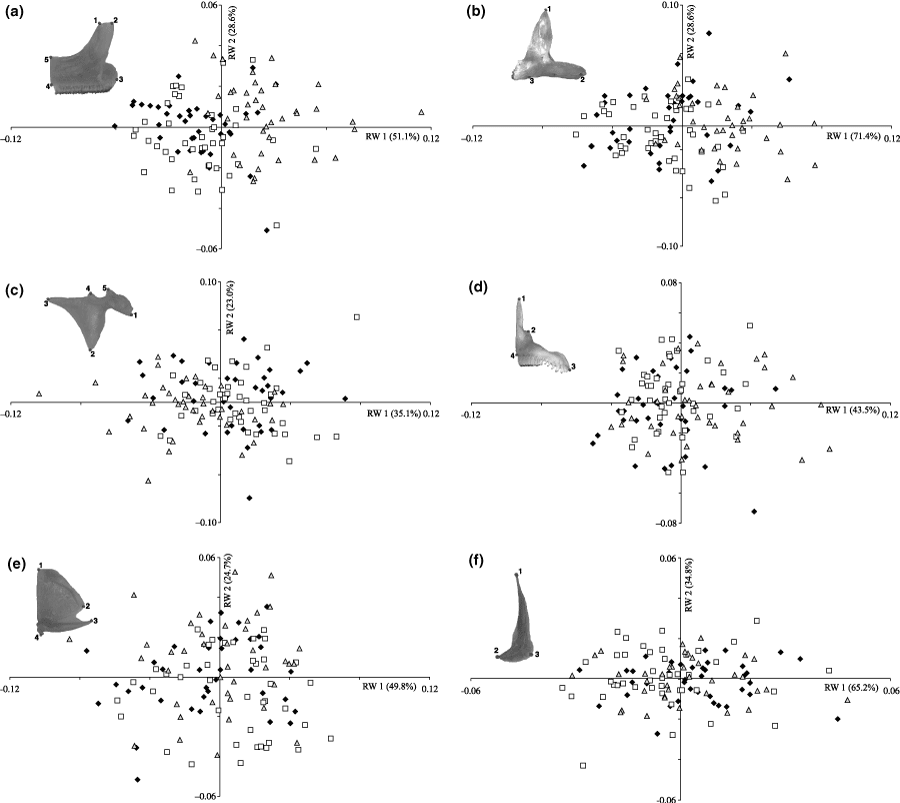
Relative warp analysis on the shape variables of the viscerocranial bones (Nakaku = △, Katoto = ♦, Mbita = ). (a) Caudal view of the dentary (5 landmarks). (b) Lateral view of the dentary (3 landmarks). (c) Lateral view of the articular (5 landmarks). (d) Lateral view of the premaxilla (4 landmarks). (e) Lateral view of the quadrate (4 landmarks). (f) Lateral view of the preopercle (3 landmarks)
The statistical analyses were performed with imp (Integrated Morphometrics Package, Buffalo, NY, USA; Sheets 2003) or TPS (Rohlf 2006, 2007). First, the raw coordinates were generated and aligned utilizing the partial Procrustes superimposition (Rohlf 1999; Slice 2001) in the subprogram CoordGen6 (coordinate generating utility program; Integrated Morphometrics Package), then a relative warp analysis (RW), which is similar to a principal component analysis of shape variables, was carried out with tpsRelw (version 1.45; TPS Software Series; Rohlf 2007). The RWs were calculated to summarize the variation among the specimens (related to their partial warp scores) in preferably few dimensions. To test for morphological variation between the three populations a canonical variate analysis (CVA) was carried out in CVAGen6 (canonical variate analysis generating program; Integrated Morphometrics Package). This CVA identified the set of axes which best discriminates among two or more groups. After computing partial warp scores to a general reference this program was also used to conduct a multivariate analysis of variance (manova), followed by a CVA. In this way, all distinct CVA axes were examined for the data at a significance level of p < 0.05 and the canonical variate scores of all analysed specimens were calculated.
