Morphometry and eye morphology in three species of Carabus (Coleoptera: Carabidae) in relation to habitat demands
Morfometria e morfologia degli occhi in tre speciedi Carabus (Coleoptera, Carabidae) in relazione alle caratteristiche dell’ habitat
Abstract
enMorphological features of three common European olfactory hunting carabid beetles, Carabus coriaceus mediterraneus Born, 1906, Carabus lefebvrei Dejean, 1826 and Carabus preslii neumeyeri Schaum, 1856, were compared. According to eye measurements, the three species are nocturnal and/or twilight hunters. They differ, however, in relative length of the antennae, relative surface area of the compound eyes, density of ommatidia and relative head width. These differences can be correlated with the species-specific habitat demands (light intensity, open land or shaded places). In particular, the greater lateral eye protrusion in C. lefebvrei corresponds to its tree-climbing habits, while the larger relative eye surface area and ommatidia density in C. coriaceus correspond to its choice of open habitats.
Riassunto
frSono state investigate le caratteristiche morfometriche e dei componenti degli occhi di tre specie di coleotteri carabidi predatori olfattivi, Carabus coriaceus mediterraneus Born (1906), C. lefebvrei Dejean (1826) e C. presliineumeyeri Schaum (1856). Le differenze dei parametri considerati, nelle tre specie in esame, sono riconducibili con le diverse caratteristiche dei diversi habitat sfruttati. In particolare, sono state riscontrate differenze nella protrusione laterale degli occhi, che è maggiore in C. lefebvrei, probabilmente perchéè l'unica delle tre specie che si arrampica sugli alberi, e nell'area delle cornee e numero di ommatidi, maggiore in C. coriaceus, specie che predilige habitat aperti.
Introduction
Morphological characters of the compound eye reflect features of the life-style of insect species (Wehner 1981), and thus can be used to infer such features (Bauer and Kredler 1993). For inhabitants of the deep litter layer and/or caves, eyes may be superfluous, or it may be sufficient to them to perceive the direction of a light source, as these species usually need to avoid bright light (for a review see Bartkowiak et al. 1991). Surface-dwelling species, however, should be able to orient themselves with respect to the surrounding vegetation and landscape features. If they are active during the night or at twilight, they should at least be capable of perceiving variations in light intensity and large silhouettes in their surroundings, to remain in their habitat or to find another into which they can migrate (Lauterbach 1964; Bathon 1973). Krumbiegel (1932) showed geographic (N/S and E/W) variation correlated to day/night activity in relation with the size of the eyes. He found measurable differences in the photophoby/diurnal activity of western and eastern populations of Carabus nemoralis Müller, 1764. The eyes of populations of East Europe were smaller and conical, the activity full nocturnal. If a species is active in the daylight, it is threatened by visual hunting predators, such as birds and other insects (e.g. Pearson 1985), and must be able to perceive small moving silhouettes. Visual hunting ground beetles, which regularly move in the light to seek prey, must not only be able to perceive predators (i.e. objects usually larger than themselves) but also to prey upon animals smaller than themselves. In addition, they should be able to guess the distance and absolute size of the prey before an attack (Bauer 1981, 1985; Schwind 1989).
Diurnal visual hunters have large, laterally protruding eyes with distinct binocular overlap of the visual fields. In nocturnal insects that detect prey chemically and by mechanical cues, the eyes are much smaller with fewer ommatidia (Bauer et al. 1998).
All species of carabid beetles show structural adaptations of their feeding apparatus indicative of their feeding habits (Forsythe 1982, 1983; Evans and Forsythe 1985). There is also a direct correlation between body form and habitat (e.g. feeding, locomotion, burrowing and flight). For instance, the Carabini are generally heavier, bulkier and stronger than other running ground beetles, but also relatively slow runners. This is reflected mainly in the size of the hind body, notably hind body depth and prothorax depth. It has been suggested that the size, bulk and strength of the Carabini may help them overcome the ‘environmental resistance’ (Heydemann 1957) of a variety of habitats and enable them to overcome larger but slower prey such as molluscs, worms, caterpillars and other slow-moving animals (Forsythe 1991).
Species that propel themselves through the litter when searching for prey generally have much larger trochanters than surface runners (Forsythe 1991).
