Phylogeny of the African representatives of the catfish family Clariidae (Teleostei, Siluriformes) based on a combined analysis: independent evolution towards anguilliformity
Phylogénie des Clariidae africaines (Siluriformes) fondée sur des analyses combinées: l’évolution indépendante vers l'anguilliforme
Abstract
enClariid catfishes span a broad range of body forms ranging between fusiform and anguilliform morphotypes. Although such variation in body shape has been observed in other families of teleost fishes, amphibians and reptiles, it is rarely as extreme as within the Clariidae. Although the Clariidae were thought to have undergone anagenetic evolution (i.e. progressive evolution within a lineage), more recent studies indicate that anguilliformity evolved several times through a process of cladogenesis (i.e. branching of evolutionary lineages). In this study, it is shown that the phylogenetic analysis of morphological data mainly gives a reflection of the cranial evolution in the Clariidae despite the use of 18 post-cranial characters (out of a total of 53 characters). A combined phylogenetic analysis of both morphological and molecular data rather suggests the derived nature of body elongation. The corresponding morphological changes that co-occur with this elongation can be regarded as an extreme case of convergent evolution at the genus level within the Clariidae.
Résumé
frLes Clariidae représentent une gamme entre des morphes fusiformes et anguilliformes. Quoique cette gamme soit observée dans des autres familles des téléostéens, des amphibies et des reptiles, elle n'est jamais si prononcée que dans les Clariidae. Même si on a pensé que les Clariidae ont subit une évolution anagénétique, les études récentes ont fournis l'hypothèse que l'anguilliformité a évoluée plusieurs fois par le processus de cladogenèse. Dans la présente étude, on montre que l'analyse phylogénétique fondée sur des caractères morphologiques, donne essentiellement une réflexion de l’évolution crânienne des Clariidae, malgré l'usage de 18 caractères postcrâniennes. L'analyse phylogénétique combinée (morphologique et moléculaire) suggère plutôt la nature dérivée de l’élongation du corps. Les changements morphologiques correspondants, qui se reproduisent avec cette élongation, peuvent être considérés comme des cas extrêmes d'une évolution convergente au niveau du genre.
Introduction
The freshwater clariids are one of the 37 catfish families within the order Siluriformes (Sabaj et al. 2004). Although some species are distributed in Syria, southern Turkey and throughout Southeast Asia, their diversity is the greatest in Africa (Teugels 1996; Teugels and Adriaens 2003). Some of the generalized, fusiform species in Africa, such as Clarias gariepinus (Burchell, 1922) are broadly distributed, whereas the anguilliform species are restricted to swampy areas in the Nilo-Sudan (Niger delta), the Lower Guinea and the Zaire (Congo River basin) ichthyological provinces (Poll 1957; Roberts 1975; Teugels 1986; Teugels et al. 1990).
Clariid catfishes are characterized by an elongate body, four pairs of barbels, and by the unique presence of a suprabranchial respiratory organ, formed by arborescent structures derived from the second and fourth gill arches (Greenwood 1961; Teugels and Adriaens 2003).
These clariids range between fusiform and anguilliform morphotypes, with Heterobranchus Geoffrey St-Hilaire, 1809 as one extreme and Dolichallabes Poll, 1942 as the most anguilliform (Pellegrin 1927). Although this type of variation has been observed in other families of teleost fishes, amphibians and reptiles (Lande 1978), it is rarely as extreme within vertebrates as within the Clariidae. Together with the elongated body, a whole set of morphological changes are observed, such as reduction and loss of the adipose fin, fusion of the dorsal, caudal and anal fins, reduction of the pectoral and pelvic fins, reduction of the eyes, reduction of the skull bones and hypertrophy of the jaw muscles (Devaere et al. 2001).
Originally, the Clariidae were thought to have undergone anagenetic evolution of body form (Pellegrin 1927), which involved sequential transformations towards increased body elongation (i.e. anguilliformity). However, this idea was doubted by Poll (1977) and recent phylogenetic studies provide support for the hypothesis that anguilliformity evolved several times within the Clariidae (Graham 1997; Teugels and Adriaens 2003; Agnèse and Teugels 2005).
Here, the evolutionary path towards anguilliformity (defined as an abdominal body depth/standard length ratio <11.6 (Devaere et al. 2005a, 2006), with elongated dorsal- and anal-fins, coupled with anguilliform locomotion) for several representatives of the Clariidae is demonstrated, using morphological and molecular data.
Materials and Methods
Osteological examinations were done primarily on specimens cleared and counter-stained for bone and cartilage following the method of Hanken and Wassersug (1981). To supplement cleared and stained specimens, some species were also radiographed or examined as dry skeletal preparations.
Materials examined
The complete list of taxa examined in this study appears in Appendix 1. Outgroup taxa are listed first, followed by the ingroup clariid species. The ingroup taxa are listed, corresponding to their degree of body elongation. All taxa were chosen with a large degree of overlap with the molecular analysis in Jansen et al. (2006). We follow the recently revised taxonomy of Devaere et al. in press. The morphological analysis presented here, contains more anguilliform species, since tissue of many anguilliform species were not available for this study. As an outgroup, a representative of the most primitive catfish family (Diplomystidae), Diplomystes chilensis (Molina, 1782) was used for the morphological analysis (Arratia 1987; Fink and Fink 1996). The morphological characters of D. chilensis are based on the descriptions by Arratia (1987). An additional outgroup for the clariids is Heteropneustes fossilis (Bloch, 1974). The Heteropneustidae are considered to be closely related to the Clariidae, some authors even place them in the Clariidae, as sister group to all other clariids (de Pinna 1993; Diogo 2005).
Molecular data
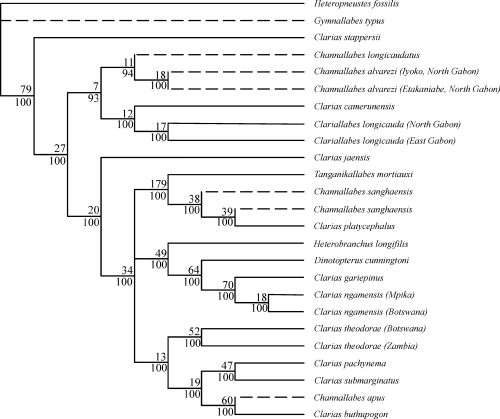
The molecular characters analysed in this study were taken directly from the study of Jansen et al. (2006). In that paper the 18S, 5.8S and 28S (partial) ribosomal genes and the ITS1 and ITS2 regions (Internal Transcribed Spacers) were analyzed. A first analysis of the 18S data established the monophyly of the ingroup. Subsequently, all data was analysed [18S-ITS1-5.8S-ITS2-28S (partial)] for four outgroup and 24 ingroup taxa. The alignment was done by ClustalW 1.8 (Thompson et al. 1997) using default parameters. The data set was improved based on secondary structure information, using Genedoc 2.6.002 (Nicholas et al. 1997) and comparing the alignment of the ITS regions with fish sequences obtained from the EMBL database. The alignment includes 2889–3110 bp in the ingroup and 2697–2861 bp in the outgroup, totalling 3649 aligned positions. The 18S data set comprised 1901 characters, 101 of which were variable and 44 of which were parsimony informative. The ITS1-5.8S-ITS2 data set included 1609 characters, 464 of which were parsimony informative. The data set was analysed using the Maximum Parsimony, Maximum Likelihood and Neighbour Joining algorithms in paup* (Swofford 2003), Bayesian inference in MrBayes 3.0b4 (Huelsenbeck and Ronquist 2001) and direct optimization in poy (Wheeler 1996). The resulting consensus tree is shown in Fig. 1.
