Population genetic study of allozyme variation in natural populations of Drosophila antonietae (Insecta, Diptera)
Estudio de la genética de poblaciones y de la variación aloenzimática en poblaciones naturales de Drosophila antonietae.
Abstract
enDrosophila antonietae is an endemic South American cactophilic species found in relictual xerophytic vegetation, mostly associated with Cereus hildmaniannus cactus. Low differentiation among populations of this species has been detected using several markers. In this work, we performed an allozyme genetic variability analysis of 11 natural populations of D. antonietae and included a discussion about the possible influences of several evolutionary processes that might be acting to maintain the pattern observed. The genetic variability of 14 isoenzyme loci was analysed and showed a high genetic diversity (average observed heterozygosity = 0.319) and a moderate genetic differentiation among populations (F statistics = 0.0723). A correlation between genetic and geographical and ecological distances was detected among pairs of populations and the regional equilibrium analysis was thus applied. This analysis resulted in Nm (number of migrants) of approximately 3.21, indicating that moderate levels of both gene flow and genetic drift occur in this species, with gene flow overlapping genetic drift. However, considering ecological features of drosophilids, we propose a hypothesis to explain the moderate differentiation encountered as a result of three different processes, or a combination of them: (1) gene flow; (2) a short period of differentiation, i.e. maintenance of ancestral polymorphism; and (3) action of natural selection. Moreover, if gene flow is present, the high genetic diversity compared with other cactophilic and non-cactophilic species could be due to differential selection in different populations followed by gene exchange among them. These factors are discussed in the light of D. antonietae’s historical and evolutionary association with the host cactus.
Resumen
esDrosophila antonietae es una especie de cacto endémica de la América del Sur. Es encontrada en vegetaciones xerofíticas relictas y generalmente está asociada al cacto Cereus hildmaniannus. Baja diferenciación entre las poblaciones de esta especie ha sido detectada usando diversos marcadores. En este trabajo fue realizado un estudio sobre la variabilidad genética aloenzimática de 11 poblaciones naturales de D. antonietae. Este estudio posibilitó la discusión sobre las posibles influencias de diversas fuerzas evolutivas que pueden estar actuando manteniendo el patrón observado. La variabilidad genética de 14 loci isoenzimáticos fue analizada y evidenció una alta diversidad genética (promedio Ho = 0.319) y diferenciación genética moderada entre las poblaciones (Fst = 0.0723). Fue detectada una correlación entre las distancias genéticas y las distancias físicas (geográfica y ecológica) entre pares de poblaciones y el teste de equilibrio regional pudo ser aplicado. Este teste resultó en un Nm de aproximadamente 3.21, indicando que niveles moderados de flujo génico y deriva genética ocurren en esta especie, con el flujo génico sobreponiéndose a la deriva genética. Sin embargo, considerando características ecológicas de drosophlideos, proponemos una hipótesis para explicar la diferenciación moderada encontrada. Esta hipótesis es el resultado de tres procesos diferentes o la combinación entre ellos: (1) flujo génico; (2) un período breve de diferenciación, o sea, la manutención de polimorfismos ancestrales; y (3) acción de selección natural. Además, si el flujo génico está presente, la alta diversidad genética comparada con otras especie de Drosophila puede deberse a la selección diferencial en las poblaciones diferentes acompañada por intercambio de material genético entre ellas. Estos factores son discutidos desde el punto de vista de la asociación histórica y evolutiva de D. antonietae con el cacto hospedero.
Introduction
Drosophila antonietae (Tidon-Sklorz and Sene, 2001) is an endemic South American species of the Drosophila repleta group. This group comprises more than 95 species that occur in different habitats (Heed 1982; Pereira et al. 1983; Wasserman 1992; Etges et al. 2001; Tidon-Sklorz and Sene 2001). More than half of these species are cactophilic, that is, they lay eggs in rotting cladodes of neotropical cacti where larvae are feeding on specific yeasts dependent on the decomposition processes (Pereira et al. 1983; Morais et al. 1994), in the cactus–yeast–Drosophila system. Therefore, cactophilic species are always found in areas where host cacti occur.
Drosophila antonietae, together with Drosophila serido (Vilela and Sene, 1977), Drosophila borborema (Vilela and Sene, 1977), Drosophila koepferae (Fontdevila et al., 1988), Drosophila seriema (Tidon-Sklorz and Sene, 1995), Drosophila gouveai (Tidon-Sklorz and Sene, 2001) and Drosophila buzzatii (Patterson and Wheeler, 1942), compose the D. buzzatii cluster. These species are cactophilic and endemic to South America, except D. buzzatii that is found on other continents where it was introduced with the Opuntia genus host cactus by human activity (Barker et al. 1985). Drosophila antonietae, more specifically, can be found in relictual xerophytic vegetations over the south and southeast regions of Brazil and north to the eastern boundary of the Argentinean Chaco, mostly associated with cacti species of the Cereus genus. Nowadays, the cactaceae distribution over South America is discontinuous because of paleoclimatic changes (Bigarella et al. 1975; Ab'Saber 1977a,b; Vanzolini 1981), which generated the current geographical isolation of the D. buzzatii species cluster, including D. antonietae (Manfrin and Sene 2006).