The members of the genus Carabus, which includes >800 species, show well-developed differentiation at population level. In some species, more than several dozen subspecies have been described. The differentiation mainly involves elytral sculpture and colour. For these characters, as well as for many morphometric parameters, random drift seems to be the driving force of differentiation below the species level (Assmann et al. 2000).
The beetles of the genus Carabus are mostly nocturnal olfactory/tactile predators that run on the surface of soil or litter (Grüm 1976). They may compete for food with similarly sized carabid beetles of the genera Calosoma and Cychrus (Grüm 1994).
According to Casale et al. (1982), many Carabus species are specialized for snail hunting. Such species have a slender forebody (‘cychrization’, e.g. Carabus cychroides Baudi, 1860) and enter the shell through the aperture to attack the gastropod.
Another important strategy is shell-breaking, as described for Carabus coriaceus Linnaeus, 1758 (Sturani 1962).
In Italy, the habitat of C. coriaceus includes various lowland and hill biotopes, including urban habitats such as parks and gardens. It is mostly diurnal in autumn (September to October), especially during rainy days (Turin et al. 2003). Carabus lefebvrei Dejean, 1826 inhabits forests and forest edges, from sea level (Quercus spp.) and foothills (Castanea) to montane forests (Fagus sylvatica at 800–1800 m asl and coniferous stands at 900–2000 m asl). This is a nocturnal species and hides under stones or climbs trees during the day. Activity begins in mid-March (Sicily) but increases in April and continues until summer (Korell 1975). In Italy, Carabus preslii Dejean & Boisduval, 1830 prefers montane forests from 500 to 1800 m asl. It occurs in deciduous (Fagetum) and coniferous forests, but is less common in broad-leafed forests (Casale et al. 1982).
In this study, we investigated three species of the genus Carabus: C. coriaceus mediterraneus Born, 1906 (Fig. 1a), C. lefebvrei Dejean, 1826 (Fig. 1b) and C. preslii neumeyeri Schaum, 1856 (Fig. 1c). These species differ greatly in colour, habitus and habitat choice. We assume that C. coriaceus is an open habitat species adapted to very high light intensity, whereas the other species prefer shaded places. We tested the hypothesis that the supposed differences in habitat demands are reflected by differences in morphology, especially the shape and size of the compound eyes.
Specimens of Carabus coriaceus mediterraneus (a), Carabus lefebvrei (b), Carabus preslii neumeyeri (c)
Materials and Methods
The sample consisted of 14 individuals (seven males and seven females) for each of the three Carabus species: C. coriaceus, C. lefebvrei and C. preslii. Specimens were collected in southern Italy (Calabria): C. coriaceus was caught (bait-traps) in open fields and pastures (Squillace, Catanzaro) in spring 2004 (250 m asl), while C. lefebvrei and C. preslii were trapped (pit-fall traps) in upland forests (Sila Mountains) between March and September 2003 (1200 m asl).
All specimens were stored in alcohol (70%). Photographs were taken with a stereoscope (Zeiss Stemi SV 11Apo) and acquired by Matrox PC-VCR software (Windows 2000). For each individual, we measured body length (mm), antenna length (mm), head width (mm), trochanter length (mm), number of ommatidia, eye protrusion (mm) (head width-eye distance, Fig. 2), eye surface area (mm2), ommatidia density (number of ommatidia/mm2 of eye surface area).
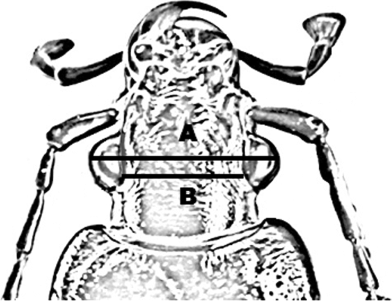
Measured traits: head width (a), eye distance (b) and eye protusion (a, b)
Relative measures of antenna length, number of ommatidia, eye surface area and eye protrusion were weighted against head width, while trochanter length was weighted against body length.
To determine the number of ommatidia and cornea size, we softened the specimens in hot potash lye for a few minutes. The cornea was removed and fixed through the following stations: distilled water, acetone, ethanol (70%), absolute ethanol and xylol. It was then spread on a microscope slide and photographed. Measurements were taken using Sigma Scan Pro 5 Software (SPSS®, Chicago, Inc.).
