Chromosomal diversity in the genus Arvicanthis (Rodentia, Muridae) from East Africa: a taxonomic and phylogenetic evaluation
Diversità cromosomica nel genere Arvicanthis (Rodentia, Muridae) dell'Africa dell'est. Una valutazione tassonomica e sistematica.
Abstract
enIn this paper we discuss the contribution of cytogenetics to the systematics of Arvicanthis in East Africa, by reviewing all the known chromosomal cytotypes of the genus in the area. We also provide G- and C-banding comparisons for two recently described karyotypes, provisionally named ANI-5 (2n = 56, NFa = 62) and ANI-6 (2n = 60, NFa = 72). This, therefore, brings the total number of known cytotypes in this area to 10. Five of these correspond to the species recognized by the latest rodent checklist, i.e. A. nairobae (2n = 62, NFa = 78), A. neumanni (2n = 52–53, NFa = 62), A. blicki (2n = 48, NFa = 62), A. abyssinicus (2n = 62, NFa = 64) and A. niloticus (2n = 62, NFa = 60–62). The taxonomic status of the remaining five cytotypes (A. cf. somalicus, 2n = 62 NFa = 62–63; ANI-5, 2n = 56, NFa = 62; ANI-6/6a 2n = 60, NFa = 72/76; ANI-7, 2n = 56, NFa = 78; and ANI-8, 2n = 44, NF = 72) is discussed. Finally, we reconstruct the phylogenetic relationships among all the known karyotypes on the basis of banding data available for the genus in Africa and show the occurrence of two main clades, each characterized by different types of chromosomal rearrangements. The times of the cladogenetic events, inferred by a molecular clock, indicate that karyotype evolution has accomplished almost all the dichotomic events from the end of the Miocene to the present day. The discovery of a large chromosomal differentiation between populations showing low genetic distances and intrapopulation chromosomal polymorphism suggests that the process of chromosomal differentiation in Arvicanthis is still ongoing and may possibly be responsible for speciation.
Riassunto
frIn questo lavoro viene discusso il contributo che la citogenetica ha fornito alla sistematica del genere Arvicanthis in Africa Orientale. Viene presentata una rassegna di tutti i dati cromosomici fino ad ora conosciuti per il genere nell'area e nuovi dati citogenetici da tre località del Kenya e una località dell'Etiopia. Inoltre, viene fornito il cariotipo bandeggiato G- e C- per due cariotipi descritti recentemente e chiamati provvisoriamente ANI-5 (2n = 56, NFa = 62) e ANI-6 (2n = 60, NFa = 72). Il numero di citotipi totali rinvenuti nell'area è pari a 10. Di questi, cinque corrispondono alle specie presenti nell'ultima revisione, vale a dire A. nairobae (2n = 62, NFa = 78), A. neumanni (2n = 53–53, NFa = 62), A. blicki (2n = 48, NFa = 62), A. abyssinicus (2n = 62, NFa = 64) and A. niloticus (2n = 62, NFa = 60–62). Viene discusso lo status tassonomico dei rimanenti 5 citotipi (A. cf. somalicus, 2n = 62 NFa = 62–63, ANI-5, 2n = 56, NFa = 62; ANI-6/6a 2n = 60, NFa = 72–76; ANI-7, 2n = 56, NFa = 78 and ANI-8, 2n = 44, NF = 72). Inoltre, sono state individuate lievi differenze cromosomiche tra A. niloticus e A. somalicus (2n = 62, NFa = 62–63) (precedentemente messo in sinonimia con A. neumanni). L'albero filogenetico costruito con tutti i cariotipi del genere in Africa mostra la presenza di due cladi principali, ciascuno caratterizzato da differenti tipi di mutazioni cromosomiche. I tempi degli eventi cladogenetici, stimati tramite orologio molecolare, indicano che l'evoluzione cromosomica ha accompagnato quasi tutti gli eventi speciativi dalla fine del Miocene ad oggi. La scoperta di un elevato differenziamento cromosomico tra popolazioni che mostrano una bassa distanza genetica e la presenza di polimorfismo intrapopolazione indicano che il processo di differenziamento cromosomico è ancora in atto e che può essere responsabile degli eventi di speciazione.
A taxonomic overview of the genus Arvicanthis
The correct evaluation of species distribution and their taxonomic status is a fundamental prerequisite in assessing biodiversity in any geographical region. This constitutes the main challenge prior to any conservation planning and/or biodiversity management. For some groups this objective is problematical due to the occurrence of cryptic species and therefore taxonomic uncertainty. In rodents this is common because of the high morphological uniformity compared to important genetic diversity (Taylor 2000; Corti 2001; Granjon and Dobigny 2003). The high frequency of cryptic species occurring in syntopy or in allopatry, makes the classical morphological approach inadequate to determine the entire rodent diversity in many geographical areas. Morphology on its own, therefore, cannot form the basis of future biodiversity studies. In the last 30 years, however, molecular and cytogenetic techniques have greatly improved the taxonomic resolution for many genera (Corti 2001). These studies have shown that species recognition requires a multidisciplinary approach based on cytogenetics (see for example Taylor 2000), the estimate of genetic divergence and DNA taxonomy (Blaxter 2004) and morphometrics (Loy et al. 2004).
The African Arvicanthine rats of genus Arvicanthis Lesson, 1842 represent an emblematic example of this kind of problem. Several species are widely distributed in all the sub-Saharan African savannahs, along the Nile valley and down to the Zambezi. Their opportunistic and generalized diet makes them very common in agricultural fields and particularly in staple crops where they cause serious pre- and post-harvest damage (Stenseth et al. 2003).
The systematics of the genus was recently improved by molecular (Ducroz et al. 1998), cytogenetic (Capanna et al. 1996; Volobouev et al. 2002b; Castiglia et al. 2003) and geometric morphometric investigations (Fadda and Corti 2001), so that now there are seven recognized species in the most recent checklist (Musser and Carleton, 2006) versus the five which had previously been accepted by the same authors (Musser and Carleton 1993).
This increase in species number depends on the studies carried out recently in West Africa. In fact, through cytogenetics and molecular genetics (Volobouev et al. 1987; Civitelli et al. 1995; Ducroz et al. 1997, 1998) four distinct species, chromosomally well differentiated, have been identified which were previously included in the single species A. niloticus (Desmarest, 1822). These karyotypic variants have been reviewed by Volobouev et al. (2002b) and assigned to A. niloticus (2n = 62; autosomal fundamental number, NFa = 62–64; Niger, Chad, Mali, Senegal, Burkina Faso, Mauritania), A. rufinus (Temminck, 1853) (2n = 62; NFa = 74; Benin), A. ansorgei Thomas, 1910 (2n = 62; NFa = 74–76; Burkina Faso, Mali, Senegal). In addition, another unidentified species has been found in the Central African Republic, which has been provisionally named ANI-2 (2n = 58; NFa = 72).