Finally, pair-wise comparisons based on the CVA-results were carried out using TwoGroup6 (Integrated Morphometrics Package) which is also part of the imp software package. Goodall’s F-test (Bonferroni-corrected) was used to test significant shape differences between the three populations. Calculations of the full and partial Procrustes distances between the means of two groups were conducted in TwoGroup6. For the two views of the dentary thin-plate spline deformation grids for the two CVA axes were generated.
Results
Relative warp analyses
Relative warp-scatter plots for the five bony structures show RW axis 2 against RW axis 1. Only the plot for the caudal view of the dentary (Fig. 2a) separated the Nakaku-population, which was placed on the positive side of RW axis 1, while the Mbita- and Katoto-population were placed on the negative side. Neither of the plots for the remaining bony elements could separate groups; the populations overlapped widely (Fig. 2).
Canonical variate analyses and Goodall’s F-test
The CVA for the dentary separated the Nakaku population from the other two (Fig. 3a and b). However, the CVA for the articular, premaxilla, quadrate and preopercle could not clearly discriminate the three populations (Fig. 3c–f). Goodall’s F-test (Table 2) was calculated to test in-between population shape differences of viscerocranial elements between populations. Lack of independence in the comparisons amongst the three populations was aligned by the use of Bonferroni correction factor.
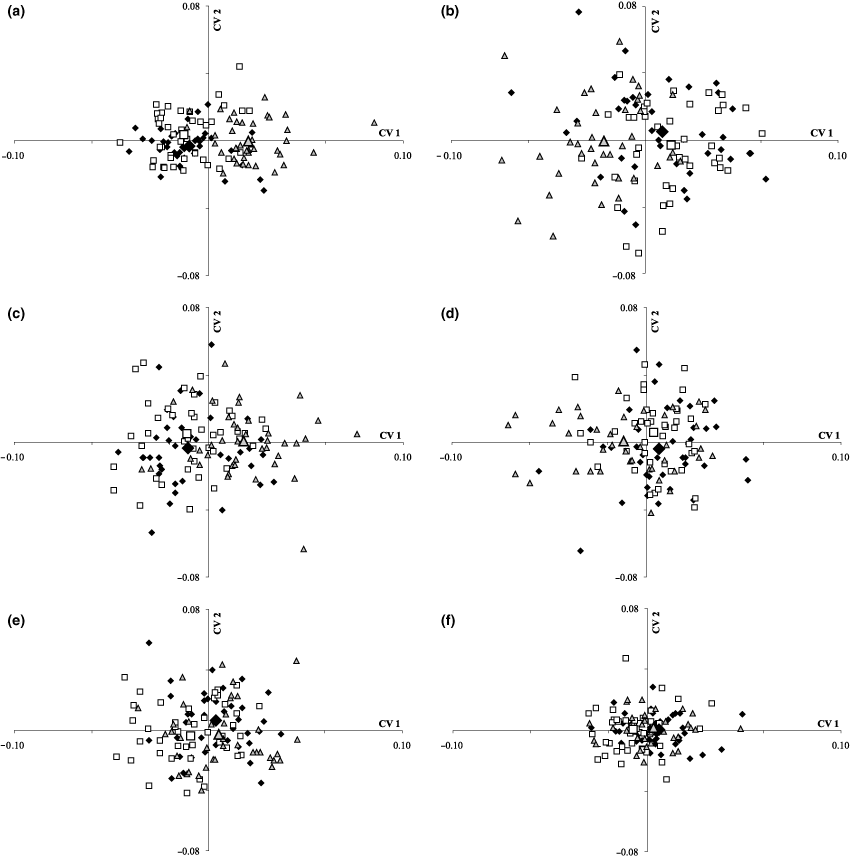
Canonical variate analysis on the shape variables of the viscerocranial bones (Nakaku = △, Katoto = ♦, Mbita = ; distribution means for each population are shown by enlarged symbols). (a) Caudal view of the dentary (5 landmarks). (b) Lateral view of the dentary (3 landmarks). (c) Lateral view of the articular (5 landmarks). (d) Lateral view of the premaxilla (4 landmarks). (e) Lateral view of the quadrate (4 landmarks). (f) Lateral view of the preopercle (3 landmarks)
| Katoto– Mbita | Katoto– Nakaku | Mbita– Nakaku | ||
|---|---|---|---|---|
| Dentary caudal view | F-score | 0.92 | 22.92 | 22.26 |
| p* | 0.48 | 0 | 0 | |
| dbm | 0.01 | 0.04 | 0.04 | |
| Dentary lateral view | F-score | 1.76 | 21.28 | 13.93 |
| p* | 0.18 | 6.55 × 10−9 | 2.79 × 10−6 | |
| dbm | 0.01 | 0.03 | 0.03 | |
| Articular | F-score | 0.59 | 6.14 | 8.19 |
| p* | 2.21 | 0.00001 | 5.61 × 10−8 | |
| dbm | 0.01 | 0.03 | 0.03 | |
| Premaxilla | F-score | 1.35 | 4.68 | 4.28 |