Monteiro (1997) studied D. gouveai and D. antonietae parapatric populations and detected that the populations of the first species are found on the top of hills associated with the Pilosocereus machrisii cactus, while the populations of the second species are present in the valleys of rivers of the Parana river basin and are associated with the Cereus hildmaniannus cactus.
Manfrin et al. (2001) analysed cytochrome oxidase I mitochondrial gene sequences of several South American species and detected that specimens of D. gouveai from Analândia-SP (Brazil) population grouped with those from D. antonietae populations. Thus, they suggested a secondary contact between these two species with hybridization and differential introgression of the mitochondrial genome, that is, introgression retaining the ancestor 2e8 chromosomal inversion of D. gouveai.
Monteiro and Sene (1995), analysing aedeagus morphology of specimens from populations along the border of the distribution of these two species, observed that each population of D. gouveai could be discriminated from all the others, suggesting absence of gene flow. They speculated that this could be due to the fact that the various populations are found at the top of geographically isolated hills. On the contrary, D. antonietae populations did not show such discrimination but the greater the distance among D. antonietae populations along rivers was, the greater was the morphological divergence among them. Therefore, they suggested the occurrence of gene flow between D. antonietae populations along the river systems.
Homogeneity for this species is also found in karyotype analyses. Only one karyotype (type V, according to Baimai et al. 1983; Kuhn et al. 1996) has been found in all the populations of D. antonietae analysed so far.
Recently, Mateus and Sene (2003) analysed the intrapopulation genetic variability of two distinct populations of D. antonietae using allozymes. Their work showed that a temporal variation in the frequencies of some allozymes studied is probably correlated with pluviometric indexes. Moreover, this species seems to have neither intrapopulational structure nor inbreeding. From this previous work and also from the homogeneity with respect to several markers found for D. antonietae populations, it was decided to study, in this work, the allozyme variability of natural populations of D. antonietae to try to understand how the low intraspecific differentiation is maintained, considering ecological features and historical aspects. The results might contribute to the general problem of differentiation and phylogenetic relationships among geographically isolated cactophilic Drosophila populations and might also help to clarify the evolutionary processes in the D. buzzatii species cluster.
Materials and Methods
Specimens were collected between 1998 and 2000 from geographically isolated natural populations of D. antonietae presented in Fig. 1. Only one collection was made in each of the areas. The collections were performed always in the hot/wet period of the year (between February and May), with the exception of Sertãozinho-SP, where it was conducted in October 1999. The method used in the collections is described in Sene et al. (1981), Mateus et al. (2006). Two types of physical distances between pairs of populations were determined: geographical distance (i.e. linear distance between pairs of populations); ecological distance (i.e. distance between pair of populations along rivers), which was determined using a map (1 : 1 000 000 scale) and a map measure tool, linking pair of populations through the Parana River and its tributary streams.
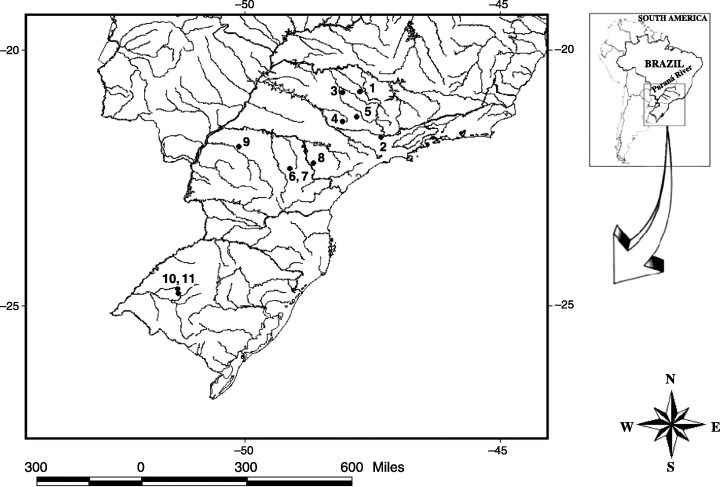
Drosophila antonietae populations collected in South America. 1, Serrana-SP – 21°15′S, 47°34′W; 2, Itatiba-SP – 22°56′S, 46°55′W; 3, Sertãozinho-SP – 21°10′S, 48°05′W; 4, Santa Maria da Serra-SP – 22°34′S, 48°12′W; 5, Itirapina-SP – 22°16′S, 47°48′W; 6, Santo Santa Rosa/Tibagi-PR – 24°37′S, 50°33′W; 7, Canyon Guartelá/Tibagi-PR – 24°32′S, 50°18′W; 8, Sengés-PR – 24°07′S, 49°23′W; 9, Cianorte-PR – 23°34′S, 52°33′W; 10 and 11, Santiago-RS – 29°12′S, 54°53′W and 29°25′S, 54°50′W, respectively
Genetic variability of populations was accessed analysing 14 enzyme loci (Me, Mdh, Est-1 and −2, Lap, Gpdh, Idh, Adh-1 and −2, Hk-1, −2 and −3, Pep and Pgm) in two electrophoretic systems (TC1 and TC2), according to Mateus and Sene (2003). The nomenclature used was that proposed by Mateus and Sene (2003), with the alleles designated by numbers in ascending order of anodal mobility. In each gel, two D. buzzatii males, from the monomorphic lineage H61F1M-A2, were used as electrophoresis control. All gels were photographed with a Canon camera using Copex Pan A.H.U. (Agfa™) or Imagelink HQ (Kodak™) 35 mm films.