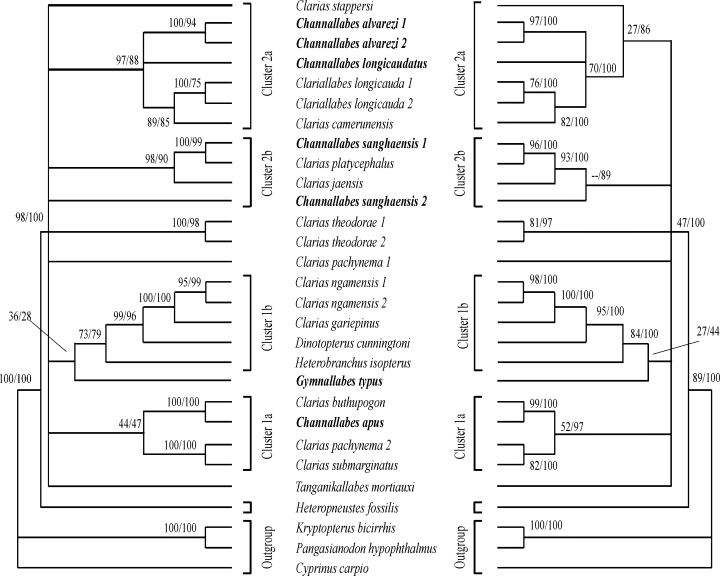
Consensus tree of four analyses (NJ, MP, ML and MrB) based on sequences of the ribosomal ITS1–5.8S–ITS2 region. Only nodes supported by all methods are shown. Left-hand side: consensus of NJ and MP (values indicate resp. bootstraps values); right-hand side: consensus of ML and MrB analyses (values indicate resp. bootstrap values and posterior probabilities). Anguilliform taxa are indicated in bold (modified after Fig. 4 in Jansen et al. 2006)
Phylogenetic analyses
For the morphological phylogenetic analysis, a maximum parsimony analysis was performed using nona (Goloboff 1998). Equally parsimonious trees were obtained using the heuristic search algorithm with sequences added randomly and tree-bisection-reconnection (TBR) branch swapping (holding all trees after each 100 × 10 replicates). To estimate the robustness of the clades recovered in the phylogenetic hypotheses, Bremer support (Bremer 1988, 1995) and bootstrap percentages [200 replications, 10 random addition sequences (RAS) per replicate] were calculated in nona based on the resulting implied alignment.
For the combined phylogenetic analysis, 1767 aligned base pairs from the ITS1-5.8S-ITS2 region were simultaneously analysed with the 53 morphological characters, under the optimality criterion of parsimony with equal weights (i.e. gaps, transitions and transversions all given a weight of 1). The parsimony analysis was conducted using direct optimization (Wheeler 1996) and iterative pass (Wheeler 2003a) as implemented in the program poy and run on the American Museum of Natural History Parallel Computing Cluster.
The analyses began by generating 30 RAS per random replicate for five replicates. These 150 RAS were improved with TBR branch swapping during the searches, an additional round of TBR branch swapping and tree fusing (Goloboff 1999) at the end. These random replicates resulted in two equally most parsimonious trees. The resulting trees were submitted to poy for further tree searching using the commands iterative pass (Wheeler 2003a). This second step of each of the analyses began by tree fusing (Goloboff 1999) the submitted topologies and it was followed by an additional round of tree fusing and TBR branch swapping to reduce heuristics in the first-step analysis.
The length of the resulting implied alignments (Wheeler 2003b) were verified in nona (Goloboff 1998) and WinClada (Nixon 2002). To estimate the robustness of the clades recovered in the phylogenetic hypotheses, Bremer supports (Bremer 1988, 1995) and bootstrap percentages (200 replications, 10 RAS per replicate) were calculated in nona based on the resulting implied alignment. Character evolution on the recovered topologies was examined using nona and WinClada.
Results
For the morphological analysis, the characters are grouped according to anatomical complex: neurocranium, jaws and hyoid (0–27), suspensorium (28–33), pectoral girdle (34–36), axial skeleton (37–44), and caudal skeleton (45–52). The consistency index (CI, Kluge and Farris 1969) is presented as a measure of homplasy for each character. The character state (e.g. 0–5) is listed as an integer in parentheses after each respective character state description. Six characters are considered additive (ordered), based on ontogenetic evidence in clariids and higher-level phylogenetic evidence in Siluriformes (0, 12, 14, 28, 31 and 32). The complete character by taxon matrix is presented in Appendix 2.
Form of the nasal bone (CI: 0.40)
In Diplomystes, Heterobranchus, Dolichallabes and both Gymnallabes species, the nasal bone is tubular (0). In all other clariids examined, the nasal has a plate-like extension (2), in Channallabes sanghaensis and Tanganikallabes this extension is present only on the lateral side of the supraorbital canal (1). Channallabes apus is polymorphic (1, 2).
Posterior margin of the mesethmoid (CI: 0.14)
Diplomystes, all Clarias, except Clarias buthupogon and Clarias theodorae, Platyclarias, Dinotopterus, Bathyclarias, Gymnallabes nops, Channallabes ogooensis, Channallabes alvarezi and Channallabes teugelsi have a mesethmoid with an indented posterior margin that contacts the anterior fontanel (0) (Fig. 2). All the rest shows a uniform posterior margin of the mesethmoid (1) (Fig. 2b).
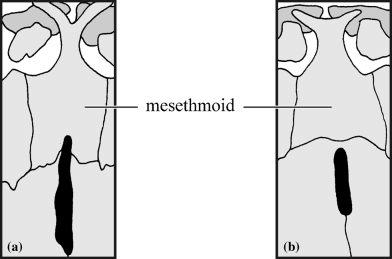
Dorsal view of the posterior margin of the mesethmoid. (a) Indented (Clarias ngamensis); (b) not indented (Clarias jaensis)
Rostral projection on the frontal (CI: 0.50)
Except for Diplomystes and Bathyclarias (0), all clariids have a rostral projection on the frontal bone (1) (Fig. 3).
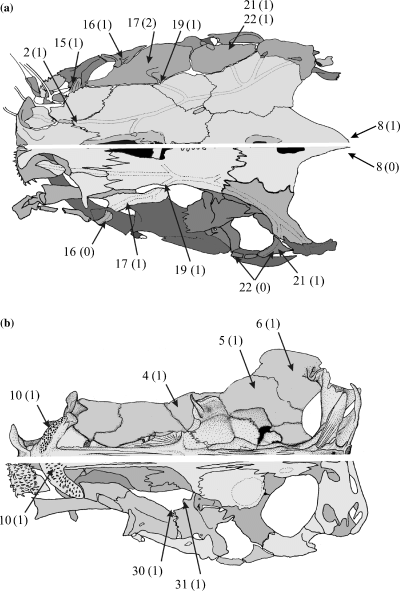
Overall view of the skull of Channallabes apus bottom and Clarias gariepinus top. (a) dorsal view; (b) ventral view. Numbers refer to morphological characters; character state is indicated between brackets
Extent of the lateral wings of the frontal (CI: 0.50)
Diplomystes, Platyallabes and C. alvarezi have a frontal that reaches laterally as far as the orbitosphenoid (0) (Fig. 4). Heterobranchus, Clariallabes, Dinotopterus, Bathyclarias and all Clarias have a frontal that is much broader than the orbitosphenoid (1) (Fig. 4b). In Heteropneustes, Platyclarias, G. nops and all Channallabes, except C. alvarezi, the lateral plates are a little broader than the orbitosphenoid (2) (Fig. 4c). In Dolichallabes and Gymnallabes typus, no lateral plate is visible (3) (Fig. 4 in Cabuy et al. 1999). Again, Channallabes apus is polymorphic (0, 2).
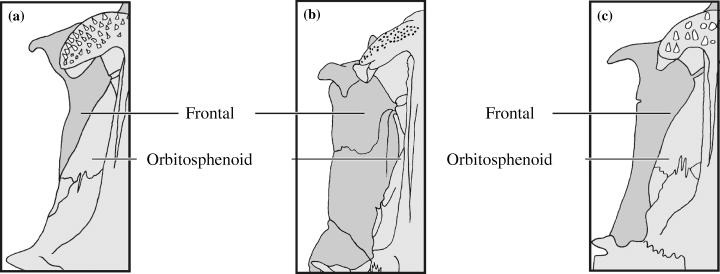
Illustration of the extent of the lateral plate-like outgrow of the frontal in relation to the orbitosphenoid. (a) lateral plate equals the outlines of the orbitosphenoid (Channallabes alvarezi); (b) lateral plate much wider than the outlines of the orbitosphenoid (Clarias gariepinus); (c) lateral plate a little wider than the outlines of the orbitosphenoid (Channallabes longicaudatus). Numbers refer to morphological characters; character state is indicated between brackets
Lateral plate of the sphenotic (CI: 0.33)
No lateral plate-like outgrow on the sphenotic is present in Diplomystes, Platyallabes, Dolichallabes, Gymnallabes, Tanganikallabes and all Channallabes (0). All other have a distinct lateral plate-like outgrow (1) (Fig. 3b).