The taxonomic resolution for the East African species is, however, more complex. Musser and Carleton (2006) recognized the occurrence of five species in this region, namely A. nairobae J. A. Allen, 1909, A. neumanni (Matschie, 1894) (A. somalicus in Musser and Carleton 1993), A. blicki Frick, 1914, A. abyssinicus (Rüppell, 1842) and A. niloticus (Table 1).
| Taxon | Type locality |
|---|---|
| A. abyssinicus (Rüppell, 1842) | Ethiopia, Simien Prov., Simien Mtns, Entschetqab |
| A. ansorgei Thomas, 1910 | Bissau Guinea, Gunnal |
| A. blicki Frick, 1914 | Ethiopia, South Chilalo Mtns, Hora Mt base camp (2743 m) |
| A. nairobae J. A. Allen, 1909 | Kenya, Nairobi |
| A. neumanni (Matschie, 1894) | Central Tanzania, Kondoa District, Barungi |
| A. niloticus (Desmarest, 1822) | Egypt |
| A. rufinus (Temminck, 1853) | S Ghana, Elmina |
There are however uncertainties regarding the taxonomy and systematics of this last species in the region: in fact, Yalden et al. (1976, 1996) maintained that an additional species occurs in Ethiopia on the basis of morphological differences, i.e. A. dembeensis (Rüppell, 1842), which in subsequent work was included in A. niloticus on the basis of chromosomal (Corti et al. 1996) and molecular homogeneity (Ducroz et al. 1998). Moreover, Orlov et al. (1992) and Bulatova et al. (2002) showed that under the ‘A. niloticus–dembeensis’ complex there are at least three cryptic taxa in Ethiopia when considering the karyotype and morphology of spermatozoa (Baskevich and Lavrenchenko 1995). Given that, there still remains some taxonomic uncertainty among the A. niloticus–dembeensis complex, we included the two taxa in A. niloticus (following Corti et al. 1996; Volobouev et al. 2002b) while waiting for an eventual clarification from additional molecular and morphological data.
Molecular-based phylogenies have also greatly improved systematics of the genus. Two well-supported clades have been identified (Ducroz et al. 1998): clade 1, with species occurring in East (A. nairobae) Central (ANI-2) and West Africa (A. rufinus and A. ansorgei); and clade 2, including East African species only, i.e. A. niloticus, A. neumanni, A. abyssinicus. The genetic differentiation between these two clades is high and their separation occurred about 5.3 Ma (Ducroz et al. 1998). These two clades are also supported by cytogenetics (Volobouev et al. 2002b): all the members of clade 1 share a reciprocal translocation between chromosomal pairs 4 and 7. This is a kind of chromosomal mutation which usually causes severe impairment during gametogenesis in structural heterozygotes (Koleva and Benova 1992; King 1993).
In Arvicanthis, chromosomal diversification is often accompanied by mtDNA divergence. For example, the sister taxa A. ansorgei/A. rufinus and A. niloticus/A. abyssinicus diverge, respectively, by 8.5–11.1% and 12.8–15.1% in the sequence of the complete cytochrome b (Ducroz et al. 1998). However, inter- and intra-specific values of divergences overlap; for example, ANI-2 and A. rufinus diverge by 5.4–5.7% only, a value that falls within the levels of divergence found in intraspecific and in interspecific comparison (Ducroz et al. 1998; Volobouev et al. 2002a). For this reason both karyotype and molecular genetics analysis are fundamental in delimiting species of Arvicanthis.
Because of the importance of karyotype assessment for both the systematics and taxonomy of the genus, we discuss in this paper all the known karyotype variability from East Africa and provide banding data on the karyotypes from three localities in Kenya and one locality in Ethiopia, two of which are new and have recently been described on the basis of standard techniques only (Corti et al. 2005). Moreover, we attempt to infer phylogenetic relationships among all the known karyotypes of Arvicanthis for which banding data are available. Finally, we discuss the contribution of cytogenetics in the systematics of Arvicanthis in this part of Africa and provide a taxonomical evaluation of the chromosomal cytotype found in the area.
Chromosomal methods and taxonomical evaluation of cytotypes
The new data provided in this paper have been obtained through the analysis of 13 specimens from one locality in Ethiopia and three localities in Kenya (Fig. 1, Table 2). Species identification was based on morphological comparisons (Yalden et al. 1976, 1996; W. Verheyen and J. Hulselmans, personal communication) and our previous chromosomal analysis (Corti et al. 1996; Volobouev et al. 2002b; Castiglia et al. 2003).
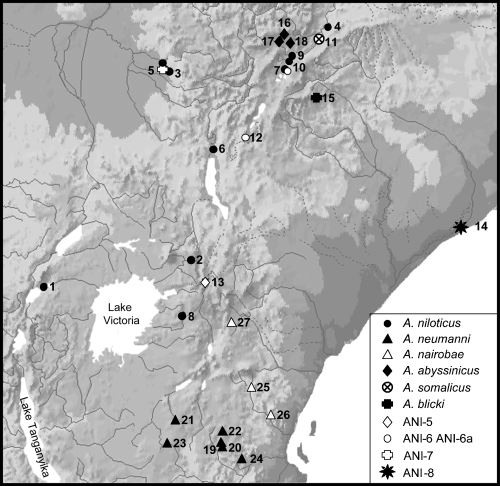
Map showing the locations of the samples for which chromosomal data are available in East Africa. Species names or acronyms are indicated. For locality numbers, see Table 1
| Species | Site | Locality, country | Latitude–longitude | ♂♂ | ♀♀ | 2n | NFa | X | Y | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| A. niloticus | 1 | Kyambara, Uganda | Equator - 29°59′E | 1 | – | 62 | 62 | SM | ? | Ducroz et al. (1998) |
| 2 | Masai Mara, Kenya | 01°01′S–35°19′E | 1 | 1 | 62 | 62 | SM | ? | Ducroz et al. (1998) | |
| 3 | Gambella, Ethiopia | 07°53′N–34°22′E | 53 | 62 | 62 | SM | M | Orlov et al. (1992) | ||
| 4 | Awash Nat. Park, Ethiopia | 09°28′N–40°18′E | 23 | 62 | 62 | SM | – | Orlov et al. (1992) | ||
| 5 | Gambela area, Ethiopia | 07°55′N–34°19′E | – | 1 | 62 | 60 | SM | – | Bulatova et al. (2002) | |
| 5 | Gambela area, Ethiopia | 07°55′N–34°19′E | 123 | 62 | 62 | SM | M | Bulatova et al. (2002) | ||
