Female mating frequency in a wild population of scorpionflies (Panorpa germanica, Panorpidae, Mecoptera)
Weibliche Paarungshäufigkeit in einer Wildpopulation von Skorpionsfliegen (Panorpa germanica, Panorpidae, Mecoptera)
Abstract
enIn this study, we investigated mating frequencies of female scorpionflies Panorpa germanica in the field using two different experimental approaches. First, the mating status of wild-caught females was estimated on the basis of sperm numbers present inside their sperm storage organs. Secondly, the number of mating partners wild-caught females must have had was inferred from mother-offspring analyses of a polymorphic microsatellite locus. Our results suggest a very low mating frequency of wild female P. germanica. Consequently, the risk of sperm competition is rather low in this species. The relevance of female mate choice in relation to the low mating frequency is discussed.
Zusammenfassung
deIn dieser Studie wurde die Paarungshäufigkeit von Freilandweibchen der Skorpionsfliege Panorpa germanica mittels zweier verschiedener experimenteller Ansätze untersucht. Zum einen wurde der Paarungsstatus von im Freiland gefangener Weibchen anhand der Menge der im Spermienspeicherorgan vorhandenen Spermien abgeschätzt. Zum anderen wurde über Mutter- und Nachkommen-Genotypisierung eines polymorphen Mikrosatelliten-Locus auf die Zahl der Paarungspartner von Freilandweibchen geschlossen. Unsere Ergebnisse deuten auf eine sehr geringe Paarungshäufigkeit der Weibchen von P. germanica im Freiland hin. Dementsprechend ist bei dieser Spezies das Risiko der Spermienkonkurrenz relativ gering. Die Bedeutung von Weibchenwahl im Zusammenhang mit der geringen Paarungshäufigkeit wird diskutiert.
Introduction
As the production of male sex cells usually involves relatively little metabolic energy investment males can often increase their reproductive output by mating with as many females as possible (Bateman 1948; Parker et al. 1972; Trivers 1972). Females on the other hand are much more limited in their gamete production and the number of sperm females obtain within one or few matings should in general be sufficient for complete fertilisation; hence a female's reproductive output should not increase with number of matings performed (Bateman 1948; Parker et al. 1972; Trivers 1972). However, in contrast to these predictions, females of many animal species mate multiply. Various explanations for why polyandry may increase female fitness in some species have been suggested (reviewed in Arnqvist and Nilsson 2000): females may for instance simply ensure the receipt of sufficient viable sperm (Walker 1980), but female reproductive success may also be enhanced through direct benefits such as nuptial gifts or indirect, genetic benefits (Ridley 1988; Zeh 1997; Tregenza and Wedell 1998, 2000; Vahed 1998; Yasui 1998; Neff and Pitcher 2005). Although there is no general explanation for the evolution of polyandry, it is clear that female mating rate plays an important role in many evolutionary processes such as sperm competition (e.g. Parker 1970; Boorman and Parker 1976; Birkhead and Møller 1998) or cryptic female choice (e. g. Eberhard 1996; Birkhead 1998). In many species the optimal mating rate will be different for males and females, triggering sexual conflict between the two sexes (e. g. Rice 1996; Markow 2002; Chapman et al. 2003). The intensity of sperm competition, for instance, largely depends on the females’ mating frequency, but certainly has strong implications for the evolution of male adaptations to increase paternity success (Stockley 1997).
Females of the scorpionfly Panorpa vulgaris Imhoff and Labram, 1836 (Mecoptera, Panorpidae), have been shown to be highly polyandrous not only under laboratory conditions but also in the field (Sauer et al. 1998, 1999). Numerous studies on the mating system of this species revealed that polyandry is beneficial for females as it mediates ‘cryptic female choice’ (sensuThornhill 1983). Just as in other Panorpa species males of P. vulgaris provide females with nutritious saliva secretions during copulation (Sindern 1996; Sauer et al. 1997, 1998; Sauer 2002) and this nuptial feeding has been shown to function as an honest signal for male quality (Fleck and Sauer 1995; Sauer et al. 1998; Kurtz and Sauer 1999) according to the ‘good genes’ models of sexual selection (Zahavi 1975; Andersson 1982). Females decide on copula duration based on the number of salivary masses they receive and because sperm is transferred continuously throughout mating males that are able to produce many ‘salivary masses’ will also transfer larger amounts of sperm than males of minor saliva secretion (Sindern 1996; Sauer et al. 1997, 1998; Sauer 2002). The number of sperm transferred in turn determines the males’ reproductive success: through a complete mixing of sperm from different males inside the female's spermatheca paternity allocation follows the ‘fair raffle principle’ (sensuParker 1990), i. e. it reflects the relative numerical representation of sperm each male contributes (Sauer et al. 1999). This cryptic female choice based on saliva secretion as an honest signal for male quality and complete sperm mixing is a safe method to discriminate between males of different qualities; however, in order to do so females of P. vulgaris always have to mate multiply.