| p* | 0.75 | 0.003 | 0.006 | |
| dbm | 0.01 | 0.02 | 0.02 | |
| Quadrate | F-score | 3.24 | 1.1 | 3.3 |
| p* | 0.03 | 1.07 | 0.03 | |
| dbm | 0.02 | 0.01 | 0.02 | |
| Preopercle | F-score | 5 | 0.75 | 1.91 |
| p* | 0.024 | 1.43 | 0.46 | |
| dbm | 0.01 | 0.003 | 0.01 |
- *Bonferroni adjusted p-value, p < 0.05.
Pair-wise comparison with Goodall’s F-test between Katoto and Nakaku and between Mbita and Nakaku showed significant differences in dentary shape (p < 0.05). As can be seen in the CVA-plot for the caudal view of the dentary, most data points of the Nakaku-population and their mean value were situated on the positive side of CV 1, while values for the Katoto- and Mbita-population were mostly placed on the negative side, and overlapped extensively with each other. Concerning the lateral view of the dentary, Nakaku was again clearly separated from the other two populations, but this time was placed more on the negative side of CV 1. Likewise, Katoto and Mbita were similar and overlapped extensively, but on the positive side of CV 1 (Fig. 3b; Ev CV 1 = 0.39 and Ev CV 2 = 0.027). For the articular the same trend as for the caudal view of the dentary was observed in that the Nakaku-population was placed on the positive side of CV 1, while Katoto and Mbita were placed on the negative side, again with greater overlap (Fig. 3c; Ev CV 1 = 0.35 and Ev CV 2 = 0.02). In case of the premaxilla, all three populations were more similar and overlapped widely (Fig. 3d; Ev CV 1 = 0.13 and Ev CV 2 = 0.03). The quadrate (Fig. 3e; Ev CV 1 = 0.14 and Ev CV 2 = 0.03) and the preopercle (Fig. 3f; Ev CV 1 = 0.11 and Ev CV 2 = 0.0) showed a different trend, in that Nakaku and Katoto were more similar to each other and placed on the positive section of CV 1, while Mbita was shifted slightly to the negative section of CV 1, as corroborated by Goodall’s F-test for the two bones (Table 2).
Thin plate spline deformation grids for the dentary
Trends of morphological divergence displayed in the CVA are shown in two sets of deformation grids for CV 1 and CV 2 (Fig. 4a and b for the caudal view and Fig. 4c and d for the lateral view of the dentary). The most significant differences in the deformation grid of the caudal view for the CV 1 were observed for the medial tip of the the ventral process (Landmark 1) and the lateral tip of the dentigerous part (Landmark 3; for landmark positions see Fig. 3a). The ventral process was shortened, whereas the dentigerous part was elongated.