| 6 | Omo Valley, Ethiopia | 05°00′N–36°07′E | ? | ? | 62 | 62 | ST | ? | Bulatova et al. (2002) | |
| 7 | Zway, Ethiopia | 07°55′N–38°43′E | 5 | – | 62 | 62 | SM | M | Present study | |
| 8 | Kitale, Kenya | 01°01′N–35°00′E | 1 | – | 62 | 62 | SM | M | Present study | |
| 9 | Koka, Ethiopia | 08°24′N–39°01′E | 2 | 1 | 62 | 62 | SM | M | Corti et al. (1996) | |
| 10 | Koka, Ethiopia | 08°13′N–38°55′E | 203 | 62 | 62 | SM | M | Baskevich and Lavrenchenko (2000) | ||
| A. somalicus 1 | 11 | Awash Nat. Park, Ethiopia | 09°00′N–40°10′E | 53 | 62 | 62–63 | SM | M | Baskevich and Lavrenchenko (2000) | |
| A. blicki | 15 | Sanetti Plateau, Bale, Ethiopia | 06°52′N–39°52′E | – | 2 | 48 | 62 | SM | – | Corti et al. (1996), Lavrenchenko et al. (1997) |
| A. abyssinicus | 16 | Sululta, Ethiopia | 09°15′N–38°43′E | 1 | 4 | 62 | 64 | SM-ST | M | Corti et al. (1996) |
| 17 | Managhesha, Ethiopia | 09°00′N–38°35′E | 5 | – | 62 | 64 | SM | M | Corti et al. (1996) | |
| 18 | Ambo, Ethiopia | 08°56′N–38°58′E | 113 | 62 | 64 | Orlov et al. (1992) | ||||
| A. neumanni | 19 | Matongolo, Tanzania | 05°46′S–36°28′E | 7 | 4 | 53–54 | 62 | SM | M | Castiglia et al. (2003) |
| 20 | Zoissa, Tanzania | 05°40′S–36°25′E | 1 | 2 | 53–54 | 62 | SM | M | Castiglia et al. (2003) | |
| 21 | Singida, Tanzania | 04°49′S–34°45′E | 1 | 1 | 54 | 62 | SM | M | Castiglia et al. (2003) | |
| 22 | Ndaleta, Tanzania | 05°14′S–36°29′E | 1 | – | 53 | 62 | SM | M | Castiglia et al. (2003) | |
| 23 | Itigi, Tanzania | 05°41′S–34°28′E | 2 | – | 53–54 | 62 | SM | M | Castiglia et al. (2003) | |
| 24 | Berega, Tanzania | 06°14′S–37°10′E | 1 | – | 54 | 62 | SM | M | Ducroz et al. (1998) | |
| A. nairobae | 25 | Luwami, Tanzania | 03°41′S–37°32′E | – | 2 | 62 | 78 | ST | – | Castiglia et al. (2003) |
| 26 | Manolo, Tanzania | 04°37S–38°13′E | 3 | 2 | 62 | 78 | ST | M | Castiglia et al. (2003) | |
| 27 | Nairobi, Kenya | 01°16′S–36°49′E | – | 1 | 62 | 78 | ST | – | Present study | |
| ANI-5 | 13 | Rongai, Kenya | 00°10′S–35°51′E | 3 | 2 | 56 | 62 | SM | M | Present study |
| ANI-6 | 7 | Zway, Ethiopia | 07°55′N–38°43′E | – | 1 | 60 | 72 | ST | – | Present study |
| ANI-6a | 12 | Gamo Gofa, Konso, Ethiopia | 05°25′N–37°20′E | 123 | 60 | 76 | Orlov et al. (1992) | |||
| ANI-7 | 5 | Gambela area, Ethiopia | 07°55′N–34°19′E | 1 | – | 56 | 78 | A | A | Bulatova et al. (2002) |
| ANI-8 | 14 | Afgoi, Somalia | 02°08′N–45°07′E | – | 1 | 44 | 722 | ? | ? | Capanna and Civitelli (1988) |
- 1These specimens were assigned to A. somalicus by Baskevich and Lavrenchenko (2000) but synonymized with A. neumanni by Musser and Carleton (2006).
- 2Fundamental number (FN).
- 3Sex not indicated.
The chromosomal species/cytotypes that did not correspond to recognized species have been named using the acronym ANI and a number, following Volobouev et al. (2002b) nomenclature for West Africa.
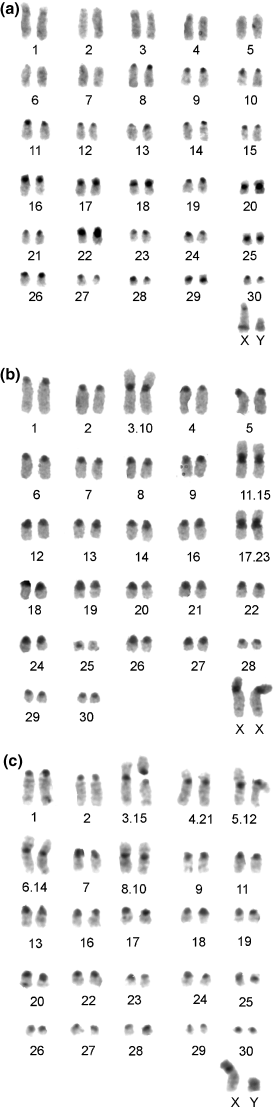
Specimens were transported to the Kenyatta University of Nairobi, Kenya, and the University of Addis Ababa, Ethiopia, for chromosome preparation. Chromosome metaphases were obtained from bone marrow following Hsu and Patton (1969). Cells suspensions in fixative were then transported to the University of Roma ‘La Sapienza’, Italy, where light-microscope preparations were made. These were stained by the Giemsa standard method (pH7). G-bands were enhanced with trypsin following the protocol of Seabright (1971). The heterochromatic portion of the genome was identified by C-banding using barium hydroxide 5% (Sumner 1972). Pictures of metaphases were collected using the Photometrics Sensys 1600 digital camera (Roper Scientific Photometrics, Tucson, AZ). Numeration of chromosomes follows that of A. niloticus, considered the ancestral karyotype of the genus (Volobouev et al. 2002b) (Fig. 2).

Comparison of autosomal G-banding patterns among different taxa of Arvicanthis from East and West Africa. a = A. rufinus; b = A. niloticus; c = A. nairobae; d = A. neumanni; e = ANI-5; f = A. blicki; g = A. abyssinicus; h = ANI-6. Lines include homologous portions. Arrows indicate the location of the centromere in the biarmed chromosomes. Only five chromosomes of ANI-6 are included in the comparison, i.e. chromosomes 1, 3, 4, 5 and the Rb(2.6) showing homology in one arm with chromosome 2 and chromosome 6 for the other arm. Numeration follows the one proposed for A. niloticus by Volobouev et al. (2002a)
All chromosomal data for the East Africa localities were reviewed from literature and our findings. Localities (Table 2) are mapped on the physical map shown in Fig. 1. These include samples from Tanzania, Kenya, Ethiopia, Uganda and Somalia.
Given the importance of chromosomal evolution for the taxonomy of the genus, we propose a taxonomical evaluation of all the undescribed East African Arvicanthis taxa, identified by distinct cytotypes.
First of all we considered chromosomal differences because they are believed to act as a post-mating isolation mechanism which depends on the type and the number of rearrangements involved (reviewed in King 1993). However, this approach has some limitations because a single hybrid individual between cytotypes has yet to be identified in Arvicanthis. For this reason, we take into account the level of chromosomal heterozygosity in putative hybrids between pairs of cytotypes and the associate level of infertility as seen in the literature in other species for the same structural levels of heterozygosity.