Lateral plate of the pterotic (CI: 0.33)
No lateral-plate like outgrow on the pterotic is present in Diplomystes, Platyallabes, Dolichallabes, Gymnallabes, Tanganikallabes and all Channallabes (0). All other have a distinct lateral plate-like outgrow (1) (Fig. 3b).
Lateral plate of the post-temporo-supracleithrum (CI: 0.25)
No lateral plate-like outgrow on the post-temporo-supracleithrum is present in Diplomystes, Platyallabes, Dolichallabes, Tanganikallabes, G. nops and Channallabes apus (0). All other taxa have a lateral plate-like outgrow (1). C. alvarezi is polymorphic (0, 1) (Fig. 3).
Posterior branch of the temporal canal in the post-temporo-supracleithrum (CI: 1)
Diplomystes and Heteropneustes have a posterior branch (0). All clariids lack the posterior branch (1).
Shape of the supraoccipital process (CI: 0.50)
Except for the rounded process in Clarias fuscus and C. gariepinus (1), all other taxa have a pointed process (0) (Fig. 3a).
Position of the posterior tip of the supraoccipital spine relative to the Weberian neural spines (CI: 1)
In Diplomystes and Heteropneustes, the posterior tip of the supraoccipital process does not reach the Weberian neural spines (0). In all clariids, the posterior tip does reach the Weberian neural spines (1).
Prevomeral tooth plates (CI: 0.33)
In Diplomystes, Heteropneustes, Platyallabes and Dolichallabes the two tooth plates remain separated in the adult stage (0). In all other taxa, both tooth plates fuse (1) (Fig. 3b).
Posterior process of the prevomer for the interdigitation with the parasphenoid (CI: 1)
Diplomystes has two, small rounded posterior processes on the prevomer (0). Heterobranchus, Dinotopterus, Bathyclarias and all Clarias species have two long processes (1). Heteropneustes, Clariallabes and all anguilliform taxa, except Platyclarias, have one long process (2). Platyclarias is polymorphic (one long process or three long processes) (2, 3).
Neurocranial articulation with the hyomandibula (CI: 0.33)
In Diplomystes, the articulation of the hyomandibula occurs through the pterosphenoid, sphenotic and the pterotic (0). In Heteropneustes, Dinotopterus, Bathyclarias, Clariallabes, Platyclarias, Dolichallabes, Gymnallabes, Channallabes and C. gariepinus, the sphenotic and pterotic articulate with the hyomandibula (1). In Heterobranchus, all Clarias, except C. gariepinus and Tanganikallabes, the articulation occurs at the level of the sphenotic only (2). Platyallabes is polymorphic (1, 2).
Epiotic (CI: 0.10)
In Diplomystes, Heterobranchus, Clarias gariepinus and C. fuscus, Clariallabes, Dolichallabes, Platyclarias, G. typus, Channallabes longicaudatus and C. teugelsi the epiotic is present (0). In Heteropneustes, Platyallabes, Bathyclarias, Dinotopterus, G. nops, most Clarias and Channallabes species, this bone is absent (1). Channallabes apus is polymorphic (0, 1).
Number of infraorbital bones (CI: 1)
Diplomystes has seven infraorbital bones (0). Heteropneustes has five infraorbitals (1). All clariids have four infraorbitals (2), except for Dolichallabes and G. nops, in which only two infraorbital bones are present (3).
Articulatory process on infraorbital III (CI: 0.28)
No articular process is present in Diplomystes, Heteropneustes, Platyallabes, Tanganikallabes and G. typus (0) (Fig. 3 in Cabuy et al. 1999; Fig. 6 in Adriaens et al. 1997). One is present, without contact with the lateroethmoid in Dinotopterus, Bathyclarias, Clarias pachynema and C. jaensis and most Channallabes, except C. sanghaensis (1). Contact is present in Heterobranchus, Clariallabes, Platyclarias, C. sanghaensis and Clarias, except C. pachynema and C. jaensis (2). Channallabes apus is polymorphic (1, 2).
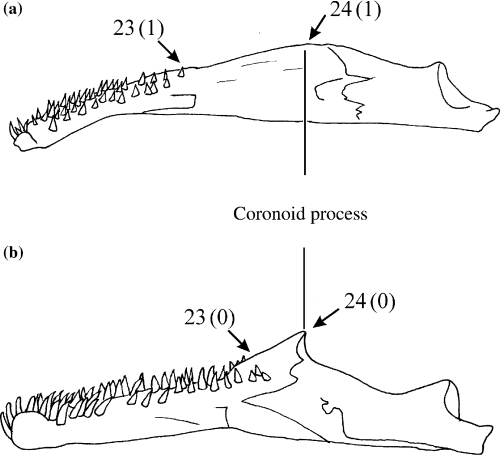
Illustration of the extent of the tooth battery and the size of the coronoid process on the lower jaw. (a) Channallabes apus; (b) Clarias gariepinus. Numbers refer to morphological characters; character state is indicated between brackets
Shape of the posteroventral infraorbital bone (CI: 0.50)
In Diplomystes, Heteropneustes, Platyallabes, Tanganikallabes, Clarias pachynema, G. typus and all Channallabes species, the posteroventral infraorbital bone is tubular (0) (Fig. 3a). In all other clariid taxa included in this study, this infraorbital is plate-like (1).
Shape of the posterodorsal infraorbital bone (CI: 0.28)
The shape of the posterodorsal infraorbital in Diplomystes, Platyallabes, Dolichallabes, Gymnallabes and Tanganikallabes is tubular (0). In Dinotopterus, Channallabes apus and C. sanghaensis and Clarias stappersii, C. pachynema, C. theodorae, C. platycephalus, the posterodorsal infraorbital is plate-like with a small supraorbital process (1) (Fig. 5a). Heteropneustes, Heterobranchus, Clariallabes, Platyclarias, Bathyclarias and all other Clarias and Channallabes species have a plate-like infraorbital with a large supraorbital process (2) (Fig. 5b).
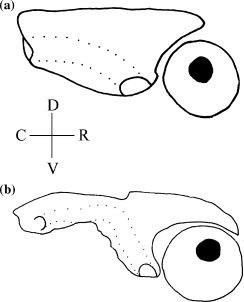
Illustration of the extent of the supraorbital process of infraorbital IV. (a) supraorbital process not reaching the rostral border of the eye (Channallabes apus); (b) supraorbital process reaching the rostral border of the eye (Channallabes alvarezi)
Position of the suprapreopercular bones (CI: 0.50)
Except for Bathyclarias and Clarias stappersii, where the suprapreopercular series are well in front, contacting the posterodorsal infraorbital bone (2) and Heteropneustes, all other Clarias species and Heterobranchus, where the suprapreopercular series is well in front but does not contact the posterodorsal infraorbital bone (1), all other taxa have a posteriorly shifted suprapreopercular series and consequently widely separated from the posterodorsal infraorbital bone (0).
Place of entrance of the infraorbital canal in the neurocranium (CI: 0.50)
In Diplomystes, Heteropneustes, Heterobranchus, Clariallabes, Dinotopterus, Bathyclarias and all Clarias species, the infraorbital canal enters the neurocranium at the level of the sphenotic (0). The canal enters at the level of the frontal (1) (Fig. 3a).
Position of the infraorbital-supraorbital canal anastomosis (CI: 0.50)
In Diplomystes, Heteropneustes, Heterobranchus, Clariallabes, Dinotopterus, Bathyclarias and all Clarias species, the anastomosis is situated in the sphenotic (0). In all other clariids, the anastomosis is situated in the frontal (1).
