International Dissemination of a High Virulence Rabbit Staphylococcus aureus Clone
Summary
High virulence rabbit Staphylococcus aureus strains cause chronic and spreading problems of mastitis, pododermatitis and subcutaneous abscesses on rabbit flock level, whereas infections with low virulence strains are limited to individual rabbits. In the present report, 13 high virulence rabbit S. aureus strains, selected out of a large collection of strains isolated in five European countries between 1983 and 2004, were genotyped using pulsed-field gel electrophoresis, spa typing, multilocus sequence typing (MLST) and accessory gene regulator (agr) group typing. Two low virulence rabbit S. aureus strains were also included in the study. The results indicate the clonal origin of high virulence rabbit S. aureus strains present in Europe. Furthermore, the results of MLST and spa typing form a basis for international epidemiology of rabbit S. aureus strains, as these DNA sequence-based typing techniques can easily be used for intercentre comparisons.
Introduction
In rabbits, Staphylococcus aureus infections lead to lesions of pododermatitis, subcutaneous abscesses and mastitis (Hermans et al., 2003). Suckling young may suffer from a pustular dermatitis and often die as a result of agalactia in mastitic does. Sporadically, abscesses in lungs, liver and uterus are observed as well. On the level of the individual animal, clinical signs are the same, no matter what S. aureus strain is involved. However, at rabbit flock level, two types of S. aureus infections can be distinguished. The first type is caused by so-called low virulence (LV) strains and is limited to individual animals, thus causing minimal economic losses. The second type of infection, caused by high virulence (HV) strains, leads to chronic problems as the infection spreads throughout the rabbitry.
Up to now, the in vitro virulence classification of rabbit S. aureus strains is performed through a combination of biotyping as described by Devriese (1984) and phage typing using bacteriophages of the international typing set for human S. aureus strains (Parker, 1962). Classical HV strains belong to the biotype ‘mixed CV-C’ and are sensitive to phages 3A/3C/55/71 of phage group II, suggesting a clonal origin (Hermans et al., 1999). Recently, however, a change occurred in this situation: next to the classic HV strains, an increasing number of aberrant strains are isolated from rabbitries facing chronic problems. These strains still belong to the biotype ‘mixed CV-C’ and still are sensitive to the phages of phage group II, but to various groups of other phages as well (Vancraeynest et al., 2004a). In 1995, an atypical isolate showing biotype ‘mixed CV-C’ and sensitive to phages 29/79/42E/92/D11/HK2, not belonging to phage group II, also caused an epidemic spread (Devriese et al., 1996).
Phage typing has been used for over 40 years to discriminate outbreak related S. aureus strains. Unfortunately, the technique has shown poor reproducibility and it does not type all isolates (Bannerman et al., 1995). Biotyping of S. aureus isolates also has some flaws: results can be discordant because of differences in test conditions, lack of standardized reagents and problematic interpretation of crystal violet tests (Hennekinne et al., 2003). However, up to recently, the combination of biotyping and phage typing proved very useful for the characterization of rabbit S. aureus isolates. The above mentioned recent changes in the phage types of HV strains altered this situation.
The growing importance of methicillin-resistant S. aureus has led to a considerable epidemiological interest in the tracking of strains to obtain a picture of the distribution of particular strains in the population and the dynamics of clonal spread (Crisostomo et al., 2001). Molecular typing methods such as pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST), spa typing and accessory gene regulator (agr) typing have pushed phage typing aside in identifying and monitoring the local and international spread of S. aureus outbreak strains (Struelens et al., 1992; Bannerman et al., 1995; Shopsin et al., 1999; Enright et al., 2000, 2002; Gilot et al., 2002; Harmsen et al., 2003).
The aim of the present study was to determine the genetic relatedness and the suggested clonality of HV rabbit S. aureus strains, isolated from different European countries over a considerable temporal scale, by characterizing them on a molecular level with PFGE, MLST, spa typing and agr typing methods.