We proceeded by matching the above identified cytotypes with the morphological differences discussed elsewhere (Yalden et al. 1976, 1996; Bekele et al. 1993; Fadda and Corti 2001; W. Verheyen and J. Hulselmans, personal communication) and, limiting to Ethiopia, also with the morphology of spermatozoa (Baskevich and Lavrenchenko 1995). Moreover, we took into account the percentage of cytochrome b sequence divergence between pairs of species calculated by Ducroz et al. (1998). For the two new cytotypes here identified, we also report the genetic divergence with their sister taxa as described by Corti (unpublished data) (Table 3).
| Taxon | 2n | nFA | Distribution | Minimum-maximum upper molar row length (mm) | Sperm head2 | Genetic differences from sister taxa (%) |
|---|---|---|---|---|---|---|
| A. abyssinicus | 62 | 64 | Ethiopia | 6.2–7.41 | L 9.4–10.5; W 3.0–3.7; H 4.2–5.5 | – |
| A. cf. somalicus | 62 | 62–63 | Ethiopia, Kenya | 5–5.91 | L 5.6–6.9; W3.5–4.2; H1.7–2.3 | – |
| A. blicki | 48 | 64 | Ethiopia | 7.8–8.91 | – | – |
| ANI-6 | 60 | 72–76 | Ethiopia (two localities) | – | L 9.5–10.6; W 3.5–4.0; H 3.8–4.8 | 6–83 |
| ANI-7 | 56 | 78 | Ethiopia (one locality) | – | L 9.6–10.2; W 2.9–3.9; H 2.2–3.6 | – |
| ANI-8 | 44 | 72 | Somalia, (one locality) | – | – | – |
| A. nairobae | 62 | 78 | Ethiopia, Kenya, Tanzania | 6.0–7.15 (6.6)6 | – | 9–104 |
| A. niloticus | 62 | 60–62 | East and West Africa | 6.2–7.21 (7.2)6 | L 9.4–11; W 3.0–4.0; H 4.0–5.1 | 8–114 |
| A. neumanni | 53–54 | 62 | Tanzania | 5.6–6.55 | – | 10–124 |
| ANI-5 | 56 | 62 | Kenya (one locality) | (6.6–6.9) 6 | – | 23 |
- 1 Yalden et al. (1976).
- 2 Baskevich and Lavrenchenko (1995): L = total length; W = width; H = length of the hook (μm).
- 3Corti (unpublished data).
- 4 Ducroz et al. (1998).
- 5Verheyen and Hulselmans, personal communication: A. nairobae, 70 specimens from Machakos (01.31S-37.16E, Kenya); A. neumanni, 37 specimens from Kujungu (05.25S-37.10E, Tanganica) and Dodoma (06.11S-35.45E, Tanzania).
- 6Specimens analysed cytogenetically in the present study.
In evaluating genetic distance as a taxonomic tool we followed some ideas by Bradley and Baker (2001) who reviewed a set of genetic distances computed from the cytochrome b sequences in four different rodent genera. They found that the average percentage in sequence divergence ranges from 0% to 6.3% at the intraspecific level, and from 2.7% to 19.2% between sister species. These values also match quite well with the values found within and among species of Arvicanthis (Ducroz et al. 1998). However, because of the overlapping divergence estimates between intraspecific and interspecific comparison, one should be cautious when using genetic distances for species identification. Nonetheless, values greater than 10–11% have usually only been found between different species in mammals (Bradley and Baker 2001).
For the taxa identified in this manner we cannot categorically confirm names because the number of forms described in the area is very high (Allen 1939), and it is impossible to give new names to newly detected taxa before comparison with type material, or extensive collections from the type localities.
Chromosomal diversity of Arvicanthis from East Africa
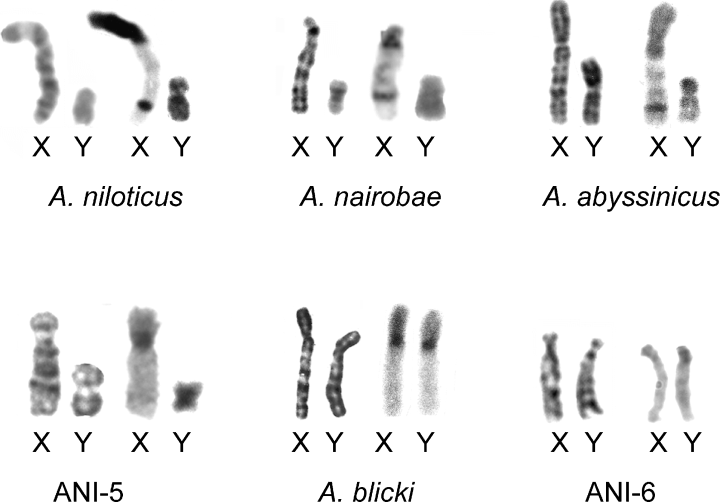
Data from the available literature and present investigation provided a total of 116 specimens from 27 localities (Table 2, Fig. 1). Excluding intra-population polymorphism, a total of 10 different cytotypes are represented. The comparisons of G-banded karyotypes for seven cytotypes is shown in Fig. 2, and a selection of the C-banded karyotype and sex chromosomes is reported in 3, 4.
C banded karyotypes of Arvicanthis: a, A. nairobae; b, ANI-5; c, A. neumanni
Selected sex chromosomes (G bands on the left and C bands on the right) from different taxa of Arvicanthis
Arvicanthis niloticus (Desmarest, 1822)
2n = 62 and NFa = 60–62. This is the most widespread karyotype as it has been found in Uganda, Kenya and Ethiopia in 10 of the 27 localities reviewed (Fig. 1, Table 2). The autosomal set is composed of all-telocentric chromosomes with the exception of a small pair of metacentrics (no. 25) (Fig. 2). This karyotype corresponds to the ones described for both A. niloticus (previously ANI-1; Volobouev et al. 1987) and A. dembeensis (Corti et al. 1996). The karyotype shows a consistent block of heterochromatin at the centromeres of all autosomes (Volobouev et al. 2002b).
The X chromosome has a short arm that is entirely heterochromatic, and the Y chromosome is entirely heterochromatic (Fig. 4). The sex chromosomes show variation in morphology: a submetacentric X chromosome has been found in several localities of Ethiopia and Kenya while Bulatova et al. (2002) found a subtelocentric configuration in a locality of the Omo Valley. Variation in the autosomal set was also found by Bulatova et al. (2002) in the Gambela area (Ethiopia), where the karyotype lacks the small metacentric pair which characterizes all the species of the genus (2n = 62; NFa = 60) (Table 2).
Arvicanthis cf. somalicus Thomas, 1903
2n = 62 and NFa = 62–63. Baskevich and Lavrenchenko (2000) found a karyotype very close to the one described for A. niloticus in specimens from the Awash National Park, Ethiopia, they identified as A. somalicus. They have also shown the presence of a small addition of heterochromatin in two pairs of autosomes. Notwithstanding the similarity of this karyotype with that of A. niloticus, an incorrect assignment of this specimens is unlikely because A. somalicus is a small species and cannot be easily mistaken for larger A. niloticus in the area. Moreover, the spermatozoon of these specimens is the most distinctive among Arvicanthis for the presence of a very short sperm head (Table 3), and this was considered sufficient to be considered as a different species from the other surrounding 2n = 62 ones (Baskevich and Lavrenchenko 1995).