Statistical analyses
Sexual dimorphism in the morphological traits was assessed in each species using the Mann–Whitney U-test (Siegel and Castellan 1988), while anova was used to test for morphological differences among species (Sokal and Rohlf 1995). Multiple comparisons (between species) were performed using the Tamhane Test for homogeneous variances not assumed.
The probability level was computed by a complete randomization method (permutation or exact test; Pexact) or by a Monte Carlo simulation based on 10 000 sampled tables (PMonteCarlo) when computation was not possible (Mehta and Patel 1996; Good 2000).
Means are reported with standard error of the mean (±SEM) throughout the text. Statistical analyses were performed with the Statistical Package for Social Sciences 10.01 (SPSS® Inc.).
Results
The three species presented clear sex differences (Table 1). In C. coriaceus, the males had significantly longer antennae (relative length) than the females (U = 4.0, W = 32.0, Pexact = 0.007). In C. preslii, the males also had significantly longer antennae but a smaller head (respectively U = 0.0, W = 28.0, Pexact = 0.001, and U = 1.0, W = 29.0, Pexact = 0.001). Most interesting, C. lefebvrei presented sexual dimorphism in eye traits: the females had a significantly higher number of ommatidia in significantly larger and more protruding eyes than the males (respectively U = 4.0, W = 32.0, Pexact = 0.007, U = 8.0, W = 36.0, Pexact = 0.038, and U = 0.0, W = 28.0, Pexact = 0.001). In C. preslii, the females also had significantly more protruding eyes (U = 8.5, W = 36.5, Pexact = 0.038), but the weighted eye protrusion did not differ between the sexes (U = 12.0, W = 40, Pexact = 0.128).
| Measures | Females | Males | U | W | P exact | ||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||||
| C. coriaceus | |||||||
| Body length (mm) | 33.743 | 0.462 | 32.786 | 0.352 | 12.0 | 40.0 | 0.128 |
| Antenna length (mm) | 13.580 | 0.274 | 14.163 | 0.155 | 12.0 | 40.0 | 0.128 |
| Number of ommatidia | 3286.500 | 97.910 | 3361.143 | 88.892 | 18.0 | 46.0 | 0.456 |
| Eye surface | 0.765 | 0.034 | 0.666 | 0.039 | 13.0 | 41.0 | 0.165 |
| Head width (mm) | 5.167 | 0.080 | 5.036 | 0.061 | 15.0 | 43.0 | 0.259 |
| Trochanter length (mm) | 2.521 | 0.046 | 2.439 | 0.033 | 13.0 | 41.0 | 0.165 |
| Weighted trochanter length | 0.075 | 0.001 | 0.074 | 0.001 | 24.0 | 52.0 | 1.000 |
| Weighted antenna length | 2.630 | 0.051 | 2.814 | 0.029 | 4.0 | 32.0 | 0.007 |
| Weighted ommatidia number | 636.512 | 18.981 | 668.759 | 22.942 | 16.0 | 44.0 | 0.318 |
| Ommatidia density (N/sqmm) | 4376.594 | 250.588 | 5148.797 | 299.186 | 12.0 | 40.0 | 0.128 |
| Weighted eye surface | 0.148 | 0.005 | 0.132 | 0.007 | 14.0 | 42.0 | 0.209 |
| Eyes protrusion/2 | 0.753 | 0.041 | 0.793 | 0.031 | 16.5 | 44.5 | 0.318 |
| Weighted eye protrusion | 0.146 | 0.008 | 0.157 | 0.006 | 16.0 | 44.0 | 0.318 |
| C. lefebvrei | |||||||
| Body length (mm) | 28.143 | 0.382 | 25.586 | 0.236 | 0.0 | 28.0 | 0.001 |
| Antenna length (mm) | 13.559 | 0.404 | 13.463 | 0.131 | 22.0 | 50.0 | 0.805 |
| Number of ommatidia | 3003.143 | 83.042 | 2654.786 | 100.526 | 4.0 | 32.0 | 0.007 |
| Eye surface | 0.499 | 0.043 | 0.372 | 0.015 | 8.0 | 36.0 | 0.038 |