Materials and Methods
Bacterial strains
The study focused on 15 rabbit S. aureus strains, which were selected out of a large collection on the basis of their respective phage types and which were isolated from rabbitries in five European countries: Belgium (n = 9), France (n = 3), Greece (n = 1), Italy (n = 1) and Spain (n = 1). There was no direct or indirect contact between these rabbitries. Strain PI 41/95, although isolated in Belgium, originated from rabbits imported from the Czech Republic (Devriese et al., 1996). The strains were isolated between 1983 and 2004 (Table 1). This collection included 13 HV strains, which were all isolated on commercial rabbitries facing chronic problems of staphylococcosis. Six of them belonged to biotype mixed CV-C and were sensitive to phages 3A/3C/55/71 of phage group II. They are further referred to as classical HV strains. Six other HV strains belonged to biotype mixed CV-C and were sensitive to phages of phage group II, but to various other phages as well. These six strains are further referred to as aberrant HV strains. One HV strain, not sensitive to phages of phage group II (biotype–phage type combination ‘mixed CV-C’–29/79/42E/92/D11/HK2) was also included in the study, and is further referred to as atypical HV strain. The two LV strains were isolated from Belgian rabbitries without S. aureus problems and belonged to other biotypes and phage types (Table 1).
| Strain | Virulence level | Biotype–phage type combination | Country of origin | Year of isolation |
|---|---|---|---|---|
| 266/3572 | High | Mixed CV-C–3A/3C/55/71 | Belgium | 1983 |
| VL 1 | High | Mixed CV-C–3A/3C/55/71 | Belgium | 1998 |
| KH 454 | High | Mixed CV-C–3A/3C/55/71 | Belgium | 1998 |
| DV 42 | High | Mixed CV-C–3A/3C/55/71 | Spain | 2004 |
| DV 58 | High | Mixed CV-C–3C/55/71 | Belgium | 2004 |
| DV 91 | High | Mixed CV-C–3A/3C/55/71 | Greece | 2004 |
| KH 119 | High | Mixed CV-C–29/3A/3C/55/71/6/42E/47/54/75/84/94 | Belgium | 1997 |
| KH 522 | High | Mixed CV-C–29/52/52A/80/3A/3C/55/71/6/42E/47/53/54/75/84 | Belgium | 1998 |
| DV 3 | High | Mixed CV-C–29/52/52A/80/3C/55/71/6/42E/47/54/75/83A/84/81/94/95 | France | 2002 |
| DV 18 | High | Mixed CV-C–29/80/3A+/3C/55/71/6/42E/47/54/75/83A/84/81/94/+ | France | 2002 |
| Ref 49 | High | Mixed CV-C–29/52+/52A±/80/3A+/3C/55/71/6/42E/47/54/75/83A/84/81/94/+ | France | 2003 |
| DV 64 | High | Mixed CV-C–29/3C/71/6±/42E/47/54/83A/94 | Italy | 2004 |
| PI 41/95 | High | Mixed CV-C–29/79/42E/92/D11/HK2 | Belgium | 1995 |
| KH 28 | Low | Mixed CV-A–29/79/85/95 | Belgium | 1997 |
| KH 202 | Low | Poultry–3A/3C/55/71 | Belgium | 1997 |
Phenotypic analysis
Biotyping was performed as described by Devriese (1984). Phage typing was performed using bacteriophages of the international typing set for human S. aureus strains and lytic reactions were examined at 100-fold routine test dilution (Parker, 1962).
Genomic analysis
All isolates were genotyped by (i) SmaI macrorestriction analysis of genomic DNA resolved by PFGE and (ii) sequence analysis of the polymorphic repeat region of the protein A gene or spa typing (Harmsen et al., 2003). PFGE patterns were classified into group and type according to the previously described nomenclature (Denis et al., 2004). Repeats and spa types were determined using Ridom Staphtype software (Ridom GmbH, Würzburg, Germany). A selected sample of strains was further analysed by MLST (Enright et al., 2000) and agr polymorphism (Gilot et al., 2002). MLST sequences were analysed using the MLST database (http://www.mlst.net) to determine MLST allelic profiles and the corresponding sequence type (ST).