Arvicanthis. cf. somalicus has been synonymized with A. neumanni by Musser and Carleton (2006). However, we follow, with caution, the interpretation of Baskevich and Lavrenchenko (2000) because the two differ in karyotype and in the shape of the skull (see below). No G-banding or molecular work has been performed on these specimens for a definitive identification.
A. abyssinicus (Rüppel, 1842)
2n = 62, NFa = 64. The karyotype of this species is known from three localities of Ethiopia (Orlov et al. 1992; Corti et al. 1996). The karyotype differs from that of A. niloticus due to a pericentric inversion in chromosome pair number 4 (Corti et al. 1996; Fig. 2). C-banding shows conspicuous blocks of heterochromatin at the centromeres of all the chromosomes, at the short arm of the X chromosome and in all the Y chromosome (shown in Corti et al. 1996). A different X chromosome has been found in heterozygous condition showing a smaller short arm (Corti et al. 1996, Fig. 4). The species is endemic to the highlands of Ethiopia where it occurs at altitudes above 1600 m (Yalden et al. 1976).
A. blicki Frick, 1914
2n = 48, NFa = 64. The karyotype of this species is known from two specimens only (Corti et al. 1996; Lavrenchenko et al. 1997). The karyotype is characterized by a reduction in diploid number following seven Robertsonian (Rb) fusions. Given that these fusions are described with a different numeration (Corti et al. 1996; Volobouev et al. 2002b), we present here the G-banded chromosomes where the numeration follows the standard of A. niloticus, following Volobouev et al. (2002b): Rb(9.15), Rb(10.19), Rb(11.16), Rb(12.17), Rb(13.22), Rb(21.26) and Rb(24.27) (Fig. 2). The C-banding shows faint spots of heterochromatin at the centromeres of all autosomes and heterochromatin is also present on the short arm of X chromosome (Corti et al. 1996).
This species is endemic to Ethiopia and confined to the moorlands of the Eastern plateau (Bale mts) above 3000 m (Yalden et al. 1976).
A. nairobae J. A. Allen 1909
2n = 62, NFa = 78. The karyotype of this species has been described from individuals caught in two localities in Tanzania (Castiglia et al. 2003). The karyotype for the type locality of the species (Nairobi, Kenya) is reported here. The autosomal set is composed of nine pairs of biarmed autosomes and 21 pairs of telocentric chromosomes which decrease in size (Fig. 2). The C-banding pattern (Fig. 3) shows an absence of heterochromatin in the two largest pairs of biarmed chromosomes (nos 1 and 3) and in five pairs of large acrocentrics. All the other chromosomes have centromeric heterochromatin. In three pairs of medium-sized submetacentrics (nos 16, 17, 22), the heterochromatic blocks entirely involve the short arms, while in the other pair (no. 18), heterochromatin is apparently limited to the centromeric area. The X chromosome is a large subtelocentric and the Y chromosome is a small subtelocentric (Fig. 4). The short arm and the proximal portion of the long arm of the X chromosome are entirely heterochromatic. The Y chromosome is entirely heterochromatic.
Comparison of the G banding pattern led to the recognition of rearrangements relative to the karyotype of A. niloticus, i.e. pericentric inversions in pairs 1, 3, 18, 20 and 29; heterochromatin additions on pairs 16 and 17 and 22 (Fig. 2) and a reciprocal translocation between chromosomes 7 and 4.
The southern and northern limits of A. nairobae are still unclear (Musser and Carleton, 2006). We found one limit in the South of Tanzania (08°15′S–34°E) at the border between the Somali-Maasai and Zambezian domains. Bulatova et al. (2002) reported it around the lower Omo Valley in Ethiopia, but with a 2n = 62, NFa = 62 that does not correspond to what has been found by us for the species. However, the authors consider the specimens analysed as A. nairobae only on the basis of the geographical position. And no morphological or other genetical analysis support it belongs to A. nairobae. Therefore, here we discuss their data in a A. niloticus framework (Table 2).
A. neumanni (Maschie, 1894)
2n = 53–54, NFa = 62. The karyotype of this species shows a widespread Rb polymorphism (Castiglia et al. 2003). The chromosomal set of the 2n = 54 specimens is composed of five pairs of biarmed chromosomes and 21 pairs of telocentric chromosomes. The reduction in diploid number of the 2n = 53 specimens is due to additional Robertsonian fusions between two telocentric chromosomes of the 2n = 54 karyotype. C-positive heterochromatic blocks occur at a paracentromeric position on all the autosomes, either biarmed or uniarmed (Fig. 3). The X chromosome is a large submetacentric and the Y is a short metacentric, which however, is larger than the smallest metacentric autosomal pair. The short arm of the X chromosome is entirely heterochromatic together with the proximal portion of the long arm. The Y chromosome is entirely heterochromatic (Fig. 4).
The arms forming the large metacentrics of A. neumanni correspond to 12 pairs of telocentrics of the A. niloticus karyotype after Robertsonian fusion: Rb(3.15), Rb(4.21), Rb(5.12), Rb(6.14), Rb(8.10) and Rb(11.20) (Fig. 2). The polymorphic Rb chromosomes are Rb(3.15) (found in four of the five localities examined) and Rb(11.20) found in a single specimen. The range of this species should be confined to Tanzania but its limits are still uncertain (see after).
ANI-5 (present study)
2n = 56, NFa = 62. This karyotype, described for the first time by standard coloration only in Corti et al. (2005) is found in the locality of Rongai, Kenya. Here we report the G and C banded karyotype (1, 2; Table 1). The karyotype is composed of 23 pairs of telocentric and four pairs of meta-submetacentric chromosomes, one of which is particularly small. C-bands show the presence of a large amount of heterochromatin in the paracentromeric areas of all the autosomes (Fig. 3). The X chromosome is a large submetacentric and the Y is a medium-sized metacentric. The short arm of the X chromosomes is entirely heterochromatic, even if its telomeric portion is less pronounced. The Y chromosome is entirely heterochromatic (Fig. 4).
The different chromosomal arms can be correctly identified by G-banding (Fig. 2) following Volobouev et al. (2002b). The three largest pairs of metacentrics are due to Rb fusion of telocentric chromosomes identified in A. niloticus. These are as follows: Rb(3.10), Rb(11.15) and Rb(17.23).