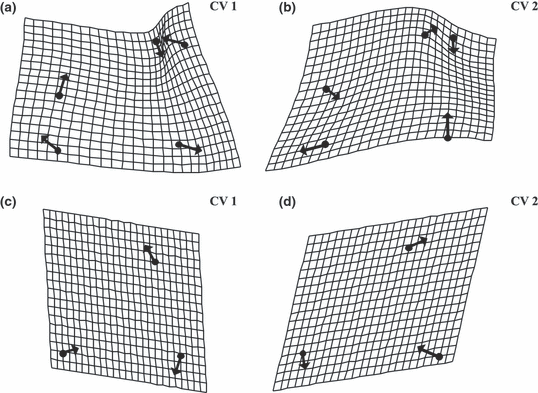
Canonical variate analysis on GM data of 120 specimens for the caudal view of the dentary based on five landmarks and shape changes in CV 1 (a) and CV 2 (b; both deformation grids with vectors, scaling factor 0.2). Canonical variate analysis on GM data of 119 specimens for the lateral view of the dentary based on three landmarks and shape changes in CV 1 (c) and CV 2 (d; both deformation grids with vectors, scaling factor 0.2)
In case of CV 2 the deformation grid of the caudal view (Fig. 4b) also showed the strongest variation for the medial tip of the ventral process (Landmark 1) and the lateral tip of the dentigerous part (Landmark 3); both process height and the overall height of the tooth-free process of the dentary was shortened. Concerning the deformation grid of the lateral view for CV 1 the tooth-bearing part, the rostral tip of the dentary, seemed to decrease (Landmark 3), while both the dentary process (the dorsal tip of the dentary process, Landmark 1) and the ventral process (the ventral-most endpoint of the mandibulary sensory canal on the ventral process, Landmark 2) seemed to elongate (Fig. 4c). The deformation grid for CV 2 showed elongation of the dentary process (Landmark 1) and compression of the ventral process (Landmark 2) and the dentigerous part (Landmark 3), as depicted in Fig. 4d. In conjunction with the CVA-scatter plots for the two views of the dentary (Fig. 3a and b), a more distinct morphology was found for the Nakaku population, in relation to the other two populations analysed.
Discussion
Methodological aspects
Our initial methodical approach was examination of the bony structures on the head in situ. Therefore, the bony structures of the head of sixty specimens were stained with alcyan blue and alizarine red (Dingerkus and Uhler 1977; Potthoff 1984). Then digital images of the lateral view of the stained heads were generated. Unfortunately, placing landmarks on scans of the non-disarticulated and glycerine-stored heads could not be carried out because the tissue was not clear enough to unambiguously define the chosen structures. Moreover, the long clearing procedure led to tissue deformations. For this reason the heads had to be fully disarticulated with KOH and trypsine in order to analyse the bones separately. The disarticulation procedure with KOH and trypsine caused partial loss of the teeth, while disarticulation by digestion with Enzyrim® led to a total loss of the teeth. For this reason neither the first nor the second method can be recommended for quantitative comparative investigations of oral and pharyngeal teeth. However, for studying the morphology of bony elements Enzyrim® is better suited, because the procedure is faster and decay is not a problem.
Landmark analysis on bony elements
Interestingly, RW analysis could not distinguish groups clearly for all five selected bony elements (Fig. 2); the populations partly overlapped. The same was found for the CVA analyses for the articular, premaxilla, quadrate and preopercle. In contrast, the CVA-analysis of both views of the dentary differentiated the Nakaku population from the two others, Mbita and Katoto (Fig. 3a and b). The Goodall’s F-tests (Table 2) showed significant differences between the three groups for both views of the dentary. Results imply a closer morphological similarity between the Mbita and Katoto which is also supported by investigations based upon the overall body shape (Maderbacher et al. 2007). This is interesting because it recently was shown that the population at Katoto comprises descendents of a secondary admixis event between the Kasakalawe–Mbita mtDNA lineage and the mtDNA lineage north of Katoto, represented here by fish from Nakaku (Sturmbauer et al. 2005; Sefc et al. 2006).
The great similarity in four of the five surveyed bony structures can be explained by the fact that all Tropheus populations occupy the same ecological niche, despite a long time of separate evolution. As alternative interpretation for the observed similarity, a relatively weak power of separation, either caused by non-optimal selection of landmarks or by non-optimal positioning of bones for the two-dimensional image, may be put forward although in general, GM is particularly useful for the discrimination of slight differences and varieties between species and populations. Thus, as perspective for future work, it may be important to further optimize and standardize the positioning of bones for image-taking as well as the positioning of the landmarks. It may also be interesting to test different two-dimensional representations from different orientations, to find the most informative image for a given structure. Alternatively, one might consider 3D-scans to cope with the problem of unequal outline projection onto 2-dimensional images. In addition, more comparative investigations using traditional and geometric morphometrics in parallel, are also suggested in order to find out the more appropriate approach for discriminating osteological structures of morphologically similar entities such as populations or sister species.