| Head width (mm) | 4.361 | 0.083 | 4.121 | 0.046 | 7.0 | 35.0 | 0.026 |
| Trochanter length (mm) | 2.030 | 0.059 | 1.904 | 0.035 | 10.5 | 38.5 | 0.073 |
| Weighted trochanter length | 0.072 | 0.002 | 0.074 | 0.001 | 18.0 | 46.0 | 0.456 |
| Weighted antenna length | 3.108 | 0.064 | 3.268 | 0.035 | 12.0 | 40.0 | 0.128 |
| Weighted ommatidia number | 688.559 | 14.242 | 643.508 | 20.508 | 11.0 | 39.0 | 0.097 |
| Ommatidia density (N/sqmm) | 6365.588 | 517.102 | 7348.191 | 421.348 | 13.0 | 41.0 | 0.165 |
| Weighted eye surface | 0.114 | 0.008 | 0.090 | 0.003 | 8.0 | 36.0 | 0.038 |
| Eyes protrusion/2 | 0.937 | 0.025 | 0.993 | 0.016 | 11.0 | 39.0 | 0.097 |
| Weighted eye protrusion | 0.215 | 0.005 | 0.241 | 0.003 | 0.0 | 28.0 | 0.001 |
| C. preslii | |||||||
| Body length (mm) | 25.000 | 0.254 | 24.229 | 0.326 | 12.0 | 40.0 | 0.128 |
| Antenna length (mm) | 12.073 | 0.290 | 12.897 | 0.198 | 6.0 | 34.0 | 0.017 |
| Number of ommatidia | 2078.214 | 94.479 | 2035.071 | 65.446 | 19.0 | 47.0 | 0.535 |
| Eye surface | 0.314 | 0.008 | 0.314 | 0.020 | 18.0 | 46.0 | 0.456 |
| Head width (mm) | 4.151 | 0.063 | 3.827 | 0.043 | 1.0 | 29.0 | 0.001 |
| Trochater length (mm) | 2.050 | 0.043 | 1.973 | 0.039 | 14.5 | 42.5 | 0.209 |
| Weighted trochanter length | 0.082 | 0.001 | 0.081 | 0.001 | 23.0 | 51.0 | 0.902 |
| Weighted antenna length | 2.915 | 0.097 | 3.370 | 0.043 | 0.0 | 28.0 | 0.001 |
| Weighted ommatidia number | 502.963 | 28.724 | 531.913 | 16.553 | 18.0 | 46.0 | 0.456 |
| Ommatidia density (N/sqmm) | 6610.511 | 265.837 | 6635.124 | 434.627 | 22.0 | 50.0 | 0.805 |
| Weighted eye surface | 0.076 | 0.003 | 0.082 | 0.005 | 13.0 | 41.0 | 0.165 |
| Eyes protrusion/2 | 0.665 | 0.045 | 0.529 | 0.030 | 8.5 | 36.5 | 0.038 |
| Weighted eye protrusion | 0.160 | 0.009 | 0.138 | 0.008 | 12.0 | 40.0 | 0.128 |
- Results of Mann–Whitney test, and significance levels estimated with a permutation procedure (Pexact). Bold, characters with significant sexual dimorphism.
anova revealed an overall significant difference among the three species for all the measured parameters (Table 2; anova, p < 0.001). The multiple comparisons showed clear differences between the three species for most of the traits (3, 4, Tamhane tests, p < 0.001), but not for two absolute measures (trochanter length and ommatidia density) and four relative ones (weighted trochanter length, weighted antenna length, weighted ommatidia number and weighted eye protrusion). Carabus coriaceus significantly differed from the other two species for weighted antenna length (P < 0.001); however, it was not significantly different from C. lefebvrei for weighted trochanter length (Tamhane test, mean difference = 0.001, p = 0.302) and weighted ommatidia number (Tamhane test, mean difference = −13.39, p = 0.884), or from C. preslii for weighted eye protrusion (Tamhane test, mean difference = 0.003, p = 0.985). Carabus lefebvrei and C. preslii significantly differed for all parameters except two-dimensional ones, trochanter length and weighted antenna length (respectively, Tamhane test, mean difference = −0.040, p = 0.739, and Tamhane test, mean difference = −0.045, p = 0.948), and one eye trait (ommatidia density, Tamhane test, mean difference = 234.07, p = 0.930).