ANI-6 (present study), ANI-6a (Orlov et al. 1992)
2n = 60 NFa = 72/76. The ANI-6 karyotype (2n = 60; NFa = 72) has been described for the first time in Corti et al. (2005), and has been found in only one female from Zway, Ethiopia (Fig. 1, Table 1). It is composed of one pair of large metacentrics, three pairs of medium/large submeta-metacentrics and of three pairs of small metacentrics. The X chromosome is a large subtelocentric with a heterochromatic short arm (Fig. 4). C-banding revealed little heterochromatin in the autosomes as only three pairs of small metacentrics had centromeric heterochromatic blocks. No heterochromatin occurred in the three pairs of large biarmed chromosomes (not shown). The G-banding pattern of this karyotype were not as clear as those of other karyograms presented here. In view of this, and the possibility that this specimen may be the only representative of a so far undescribed species, only the banded chromosomes labelled as ‘h’ in Fig. 2 are presented. Five chromosomal rearrangements relative to A. niloticus can be recognized. These are: Rb(2.6), an inversion in pairs 1 and 3 and a reciprocal translocation between pairs 7 and 4. Another four pairs of biarmed chromosomes occurred in this karyotype indicating the presence of other unidentified rearrangements compared with A. niloticus. A similar karyotype (named here as ANI-6a; 2n = 60 NFa = 76) was found by Orlov et al. (1992) who described it by standard staining. Differences between ANI-6 and ANI-6a probably consist in an addition of heterochromatin or inversion in two autosomal pairs (Orlov et al. 1992).
ANI-7 (Bulatova et al. 2002)
2n = 56, NFa = 78. This karyotype has been reported by Bulatova et al. (2002) in the Gambela area (Ethiopia). It is characterized by two pairs of large metacentrics and 10 pairs of sub- and small metacentrics. The remaining pairs of chromosomes are telocentrics. Some of these biarmed chromosomes show heterochromatic addition to the small arms. The X and Y chromosomes are telocentrics. Due to the lack of G-banding, it was impossible to ascertain chromosomal homologies between this karyotype and the others known for the genus.
Bulatova et al. (2002) did not attribute these samples to any known taxon.
ANI-8 (Capanna and Civitelli 1988)
2n = 44, Fn = 72. This karyotype was described on the basis of a single female from Afgoi, Somalia (Capanna and Civitelli 1988) (Fig. 1, Table 1). This specimen on the basis of linear measurement could be recognized as A. niloticus (see later). However, it is characterized by a strong reduction in diploid number that allows the allocation to a different taxon. The X chromosome could not be positively identified. Should the X chromosome be biarmed the NFa would be 68. This karyotype suggests the occurrence of at least eight Rb fusions, and four rearrangements which can be inversions or heterochromatin additions/deletions.
Phylogenetic relationships
Chromosomal rearrangements have been used as characters to hypothesize phylogenetic relationships among cytotypes. In this analysis we added the ANI-5 and ANI-6 and A. nairobae karyotypes to the seven used by Volobouev et al. (2002a) to infer phylogenetic relationships. As an outgroup, the karyotype of Lemniscomys zebra was used as it shows a complete banding homology with the chromosomes of Arvicanthis (Castiglia et al. 2003). Therefore, it is suitable to identify the direction of chromosomal mutations. We identified 36 chromosomal rearrangements (Fig. 2) that were coded in this analysis following Dobigny et al. (2004). The matrix of chromosomal characters (Table 4) was analysed by maximum parsimony using the heuristic search option in PAUP 4.0 (Swofford 1998).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. niloticus (ANI-1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | e |
| A. niloticus (ANI-1b) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | e |
| ANI-2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | e |
| A. ansorgei | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | e |
| A. rufinus | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | e,c |
| ANI-6 | 0 | 0 | 1 | 1 | 1 | ? | 0 | ? | ? | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | f |
| A. neumanni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | b |
| A. abyssinicus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | d |
| A. nairobae | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | b |
| A. blicki | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | d |
| ANI-5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | f |
| Lemniscomys zebra | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | a |
- The chromosomal transformations are coded as follows: 1, inv(30); 2, Rb[1; t rcp(4;7)]; 3, Rb(2;6); 4, inv (3); 5, t rcp(4.7); 6, inv(20); 7, h add/del(22); 8, inv(28); 9, inv(29); 10, inv (1); 11, in(2); 12 h add/del (17); 13 h add/del(1); 14 h add/del(2); 15, Rb(3;15); 16, Rb(4;21); 17, Rb(5;12); 18, Rb(6;14); 19, Rb(8;10); 20, Rb(11;20); 21, inv(4); 22, h add/del(16); 23, inv(18); 24, Rb(9;15); 25, Rb(10.19); 26, Rb(11.16); 27, Rb(12;17); 28, tan fus/fis (1.20); 29, tan fus/fis (6.24); 30 tan fus/fis (7.11); 31, Rb(30.29); 32, Rb(3.10); 33, Rb(11.15); 34, Rb(17.23); 35, Rb(13;22). 36, Rb(24;27). Inv = pericentric inversion; Rb = Robertsonian fusion; t rcp = reciprocal translocation; h add/del = heterochromatic addition/deletion; tan fus/fis = tandem fusion-fission. References: a, Castiglia et al. 2002; b, Castiglia et al. 2003; c, Civitelli et al. 1995; d, Corti et al. 1996; e, Volobouev et al. 2002a; f, present study.
Ten informative characters were found. The most parsimonious tree found is shown in Fig. 5 (tree length = 39; consistency index = 0.92, and excluding uninformative characters = 0.77). Two major clades occur, corresponding to clades 1 and 2 as identified by Ducroz et al. (1998) through molecular phylogenetics.
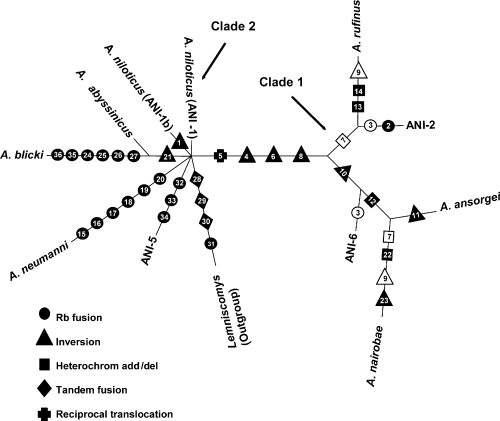
Most parsimonious tree based on chromosomal rearrangements in Arvicanthis. The tree has been outgroup rooted on Lemniscomys zebra. Character coding and rearrangement numbering is represented in Table 2. The different kinds of chromosomal rearrangements are represented in full symbols, homoplasies in open symbols
Clade 1 includes A. ansorgei, A. rufinus, A. nairobae, ANI-2 and ANI-6; A. nairobae clusters with A. ansorgei, while ANI-2 with A. rufinus. ANI-6 has a basal position relative to A. ansorgei and A. nairobae. Clade 2 comprises A. niloticus, A. neumanni, A. abyssinicus, A. blicki, and ANI-5. In this clade only A. blicki and A. abyssinicus cluster together, the other cytotypes having a bush-like unresolved relationship according to chromosomal characters.
Three homoplasies occur in clade 1 and none in clade 2 (Fig. 5): the first is due to Rb(2.6), occurring independently in ANI-2 and ANI-6; the second is the independent occurrence of a small heterochromatic arm in chromosome 22 of A. nairobae and the common ancestor of A. rufinus and ANI-2; the third is the independent occurrence of inversion on small autosome number 29 in A. nairobae and A. rufinus.