Morphological differentiation of species and populations on the basis of bony structures
Fryer and Iles (1972) observed that the head region, especially the viscerocranium, varied to a great extent in cichlid fishes as recently corroborated by a stringent analysis of Chakrabarty (2005). It was suggested this was due to strong natural selection on those morphological structures immediately responsible for trophic niche separation. Meanwhile, several morphological characters in the cichlid head have been classified that predict feeding performance of the biting mode (Liem 1973, 1991; Otten 1983; Meyer 1989; Bouton et al. 2002a,b). Recent hybridization experiments on two eco-morphologically distinct epilithic algae feeding cichlids of Lake Malawi attributed the observed marked differences in certain bony elements of the viscerocranium to distinct trophic specialization (Albertson and Kocher 2001). These differences were shown to be genetically controlled via quantitative trait loci-analyses (Albertson et al. 2003a). In this study, we analysed three populations of the Lake Tanganyika rock-dwelling cichlid T. moorii which – unlike the previously analysed Lake Malawi cichlids – are eco-morphologically equivalent and remained morphologically similar despite considerable evolutionary age and colour variation among populations (Sturmbauer and Meyer 1992). A parallel study on the overall morphology of these three populations established considerable morphological variation, even if the fish live in similar shore habitats and occupy the same trophic niche (Maderbacher et al. 2007). Five bones of the viscerocranium, for which adaptive significance was demonstrated (Albertson and Kocher 2001; Bouton et al. 2002a,b), were selected for our landmark-based geometric morphometric analysis, in order to investigate whether the three consecutive populations were also distinguishable via osteological structures of the viscerocranium. Specifically, we focused on the lower jaw (dentary and its complementary part, the articular), the premaxilla (part of the upper jaw) and on quadrate and preopercle. Important indicators for the biting mode are for example a short ascending arm of the dentary (Otten 1983), a wide, robust lower jaw (Liem 1991), an obtuse angle formed between the two arms of the premaxilla (Otten 1983) and a short load-bearing region of the ascending arm of the premaxilla. In our study it turned out that within the species level – despite long-term separation – most bony structures are highly conserved in evolutionary terms. Only the dentary, the tooth-bearing anterior part of the lower jaw, showed significant shape variation between the three populations analysed. The two populations belonging to two major mtDNA lineages with a divergence time of 0.55 to 1.1 million years age (Baric et al. 2003; Sturmbauer et al. 2005) were more distinct from each other than the natural hybrid population of the two lineages.
Evolutionary implications
While it is well established that head morphology and the underlying bony structures can vary tremendously among species and genera of cichlid fishes and that they are highly indicative for particular ecological and trophic specializations, it was so far assumed that they are the same or at least highly similar among populations. However, one has to consider the time of evolution available in a species flock. The flocks of cichlid fishes in the three East African Great Lakes, Victoria, Malawi and Tanganyika are of substantially different age. Lake Victoria is certainly less than 200 000-year old, its majority of species perhaps even 12 000 years (Johnson 1996). Lake Malawi is about 1 million-year old, while the Lake Tanganyika flock may go back 5–6 million years, perhaps even 9- to 12-million years. Greenwood (1984) argued that evolutionary age is reflected in the degree of morphological divergence observed in the three species flocks, in that Lake Victoria species are morphologically most similar and vary continuously, while Lake Tanganyika species are so highly distinct from each other that intermediates are lacking, and Lake Malawi cichlids being intermediate. Thus, it was suggested that the adaptive radiation in Lake Tanganyika is already in a highly mature stage, in which a densely packed species community reduces the probability for further eco-morphological innovation, so that speciation mostly proceeds allopatrically without further eco-morphological change (Sturmbauer 1998). It was, however, shown that not all Lake Tanganyika-species subdivided into populations are homogeneous. Considerable morphological differences among populations of eretmodine cichlids were found, sometimes even indicating parallel evolution (Rüber et al. 1999). Other species show considerable geographic variation, but mostly in colour. This is especially true for the genus Tropheus, for which about 120 colour morphs are known (Schupke 2003). Local adaptation causing population differentiation was suggested to be a major driving force of speciation during the process of adaptive radiation. All allopatric models involve local differences in environmental conditions, in that abiotic or biotic influences cause differential natural selection. So, it was important to test, to which extent morphological structures can vary among populations, in particular those that are considered of selective relevance.