The genetic and statistic analyses were performed using three software programmes: TFPGA (Miller 1997) – for allele frequencies and polymorphic loci proportions, Hardy–Weinberg equilibrium test using Markov Chain method (10 batches and 2000 permutations per batch) and Mantel test (with 2000 random permutations); GDA (Genetic Data Analysis) (Lewis and Zaykin 2001) – for heterozygosity estimations, Nei's distances and similarities, Wright's F statistics and neighbour-joining analysis; Microcal Origin (version 3.54; – Copyright© 1991–1994 Microcal Software, Inc., 1 Roudhouse Plaza, Northampton, MA, USA) – for Regional Equilibrium test, performed according to Hutchison and Templeton (1999). Neighbour-joining analysis using distance estimations and Wright's Fst were conducted excluding populations with small n, Guartelá-PR and Sengés-PR and also excluding monomorphic or almost monomorphic loci, Gpdh, Hk-1, Idh and Mdh. We decided to classify the genetic differentiation through Wright's Fst using the qualitative guideline proposed by Wright (1978) as ‘low’, ‘moderate’, ‘high’ and ‘very high’.
Two bottleneck tests were performed using BOTTLENECK software (Cornuet and Luikart 1996, http://www.montpelier.inra.fr/URLB/bottleneck/bottleneck.html) in order to check the correlation between genetic variability constraints with the genetic diversity indexes. The first evaluation was performed using the Wilcoxon sign rank test under the infinite alleles model. This statistical method is based on the fact that reductions in effective population size results in unequal losses of alleles and heterozygosity. Because allele numbers are reduced more quickly than gene diversity, a recent bottleneck can be detected by comparing the two tests and by looking whether there is gene diversity excess (H excess). The second test in BOTTLENECK plots allele frequency distribution and compares it with the expected L-shaped distribution. A population that went through a bottleneck is likely to exhibit a mode shift in the frequency distribution of alleles.
Results
From the 11 D. antonietae populations (528 specimens) analysed, 10 enzyme systems allowed the detection of 14 loci and 46 alleles. Table 1 shows the allele frequencies and genetic variability values of the collected populations. All polymorphic loci (10 of 14, i.e. approximately 71.43%) showed departure from the Hardy–Weinberg equilibrium. The frequency of polymorphic loci (P0.95) ranged from 43% in Guartelá-PR to 79% in population 1 from Santiago-RS. The mean observed heterozygosity (Ho) varied between 0.204 and 0.452 (Guartelá-PR and population 1 from Santiago-RS, respectively). The mean expected heterozygosity varied between 0.318 and 0.462 (Serrana-SP and population 1 from Santiago-RS, respectively).