Shape of the suprapreopercular bone (CI: 0.50)
Except for Diplomystes and Platyallabes (tubular) (0), all other taxa have a plate-like suprapreopercular bone (1) (Fig. 3).
Number of suprapreopercular bones (CI: 0.16)
Diplomystes, Platyallabes, Platyclarias, Dolichallabes, Gymnallabes and Tanganikallabes have more than one suprapreopercular bone (0). All other taxa have only one suprapreopercular bone (1). Channallabes apus and C. alvarezi are polymorphic (0, 1) (Fig. 3).
Extent of the tooth battery on the lower jaw (CI: 0.50)
The tooth battery of Diplomystes, Heteropneustes, Clariallabes, Clarias stappersii, C. platycephalus, Tanganikallabes and all anguilliform clariids runs posteriorly up to the coronoid process (0) (Fig. 6b). With the other clariids, the tooth battery does not run that far (1) (Fig. 6a).
Coronoid process (CI: 0.20)
The coronoid process in Diplomystes, Heteropneustes, Clariallabes, Dolichallabes, Gymnallabes, Tanganikallabes, all Channallabes species and Clarias fuscus, C. theodorae and C. platycephalus is well developed (0) (Fig. 6a). In all other taxa, the coronoid process is hardly distinguishable (1) (Fig. 6b).
Shape of the distal tip of the posterior process of the parurohyal (CI: 0.14)
The caudal tip of the medial process of parurohyal is pointed in Diplomystes, Dolichallabes, Gymnallabes, Bathyclarias, Clarias pachynema, C. buthupogon, C. ngamensis, C. jaensis, C. camerunensis and Channallabes sanghaensis, C. ogooensis, C. teugelsi (0). Forked in Heteropneustes, Heterobranchus, Platyclarias, C. gariepinus, C. fuscus, C. theodorae, C. platycephalus and C. alvarezi (1). Flat in Clariallabes, Tanganikallabes, Clarias stappersii (2). Platyallabes is polymorphic (0, 2).
Spot on the dorsal neurocranium (CI: 0.50)
Except for the unpigmented, clear spot in Platyclarias and Channallabes teugelsi, C. longicaudatus, C. ogooensis (1), all other taxa have no spot on the skull roof (0) (Fig. 7).
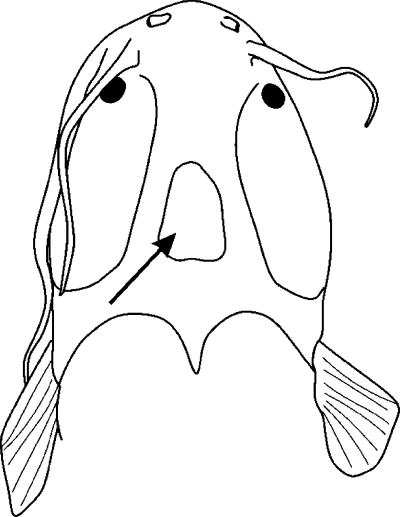
Head of Channallabes teugelsi in dorsal view: arrow indicates the position of the pale spot on the head roof
Contralateral parts of the pre-fontanel supraoccipital bone fused (CI: 1)
Except for the separated contralateral parts of the supraoccipital bone in Dolichallabes (1), all other taxa have fused contralateral parts of the supraoccipital bone (0).
Entopterygoid-quadrate connection (CI: 0.25)
There is no contact between the entopterygoid and the quadrate in Diplomystes, Heteropneustes, Clariallabes, Platyclarias, Dolichallabes, Gymnallabes, Dinotopterus, Bathyclarias, Clarias fuscus, C. ngamensis, C. stappersii, C. platycephalus and all Channallabes except C. apus and C. sanghaensis (0) (Fig. 8b,c). In Heterobranchus, Tanganikallabes, Platyallabes and all other Clarias species, there is limited contact between the entopterygoid and the quadrate (1) (Fig. 8a). In Channallabes apus and C. sanghaensis, there is an additional clear interdigitation zone present between the two suspensorial bones (2) (Fig. 8d).
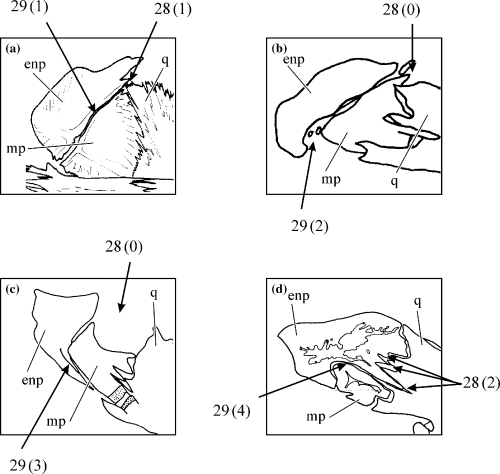
Lateral side of the entopterygoid, metapterygoid and quadrate of the left suspensorium. (a) Clarias gariepinus (modified from Adriaens and Verraes 1998); (b) Platyclarias machadoi (modified from Devaere et al. in press); (c) Gymnallabes typus; (d) Channallabes apus (modified from Devaere et al. 2001). Enp, entopterygoid; mp, metapterygoid, q, quadrate. Numbers refer to morphological characters, character state is indicated between brackets
Entopterygoid-metapterygoid contact (CI: 0.44)
In Diplomystes, contact of the entopterygoid with the metapterygoid is limited to the dorsal side of the latter bone (0). In Heteropneustes, Heterobranchus, Tanganikallabes, G. nops, C. alvarezi and most Clarias species, there is contact with the rostrodorsal margin of the metapteygoid (1) (Fig. 8a). In the other Clarias species (C. stappersii and C. platycephalus), Platyclarias, Dolichallabes, Dinotopterus, Bathyclarias, C. teugelsi, C. ogooensis, C. longicaudatus there is restricted contact with the ventral side, besides the full contact with the rostrodorsal side (2) (Fig. 8b). Entopterygoid with ventral process borders the ventral side of the metapterygoid in Platyallabes, G. typus (3) (Fig. 8c). The metapterygoid is completely enclosed dorsally by the entopterygoid in C. sanghaensis (4) (Fig. 8d). Channallabes apus is polymorphic for this character (3, 4).
Dorsal border of membranous plate of quadrate and hyomandibula (CI: 0.33)
Except for the Platyclarias, Bathyclarias and C. gariepinus, where the border is straight (0), all the taxa have an indented dorsal border of the membranous plate of quadrate and hyomandibula (1) (Fig. 3).
Rostral process on the hyomandibula (CI: 0.15)
The rostral process on the hyomandibula is absent in Diplomystes, Heteropneustes, Clariallabes, Platyclarias, Platyallabes Gymnallabes nops and C. theodorae (0) (Fig. 3). A small rostral process is present in Heterobranchus, Dolichallabes, Bathyclarias, C. sanghaensis, Clarias pachynema, C. buthupogon, C. camerunensis, C. stappersii, C. platycephalus (1). In Tanganikallabes, Dinotopterus, C. gariepinus, C. ngamensis, C. jaensis, C. ogooensis, C. longicaudatus, C. alvarezi, C. teugelsi, a large process is present (2). G. typus and Channallabes apus are polymorphic (1, 2).
Number of processes present on the anterior side of the hyomandibula (CI: 0.33)
Diplomystes shows no anterior extended process (0). Heteropneustes, Platyclarias and Dolichallabes have one process (1), Heterobranchus, Clariallabes, Gymnallabes, Tanganikallabes, Dinotopterus, Bathyclarias, all Channallabes, except C. sanghaensis and most Clarias taxa have two processes (2). Platyallabes, C. sanghaensis, Clarias pachynema, C. buthupogon, C. camerunensis have three anteriorly extended processes on the hyomandibula (3). C. apus is polymorphic (2, 3) (Fig. 6 in Devaere et al. 2001).