In contrast to P. vulgaris males of the related species P. germanica Linnaeus, 1758, produce pheromones in order to attract females (Kock et al. 2007). These pheromones could in addition function as male quality trait and female mate choice may therefore take place already prior to copulation. If so, polyandry does not constitute a precondition for female mate choice in this species.
In laboratory investigations on life history traits of P. germanica a mean lifetime mating frequency of 2–3 copulations per female was observed; about 27% mated only once, but a total number of up to eight matings per female was also observed (Gerhards 1999). In contrast, males generally adopted a polygynous mating strategy, but overall showed a higher variance in mean lifetime mating frequency than females (Gerhards 1999).
The aim of the present study is to estimate the mating frequency of females of P. germanica in a natural population in the field. This investigation is essential, as we presume the mating frequency of wild females to be lower than observed under laboratory conditions. The density of individuals is usually higher in laboratory experiments than in the field, resulting in increased encountering probabilities. Effects of density on female remating frequency have been shown for other insect species (e. g. for Drosophila ananassae, Singh and Singh, 2001). Furthermore, males of P. germanica release pheromones in order to attract females (Kock et al. 2007) and the females’ willingness to mate could also be affected by high concentrations of male pheromone in the laboratory. We applied two different experimental approaches to estimate female mating frequencies in a natural population. First, the mating status of wild females collected at different points of time during the season was estimated on the basis of sperm numbers present inside the females’ sperm storage organs. Secondly, the number of mating partners of wild-caught females was inferred from mother-offspring analyses using a polymorphic microsatellite locus. Taken together these two approaches allow us to draw clear conclusions about the level of polyandry in P. germanica in the field.
Methods
Inferring female mating status from the number of sperm present inside the spermatheca
In order to estimate mating frequencies of wild females of P. germanica adult females were collected in spring 1998 at a collection site near Bonn, Germany. Collecting took place every 2–5 days throughout the whole flying season (see Table 1). In total 89 females were captured between May 9 and June 5. We estimated the current mating status of each of the collected females by counting the number of sperm present inside their spermathecae and comparing this number to the average ejaculate size of wild-caught males.
| Sampling date | Sample size | Duration of copulations with virgin females [min] | Number of sperm transferred |
|---|---|---|---|
| Wild-caught males | |||
| 45 | 435 ± 94 | 1248 ± 630 | |
| Wild-caught females | Number of sperm in the spermatheca | Estimated number of matings | |
| 9 May | 2 | 528 ± 197 | 0.42 ± 0.16 |
| 13 May | 17 | 957 ± 652 | 0.77 ± 0.52 |
| 16 May | 10 | 1135 ± 723 | 0.91 ± 0.58 |
| 18 May | 9 | 626 ± 294 | 0.50 ± 0.24 |
| 23 May | 9 | 1237 ± 627 | 0.99 ± 0.50 |
| 26 May | 10 | 1910 ± 1116 | 1.53 ± 0.95 |
| 31 May | 11 | 1179 ± 765 | 0.95 ± 0.61 |
| 5 June | 1 | [3820] | [3.06] |
| Total | 69 | 1179 ± 851 | 0.94 ± 0.68 |
- Values are given as Mean (±SD)
Sperm numbers were determined by staining and subsequently counting the sperm the females’ spermathecae contained (see Sauer et al. 1997): females were anaesthetised using CO2 and the spermatheca was dissected. A DNA-specific fluorochrome dye (DAPI = 4′, 6-diamidino-2-phenylindole; Cal Biochem GmbH, Frankfurt, Germany; concentration 5 g ml−1 0.1 molar, pH7 Tris-buffer) was used to stain the DNA-carrying regions of the spermatozoa, so that sperm could be counted using an Orthoplan-Fluorescence Microscope (magnification 200–400×, Leitz, Wetzlar, Germany).