| Alleles | SAN1 | SAN2 | SER | ITA | SRT | SMS | ITI | SSR | GUA | SEN | CIA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adh-1 | (n = 13) | (n = 09) | (n = 95) | (n = 20) | (n = 83) | (n = 93) | (n = 82) | (n = 48) | (n = 07) | (n = 04) | (n = 17) |
| 1.05 | 0.154 | 0.111 | 0.079 | 0.050 | 0.006 | 0.011 | 0.012 | ||||
| 1.00 | 0.846 | 0.889 | 0.853 | 0.900 | 0.964 | 0.968 | 0.988 | 1.000 | 1.000 | 1.000 | 1.000 |
| 0.95 | 0.063 | 0.050 | 0.030 | 0.021 | |||||||
| 0.90 | 0.005 | ||||||||||
| Adh-2 | (n = 14) | (n = 15) | (n = 95) | (n = 20) | (n = 92) | (n = 96) | (n = 89) | (n = 48) | (n = 09) | (n = 04) | (n = 18) |
| 1.05 | 0.179 | 0.079 | 0.050 | 0.027 | 0.057 | 0.034 | |||||
| 1.00 | 0.821 | 1.000 | 0.826 | 0.750 | 0.935 | 0.943 | 0.966 | 1.000 | 1.000 | 1.000 | 1.000 |
| 0.95 | 0.026 | 0.150 | 0.016 | ||||||||
| 0.90 | 0.069 | 0.050 | 0.022 | ||||||||
| Est-1 | (n = 23) | (n = 20) | (n = 96) | (n = 20) | (n = 94) | (n = 96) | (n = 96) | (n = 48) | (n = 09) | (n = 04) | (n = 19) |
| 1.15 | 0.087 | 0.250 | 0.016 | 0.090 | 0.010 | 0.005 | 0.125 | 0.125 | |||
| 1.10 | 0.435 | 0.275 | 0.021 | 0.025 | 0.234 | 0.338 | 0.151 | 0.479 | 0.389 | 0.250 | 0.447 |
| 1.05 | 0.087 | 0.225 | 0.026 | 0.037 | 0.099 | 0.052 | 0.278 | 0.125 | 0.053 | ||
| 1.00 | 0.369 | 0.225 | 0.937 | 0.975 | 0.676 | 0.615 | 0.745 | 0.344 | 0.333 | 0.500 | 0.500 |
| 0.95 | 0.022 | 0.025 | |||||||||
| Est-2 | (n = 23) | (n = 21) | (n = 96) | (n = 20) | (n = 95) | (n = 89) | (n = 92) | (n = 48) | (n = 07) | (n = 03) | (n = 19) |
| 1.15 | 0.071 | ||||||||||
| 1.10 | 0.130 | 0.119 | 0.183 | 0.075 | 0.174 | 0.185 | 0.206 | 0.021 | 0.143 | 0.333 | 0.184 |
| 1.05 | 0.304 | 0.310 | 0.333 | 0.225 | 0.300 | 0.421 | 0.337 | 0.156 | 0.500 | 0.237 | |
| 1.00 | 0.544 | 0.452 | 0.479 | 0.500 | 0.521 | 0.315 | 0.413 | 0.521 | 0.215 | 0.334 | 0.474 |
| 0.95 | 0.022 | 0.119 | 0.005 | 0.200 | 0.005 | 0.079 | 0.044 | 0.302 | 0.071 | 0.333 | 0.105 |
| Gpdh | (n = 16) | (n = 18) | (n = 95) | (n = 20) | (n = 77) | (n = 49) | (n = 88) | (n = 38) | (n = 04) | (n = 02) | (n = 18) |
| 1.05 | 0.344 | 0.083 | |||||||||
| 1.00 | 0.656 | 0.917 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Hk-1 | (n = 06) | (n = 05) | (n = 85) | (n = 13) | (n = 47) | (n = 72) | (n = 58) | (n = 48) | (n = 08) | (n = 03) | (n = 13) |
| 1.00 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Hk-2 | (n = 14) | (n = 12) | (n = 89) | (n = 17) | (n = 44) | (n = 70) | (n = 65) | (n = 48) | (n = 08) | (n = 02) | (n = 17) |
| 1.10 | 0.036 | 0.042 | 0.021 | 0.147 | |||||||
| 1.05 | 0.321 | 0.250 | 0.191 | 0.206 | 0.409 | 0.507 | 0.308 | 0.458 | 0.562 | 0.529 | |
| 1.00 | 0.643 | 0.625 | 0.803 | 0.559 | 0.591 | 0.443 | 0.646 | 0.521 | 0.438 | 1.000 | 0.324 |
| 0.95 | 0.083 | 0.006 | 0.235 | 0.050 | 0.046 | ||||||
| Hk-3 | (n = 12) | (n = 12) | (n = 84) | (n = 16) | (n = 25) | (n = 57) | (n = 61) | (n = 48) | (n = 09) | (n = 0) | (n = 15) |
| 1.00 | 1.000 | 0.958 | 0.982 | 0.875 | 1.000 | 0.956 | 0.779 | 1.000 | 1.000 | 0.967 | |
| 0.95 | 0.042 | 0.018 | 0.125 | 0.018 | 0.221 | ||||||
| 0.90 | 0.026 | 0.033 | |||||||||
| Idh | (n = 23) | (n = 21) | (n = 96) | (n = 20) | (n = 96) | (n = 88) | (n = 92) | (n = 48) | (n = 09) | (n = 04) | (n = 19) |
| 1.02 | 0.348 | ||||||||||
| 1.00 | 0.652 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.750 | 1.000 |