Number of processes present on the posterior side of the hyomandibula (CI: 0.36)
Diplomystes, Heteropneustes, Heterobranchus, Dinotopterus, Bathyclarias, C. gariepinus, C. ngamensis and C. stappersii show no posterior process (0) (Fig. 6 in Devaere et al. 2001). Only one process is present in Platyclarias, Clarias jaensis and C. platycephalus (1). Channallabes ogooensis and Clarias fuscus have two processes, with a small anterior one (2). Clariallabes, Platyallabes, Dolichallabes and C. teugelsi posses two extended posterior process (3). Gymnallabes, Tanganikallabes, C. sanghaensis, C. longicaudatus, C. alvarezi and Clarias pachynema, C. buthupogon, C. camerunensis and C. theodorae have three posterior processes caudally increasing in length (5). C. apus is polymorphic and has either two (3) or three (4) extended process.
Anterior process on the cleithral bone (CI: 0.25)
An anterior process on the cleithral bone is absent in Diplomystes, Heteropneustes, Platyallabes, Dinotopterus and Clarias fuscus (0). All other clariids have a distinct process on the anterior side of the cleithrum (1) (Fig. 9).
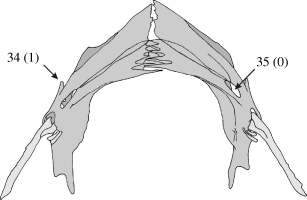
Ventral view of the pectoral girdle of Channallabes teugelsi. Numbers refer to morphological characters; character state is indicated between brackets
Fenestra between the coracoid and cleithral bone (CI: 0.33)
Diplomystes and most clariids have a fenestra between the scapulocoracoid and the cleithrum (0), Heteropneustes, Channallabes apus and Clarias platycephalus lack the fenestra (1) (Fig. 9).
Pectoral spine serrations (CI: 0.27)
Serrations are present only on the posterior edge of the pectoral spine in Diplomystes, G. nops and C. alvarezi (0). On the anterior edge in Heterobranchus, Heteropneustes, Dinotopterus, Bathyclarias, C. gariepinus, C. fuscus, C. ngamensis, C. camerunensis (1). Present on both edges in Clariallabes, G. typus, C. buthupogon, C. pachynema, C. stappersii, C. theodorae, C. sanghaensis, C. ogooensis, C. longicaudatus (2). Serration are absent on both edges in Platyclarias, Platyallabes (3). Polymorphism present in Tanganikallabes (0, 2), Clarias platycephalus (1, 3), C. jaensis (2, 3).
Size of the parapophyseal foramina on the first precaudal vertebra (CI: 0.22)
Small foramina in Heterobranchus, Clariallabes, Dolichallabes, Gymnallabes, Dinotopterus, Bathyclarias and C. gariepinus, C. fuscus, C. stappersii, C. theodorae and C. platycephalus (0) (Fig. 10a). Large foramina present in Platyclarias, all Channallabes species and Clarias pachynema, C. buthupogon, C. ngamensis, C. jaensis, C. camerunensis (1) (Fig. 10b). Very large foramina are present in Heteropneustes, Platyallabes, Tanganikallabes (2) (Fig. 10c). Channallabes apus is polymorphic (0, 1).
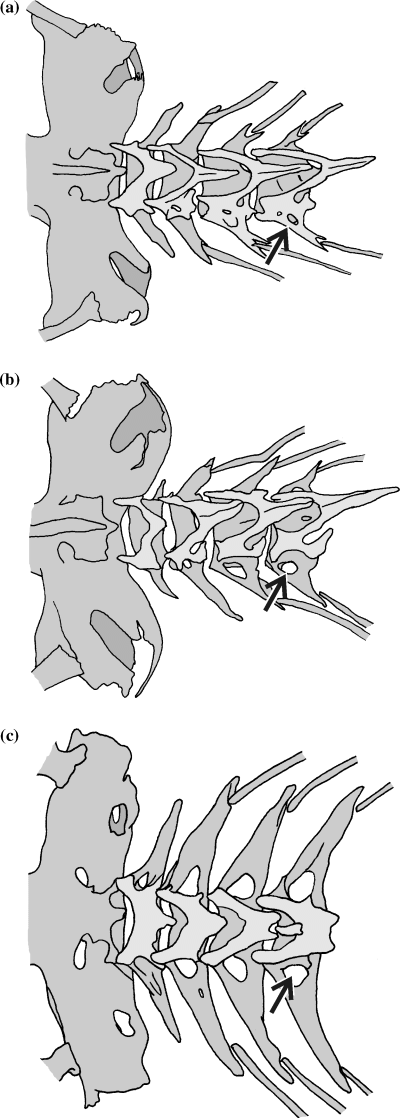
Illustration of the size of the foramen at the bases of the parapophyses of the fourth post-Weberian vertebra in (a) small (Channallabes sanghaensis); (b) large (Channallabes apus); and (c) very large (Platyallabes tihoni)
Lateral process on the first dorsal-fin pterygiophore (CI: 0.25)
Except for the presence of a lateral process on the first dorsal-fin pterygiophore in Heterobranchus, Platyallabes, Dinotopterus, G. typus and Clarias stappersii (1), all other taxa lack this lateral process (0).
Lateral process on the second dorsal-fin pterygiophore (CI: 0.12)
In Diplomystes, Platyclarias, Platyallabes, Dolichallabes, Dinotopterus, C. alvarezi and C. longicaudatus, a lateral process is absent on the second dorsal-fin pterygiophore (0). Heteropneustes and all other clariid representatives show a lateral process on the second dorsal-fin pterygiophore (1). Channallabes apus and C. teugelsi are polymorphic.
Anterior process on the second dorsal-fin pterygiophore (CI: 0.16)
An anterior process is absent in Diplomystes, Platyclarias, Clarias pachynema, C. buthupogon, C. jaensis, C. camerunensis and in Channallabes longicaudatus and C. alvarezi (0). For Heteropneustes and all other clariids studied in this analysis, the process is present (1). Channallabes apus and C. teugelsi are polymorphic.
Position of the first dorsal-fin pterygiophore (CI: 0.27)
In Diplomystes, Heteropneustes, Heterobranchus, Dolichallabes, G. nops and C. sanghaensis, the dorsal-fin originates between the sixth and eighth vertebra (0). In Tanganikallabes, Dinotopterus, G. typus and Clarias stappersii, C. jaensis, C. theodorae, C. platycephalus, the dorsal-fin originates between the fifth and sixth vertebrae (1). Platyallabes, Bathyclarias and C. gariepinus, C. fuscus, C. pachynema, C. buthupogon, C. ngamensis, the origin lies anterior to the process of the fifth vertebra (2), while in C. alvarezi, C. ogooensis, C. longicaudatus and C. teugelsi the origin lies posterior to the eighth vertebra (3). Channallabes apus is polymorphic (0, 4).
Adipose fin (CI: 0.25)
An adipose fin is only present in Diplomystes, Heterobranchus, Dinotopterus and Clarias ngamensis (0). All the other taxa lack an adipose fin (1).
First post-Weberian vertebra with ribs (CI: 0.25)
Heteropneustes, Heterobranchus, Bathyclarias always have ribs on both sides on the first post-Weberian vertebra (0). All other clariids lack such ribs (1). Platyclarias, C. teugelsi and C. apus are polymorphic (0, 1) (Fig. 10).
Length of the last precaudal neural spine (CI: 0.33)
Only the neural spines in Diplomystes, Heterobranchus, Bathyclarias and C. gariepinus are elongated, reaching the adipose fin or dorsal part of the body (1). In all other taxa, the neural spines do not reach the dorsal part of the body (0).
Neural spine of the second preural vertebra (CI: 0.28)
The neural spine of Diplomystes, Heteropneustes, Heterobranchus, Platyclarias, Tanganikallabes, Dinotopterus, Bathyclarias, G. nops and all Clarias taxa, except C. gariepinus, is elongated and distally widened (0) (Fig. 11). Platyallabes, C. gariepinus and Channallabes apus show a non-elongated, spiny neural spine (1), while Dolichallabes, all other Channallabes species and G. typus have an elongated and spiny neural spine (2).