| Measures | Species | F | df | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Carabus coriaceus | Carabus lefebvrei | Carabus preslii | |||||||
| Mean | SEM | Mean | SEM | Mean | SEM | ||||
| Body length (mm) | 33.264 | 0.309 | 26.864 | 0.415 | 24.614 | 0.226 | 189.53 | 2 | 0.000 |
| Antenna length (mm) | 13.871 | 0.171 | 13.511 | 0.204 | 12.485 | 0.204 | 13.79 | 2 | 0.000 |
| Number of ommatidia | 3323.821 | 64.365 | 2828.964 | 79.102 | 2056.643 | 55.535 | 90.74 | 2 | 0.000 |
| Eye surface | 0.716 | 0.028 | 0.435 | 0.028 | 0.314 | 0.010 | 74.55 | 2 | 0.000 |
| Head width (mm) | 5.101 | 0.052 | 4.241 | 0.056 | 3.989 | 0.058 | 111.00 | 2 | 0.000 |
| Trochanter length (mm) | 2.480 | 0.029 | 1.967 | 0.037 | 2.011 | 0.030 | 77.35 | 2 | 0.000 |
| Weighted trochanter length | 0.075 | 0.001 | 0.073 | 0.001 | 0.082 | 0.001 | 26.66 | 2 | 0.000 |
| Weighted antenna length | 2.722 | 0.038 | 3.188 | 0.041 | 3.143 | 0.081 | 20.37 | 2 | 0.000 |
| Weighted ommatidia number | 652.635 | 14.987 | 666.034 | 13.524 | 517.438 | 16.424 | 29.93 | 2 | 0.000 |
| Ommatidia density (N/sqmm) | 4762.696 | 215.905 | 6856.890 | 348.200 | 6622.818 | 244.770 | 17.34 | 2 | 0.000 |
| Weighted eye surface | 0.140 | 0.005 | 0.102 | 0.005 | 0.079 | 0.003 | 46.56 | 2 | 0.000 |
| Eyes protrusion/2 | 0.773 | 0.025 | 0.965 | 0.016 | 0.597 | 0.032 | 52.51 | 2 | 0.000 |
| Weighted eye protrusion | 0.152 | 0.005 | 0.228 | 0.004 | 0.149 | 0.007 | 68.39 | 2 | 0.000 |
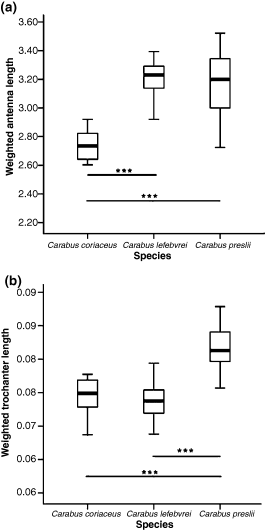
Measured traits: (a) weighted antenna length (mm), (b) weighted trochanter length (mm)
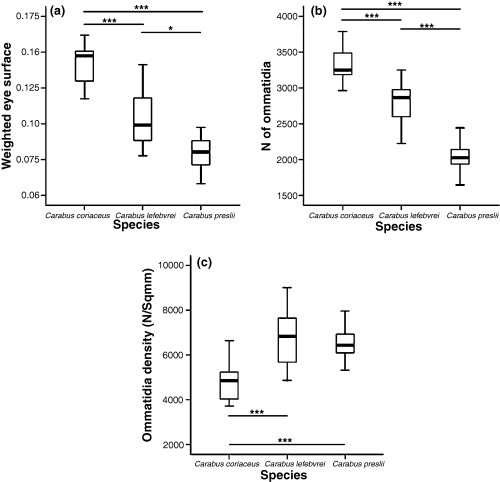
Measured traits: (a) weighted eye surface area (mm2), (b) number of ommatidia, (c) weighted ommatidia density (N/sqmm)
Discussion
The three species present differences in both body and eye traits. All three species are olfactory hunters, but their different life-styles have influenced body and eye characteristics: C. coriaceus and C. lefebvrei can be included in the second group of Bauer and Kredler (1993), which includes species with no preferred activity period (i.e. active by day and night, but preferably at twilight), while C. preslii belongs to the third group of nocturnal species (Bauer and Kredler 1993).