| 0.95 | 0.250 | S | |||||||||
| Lap | (n = 23) | (n = 21) | (n = 96) | (n = 20) | (n = 94) | (n = 96) | (n = 95) | (n = 48) | (n = 09) | (n = 04) | (n = 19) |
| 1.05 | 0.283 | 0.071 | 0.109 | 0.175 | 0.064 | 0.234 | 0.216 | 0.073 | 0.055 | 0.125 | |
| 1.00 | 0.369 | 0.595 | 0.688 | 0.600 | 0.750 | 0.568 | 0.611 | 0.729 | 0.500 | 0.625 | 0.684 |
| 0.98 | 0.087 | 0.167 | 0.005 | 0.075 | 0.043 | 0.115 | 0.026 | 0.042 | 0.167 | 0.125 | 0.105 |
| 0.95 | 0.261 | 0.167 | 0.198 | 0.150 | 0.143 | 0.083 | 0.147 | 0.156 | 0.278 | 0.125 | 0.211 |
| Mdh | (n = 23) | (n = 20) | (n = 95) | (n = 20) | (n = 79) | (n = 48) | (n = 55) | (n = 21) | (n = 03) | (n = 02) | (n = 14) |
| 1.00 | 1.000 | 1.000 | 0.963 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 0.95 | 0.037 | ||||||||||
| Me | (n = 22) | (n = 19) | (n = 96) | (n = 20) | (n = 82) | (n = 95) | (n = 71) | (n = 48) | (n = 09) | (n = 04) | (n = 19) |
| 1.05 | 0.500 | 0.158 | 0.094 | 0.274 | 0.184 | 0.141 | 0.136 | 0.056 | 0.375 | 0.421 | |
| 1.00 | 0.409 | 0.500 | 0.568 | 0.550 | 0.482 | 0.542 | 0.556 | 0.510 | 0.833 | 0.375 | 0.474 |
| 0.95 | 0.091 | 0.342 | 0.338 | 0.450 | 0.244 | 0.274 | 0.303 | 0.354 | 0.111 | 0.250 | 0.105 |
| Pep | (n = 14) | (n = 17) | (n = 96) | (n = 20) | (n = 89) | (n = 94) | (n = 88) | (n = 48) | (n = 09) | (n = 04) | (n = 19) |
| 1.05 | 0.607 | 0.206 | 0.005 | 0.247 | 0.447 | 0.216 | 0.208 | 0.625 | 0.553 | ||
| 1.00 | 0.393 | 0.794 | 0.995 | 1.000 | 0.753 | 0.553 | 0.784 | 0.792 | 1.000 | 0.375 | 0.447 |
| Pgm | (n = 18) | (n = 14) | (n = 95) | (n = 20) | (n = 80) | (n = 39) | (n = 73) | (n = 45) | (n = 08) | (n = 03) | (n = 15) |
| 1.05 | 0.033 | ||||||||||
| 1.00 | 0.333 | 0.214 | 0.647 | 0.750 | 0.662 | 0.756 | 0.706 | 0.789 | 0.812 | 0.500 | 0.734 |
| 0.95 | 0.667 | 0.750 | 0.353 | 0.225 | 0.313 | 0.218 | 0.267 | 0.167 | 0.125 | 0.500 | 0.200 |
| 0.90 | 0.036 | 0.025 | 0.025 | 0.026 | 0.027 | 0.044 | 0.063 | 0.033 | |||
| P 0.95 | 0.79 | 0.64 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.50 | 0.43 | 0.54 | 0.50 |
| H o | 0.452 | 0.393 | 0.281 | 0.260 | 0.315 | 0.344 | 0.272 | 0.312 | 0.204 | 0.333 | 0.348 |
| H e | 0.4621 | 0.429 | 0.318 | 0.366 | 0.367 | 0.409 | 0.3931 | 0.3511 | 0.328 | 0.453 | 0.3971 |
| AFD | SM | L-sh | L-sh | L-sh | L-sh | L-sh | L-sh | L-sh | L-sh | SM | SM |
- SAN1, population 1 from Santiago-RS; SAN2, population 2 from Santiago-RS; SER, Serrana-SP; ITA, Itatiba-SP; SRT, Sertãozinho-SP; SMS, Santa Maria da Serra-SP; ITI, Itirapina-SP; SSR, Salto Santa Rosa/Tibagi-PR; GUA, Canyon Guartelá/Tibagi-PR; SEN, Sengés-PR; CIA, Cianorte-PR; n, number of specimens analysed; P0.95, proportion of polymorphic loci; Ho, mean observed heterozygosity; He, mean expected heterozygosity; AFD, allele frequency distribution; L-sh, L-shaped distribution; SM, shifted mode.
- Numbers in bold represent frequencies that are not in Hardy–Weinberg equilibrium.
- 1Significant H excess.
The bottleneck test detected statistically significant H excess in four populations (population 1 from Santiago-RS, Itirapina-SP, Salto Santa Rosa/Tibagi-PR and Cianorte-PR) and allele frequency distribution mode shift occurred in three populations (population 1 from Santiago-RS, Sengés-PR and Cianorte-PR). These results did not demonstrate any correlation with the differentiation status or the genetic diversity of the populations.