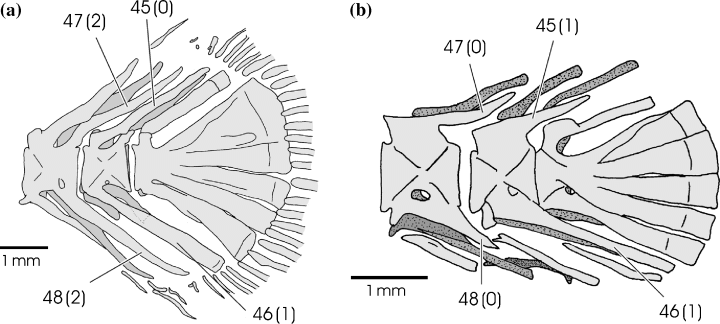
Overall view of the caudal skeleton of (a) Platyclarias machadoi and (b) Platyallabes tihoni. Numbers refer to morphological characters; character state is indicated between brackets
Haemal spine of the second preural vertebra (CI: 1)
All taxa, except Dolichallabes and G. nops (0), have distally widened haemal spines on the second preural vertebra (1). Channallabes apus is polymorphic (0, 1) (Fig. 11).
Neural spine of the third preural vertebra (CI: 33)
Dolichallabes and Platyallabes have no elongated, spiny neural spine (0). The neural spine of Heteropneustes, Heterobranchus, Dinotopterus, Bathyclarias, G. nops and C. gariepinus, C. fuscus, C. ngamensis is elongated, and distally widened (1). Platyclarias, G. typus, all Channallabes species, except C. apus and Clarias pachynema, C. buthupogon, C. jaensis, C. camerunensis, C. theodorae, C. platycephalus, on the other hand, have an elongated, spiny neural spine on the third preural vertebra (2). Channallabes apus is polymorphic (0, 1) (Fig. 11).
Haemal spine of the third preural vertebra (CI: 0.33)
Platyallabes, Dolichallabes, Clarias jaensis and C. camerunensis have a non-elongated haemal spine (0), the haemal spine of the third preural vertebra in Heteropneustes, Heterobranchus, Dinotopterus, Bathyclarias, C. gariepinus, C. fuscus and C. ngamensis is elongated and distally widened (1). All the Channallabes species, Platyclarias, G. typus, Tanganikallabes, Clarias pachynema, C. buthupogon, C. theodorae and C. plathycephalus have an elongated, spiny haemal spine (2). Again, Channallabes apus is polymorphic (0, 2) (Fig. 11).
Hypurapophyses on the parhypural (CI: 1)
In contrast to Diplomystes and Heteropneustes (0), all clariids lack hypurapophyses on the parhypural (1).
Hypurals 1–2 (CI: 0.25)
The hypurals one and two are not fused in Diplomystes, Heteropneustes, Platyallabes, Tanganikallabes, Dinotopterus, Bathyclarias, G. typus, all Clarias and Channallabes species, except C. longicaudatus (0). In Heteropneustes, Dolichallabes, G. nops, Channallabes longicaudatus, the two hypurals are fused (1). Platyclarias and Channallabes apus are polymorphic (0, 1).
Hypurals 3–4 (CI: 0.33)
The hypurals three and four are not fused in Diplomystes, Heteropneustes, Platyallabes, Platyclarias, Tanganikallabes, Dinotopterus, Bathyclarias, G. typus, all Clarias and Channallabes species, except C. longicaudatus (0). In Heteropneustes, Dolichallabes, G. nops, Channallabes longicaudatus, the two hypurals are fused (1). Channallabes apus is polymorphic (0, 1).
Hypurals 4–5 (CI: 0.25)
The hypurals four and five are not fused in Diplomystes, Heteropneustes, Platyallabes, Tanganikallabes, Dinotopterus, Bathyclarias, G. typus, all Clarias and Channallabes species, except C. longicaudatus (0). In Heteropneustes, Dolichallabes, G. nops, Channallabes longicaudatus, the two hypurals are fused (1). Platyclarias and Channallabes apus are polymorphic (0, 1).
The morphological analysis resulted in two most parsimonious trees with a length of 237 steps, a consistency index (CI, Kluge and Farris 1969) of 0.35, and a retention index (RI, Farris 1989) of 0.66. A strict consensus of both trees is presented in Fig. 12. All of the African Clariidae are grouped with reasonable support (Fig. 12). This node has a bootstrap support value of 82 and a Bremer support value of 5. Eight synapomorphies support this node: no posterior branch of the temporal canal in the post-temporo-supracleithrum (7–1), the posterior tip of the supraoccipital process reaches the Weberian neural spines (9–1), fused prevomeral tooth plates (10–1), the number of infraorbital bones is less than five (14–2, 3), the entrance of the infraorbital canal and the infraorbital-supraorbital canal anastomosis lies in the frontal (19–1, 20–1) anterior process on the cleithral bone is present (34–1) and no hypurapophyses are present on the parhypural (49–1).
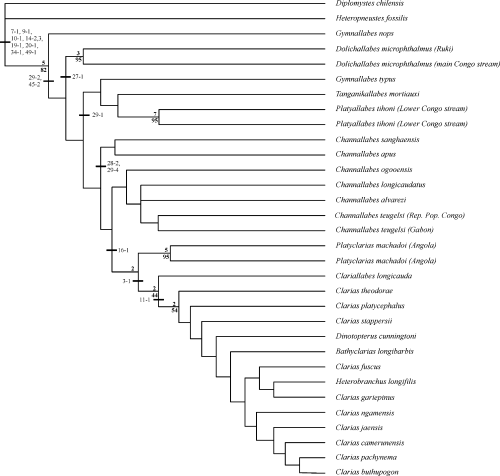
Strict consensus tree of both equally most parsimonious trees (TL: 237 steps, CI: 0.35, RI: 0.66) from parsimony analysis of 53 morphological characters for clariid fishes and two outgroup. The number above the base of a node indicates the Bremer support, different from one and the number below indicates the bootstrap support (above 40) for the respective node. Non-homoplasious characters that support nodes are indicated by their respective character number, followed by the character state
The included species of the genus Clarias, together with Heterobranchus longifilis, Bathyclarias longibarbis and Dinotopterus cunningtoni form a clade that is separated from all the anguilliform representatives. This node has a bootstrap support value of 54 and a Bremer support value of 2. The non-homoplasious character that supports this node is the presence of two long posterior processes on the prevomer (11–1).
Clariallabes longicauda is placed in between the fusiform and more anguilliiform taxa in the phylogenetic analysis, and this corresponds with its intermediate degree of body elongation. Although, the bootstrap support for this node is just below 50 (44), with a Bremer support value of 2, it is supported by a non-homoplasious character: the presence of a very broad lateral plate on the frontal (3–1) Tanganikallabes is incorporated along with the other anguilliform taxa. Furthermore, we see that of those species where different populations were represented, these operational taxonomic units (OTUs) cluster together and are well supported (bootstrap value greater than 95 for the C. teugelsi, Dolichallabes microphthalmus, Platyallabes tihoni and Platyclarias machadoi populations).
This analysis shows the paraphyletic nature of the genera Channallabes and Clarias. Two distinct clades containing Channallabes species are present. The first contains just C. apus and C. sanghaensis and this is sistergroup to a clade containing a second clade of more fusiform Channallabes species plus a large clade containing Bathyclarias, Clarias, Clariallabes, Dinopterus, Heterobranchus and Platyclarias. Within this last clade, Bathyclarias, Dinopterus and Heterobranchus are nested among Clarias species. Finally, a close relation between C. gariepinus and H. longifilis, as indicated earlier in Teugels et al. (1992) and Agnèse and Teugels (2001) is supported.
The combined analysis of all 1767 molecular and 53 morphological characters resulted in two most parsimonious trees with length of 2320 steps, consistency index (CI, Kluge and Farris 1969) of 0.74 and retention index (RI, Farris 1989) of 0.78, when only informative characters were retained. A strict consensus of both trees is presented in Fig. 13. Again, the monophyly of the African Clariidae is strongly supported. On the other hand, Clarias again is paraphyletic, with representative species spread over several clades. Channallabes also appears paraphyletic, but now contains a strongly supported Lower Guinea clade, as suggested in another study (Devaere et al. in press). In contrast the two Congo species of Channallabes in the combined analysis appear separately in the phylogeny and are most closely related to two different Clarias species. In summary this phylogeny reveals the independent evolution of an elongated body form in four clades, in each case with similar levels of overall body elongation.