The genus Tropheus– despite considerable morphological stasis – was shown to be about equally old as the entire mbuna clade of Lake Malawi (Sturmbauer and Meyer 1992), so that one can postulate a long period of allopatric evolution in the absence of substantial morphological change, due to the action of stabilizing selection keeping species in the niches they invaded. Thus, the here observed significant differences in the shape of the dentary are interesting in the light that all other bones remained more similar among the surveyed populations. Indeed, the observed difference may indicate differential selective forces, or be the result of neutral drift within the constraints enforced by stabilizing selection. Given that these populations for a long time live in the same niche and the same type of habitat, it is indeed possible that morphology of specialization-relevant structures can vary slightly within the freedom granted by stabilizing natural selection, so that populations remain competitive on the community level for the niche they once occupied.
Taken together with the results recently obtained for the overall morphology of these three populations (Maderbacher et al. 2007), it seems that overall morphology may drift considerably under stabilizing selection. Most but not all bony structures in the head with established selective relevance for trophic specialization seem indeed quite static in terms of evolutionary change. A combined morphometric and population genetic analysis is needed to explore these perspectives further.
Acknowledgements
We would like to thank P. Ngalande, H. Phiri and D. Sinyinza, Department of Fisheries, Ministry of Agriculture and Cooperatives, Republic of Zambia, for their support during field work. The study was financed by the Austrian Science Fund project number P17680-B06.
Zusammenfassung
Anwendung geometrischer Morphometrie (GM) auf Knochen des Viscerocraniums bei drei Populationen von Tropheus moorii, einer im Lake Tanganyika endemischen Cichlidenart
Der Tanganyikasee ist eines der ökologisch komplexesten Süßwasserhabitate der Erde. Er zeichnet sich durch eine große Anzahl von endemischen Buntbarsch-Arten aus, die eine Vielfalt ökologischer Nischen besetzt haben. Viele Arten sind ihrerseits in genetisch und phänotypisch unterschiedliche Populationen unterteilt. Ein gutes Beispiel für dieses Phänomen ist die Gattung Tropheus. Diese Studie beschreibt die Unterschiede zwischen drei genetisch diferenzierbaren, aber morphologisch ähnlichen Populationen von Tropheus moorii in spezialisierungs-relevanten Knochen des Kieferapparates mit Hilfe geometrischer Morphometrie (GM). Knochenelemente spielen bei morphologischen Untersuchungen eine wichtige Rolle; schließlich kann man äußerliche Formveränderungen oft auf Unterschiede in bestimmten Knochenstrukturen zurückführen. Für die Analyse wurden zunächst die Köpfe der untersuchten Individuen vollständig disartikuliert, danach die einzelnen Knochen standardisiert digital photographiert und ein geeignetes Landmark-Set für jedes einzelne Knochenelement entwickelt. Nur im Fall des Dentale zeigen sich signifikante morphologische Unterschiede zwischen den drei untersuchten Populationen. Nachdem sie dieselbe trophische Nische besetzen und in ähnlichen aber nicht gleichen Habitaten leben, kann der beobachtete Formunterschied im Dentale entweder auf den Einfluss unterschiedlich gerichteter Selektion aufgrund feiner Habitats-Unterschiede oder auf neutrale Drift zurückzuführen sein. Das Fehlen von morphologischen Unterschieden in den anderen Knochen könnte dagegen auf die Wirkung von stabilisierender Selektion hinweisen.