Differences in antenna length may reflect different sensory abilities/habits, as the antennae are usually shorter in visual hunters than in tactile hunters (Bauer and Kredler 1993). The relative length of the antenna (Fig. 3a) is bigger in C. preslii and C. lefebvrei than in C. coriaceus, suggesting that the first two species are prevalently tactile hunters.
We also found that C. coriaceus and C. lefebvrei have shorter trochanters than C. preslii (Fig. 3b). According to Forsythe (1991), surface runners are equipped with short trochanters, in contrast to ‘wedge pushers’ which seem to have relatively long and broad trochanters, enabling them to push their body under the litter layer in search of prey (Bauer and Kredler 1993). Hence our results suggest marked surface habits in C. coriaceus and C. lefebvrei (in contrast to C. preslii), confirming what is known about the ecology of the species (Turin et al. 2003).
We then observed that C. coriaceus has larger eyes than the other two species, suggesting partial visual hunter habits, since visual hunters generally have larger eyes than tactile hunters (Bauer and Kredler 1993).
There are more clearly defined differences in the number of ommatidia (Fig. 4b). Indeed, visual hunters generally have about 50% more ommatidia than tactile hunters (Bauer et al. 1998). The number of ommatidia is much larger in C. coriaceus than in C. lefebvrei and C. preslii, all three species differing significantly for this parameter. This confirms that C. coriaceus is more adapted to open habitats than the other two species.
The number of ommatidia per unit surface area of the eye (ommatidia density) varies even within the same genus, and it is probably an adaptation to the light conditions in the species-specific habitat. The number of ommatidia cannot be derived automatically from the eye surface area. The mean diameter of the facet lenses may vary between species, even if identical parts of the eye are compared. One explanation for this is that to achieve optimal contrast sensitivity the lens size is adapted to the mean light intensity under which the eyes have to function (Bauer and Kredler 1993).
Carabus coriaceus has a larger relative surface area of the eyes and a higher number of ommatidia than C. lefebvrei and C. preslii, while its ommatidia density (number/mm2 of surface area) is lower than in the other two species. The latter finding contrasts with the trend of the other parameters (surface area and number of ommatidia).
Carabus lefebvrei shows greater eye protrusion than the other two species. Laterally, protruding compound eyes favour peripheral vision and may be associated with an array of ommatidia improving resolution in the frontal visual field (e.g. Burkhardt and de la Motte 1983). However, they may also hinder the movements of the beetle between plants and soil structures. Perhaps this finding can be explained by the fact that C. lefebvrei is the only one of the three species that climbs trees and probably also hunts on them.
In visual hunters, the eyes not only protrude laterally but also frontally above the bases of the antennae. In nocturnal species, the antennae are inserted more in front of the eyes, the front edges of which are often curved around the antennal bases. In this position, the antennae hinder frontal vision, indicating that, for this group, mechanical and chemical cues are much more important in detecting nearby objects (Bauer and Kredler 1993). The eye-antenna angle in the three species does not differ greatly (C. coriaceus mean 38.50°, C. lefebvrei mean 27.28°, C. preslii mean 36.34°) and is never >50°, a value found only in visual hunters (Bauer and Kredler 1993).
The three species also differ in the extent of sexual dimorphism. The males of C. coriaceus and C. preslii have significantly greater relative antenna length than the females. This is found in most Carabidae and is likely related to the fact that males, rather than females, actively search for sexual partners (Bauer et al. 1998). Sexual dimorphism is even greater in C. lefebvrei, with the females having a significantly higher number of ommatidia and larger and more protruding eyes than males. This may reflect niche differentiation within the species, although no other data are available in this regard.
Our results confirm that morphological measurements, especially of the compound eyes, can be considered sensitive indicators of different habitat demands among closely related species.
Acknowledgements
The authors thank Prof. T. Bauer for the cornea extraction technique and for valuable suggestions, Dr A. Mazzei and Dr M. Sapia for collecting the specimens and two anonymous reviewers for their useful comments.