The mean ejaculate size of wild males was determined by collecting adult males in the field at the same time as the females and mating them each to a lab-reared virgin female (for details about lab-breeding see Sauer 1970, 1977; Kock et al. 2006). After copulation the spermatheca of each of these test-females was dissected and the number of sperm having been transferred was counted. The mean ejaculate size of wild-caught males was calculated on the basis of 45 test-copulations.
Some of the sperm preparation samples were counted twice in order to test for repeatability of sperm counting. Out of a total number of 104 samples 18 were counted twice (10 of 69 from wild-caught females and eight of 45 from test-females). As a linear regression approved high repeatability (F17 = 611.4, P < 0.001; R2 = 0.97), we take our method as being reliable. For any further analyses we always used the sperm numbers obtained at first counts.
Inferring female mating status from mother-offspring analyses of a polymorphic microsatellite locus
Female mating frequency was estimated via mother-offspring analyses of a polymorphic microsatellite locus named PG2 (Kock et al. 2006). To estimate the degree of polymorphism at this locus within our study population we collected 206 adult P. germanica (109 females and 97 males) at our collection site near Bonn, Germany, and determined their genotypes with respect to the PG2-locus (for methodology see Kock et al. 2006).
For mother-offspring microsatellite analyses we collected adult female P. germanica at different points of time during the flying season in spring 2003 as well as in spring 2004 (see Table 2) from our study population. These wild-caught females were then kept in the laboratory separately in plastic boxes (10 cm × 10 cm × 6 cm), each containing moist tissue paper as water supply and a small Petri dish (Ø 5 cm) filled with moist peat as substrate for oviposition. Food (segments of last instar larvae of Tenebrio molitor) was provided ad libitum. For further development all eggs laid were transferred into small Petri dishes (Ø 5 cm) containing moist tissue paper. We checked the clutches daily and transferred any hatched larvae into 100% ethanol until used for microsatellite analysis. Females were allowed to lay eggs until they died and were then stored at −80°C until DNA-extraction. Many of our approximately 100 wild-caught females did not lay any eggs at all or did not achieve any egg hatching success. Therefore, only 27 families were analysed. In total 482 larvae were genotyped at the polymorphic microsatellite locus PG2. Having identified the mother's and offspring's genotypes with respect to the PG2-locus, a minimum number of fathers can be inferred from the number of paternal alleles (alleles different to the mother's genotype) found among the offspring of a given female.
| Wild-caught females | Number of larvae screened for paternal alleles | Number of females with a minimum number of | |||
|---|---|---|---|---|---|
| Sampling date | Sample size | Total number of larvae | Number of larvae per female alleles), mean (±SD) | One mate (up to two paternal alleles) | Two mates (up to four paternal alleles) |
| 4 May 2003 | 1 | 17 | – | 1 | |
| 8 May 2003 | 1 | 30 | 1 | – | |
| 12 May 2003 | 2 | 74 | 37.0 ± 24.0 | 2 | – |
| 18 May 2003 | 1 | 16 | – | 1 | |
| 14 May 2004 | 1 | 8 | 1 | – | |
| 18 May 2004 | 8 | 80 | 10.0 ± 2.9 | 7 | 1 |
| 22 May 2004 | 1 | 11 | 1 | – | |
| 25 May 2004 | 4 | 68 | 17.0 ± 5.2 | 3 | 1 |
| 30 May 2004 | 4 | 71 | 17.7 ± 3.2 | 3 | 1 |
| 2 June 2004 | 1 | 5 | 1 | – | |
| 7 June 2004 | 1 | 20 | 1 | – | |
| 14 June 2004 | 2 | 37 | 18.5 ± 2.1 | 2 | – |
| Total | 27 | 437 | 16.2 ± 9.6 | 22 | 5 |
Results
Mating status of wild-caught females inferred from sperm numbers
In total 89 wild-caught females were checked for numbers of sperm present inside their sperm storage organs. Of these 89 females 20 were found to be virgins as no sperm could be detected inside their reproductive tracts, whereas the remaining 69 females showed variable numbers of sperm inside their spermatheca, ranging from 97 up to 4286 spermatozoa. Table 1 presents mean sperm numbers for each of our sampling days separately as well as the mean number of sperm per spermatheca taking all 69 inseminated females together.