Appendix
Appendix
| Pop. | Sex | ID | SL | Pop. | Sex | ID | SL | Pop. | Sex | ID | SL | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Katoto | m | T05/9/I3 | 84.2 | 1 | Mbita | m | T05/10/E6 | 77.55 | 1 | Nakaku | m | T05/9/A1 | 82.5 |
| 2 | Katoto | m | T05/9/I6 | 82.36 | 2 | Mbita | m | T05/10/E7 | 72.71 | 2 | Nakaku | m | T05/9/A8 | 82.15 |
| 3 | Katoto | m | T05/9/I8 | 82.56 | 3 | Mbita | m | T05/10/E9 | 77.53 | 3 | Nakaku | m | T05/9/A9 | 81.32 |
| 4 | Katoto | m | T05/9/I10 | 75.09 | 4 | Mbita | m | T05/10/E10 | 70.97 | 4 | Nakaku | m | T05/9/A10 | 84.4 |
| 5 | Katoto | m | T05/9/J1 | 72.59 | 5 | Mbita | m | T05/10/F1 | 76.7 | 5 | Nakaku | m | T05/9/B1 | 84.7 |
| 6 | Katoto | m | T05/9/J2 | 83.8 | 6 | Mbita | m | T05/10/F2 | 77.74 | 6 | Nakaku | m | T05/9/B3 | 78.35 |
| 7 | Katoto | m | T05/9/J10 | 76.45 | 7 | Mbita | m | T05/10/F3 | 74.32 | 7 | Nakaku | m | T05/9/B7 | 77.71 |
| 8 | Katoto | m | T05/10/A1 | 73.77 | 8 | Mbita | m | T05/10/F4 | 78.54 | 8 | Nakaku | m | T05/9/B8 | 82.79 |
| 9 | Katoto | m | T05/10/A2 | 78.39 | 9 | Mbita | m | T05/10/F7 | 74.27 | 9 | Nakaku | m | T05/9/B10 | 78.78 |
| 10 | Katoto | m | T05/10/A4 | 76.71 | 10 | Mbita | m | T05/10/F9 | 74.04 | 10 | Nakaku | m | T05/9/C1 | 79.18 |
| 11 | Katoto | m | T05/10/A5 | 77.62 | 11 | Mbita | m | T05/10/G1 | 71.08 | 11 | Nakaku | m | T05/9/C2 | 81.71 |
| 12 | Katoto | m | T05/10/A9 | 69.94 | 12 | Mbita | m | T05/10/G2 | 69.2 | 12 | Nakaku | m | T05/9/C4 | 61.21 |
| 13 | Katoto | m | T05/10/B1 | 66.67 | 13 | Mbita | m | T05/10/H3 | 66.25 | 13 | Nakaku | m | T05/9/C5 | 75.57 |
| 14 | Katoto | m | T05/10/B3 | 59.94 | 14 | Mbita | m | T05/10/H4 | 67.21 | 14 | Nakaku | m | T05/9/C7 | 81.59 |
| 15 | Katoto | m | T05/10/B4 | 57.81 | 15 | Mbita | m | T05/10/H5 | 65.31 | 15 | Nakaku | m | T05/9/C8 | 67.97 |
| 16 | Katoto | m | T05/10/B7 | 73.08 | 16 | Mbita | m | T05/10/H6 | 68.02 | 16 | Nakaku | m | T05/9/D2 | 77.18 |
| 17 | Katoto | m | T05/10/C5 | 73.84 | 17 | Mbita | m | T05/10/H8 | 66.4 | 17 | Nakaku | m | T05/9/D4 | 69.83 |
| 18 | Katoto | m | T05/25/A7 | 72.04 | 18 | Mbita | m | T05/10/H9 | 66.86 | 18 | Nakaku | m | T05/9/D5 | 72.61 |
| 19 | Katoto | m | T05/9/I1 | 85.87 | 19 | Mbita | m | T05/10/H10 | 74.46 | 19 | Nakaku | m | T05/9/D6 | 73.14 |
| 20 | Katoto | f | T05/9/I2 | 84.64 | 20 | Mbita | m | T05/10/D6 | 76.52 | 20 | Nakaku | m | T05/9/E7 | 66.65 |
