The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion
Summary
Impairment of segmentation during embryonic development leads to congenital fusion of vertebrae. Nevertheless, vertebral fusion can also occur during post-embryonic life. Fusion can cause reduction in mobility and may be pathological, but it can also be part of normal development and mechanically required, such as in the teleost caudal skeleton, or in the tetrapod sacrum. Using a series of closely spaced ontogenetic stages of zebrafish, stained for mineralized (Alizarin red) and cartilaginous (Alcian blue) structures, we have characterized all fusions occurring during the formation of the caudal skeleton. The urostyle results from the vertebral fusion of the compound centrum preural1-ural1 [PU1++U1] and ural2 [U2+]. Based on developmental and morphological characters: (i) number of vestigial haemal arches, (ii) occasional presence of a haemal arch rudiment, (iii) occasional individuals with separate centra rudiments or distinct mineralization time points, and (iv) evidence for internal separation, we propose that the urostyle forms as a fusion product of five, and not three vertebral centra, as previously described. The last fusion to occur in development, between the compound centrum [PU1++U1] and U2+, is a relatively slow process that typically occurs in Cypriniformes and Salmoniformes and is therefore considered reliable to monitor the fusion process. The vertebrae adjacent to the urostyle, preurals 2 and 3, are highly susceptible to fusion, and thus inadequate as a negative control to fusion, in contrast to trunk vertebrae, where fusion is never observed. With this we have established the basis for a new model to study vertebral fusion and to unravel cellular and molecular events underlying this process.
Introduction
The vertebral column, or spine, is a complex and vital structure providing support for the body axis and protection for the postcranial nervous system and major blood vessels.
Segmentation of the vertebrate body axis underlies the formation of vertebrae, a series of similar anatomical units made from cartilage and/or bone. Vertebrae are composed of centra, and of neural and haemal arches. Vertebrae are separated by notochord-based intervertebral tissue (Grotmol et al., 2003; Turnpenny et al., 2007). The formation of segmentally arranged vertebrae is intrinsically associated with two main structures, the notochord and the somites (Hall, 1975; Kaplan et al., 2005). When segmentation is partially impaired during embryonic development it may result in congenital fusion of vertebrae and consequent reduction of mobility (Kaplan et al., 2005). Vertebral fusion can also occur during post-embryonic life. Fusion may have an unknown ethiology or may be the result of injuries, juvenile rheumatoid arthritis, or infections such as tuberculosis (Peh, 2004; Witten et al., 2006).
The vertebral column in teleosts, as in zebrafish, is essentially divided in two main regions, the trunk vertebrae that define the abdominal region, and the caudal vertebrae located caudal to the abdominal cavity (Meunier and Ramzu, 2006). The caudalmost vertebrae are modified to support the caudal fin (Bird and Mabee, 2003). They carry modified haemal and neural arches (the hypurals and epurals, respectively) and individual vertebral body anlagen fuse to form the urostyle complex (Gosline, 1997; Bird and Mabee, 2003), a process analogous to sacrum development in tetrapods. While fusion of caudal vertebrae is considered as a physiological and mechanical requirement for normal development, zebrafish almost never display fusion of the trunk vertebral bodies (Ferreri et al., 2000), as opposed to farmed fish, such as salmonids, where trunk vertebral fusion is a common malformation (Witten et al., 2006, 2009; Gjerde et al., 2005; Schultze and Arratia, 1988).
Recent studies in zebrafish have linked genetic factors to a fusion-like phenotype caused by the over mineralization of vertebrae (König et al., 1999; Spoorendonk et al., 2008). Impairment of segmentation is another cause that can lead to vertebral body fusion. Although the role of somites and notochord in fusion has been discussed (Hall, 1975; Witten et al., 2005) many factors underlying the formation or the non-formation (fusion) of segmentally arranged vertebrae in zebrafish are still unclear.
Most studies regarding the caudal skeleton of actinopterygians have focused on systematics and phylogeny, related to the characterization of the composition of the caudal skeleton (Gosline, 1960; Nybelin, 1963; Patterson, 1968; Eastman, 1979; Schultze and Arratia, 1986; Fink and Fink, 1996; Sanger and McCune, 2002). Basal actinopterygians, with a polyural caudal skeleton (several ural centra, cf. Schultze and Arratia, 1988), have relatively unmodified caudal vertebral elements with a direct one-to-one relationship between neural arch, centrum and hypural (Nybelin, 1963; Schultze and Arratia, 1986, 1988). In teleosts with a diural skeleton (two ural centra, cf. Schultze and Arratia, 1988), such as zebrafish, we can deduce the product of vertebral fusion and estimate the number of fused elements, based on the number of hypurals, independently of whether the fusion is the result of a past evolutionary process or if it can still be observed during development.
The aim of the present work is the characterization of all fusions occurring during the development of the zebrafish caudal skeleton, which is the foundation for a new model to study vertebral fusion and to unravel cellular and molecular events underlying pathological disruption of vertebral identity.