Nei (1972) genetic identities (I) and distances (D) between pairs of populations are presented in Table 2. The most similar populations were Itirapina-SP and Sertãozinho-SP (D = 0.0190; I = 0.9811) and the most divergent were the low n Guartelá-PR and Sengés-PR populations (D = 0.3239; I = 0.7234), followed by Itatiba-SP and population 1 from Santiago-RS (D = 0.2159; I = 0.8059).
| Population | SAN1 | SAN2 | SER | ITA | SRT | SMS | ITI | SSR | GUA | SEN | CIA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SAN1 | **** | 0.0762 | 0.1610 | 0.2159 | 0.0869 | 0.0863 | 0.1192 | 0.1239 | 0.1817 | 0.1876 | 0.0784 |
| SAN2 | 0.9267 | **** | 0.1008 | 0.1389 | 0.0690 | 0.1028 | 0.0866 | 0.0800 | 0.1119 | 0.1976 | 0.1221 |
| SER | 0.8513 | 0.9041 | **** | 0.0219 | 0.0324 | 0.0795 | 0.0271 | 0.0852 | 0.0989 | 0.2310 | 0.1351 |
| ITA | 0.8059 | 0.8703 | 0.9784 | **** | 0.0545 | 0.0862 | 0.0353 | 0.0843 | 0.1102 | 0.2631 | 0.1499 |
| SRT | 0.9168 | 0.9333 | 0.9611 | 0.9469 | **** | 0.0247 | 0.0190 | 0.0311 | 0.0687 | 0.1869 | 0.0394 |
| SMS | 0.9173 | 0.9023 | 0.9236 | 0.9174 | 0.9756 | **** | 0.0303 | 0.0406 | 0.0611 | 0.2014 | 0.0277 |
| ITI | 0.8876 | 0.9171 | 0.9732 | 0.9653 | 0.9811 | 0.9701 | **** | 0.0506 | 0.0669 | 0.1600 | 0.0722 |
| SSR | 0.8835 | 0.9231 | 0.9183 | 0.9191 | 0.9694 | 0.9602 | 0.9507 | **** | 0.0547 | 0.2121 | 0.0463 |
| GUA | 0.8339 | 0.8942 | 0.9058 | 0.8957 | 0.9336 | 0.9408 | 0.9353 | 0.9468 | **** | 0.3239 | 0.0943 |
| SEN | 0.8289 | 0.8207 | 0.7937 | 0.7686 | 0.8296 | 0.8176 | 0.8521 | 0.8089 | 0.7234 | **** | 0.1998 |
| CIA | 0.9246 | 0.8850 | 0.8736 | 0.8608 | 0.9614 | 0.9727 | 0.9303 | 0.9547 | 0.9100 | 0.8189 | **** |
- ****, no distance between the same sample/population.
The Wright's F statistics analysis (Table 3) showed a significant deviation from zero and indicated a moderate genetic differentiation (average Fst = 0.0723 for all loci) among all populations. The fixation index of Wright revealed a high deficiency of heterozygotes in all populations (Fis = 0.1590) and in the total (Fit = 0.2199), both values also significantly differed from zero.
| Loci | Fis | Fit | Fst |
|---|---|---|---|
| Adh-1 | 0.2298 | 0.2635 | 0.0437 |
| Adh-2 | 0.3126 | 0.3465 | 0.0493 |
| Est-1 | –0.0601 | 0.1052 | 0.1559 |
| Est-2 | 0.2265 | 0.2472 | 0.0267 |
| Hk-2 | 0.0934 | 0.1614 | 0.0750 |
| Hk-3 | 0.4559 | 0.5190 | 0.1159 |
| Lap | –0.0120 | 0.0194 | 0.0311 |
| Me | 0.2900 | 0.3078 | 0.0251 |
| Pep | 0.3723 | 0.4829 | 0.1763 |
| Pgm | 0.0806 | 0.1475 | 0.0728 |
| All loci | 0.1590 | 0.2199 | 0.0723 |
| 95% CI | |||
| Minimum | 0.0666 | 0.1330 | 0.0398 |
| Maximum | 0.2623 | 0.3238 | 0.1146 |
- CI, confidence interval of significance level.
The neighbour-joining analysis using Nei (1972) genetic distances (Fig. 2) showed a separation of the populations of the upper Parana–Uruguay Rivers basin (clade A) from the lower basin populations (clade B). Furthermore, all São Paulo State populations were grouped in one clade (D), with exception of Santa Maria da Serra-SP, which was grouped with the Parana State populations (clade C).