Furthermore, the isolates were checked for mecA presence using the mec-nuc duplex polymerase chain reaction as described by Brakstad et al. (1994).
Results
SmaI macrorestriction analysis of the six classical and the six aberrant HV rabbit S. aureus isolates revealed an identical PFGE type, designated N2. PFGE pattern U showed a difference of seven DNA fragments with the pattern N2 and was recovered in the atypical HV rabbit S. aureus isolate PI 41/95 (Fig. 1). PFGE profiles generated in the LV isolates KH 28 and KH 202 were distinct (Y3 and T1) and markedly different from the N2 and U patterns (Fig. 1). MLST, spa typing and agr analysis confirmed PFGE results. Of the 12 HV strains displaying the N2 PFGE type, nine strains showed spa type t645 (Fig. 1). The three other N2 PFGE type strains presented spa types differing in their allelic profile by a single nucleotide mutation in one repeat (t738, t741) or insertion of two repeats (t272) (Fig. 1). The N2 PFGE type HV isolates that were analysed by MLST and agr typing, all displayed ST121/agr group 4. The atypical HV strain PI 41/95, displaying PFGE type U, had a spa allelic profile t742 differing from spa type t645 by deletion of one repeat (repeat 13) and insertion of five consecutive repeats (17–17–17–23–24) at the end of the polymorphic region. PI 41/95 belonged to agr group 2 and showed ST425 which was clearly distinct from ST 121. The two LV strains showing T1 or Y3 PFGE types presented spa t181/agr 2 and spa t745/agr 3, respectively (Fig. 1). None of the rabbit S. aureus strains contained the mecA gene.
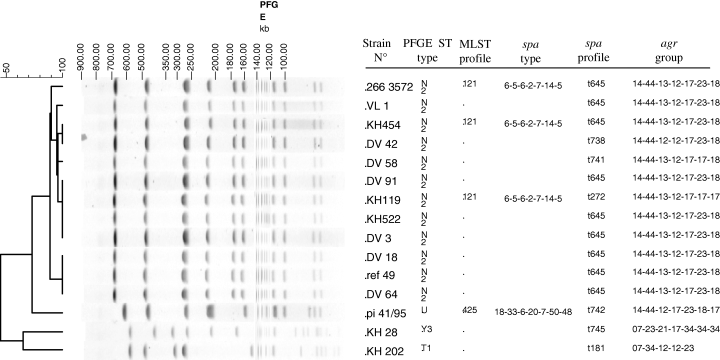
Molecular typing results of rabbit Staphylococcus aureus strains.
Discussion
This study indicates the international dissemination of a HV rabbit S. aureus clone in Europe since at least 1983. This is in agreement with the findings of Hermans et al. (2000), who found that HV rabbit S. aureus strains display an identical randomly amplified polymorphic DNA (RAPD) pattern, and with previous studies showing that these strains have a number of virulence factors in common (Vancraeynest et al., 2004b, 2006).
Many studies have proven the advantages of molecular typing methods compared with phenotypic methods, particularly in terms of reproducibility, discrimination and epidemiological concordance (Struelens et al., 1992; Bannerman et al., 1995). In this study, both the six classical and six aberrant HV rabbit S. aureus strains, showed a single PFGE type N2. The fact that S. aureus strains belonging to different phage types result in one PFGE type is in agreement with previous findings in human S. aureus strains (Schlichting et al., 1993) and demonstrates limitation and potential instability of phage typing for the characterization of S. aureus strains associated with chronic infections in rabbits. However, the main HV rabbit S. aureus phage type was unaltered during the whole study period. Thus, as a rule, phage typing can still contribute to the in vitro virulence determination of rabbit S. aureus strains at a local level.