Throughout the season a number of 45 males were also caught in the field in order to perform test-copulations with virgin females in the laboratory for estimating the mean ejaculate size of wild males. Table 1 gives the mean duration of these test-copulations together with the mean number of sperm transferred. Based on the mean ejaculate size of 1248 spermatozoa per copulation we estimated the number of copulations each of the wild-caught females has had before being captured. Estimated numbers of matings ranged from 0.08 to 3.34 copulations per female. Estimated mean numbers of matings of wild-caught females for each of our sampling days as well as the overall estimated mean number of copulations per female are presented in Table 1. These data suggest a predominant monandry of females in the field, as estimated numbers of copulations remain small throughout the whole flying season, with most females presumably having had only a single copulation even at the end of season. However, as ejaculate size varied considerably between males and a few very large ejaculates might have led to a serious underestimation of female mating frequency, we took a closer look at the frequency distributions of ejaculate sizes and the amount of sperm encountered inside the females’ reproductive tracts as presented in Fig. 1. Again we conclude that our data are in good accordance with the assumption of a very low mating frequency of female P. germanica in the field.
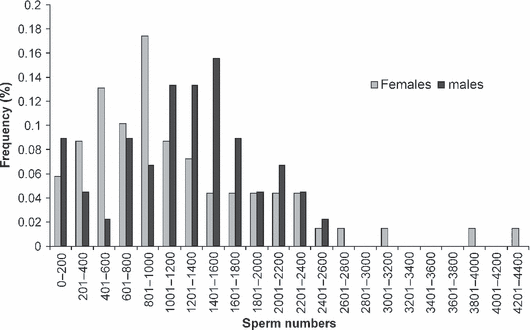
Frequency distributions of sperm numbers transferred by males within a single test-copulation and retrieved from wild-caught females
Yet, our data also indicate that some females do mate more than once. In this case, however, mating frequency apparently does not exceed two or three copulations per female.
Remating rates inferred from mother-offspring analyses of a microsatellite locus
To estimate the degree of polymorphism of the PG2-locus a number of 206 adult scorpionflies collected from our study population was genotyped and seven different alleles could be identified occurring with varying frequencies (Fig. 2). Seventy-four individuals showed two different alleles whereas only a single allele could be identified for the other 132 animals. However, from previous studies involving paternity analyses based on the PG2-locus (Kock et al. 2006) we know that alleles that do not amplify occur regularly; hence individuals showing only one allele can still be heterozygous with one allele failing to get amplified. Thus, the exact degree of heterozygosis cannot be determined.
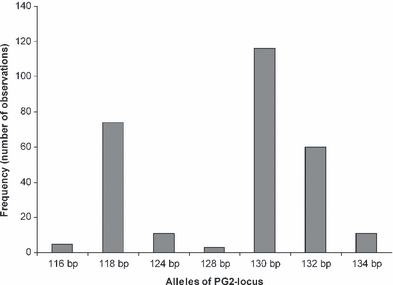
Frequencies of different alleles of the PG2-microsatellite locus observed within a wild population of P. germanica near Bonn, Germany. bp = base pairs
For estimating female mating frequency a total number of 437 larvae obtained from 27 different wild caught-females were screened for paternal alleles, i. e. alleles that differ from the mother's genotype (Table 2). On average 16.2 (SD = 9.6) larvae per female were genotyped. Table 2 summarises our results obtained from microsatellite analyses. In 22 of these 27 cases (i.e. in 81%) we found that one male possibly fathered all offspring. Solely five cases could be identified in which at least two males were involved. A minimum number of more than two mates could not be detected. The five cases in which two or more fathers were found to be involved were evenly distributed over the whole period of collecting; the mating status of fertilised females did not relate to progression of season or age of the females, respectively. Again our data suggest that females of P. germanica mate only very few times under natural conditions in the field. Still, the exact number of fathers cannot be ascertained unambiguously as males might share the same alleles with respect to the PG2-locus and therefore cannot be discriminated. This is especially true for the most common allele 130 bp (see Fig. 2). Female mating frequency will therefore be underestimated to some degree.