Given the generally recognized polymorphism of heterochromatic regions, especially short arms, and the repetitive nature of heterochromatin, such a character is likely to be a source of homoplasy. Moreover, we cannot ignore the possibility of independent Rb fusion and pericentric inversion, in such a highly chromosomally diversified genus.
The chromosomal phylogenetic hypothesis illustrated in Fig. 5 shows how the two clades are characterized by different types of chromosomal rearrangements. In clade 2, Rb fusions appear repeatedly in different lines and predominate over any other kind of chromosomal rearrangement. There are no shared Rb fusions among lines of this clade, and several cases of monobrachial homology occur (Table 4). This strongly suggests a cytotype radiation from an ‘all telocentric’ condition. On the other hand, inversions, additions of heterochromatic small arms and Rb fusions characterize chromosomal evolution within clade 1.
The differences in the type of chromosomal mutation between the two clades could depend on the different molecular architecture of centromeric heterochromatin involved in various types of chromosomal mutations (Garagna et al. 1995; Redi et al. 2001). All the karyotypes of clade 2, with the exception of A. blicki, show conspicuous blocks of heterochromatin at the centromere (Corti et al. 1996; this study), but this is more variable and does not constitute a common pattern for those of clade 1. It is impossible, at present, to determine whether this reflects differences at the molecular level.
Redi et al. (2001) reviewed the role of heterochromatin as a molecular drive for structural rearrangements of the karyotype such as during the Robertsonian formation of metacentrics (Garagna et al. 1995, 2001). Heterochromatin transfer among homologous and non-homologous chromosomes with repetitive DNA intragenomic movements suggests that repetitive DNA families may have been involved at least in Robertsonian processes (see Garagna et al. 1995 and Hamilton et al. 1990 for rodents; Wichman et al. 1991 for equids) and might be related to speciation.
Intraspecific sex chromosome polymorphism due to heterochromatic addition or deletion has been found in many species of the genus: A. niloticus (Bulatova et al. 2002; Volobouev et al. 2002b), A. abyssinicus (Corti et al. 1996) and A. rufinus (Civitelli et al. 1995); thus, this variation in sex chromosomes cannot be easily used as a taxonomic marker. Nonetheless, we cannot completely exclude a functional consequence of these chromosomal rearrangements. As a matter of fact, they may have some effect in the pairing between X and Y chromosomes (Stitou et al. 2000). However, other functional roles may be possible. For example, in populations of A. rufinus from Benin, the occurrence of different Xs significantly affects the genome size in the specimens, thus may affect the metabolic properties at the cellular level (Garagna et al. 1999).
Implications of chromosomal variation for the taxonomy of East African Arvicanthis
Five of the identified cytotypes correspond to the species reported by Musser and Carleton (2006), i.e. A. nairobae, A. neumanni, A. blicki, A. abyssinicus and A. niloticus. All these cytotypes are distinguishable by their own chromosomal (Corti et al. 1996; Castiglia et al. 2003), genetic (Capula et al. 1997; Ducroz et al. 1998) and morphological (Yalden et al. 1976, 1996; Baskevich and Lavrenchenko 1995; Corti and Fadda 1996; Fadda and Corti 1998) differences (Table 3). The taxonomic status of A. cf. somalicus and of the cytotypes named ANI-5, ANI-6/6a, ANI-7 and ANI-8 merits a more thorough discussion.
Musser and Carleton (2006) synonymized A. somalicus with A. neumanni from Tanzania. We prefer here to retain the taxon name somalicus because it presents some important differences. First, the two taxa show a different karyotype, where A. neumanni has four to six additional Rb fusions in Tanzania. Putative F1 hybrids between these two taxa might carry from four to six trivalents at meiotic diakinesis. This meiotic configuration usually implies high levels of ipofertility in house mouse hybrids (Garagna et al. 1990; Wallace et al. 2002). Moreover, Fadda and Corti (2001) found a different skull shape in the Tanzanian sample compared with the Kenyan, Somalian and Ethiopian samples. Only molecular analysis or breeding data may help to understand if A. somalicus from Ethiopia is a chromosomal form of A. neumanni or that it represents a distinct species. If the Tanzanian population is a different species, A. neumanni would be the correct name for it, and A. somalicus would extend its range from Kenya to Somalia, south Ethiopia and south-east Sudan (Musser and Carleton, 2006).
The cytotype ANI-6 from Ethiopia (2n = 60 NFa = 72, Fig. 1, Table 1) is very similar to the one described by Orlov et al. (1992) (standard techniques only; ANI-6a, 2n = 60, NFa = 76) in the Gambela region, which is approximately 300 km from Zway. The authors, when considering the external morphology, decided these specimens are similar to A. niloticus, although they differ slightly in the morphology of the spermatozoa (Baskevich and Lavrenchenko 1995) (Table 3). The G- banding pattern here presented clearly places ANI-6 within clade 1 and not in clade 2 as A. niloticus, as there is the same reciprocal translocation between chromosomes 4 and 7 characterizing this clade (see introduction). For this reason here we confirmed the high chromosomal divergence between ANI-6 and A. niloticus. Moreover, the analysis of the sequences of the entire cytochrome b of these specimens shows that the closer representative of this clade is A. ansorgei (from Senegal and Burkina Faso), which diverged by 6–8% (Corti, unpublished data), a moderately high level of divergence in a sequence of cytochrome b. Recognized differences with the karyotype of A. ansorgei include one Rb fusion and one inversion. However, ANI-6 shows another six unidentified pairs of biarmed chromosomes. Due to this uncertainness in the interpretation of karyotypes, it is difficult to evaluate the effect of the observed chromosomal differences on fertility of putative hybrids. Therefore, additional data are required to taxonomically evaluate this taxon correctly.
Orlov et al. (1992) recognized the large metacentric of ANI-6a as Rb(1.7), but from their picture no categorical identification of telocentrics involved in the fusion is possible. Therefore, it cannot be excluded that the chromosome recognized as Rb(1.7) is, in fact, Rb(2.6). Were this to be the case, then differences between ANI-6 and ANI-6a would be minimal and probably represented by an inversion or addition of heterochromatic material, showing evidence of chromosomal variation within this undescribed taxon. This same Rb(2.6) chromosome is also shared by ANI-2 from the Central African Republic (Table 2). However, in our phylogenetic tree (Fig. 5) it has been hypothesized that its occurrence in these two cytotypes represents an independent event, although a less parsimonious solution could also depict the ‘true’ phylogeny.
No G-banding, morphological and molecular data are available for the cytotypes named ANI-7 and this makes it even more difficult to establish its taxonomic status. The presence of large metacentrics in ANI-7 could relate this cytotype to ANI-6, but the sperm head of these specimens is rather distinctive with a short hook and this may indicate specific distinction (Baskevich and Lavrenchenko 1995, Table 3). Another possible relative cytotype is ANI-2 from the Central African Republic, also characterized by large metacentrics. At the moment the relationship between this cytotype and the eastern and central relatives is unclear. However, it seems reasonable to suppose that ANI-7 represents an additional species for the East African Arvicanthis fauna.