Pulsed-field gel electrophoresis is a highly discriminative technique that indexes rapid accumulation of genetic variation (Enright et al., 2000), and which has been used successfully to analyse local outbreaks or centre-to-centre transmission events (Denis et al., 2004; Malachowa et al., 2005). Therefore, it is remarkable that S. aureus strains, which were isolated over a period of 21 years in five different countries, belong to the same PFGE type.
The PFGE profile of strain PI 41/95 (U) showed seven DNA fragments of difference with the patterns of other HV isolates (PFGE type N2). Thus, according to Tenover et al.’s (1995) criteria, strain PI 41/95 can be considered unrelated to the other HV strains. MLST, agr typing and spa typing also showed differences. Previous studies have already demonstrated that this strain is peculiar: (i) opposed to the strains of PFGE type N2, which are still causing problems today, it was isolated only once (Devriese et al., 1996); and (ii) it also shows differences with other HV strains on the level of virulence factors (Vancraeynest et al., 2004b, 2006).
Multilocus sequence typing analysis allows comparison and tracing of STs worldwide. The ST121 recovered in the HV rabbit S. aureus strains was previously described in human methicillin-sensitive S. aureus isolates recovered from widely diverse European countries like Belgium, Germany, Portugal, the UK and Sweden, but it was also isolated in Nigeria (Enright et al., 2000; Adesida et al., 2005; Aires de Sousa et al., 2005; Hallin et al., 2005). Although molecular typing methods already demonstrated the transmission of epidemic clones between animals and humans (Weese et al., 2005), there have been no studies regarding the occurrence of ST121 in people closely involved in rabbit production.
Polymorphism in the agr locus led to the classification of S. aureus into four groups (Gilot et al., 2002). Most bovine and human S. aureus strains have been assigned to agr group 1 or 2 (Gilot et al., 2002; Gilot and van Leeuwen, 2004; Aires de Sousa et al., 2005). In our study, the N2 PFGE type HV strains showed the agr type 4 rarely found in these previous studies. In humans, agr group 4 strains were associated with generalized exfoliative syndromes (Jarraud et al., 2002). However, HV rabbit S. aureus strains do not contain the genes encoding the exfoliative toxins A and B (Vancraeynest et al., 2006).
Staphylococcus aureus is an organism with a highly clonal population structure (Musser et al., 1990; Enright et al., 2000; Feil et al., 2003). Whether certain S. aureus lineages have a higher capacity of causing disease than others is a point of discussion in current literature. Booth et al. (2001) found differences in the frequencies of specific lineages when comparing samples of clinical and carried human isolates. However, Feil et al. (2003) denied the existence of hypervirulent S. aureus clones in humans. Vautor et al. (2003) found that one predominant S. aureus clone is responsible for the majority of mastitis cases in dairy sheep, suggesting that this clone may have special properties to overcome host defence mechanisms. A similar study by Rodgers et al. (1999) demonstrated that one predominant S. aureus strain type is associated with clinical disease in broiler flocks. It should be noted that the two latter studies were limited to certain geographical regions. Bovine S. aureus isolates, however, have been studied on an intercontinental level (Kapur et al., 1995; Zadoks et al., 2002; Smith et al., 2005). These studies demonstrated that a relatively low number of specialized clones are responsible for the majority of bovine intramammary infections. This is in agreement with the present study, which showed that the HV rabbit S. aureus clone, responsible for chronic problems of staphylococcosis on rabbitry level, is disseminated internationally.
In conclusion, this study shows that chronic problems of staphylococcosis in rabbitries are caused by clonal S. aureus strains, which are disseminated internationally. As was to be expected, genotyping techniques applied here performed better at characterizing various rabbit S. aureus strains than phage typing. Techniques like MLST and spa typing will surely be of future use in the epidemiology of rabbit S. aureus strains, as they result in data which can easily be compared between laboratories, on an international level. The ST121 and the spa types of the HV strains, as provided in the present study, may form a basis for these comparisons.
Acknowledgements
This work was supported by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen) to Dieter Vancraeynest.