Discussion
Estimated mating frequencies of wild-caught females
The numbers of sperm stored in wild-caught females at different points of time during the flying season indicates that females of P. germanica were mostly monandrous or only slightly polyandrous. Admittedly, this method has its limitations in revealing the real number of matings a female was engaged in before being captured. The wild-caught males showed considerable variation in the amount of sperm they transferred in test-copulations in the laboratory. Thus, inferring a female's mating status from the number of sperm in store compared to the mean ejaculate size of wild-caught males can only be an approximation to the real situation. It is needless to say that the actual number of copulations can only be an integer and of course no smaller than one if only inseminated females are considered. However, many females had much fewer sperm in store than a wild male on average transferred in an undisturbed copulation. Since sperm is transferred continuously throughout copulation (Gerhards 1999) very small numbers of sperm indicate mating events of short duration. Compared to copulations taking place in the laboratory an early termination might occur more frequently under field conditions because of various possible sources of disturbance (e. g. predators, conspecifics, weather conditions). Consequently, the actual mean sperm transfer with respect to natural copulations could be somewhat smaller than calculated and the female mating status might be underestimated in some cases.
Additionally, we cannot tell the oviposition history of wild-caught females. Continuous usage of sperm as eggs get fertilised will obviously reduce the number of sperm in storage with time. Under laboratory conditions (i. e. no predation, no disturbance and high food supply) females lay up to about 100 eggs during their lifetime (D. Kock, personal observation). This number will be much lower in the field due to a limited availability of food and higher risk of predation. Comparing this number to the mean number of 1179 spermatozoa females had in store and the wild males’ mean ejaculate size of 1248 spermatozoa the usage even of about 100 spermatozoa is negligible for estimating the number of matings a female has had. However, removal of rival sperm by males or a depletion or loss of sperm from the spermatheca could in addition lead to lower numbers of sperm in store than a female has initially received (Tsubaki and Yamagishi 1991; Yamagishi et al. 1992). Yet, removal of rival sperm has not been found in scorpionflies (K. P. Sauer, personal observation). Furthermore, we could show that sperm is present and fully viable inside the female's reproductive tract for at least 7 or 8 days after mating and high egg hatching rates (>50%) are regularly achieved for more than two weeks after a single copulation (Kock and Sauer 2007). We do realise that our sperm counts will in some cases somewhat underestimate the total number of sperm a female has received before being captured, but we are still confident about our conclusion that female P. germanica will in general either behave monandrous or will adopt only a slightly polyandrous mating strategy.
In addition, the mother-offspring microsatellite analyses gave results that are in good accordance with the results obtained through determination of sperm numbers. Mother-offspring analysis of microsatellite loci has recently been applied in several studies investigating female mating behaviour in the field (e. g. Sauer et al. 1999; Bonizzoni et al. 2002; Bundgaard et al. 2004; Kraaijeveld et al. 2005). However, the actual number of matings will be underestimated for some females, as different males sharing the same alleles cannot be discriminated. This becomes especially relevant if only a single locus is considered. But as no other than our PG2-microsatellite system has been established for P. germanica so far and markers used for related species such as P. vulgaris (see Epplen et al. 1998; Sauer et al. 1999) do not work in our species, there is no circumventing of this problem to date. Still, if female P. germanica generally adopted a polyandrous mating strategy, one would expect to reveal more than two fathers at least in some cases.
Taking the results of our two experimental approaches together allows us to draw clear conclusions about the mating frequencies of females of P. germanica in the field. In both cases we found that most females had in all likelihood performed only a single copulation. Some had presumably mated twice and a few probably even thrice. But there are no hints that any female had performed more than three copulations. We therefore conclude that in P. germanica most females are monandrous in the field, whereas some adopt a slightly polyandrous mating strategy. Female mating frequencies of up to eight copulations like Gerhards (1999) observed are most certainly attributable to unnatural laboratory conditions and thus constitute an artefact. We assume that in laboratory experiments the mating frequency of female P. germanica is increased due to high densities resulting in high encountering probabilities of males and females and high concentrations of male pheromone. Although increased densities did not increase remating frequency in laboratory populations of Drosophila melanogaster (Gromko and Gerhart, 1984) there is evidence that density affects female remating rate in another Drosophila species (D. ananassae, Singh and Singh, 2001). Kraaijeveld et al. (2005) on the other hand showed that changing sex ratios in laboratory populations affects female remating in Mediterranean fruit flies (Ceratitis capitata). They inferred the remating rates of wild females from mother-offspring microsatellite analyses, which were found to be in accordance with results from their laboratory experiments. Yet, the authors did not report total numbers of matings females performed during lifetime, but only if remating (i. e. more than one copulation) occurred. A study on mating frequencies in a natural population of D. buzzatii revealed lower mating frequencies in the field (Bundgaard et al. 2004) than previously observed in laboratory experiments (Bundgaard and Barker 2000).