| 21 | Katoto | f | T05/9/I4 | 78.23 | 21 | Mbita | m | T05/10/D5 | 74.71 | 21 | Nakaku | f | T05/9/A3 | 81.83 |
| 22 | Katoto | f | T05/9/I5 | 74.44 | 22 | Mbita | f | T05/10/E1 | 74.67 | 22 | Nakaku | f | T05/9/A5 | 84.5 |
| 23 | Katoto | f | T05/9/I7 | 74.24 | 23 | Mbita | f | T05/10/E2 | 76.44 | 23 | Nakaku | f | T05/9/A6 | 87.86 |
| 24 | Katoto | f | T05/9/I9 | 78.04 | 24 | Mbita | f | T05/10/E3 | 79.71 | 24 | Nakaku | f | T05/9/B4 | 84.62 |
| 25 | Katoto | f | T05/9/J5 | 71.15 | 25 | Mbita | f | T05/10/E4 | 76.46 | 25 | Nakaku | f | T05/9/B9 | 74.79 |
| 26 | Katoto | f | T05/9/J6 | 78.54 | 26 | Mbita | f | T05/10/E5 | 82.41 | 26 | Nakaku | f | T05/9/C3 | 76.6 |
| 27 | Katoto | f | T05/9/J9 | 79.2 | 27 | Mbita | f | T05/10/E8 | 75.24 | 27 | Nakaku | f | T05/9/C6 | 75.68 |
| 28 | Katoto | f | T05/10/A3 | 75.79 | 28 | Mbita | f | T05/10/F5 | 78.87 | 28 | Nakaku | f | T05/9/C9 | 82.91 |
| 29 | Katoto | f | T05/10/A6 | 70.31 | 29 | Mbita | f | T05/10/F8 | 67.39 | 29 | Nakaku | f | T05/9/D1 | 84.49 |
| 30 | Katoto | f | T05/10/A7 | 67.34 | 30 | Mbita | f | T05/10/F10 | 71.08 | 30 | Nakaku | f | T05/9/D3 | 79.08 |
| 31 | Katoto | f | T05/10/A8 | 72.68 | 31 | Mbita | f | T05/10/G3 | 76.54 | 31 | Nakaku | f | T05/9/D7 | 64.74 |
| 32 | Katoto | f | T05/10/B5 | 74.33 | 32 | Mbita | f | T05/10/G4 | 71.26 | 32 | Nakaku | f | T05/9/D8 | 67.68 |
| 33 | Katoto | f | T05/10/B6 | 71.2 | 33 | Mbita | f | T05/10/G5 | 77.44 | 33 | Nakaku | f | T05/9/D9 | 64.37 |
| 34 | Katoto | f | T05/10/B2 | 68.18 | 34 | Mbita | f | T05/10/G6 | 77.18 | 34 | Nakaku | f | T05/9/E1 | 61.15 |
| 35 | Katoto | f | T05/10/C6 | 73.68 | 35 | Mbita | f | T05/10/G7 | 72.22 | 35 | Nakaku | f | T05/9/E4 | 62.98 |
| 36 | Katoto | f | T05/10/C7 | 75.56 | 36 | Mbita | f | T05/10/G9 | 68.55 | 36 | Nakaku | f | T05/9/E5 | 61.54 |
| 37 | Katoto | f | T05/11/A6 | 79.03 | 37 | Mbita | f | T05/10/G10 | 69.63 | 37 | Nakaku | f | T05/9/E6 | 64.07 |
| 38 | Katoto | f | T05/11/A7 | 72.99 | 38 | Mbita | f | T05/10/I1 | 77.23 | 38 | Nakaku | f | T05/9/H5 | 72.36 |
| 39 | Mbita | f | T05/10/I2 | 73.08 | 39 | Nakaku | f | T05/9/H6 | 72.74 | |||||
| 40 | Mbita | f | T05/11/A9 | 77.77 | 40 | Nakaku | f | T05/9/B6 | 83.02 | |||||
| 41 | Mbita | f | T05/11/A10 | 79.43 | ||||||||||
| 42 | Mbita | f | T05/10/D4 | 69.99 |