Strict consensus of both equally most parsimonious trees (TL: 2315 steps, CI: 0.74, RI: 0.78) from parsimony analysis of 1767 aligned nucleotides and 54 morphological characters for clariid fishes and two outgroup. The number above the base of a node indicates the Bremer support and the number below indicates the bootstrap support for each resolved node
Discussion
Polymorphism
Several elongated clariids show intraspecific variation in several of the characters examined in this study (Channallabes apus, C. alvarezi, C. teugelsi, G. typus, Platyclarias machadoi and Platyallabes tihoni). Therefore, additional biometric and meristic research was performed on each of these species. Results of these extended studies however did not support recognizing any additional natural groups or new species (Devaere et al. 2004, 2005a,b, 2006, in press). The problem of polymorphism and intraspecific variation in clariids previously been discussed in earlier studies (Adriaens et al. 2002; De Schepper et al. 2004; Devaere et al. 2004, 2005a,b).
Evolutionary polarization of body elongation
The morphological analysis suggests that body elongation is plesiomorphic within the African representatives of the Clariidae, with a transition from anguilliform representatives to fusiforms, over an intermediate situation (Clariallabes longicauda). Only Tanganikallabes mortiauxi does not fit this trend, although it has a more fusiform body, it clusters with the anguilliform taxa. However, the outgroup taxa used in this analysis imply a reversal to a fusiform ‘Bauplan’ (both Diplomystes and Heteropneustes are not elongated). Furthermore, the sister group of the Clariidae and Heteropneustidae is fusiform (de Pinna 1993; Diogo 2005). It is known that an expansion of the Hox gene expression domains along the body axis, both accounts for elongation of the body as well as the generally co-occurring loss of limbs (Cohn and Tickle 1999). The reverse process, on the other hand, is less unambiguously documented. A number of authors have discussed and disputed the possible reappearance of well-developed hind limbs among the primitive lineages of snakes (Zaher and Rieppel 1999; Greene and Cundall 2000; Lee et al. 2000). While many examples can be given of elongation processes in several groups (Lande 1978; Caroll 1988), none are found with such a reversal.
An explanation for this strange topology, however, could be that it is biased by certain aspects of the morphology. Looking at the data matrix, it seems that the topology indeed is merely a reflection of the cranial evolution in the Clariidae. Together with elongation of the body, a whole set of morphological features covary in the cranial region (Cabuy et al. 1999; Devaere et al. 2001). The cranial changes include reduction of many bones, such as the suprapreopercular and infraorbital bones. These tubular lateral bones are considered plesiomorphic, as, for instance, within the Siluriformes only in Heteropneustes, Uegitglanis, Heterobranchus and Clarias an enlarged infraorbital IV is present (Diogo 2005). This, however, means that in the Clariidae the large skull morphology is plesiomorphic, since the closest sister-group, the Heteropneustidae, shows a robust skull (see below). The skull of the anguilliform taxa resembles that of the primitive D. chilensis, e.g. the absence of a sphenotic and pterotic lateral plate as well as the tubular suprapreopercular and infraorbital series.
The ambiguous nature of this morphology-based phylogeny could be tested, using the molecular phylogeny presented in Jansen et al. (2006). In that study, four clades were distinguished, along with a surprising distribution of anguilliform taxa. Given the molecular phylogeny, body elongation would have arisen independently from three different Clarias lineages. Consequently, when considering the high number of shared morphological traits in anguilliform taxa (see above), this topology would suggest a high degree of homoplasic evolution.
Subsequently, the combined analysis of both morphological and molecular data sets presented here might clear this up (Fig. 13). Anguilliformity still seems to be plesiomorphic within the Clariidae given of the basal position of a fusiform G. typus. On the other hand, body elongation still is the result of parallel evolution, with the elongate Channallabes taxa arising from three different fusiform Clarias ancestors.
The basal position of G. typus is unexpected, but may be explained. First, when mapping the degree of anguilliformity on the tree, the common ancestor of G. typus and all other clariids cannot be determined unambiguously (Fig. 14). Additionally, incorporating the biogeography of the different OTUs, G. typus is the only representative from the Niger delta, whereas all other anguilliform taxa are from the Congo basin and Lower Guinea ichthyological province. Secondly, for the reason mentioned above, it seems very unlikely that anguilliformity is plesiomorphic in clariids. In the analysis of Agnèse and Teugels (2005), based on Cytochrome b sequence data, G. typus groups with Clarias ebriensis and C. pachynema, where the former also occurs in the Niger River delta. This could mean that G. typus could be considered as an anguilliform branch of an early West African lineage, with a non-anguilliform ancestor. Other studies have suggested alternative positions for G. typus within the Clariidae, so at present no consensus can has been reached (Graham 1997; Teugels and Adriaens 2003).
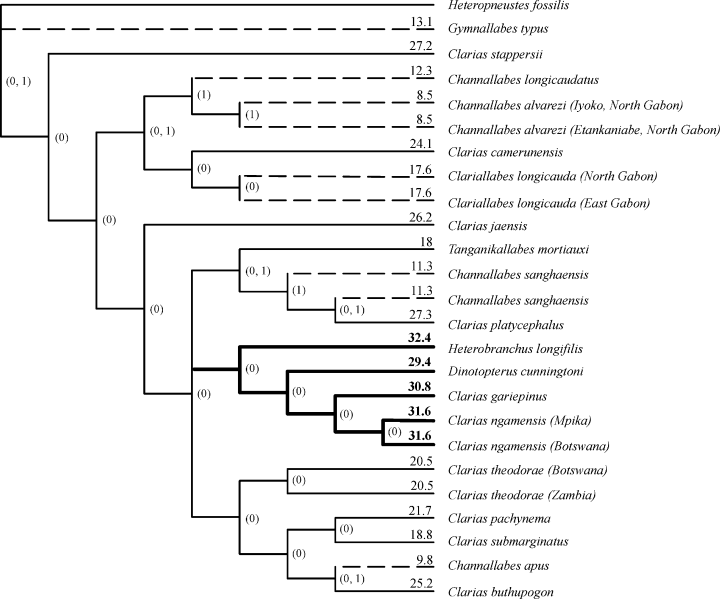
Mapping anguilliformity on the strict consensus of both equally most parsimonious combined morphological and molecular trees. 0, non-anguilliform state; 1, anguilliform state. The number at the end of each branch indicates the average skull length (% SL). Elongated taxa are indicated by striped branches, ‘long headed’ taxa are indicated by bold branches
Morphological co-evolution with body elongation
Even though the degree of anguilliformity (see definition in Introduction) may be unique in the clariids, body elongation itself is not unique, but has occurred several times throughout vertebrate evolution, in other teleosts, amphibians and reptiles (Gans 1975; Lande 1978; Nelson 1994). This elongation is observed to sometimes co-occur with a whole set of morphological changes, e.g. increase in the number or size of vertebrae, limblessness, reduction of the eyes, and/or increasing rigidity of the skull (Gans 1975; Lande 1978).
Co-occurrence of similar traits is also observed in clariids. Elongated clariids have a significantly higher number of vertebrae (Devaere et al. 2001, 2004). Although all Clariidae are diagnosed with small eyes, the eyes of the anguilliform species are clearly smaller and sometimes even completely absent (Devaere et al. 2005a). It is further shown that elongation in clariids co-occurs with a loss of paired fins (Adriaens et al. 2002). Although, the general skull morphology in elongated clariids shows a reduction or even loss of some bones (e.g. lateral bones), there is an increased rigidity, through the outgrowth of several interdigitation zones (Devaere et al. 2001). Many of these characters also seem to determine the topology of the morphology based phylogeny (e.g. characters 3, 4, 5, 11, 16 and 18) and lead to the grouping of the elongated species. In the combined analysis, however, the correlated morphological changes appear to be the result of convergent evolution and this on a genus level. Similar convergent evolution in vertebrates has occurred several times on a higher taxonomic level (Gans 1975; Lande 1978; Nelson 1994). Furthermore, the presence or absence of the paired fins appeared to differ on a population level, indicating the existence of a high level of phenotypic plasticity of traits that were generally considered as stable on a micro-evolutionary level, and even up to some degree on a macro-evolutionary scale (Adriaens et al. 2002).