Female mating frequency, female mate choice, and the relevance of sperm competition
A very low level of polyandry is a characteristic in which the mating system of P. germanica differs greatly from those of related species such as P. vulgaris (Sauer et al. 1998, 1999), P. communis Linnaeus, 1758, (Aumann 2000), or P. cognata Rambur, 1842, (Engqvist and Sauer 2001, 2003). For P. vulgaris it has been shown that polyandry is beneficial for females as it forms a precondition for cryptic female choice (Sauer et al. 1997, 1998, 1999). In contrast to P. vulgaris female mate choice in P. germanica possibly takes place already prior to copulation based on male pheromone production (Kock et al. 2007). Thus, females of P. germanica do not need to mate multiply for getting high quality males to sire their offspring and may therefore mostly behave monandrous.
Clearly, the low level of polyandry in P. germanica has implications for the relevance of sperm competition in this species. Together with the low remating rate of females the risk of sperm competition is also low. In most cases males obtaining a copulation will remain the only mate of the according female. Males on the other hand are polygynous (Gerhards 1999; and D. Kock, personal observation). As a consequence competition between males in terms of mate acquisition should be substantial. This assumption gets support from the observation that variance in mating frequency is much higher for males than for females (Gerhards 1999; and D. Kock, personal observation). Furthermore, males obtaining no copulations at all are not uncommon. Apart from precopulatory female choice based on pheromone production males of P. germanica always have to offer salivary masses already prior to copulation in order to initiate a copulation (D. Kock, personal observation).
Compared to other species (e. g. for P. vulgaris see Sauer et al. 1998) mating takes rather long in P. germanica: the mean duration of test-copulations performed by wild-caught males with virgin females was 435 min (SD = 94). Interestingly, Gerhards (1999) found that the longer the first copulation of a female the less likely she will mate again. The extreme copulation durations we observe in P. germanica may thus constitute an adaptation of males reducing the risk of sperm competition. However, from our study we know that some females do mate more than once. In case of doubly mated females paternity outcome largely relates to the relative copulation duration of both males, but with the second male on average achieving a slightly greater fertilisation success than its copulation duration would predict (Kock et al. 2006). Apparently, this second male advantage is not caused by natural death, loss, or depletion of sperm, but might be attributable to some sperm stratification effects (Kock and Sauer 2007). Anyhow, because of this second male advantage the first male should have an even stronger interest in preventing the according female from remating than in case of a ‘fair raffle’(sensuParker 1990).
Thus, males of P. germanica should always aim to achieve copulations of maximum duration to reduce the probability of female remating in the first place, but also to increase paternity success in the event of sperm competition. Long matings can be achieved through high saliva secretion: females adjust copula duration to the number of salivary masses they receive (Gerhards 1999) and terminate copulation when the male ceases to produce salivary masses (D. Kock, personal observation). As the capability of saliva secretion of male scorpionflies depends on male condition (Fleck et al. 1994; Fleck and Sauer 1995) female P. germanica choose for male quality not only precopulatory (pheromone production and offering a salivary mass to initiate copulation) but also during mating based on the quantity of salivary secretion. Male mating effort (sensuSimmons and Parker 1989) is therefore extensive in this species and female mate choice imposes great selection pressure on males.
Acknowledgements
We thank Sonja von Zeddelmann and Alexander Weiß for providing data from their Diploma theses. The ‘Studienstiftung des deutschen Volkes’ we thank for the provision of a full Ph.D. scholarship. This project was supported by the ‘Deutsche Forschungsgemeinschaft’ [SA 259/7–1 to 4].