ANI-5 from Rongai differs from all other known karyotypes. Chromosomes 4 and 7 are present in the ancestral state (i.e. they are not involved in reciprocal translocation) and this makes it possible to place this species within the group of the strictly East African species, together with A. niloticus, A. abyssinicus, A. blicki and A. neumanni (clade 2, following Ducroz et al. 1998). Its diploid number and NFa resemble the karyotype of A. neumanni (2n = 53–54; NFa = 62; Castiglia et al. 2003). However, the G-banding revealed several differences. As a matter of fact, there are no metacentrics shared with other cytotypes and, among these, Rb(3.15) of A. neumanni shows a monobrachial homology with metacentrics Rb(3.10) and Rb(11.15) of ANI-5. The metacentrics of ANI-5 are likely to have arisen directly from an ancestral ‘all-telocentrics’ karyotype as for A. niloticus (see later). The length of the upper molar row, a good indicator of dimension in these specimens (6.6–6.9) falls within the range known for A. niloticus (6.2–7.2) (Table 3).
The analysis of the sequences of the entire cytochrome b of these specimens showed a 2.5% divergence from the 2n = 62 NFA = 62 A. niloticus from Kitale (Corti, unpublished data). This value falls within the range of intraspecific variation. The low distance between ANI-5 and A. niloticus is considerable, and suggest a fast chromosomal evolution in these species.
In putative hybrids with A. niloticus the three Rb fusions in a heterozygous condition would not severely impair gametogenesis and theoretically would not represent a strong post-zygotic isolation barrier, as occurs in other rodent species (see for example, Castiglia and Capanna 2000). However, only through the analysis of structural heterozygotes from an eventual hybrid zone between ANI-5 and A. niloticus (the distance between Rongai and Kitale is about 160 km) may assess the taxonomic status of this cytotype that easily represent a chromosomal race within A. niloticus.
An extreme reduction in diploid number was found by Capanna and Civitelli (1988) in one female from Somalia (ANI-8; 2n = 44). In Somalia two morphospecies of Arvicanthis occur, a large one identified as A. niloticus and a small one identified as A. somalicus. The specimen analysed by Capanna and Civitelli clearly falls in the first group based on the large size of the body and the skull. Chromosomal differences relative to the 2n = 62 A. niloticus include eight fusions and other unidentified rearrangements. Putative hybrids between these specimens and A. niloticus should have a severe impairment at gametogenesis leading to complete sterility in some cases (Gropp et al. 1982; Hauffe and Searle 1998; Castiglia and Capanna 2000). For this reason we follow Capanna and Civitelli (1988) suggesting that this specimen may represent a different unidentified species.
Times of chromosomal evolution
It is believed that the diversification of African mammal species has been caused by cycles of disruption and coalescence among biomes from the Pliocene onwards (Grubb 1999). Chromosomal phylogeny in Arvicanthis, however, suggests an intricate relationship pattern between geography and phylogeny, with members of the different clades coexisting in the same area, even in strict syntopy (for example see the locality of Zway and the Gambela region in Ethiopia; Fig. 1, Table 2). Moreover, current knowledge regarding the real ranges of these chromosomal species is still too scarce so that limited hypotheses on the biogeographical causes of variation are possible.
The richness of species and karyotypes in this part of Africa is higher relative to West Africa, with members of both clades occurring. This reinforces the view that savannas and highlands surrounding the Rift Valley represent the main, if not the original, centre of diversification of the genus, although, for the moment, no older fossils than the Omo Valley specimen (3 Myr; Wesselman 1984) have been found.
Based upon complete mitochondrial DNA cytochrome b sequences (Ducroz et al. 1998), the deepest divergence time estimated within Arvicanthis is 5.3 Myr (end of the Miocene), during which, therefore, the split between clades 1 and 2 occurred. The within-clade diversification is more recent and the causes of chromosomal diversification and cladogenesis are plausibly related to the geological and climatic events that characterized East Africa during the late Pliocene and early Pleistocene (Ducroz et al. 1998). As a matter of fact, Arvicanthis occurs in all open habitats, from the very arid conditions of Somalia to the moister Guinean region, and from sea level to the altitudes of the Bale massif, showing patterns of adaptation to these very diverse conditions (Fadda and Corti 2001).
Molecular phylogenetics support an earlier divergence of the lines of clade 1, as well as an overall lower rate of chromosomal evolution with respect to the lineages forming clade 2, where chromosomal radiation occurred in more recent times (Ducroz et al. 1998). The discovery of chromosomal differences between A. niloticus and ANI-5 (with three fixed Rb metacentrics) coupled with low genetic distances between taxa indicates that the process of differentiation in Arvicanthis may be much more recent.
Furthermore, the widespread Rb polymorphism in A. neumanni from Tanzania (Castiglia et al. 2003) indicates that factors other than geographical isolation may be driving current chromosomal evolution in the genus. It has been suggested that a new chromosomal mutation could spread and become fixed without any form of geographical isolation; this can be due to population structure and/or some kind of superiority of the new chromosomal variant (Pardo-Manuel de Villena and Sapienza 2001; Ratkiewicz et al. 2002).
Concluding remarks
Ten different cytotypes have been found in East African Arvicanthis, against the five species listed by Musser and Carleton in the area. Concerning the remaining five cytotypes, four (A. cf. somalicus, ANI-6, ANI-7, ANI-8) show a level of chromosomal, genetic and morphological diversification that could suggest a specific status; one of the cytotypes (ANI-5) is likely to be a chromosomal race because of the low divergence in mtDNA sequences compared with A. niloticus. This diversity must still be regarded as an underestimate of the real species number as few samples have been studied compared with the suitable areas for the genus in this part of Africa. This taxonomical complexity has, without doubt, an impact on applied studies such as ecology or parasitology. The syntopy of cryptic species occurring in two localities (Fig. 1, Table 1) shows the need to investigate undiscovered areas and the ecology of different species. Nonetheless, karyotype analysis is essential to determine the entire diversity in the genus Arvicanthis, given that populations with low molecular divergence show different karyotypes. Therefore, this must also be evaluated to establish a correct taxonomy.
Acknowledgements
This paper is dedicated to Walter Verheyen, recently deceased, who dedicated his scientific life to the taxonomy of African rodents. We express our gratitude to those who helped us in this research in Ethiopia (Goitom Redda), Tanzania (A.W. Massawe, L. Mulungu, G. Mgode K. Kessy, T. Protas), Kenya (D. Ojwang, B. Agwanda) and Italy (E. Capanna, C. Fadda). Thanks are extended to W. Verheyen and J. Hulselmans for providing original information on the length of upper molar row of Tanzanian Arvicanthis and to G. Contrafatto and an anonymous referee for helpful comments and suggestions. This work was supported by grants ‘Programmi d'Ateneo 60%’, ‘MURST 40%’, ‘STAPLERAT: Protecting staple crops in eastern Africa: integrated approaches for ecologically based field rodent pest management’ (European Union) (M.C.).