Furthermore, Rieppel (1996) stated that in lizards, body elongation and limb reduction are generally correlated with miniaturization. Compared with species of Dinotopterus, Clarias and Heterobranchus, the skulls of the anguilliform species are very small and morphological transformations related to miniaturization are not excludible. When plotting the average skull length (% SL) on the combined tree (Fig. 14) of all the taxa, we see the clades that give rise to anguilliformity include those Clarias species with the shortest relative skull length. The clade including H. longifilis, D. cunningtoni, C. gariepinus and C. ngamensis, however, incorporates the species with the longest relative skull length (indicated with bold values and branches) (measurements from Boulenger 1906; Poll 1943; Teugels 1986).
Acknowledgements
The authors wish to thank Leo Smith (AMNH) for the assistance with the analysis in POY and the use of the Parallel computing Cluster and Jan De Laet (KBIN) for some additional information. We thank Jean-François Agnèse (University of Montpellier, France), Roger Bills and Paul Skelton (South African Institute for Aquatic Biology, South Africa) and Royal Museum of Central Africa (Belgium) for providing tissue samples. Special thanks to M. Stiassny, C. Hopkins, J. Okouyi (M'Passa, Makokou) and the I.R.A.F. (Gabon) for assistance during collecting efforts. We are grateful to Miguel Parrent (MRAC) for his help with the collections. Research was funded by the BOF of the Ghent University (project 01104299 and 01103401) and the FWO (project G.0388.00).
Appendix
Appendices
Appendix 1 List of taxa included in the morphological study. Institutional abbreviations follow Leviton et al. (1985).
Appendix 2 Morphological character matrix used in phylogenetic analysis. Key to symbols: *0 and 1; $ = 0 and 2; / = 0 and 3; & = 1 and 2; # = 2 and 3; + = 3 and 4; £ = 3, 4 and 5; – = not applicable; ? = uncertainty.
| Family | Species | n | Museum record |
|---|---|---|---|
| Outgroup | |||
| Diplomystidae | Diplomystes chilensis (Molina, 1782) | 1 | Based on Arratia (1987) |
| Heteropneustidae | Heteropneustes fossilis (Bloch, 1794) | 1 | AMNH 178876 SW |
| Ingroup | |||
| Clariidae | Heterobranchus longifilis Valenciennes 1840 | 1 | AMNH 30545W |
| Dinotopterus cunningtoni Boulenger 1906 | 1 | MRAC dried specimen | |
| Bathyclarias longibarbis (Worthington 1933) | 1 | Based on Anseaume and Teugels (1999) | |
| Clarias gariepinus (Burchell 1922) | 1 | Based on Adriaens and Verraes (1998) | |
| Clarias fuscus (Lacepède 1803) | 1 | AMNH 10371 | |
| Clarias pachynema Boulenger 1903 | 1 | MRAC 90-029-P-393-394 | |
| Clarias buthupogon Sauvage 1879 | 1 | MRAC 90-029-P-230-231 | |
| Clarias ngamensis Castelnau 1861 | 1 | MRAC P47430 | |
| Clarias jaensis Boulenger 1909 | 1 | MRAC A0-049-P-120 | |
| Clarias camerunensis Lönnberg 1895 | 1 | MRAC A0-048-P-1826-1827 | |
| Clarias stappersii Boulenger 1915 | 1 | MRAC MRAC P83367 | |
| Clarias theodorae Weber 1898 | 1 | MRAC 94-019-P-1002 | |
| Clarias platycephalus Boulenger 1902 | 1 | MRAC 98-029-P-0912 | |
| Clariallabes longicauda (Boulenger, 1902) | 1 | MRAC 77-32-P-85 | |
| Tanganikallabes mortiauxi Poll 1943 | 1 | MRAC 130952-970 | |
| Platyclarias machadoi Poll 1977 | 2 | MRAC 78-6-P-1348-364 | |
| Platyallabes tihoni Poll 1977 | 2 | MRAC 125345-349 | |
| Channallabes apus (Günther 1873) | 5 | MRAC 97-056-P-0001-0003, 88-25-P-2192-227, 88-01-P-1976-1992, 67763-777, 162095-100 (represented as one OTU) | |
| Channallabes sanghaensis Devaere, in press | 1 | MRAC A4-31-P-171-183 | |
| Channallabes ogooensis Devaere, in press | 1 | MRAC A4-31-P-165-169 | |
| Channallabes longicaudatus (Pappenheim 1911) | 1 | MRAC A4-31-P-163-164 | |
| Channallabes alvarezi (Roman 1980) | 1 | MRAC A4-31-P-1-13 | |
| Channallabes teugelsi Devaere, in press | 2 | MRAC 78-22-P-1047-1050, 75-24-P-683-693 | |
| Gymnallabes typus Günther 1867 | 1 | MRAC 97-030-P-0010 | |
| Gymnallabes nops Roberts and Stewart 1976 | 1 | MCZ 50298 | |
| Dolichallabes microphthalmus Poll 1942 | 2 | MRAC 79258-260, 62407 | |
| 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | ||||||||||||||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |||||||||||||||||||||||||||||||||||||||||||
| Diplomystes chilensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | ? | ? | 0 | 0 | 0 | 0 |
| Heteropneustes fossilis | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| Heterobranchus longifilis | 2 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | ? | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Dinotopterus cunningtoni | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Bathyclarias longibarbis | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Clarias gariepinus | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Clarias fuscus | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | ? | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Clarias pachynema | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 5 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Clarias buthupogon | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 5 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Clarias ngamensis | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 1 | ? | ? | ? | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Clarias jaensis | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | $ | 1 | 0 | ? | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Clarias camerunensis | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 5 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Clarias stappersii | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ? | ? | ? | ? | 1 | 0 | 0 | 0 |
| Clarias theodorae | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 5 | 1 | 0 | 2 | 0 | ? | ? | ? | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Clarias platycephalus | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | & | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Clariallabes longicauda | 2 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 3 | ? | 0 | 2 | 0 | ? | ? | ? | ? | 1 | ? | 0 | ? | ? | ? | ? | 1 | ? | ? | ? |
| Tanganikallabes mortiauxi | 1 | 1 | 1 | ? | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | ? | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 5 | 1 | 0 | / | 2 | – | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Platyclarias machadoi (Angola) | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | * | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 |
| Platyclarias machadoi (Angola) | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | # | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 0 |
| Platyallabes tihoni (lower Congo stream) | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 3 | 3 | 0 | 0 | 3 | 2 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Platyallabes tihoni (lower Congo stream) | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | & | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ? | ? | 1 | 0 | 3 | 3 | 0 | 0 | 3 | 2 | ? | ? | ? | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Channallabes apus | * | 1 | 1 | $ | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | * | 2 | & | 0 | 1 | 0 | 1 | 1 | 1 | * | 0 | 0 | 2 | 0 | 0 | 2 | + | 1 | & | # | £ | 1 | 1 | ? | * | 0 | * | * | / | 1 | * | 0 | 1 | * | * | $ | 1 | * | * | * |
| Channallabes sanghaensis | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 1 | 1 | 3 | 5 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Channallabes ogooensis | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Channallabes longicaudatus | 2 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | ? | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 5 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 1 | 1 | 1 |
| Channallabes alvarezi | 2 | 0 | 1 | 0 | 0 | 0 | * | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | * | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 5 | 1 | * | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Channallabes teugelsi (Rep. Pop. Congo) | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 3 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | ? | ? | ? | ? | 1 | ? | ? | ? |
| Channallabes teugelsi (Gabon) | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 3 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Gymnallabes typus | 0 | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | & | 2 | 5 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Gymnallabes nops | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 3 | – | – | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 5 | 1 | 0 | 0 | 0 | ? | ? | ? | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Dolichallabes microphthalmus (Ruki) | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 3 | – | – | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 3 | 1 | 0 | – | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Dolichallabes microphthalmus (main Congo stream) | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 3 | – | – | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | ? | 2 | 1 | 1 | 1 | 3 | 1 | 0 | – | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |