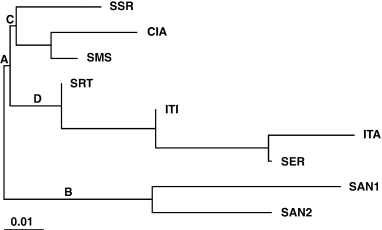
Neighbour-joining clustering analysis of Drosophila antonietae collections using Nei (1972) distances. SER, Serrana-SP; ITA, Itatiba-SP; SRT, Sertãozinho-SP; SMS, Santa Maria da Serra-SP; ITI, Itirapina-SP; SSR, Santo Santa Rosa/Tibagi-PR; CIA, Cianorte-PR; SAN1, population 1 from Santiago-RS; SAN2, population 2 from Santiago-RS
Before performing the regional equilibrium test, a Mantel test analysis was carried out, correlating the genetic distances matrix (Fst) with geographical distances and ecological distances between pair of populations. This test resulted in a positive and statistically significant correlation between genetic distances and both physical distances (geographical: r = 0.4632, p = 0.0120; ecological: r = 0.3964, p = 0.0160). Thus, the regional equilibrium test was conducted and both correlation graphics demonstrated a linear correlation showing a pattern congruent with the regional equilibrium (case I, according to Hutchison and Templeton 1999). Fig. 3 presents the result of ecological and genetic distance correlation.
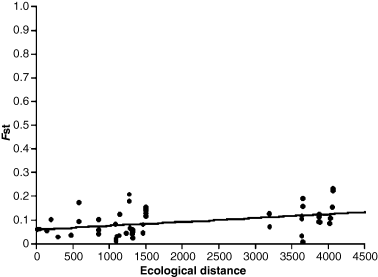
Correlation between Fst (Weir and Cockerham 1984) with ecological distances for all pairwise collections. Ecological distances in kilometres
Discussion
Interpopulational allozyme analyses of D. antonietae showed that all polymorphic loci (10 of 14 analysed) departed significantly from Hardy–Weinberg equilibrium (Table 1). As argued by Mateus and Sene (2003), this result could be due to several factors, such as natural selection, genetic drift, inbreeding and gene flow. They discussed only the influence of the first three processes, of which they discarded genetic drift and inbreeding, but a possible natural selection on allozyme variation was detected (association with rain precipitation).
The genetic variability analysis of D. antonietae populations (Table 1) revealed a much higher average Ho = 0.319 when compared with the Ho for non-cactophilic (Ho = 0.160) and other cactophilic (Ho = 0.087) Drosophila species, according to allozyme data from Zouros (1973), Johnson (1974), Barker and Mulley (1976) and Moraes and Sene (2002). Higher Ho values in two D. antonietae populations were detected earlier by Mateus and Sene (2003). Therefore, despite Barker and Mulley (1976) having suggested that low levels of genetic variability are characteristic of cactophilic Drosophila because of their ecological specificity associated with habitat patchiness, this does not appear to be the case for D. antonietae. In fact, Mateus and Sene (2003) did not detect intrapopulational structuration for this species in their analysis.
How could the higher genetic diversity be maintained in the geographically isolated D. antonietae populations? Thomas and Barker (1990) proposed that habitat selection in a population with breeding structure could be a strong force in the maintenance of variability if isolation between the subpopulations were sporadically interrupted. Amos and Harwood (1998) postulated that with restricted genetic flow, the frequencies of some rare alleles could rise in a subpopulation through random processes, and the total variability in the population should increase if isolation ends.
Regarding D. antonietae, Monteiro and Sene (1995) have suggested the occurrence of gene flow as the most likely explanation for the low differentiation of aedeagus morphology of specimens from different geographically isolated populations. Gene flow and genetic drift can be indirectly recognized using allozyme genetic analyses by the calculation of Fst index, a method that is traditionally performed in an attempt to estimate the effects of these evolutionary processes in natural populations (McCauley 1994; Rousset 1997; Whitlock and McCauley 1999; Austin et al. 2004). Thus, we will use the Fst index to estimate levels of gene flow and genetic drift for D. antonietae.
Results from Wright's Fst (Table 3) showed a moderate genetic differentiation among populations (Fst = 0.0723), with a high deficiency of heterozygotes in each of the populations (Fis = 0.1590) and over all populations (Fit = 0.2199). This high deficiency is probably because of null alleles, Wahlund effect or selection against heterozygotes as discussed by Mateus and Sene (2003). The moderate genetic differentiation was also observed when the Nei (1972) analyses were applied (Table 2). Although D. antonietae populations were very similar to each other, the comparison of Nei (1972) interpopulation identities with intrapopulation temporal values (Mateus and Sene 2003) demonstrated that the differentiation among populations is, overall, higher than a simple over time differentiation.
The neighbour-joining analysis using Nei (1972) distances showed a certain separation between upper and lower river basin populations (Fig. 2), showing a possible relationship between genetic distance and geographical distances, despite the moderate Fst differentiation. This correlation was confirmed by the Mantel test comparing matrixes of pairwise population genetic distance (Fst) and physical distances (geographical and ecological). As this correlation was confirmed, Hutchison and Templeton (1999) regional equilibrium analysis could be applied. According to these authors, traditional attempts to relate Fst estimatives with gene flow and genetic drift using Wright (1931) formula Fst = 1/(4 Nm + 1) are inappropriate, because most natural populations are not in equilibrium (McCauley 1993), as assumed by the island model on which the equation is based. The regional equilibrium model allows to study any population of a species before Wright's equation is applied. The regional equilibrium is based on the stepping-stone population structure model, checking the correlation between physical and genetic distances of a pair of populations.
Our results confirmed that D. antonietae populations are in regional equilibrium as detected by the Mantel test and the Fst and physical (geographical and ecological) distances linear regression plots, which depicted a correlation as expected in Case I described by Hutchison and Templeton (1999). Therefore, it was not possible to reject the null hypothesis of regional equilibrium for D. antonietae populations. Thus, Fst estimates can be properly associated with gene flow and genetic drift, using Wright's equation. Drosophila antonietae Fst was 0.0723 (95% CI = 0.0398–0.1146; Table 3), generating an Nm of approximately 3.21. Kimura and Weiss (1964) showed that when Nm > 4, the effects of gene flow are much higher than those from genetic drift and the populations are considered panmictic. On the contrary, when Nm is much smaller than 1, genetic drift overlaps gene flow and strong differentiation occurs among populations. The Nm calculated for D. antonietae populations lies between the two extremes, but closer to the upper limit, indicating that moderate levels of both gene flow and genetic drift occur in this species, with gene flow probably overlapping genetic drift.
Our data are consistent with the use of the isolation by distance population genetic structure model to infer the interaction between gene flow and genetic drift. However, dispersion in Drosophila seems to be limited to the locality for cactophilic flies, as egg laying and larval feeding sites are restricted to rotting cacti cladodes (Johnston and Heed 1975,1976; Johnston and Templeton 1982; Templeton and Johnston 1982; Morais et al. 1994; Moraes and Sene 2002; Mateus and Sene 2003). Although the gene flow might be allowed to explain genetic similarity, it seems not to be the case for D. antonietae, considering that the localities studied are, at least for the present time, geographically isolated and that drosophilids do not disperse too far from the xerophytic vegetation (Markow and Castrezana 2000).
According to a number of observations (Bigarella et al. 1975; Ab'Saber 1977a,b; Vanzolini 1981), several glaciation cycles occurred in the Pliocene and Quaternary period, which could be responsible for the retraction of xerophytic vegetation to dry area centres (Caatinga and Chaco) during the hot/wet phases. Between these areas, this vegetation became restricted to the top of hills, because it was adapted to dryer areas, as is specifically the case of P. machrisii cactus, or alternatively to the woodlands of shore and river valleys with low soil moisture, as it is the case of C. hildmaniannus. During cold/dry periods, cacti populations could have expanded their distribution, and cactophylic Drosophila probably followed these expansion/retraction cycles (Manfrin and Sene 2006).
The ecological association of D. antonietae with a cactus adapted to a more humid region (river valleys) has resulted in slower vicariant events for both cacti and fly populations, creating conditions that allowed gene flow, as proposed by Monteiro and Sene (1995). Furthermore, geomorphologic data describe at least four major glaciation cycles in the Quaternary period (Ab'Saber 1977a,b), which allowed secondary contacts between populations beyond the slow vicariant event, which helped to maintain the similarity.
Thus, the moderate genetic differentiation observed for D. antonietae could be the result of three different processes, or a combination of them: (1) the introduction of a new genetic variability in populations through gene flow is possibly sometimes occurring or occurred from time to time in the past, homogenizing the total variability in populations; (2) a short period of differentiation passed after the vicariance caused by the xerophytic vegetation retraction, i.e. maintenance of ancestral polymorphism and (3) natural selection is eliminating the new disadvantageous alleles, preventing genetic divergence among populations. Moreover, if gene flow is present in this species, the higher genetic diversity found, compared with non-cactophilic and other cactophilic species, could be due to differential selection of different populations, followed by genetic variability exchange among them. In conclusion, the isoenzyme data from D. antonietae were important to contribute to a better understanding of the evolutionary history of geographically isolated natural populations.
Acknowledgements
FAPESP provided a PhD fellowship for Rogério P. Mateus (Proc. number 97/13822-4 and RTB 1998/1790-3). This work was also supported by CNPq, CAPES, FINEP, FAEPA and USP. We thank Adriana C. Morales for Santiago-RS alloenzyme data, Prof. Eucléia P.B. Contel for use of her allozyme laboratory, Paulo R. Epifânio and Elisabete M.S.B. Beira for technical support, Luciana P. B. Machado, the two anonymous referees and Prof. Dr Diether Sperlich for their useful suggestions.