Materials and methods
Specimens examined
In order to determine a timeline of events for caudal vertebral fusion, an ontogenic series of Danio rerio was studied. Zebrafish eggs were obtained from natural spawning of wild-type breeding fish and larvae were maintained and raised by standard methods, according to Westerfield (2000). Larvae and juveniles were collected at size intervals of 0.1 mm, between 4.0 and 12.0 mm of total length (TL).
Specimen preparation
A total of 361 specimens were euthanized with a lethal dose of MS222 (Sigma, St. Louis, MO) and fixed for 24 h in 4% buffered paraformaldehyde at 4°C. For whole mount analysis of caudal skeletal structures, specimens were double stained with Alizarin red S for mineralized structures and Alcian blue for cartilage, adapted from the acid free method of Walker and Kimmel (2006). We selected a concentration of 60 mm of MgCl2 and a 0.1% final concentration of Alizarin red instead of 0.05%.
Terminology
Nomenclature for the vertebral column was adapted from Meunier and Ramzu (2006). For the caudal skeleton the nomenclature follows that of Patterson (1968) adapted by Arratia and Schultze (1992). The use of the terms vestiges and rudiments follows Hall (2003).
In order to clarify some terms, we will briefly describe the main structures involved. Preural centra are vertebral bodies carrying normal or little modified haemal arches and spines, while ural centra carry hypurals and epurals. The hypurals are transformed haemal arches supporting one or more lepidotrichia (or fin rays) and making contact with the notochordal axis. An epural is a dorsal element interpreted as an ural neural spine which has become detached from its neural arch. Uroneurals are modified neural arches that form a pair of elongated bones, extending from the preural 1 neural arch to the posterior tip of the notochord. The urostyle is the compound element of the vertebral column composed of preural 1 and all urals.
Results
The caudal fin endoskeleton in zebrafish, as described in previous works (Bird and Mabee, 2003) is a complex structure. It comprises the ural centra associated with five vestigial haemal arches, termed hypurals, preural 1 with its respective neural and haemal (parhypural) arches, and haemal and neural arches and spines of the second and third preural vertebrae (Fig. 1). It also includes one pair of uroneurals and one epural, elements regarded as neural arch vestiges (Witten and Huysseune, 2007). The urostyle results from the fusion of the compound centrum preural1-ural1 (PU1+U1) and ural2 (U2). A detailed analysis of our closely spaced ontogenetic series has revealed features not previously identified. Below we will focus on the composition of the centra, dissecting PU1, U1 and U2 and their fusion status.
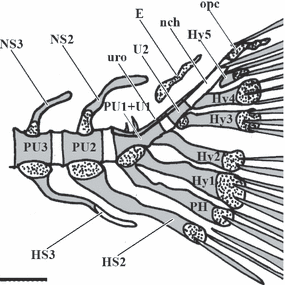
Lateral view of an 8.0 mm zebrafish Danio rerio caudal skeleton.; E, epural; HS2 and 3, Haemal spines of Preural 2 and 3; Hy1-5, Hypurals 1–5; nch, notochord; NS3 and 2, Neural spines of Preural 3 and 2; opc, opistural cartilage; PH, parhypural; PU1+U1, compound centrum preural 1 and ural 1; PU2-3, Preurals 2 and 3; U2, ural 2; uro, uroneural; scale bar = 0.2 mm
The complex PU1+U1 bears three transformed haemal arches (parhypural and hypurals 1 and 2) and frequently three rudimentary neural spines. During ontogeny, U1 is the first to mineralize (5.5 mm) in a ventral to dorsal direction. Before completion of mineralization of U1, PU1 also mineralizes (5.5–6.0 mm) as an extension of U1 leaving no internal boundaries that might suggest centra fusion (Fig. 2a). Although this was the general pattern, occasionally (4% of all individuals analyzed) PU1 and U1 were identified as two separate elements, with subsequent fusion occurring in a dorsal to ventral direction (Fig. 2b).

Different developmental stages with distinct fusion characters. (a): Early formation of preural 1 (PU1, white arrowhead), with ural 1 (U1) and U2 already mineralized (6.0 mm TL). (b): Mineralized connection (dorsally) between PU1 and U1, otherwise clearly separated (black arrowhead) (7.0 mm TL). (c): Development of hypural 3 (Hy3, black arrow) with the formation of an extra cartilaginous structure on the ventral side (black arrowhead) (5.2 mm TL). (d): Hy3 shows a broad base (whilte arrow) and U2 contains a vertical internal line (marked by black arrowhead) (7.5 mm). (e): Fusion (black arrowhead) between the compound centrum [PU1+U1] and U2 (8.9 mm). (f): Evidence for PU2 as a fusion product from the presence of two neural spines (black arrows) related to a single centrum (10.0 mm). PH, parhypural. Scale bar = 0.15 mm
Generally U2 mineralizes after PU1+U1 around 6.2 mm, and is associated with hypural 3 but no visible neural arch. Occasionally (15% of the individuals) hypural 3 was formed either by a complete hypural and an extra cartilaginous structure on the ventral side or from a broad base (Fig. 2c,d). A single specimen had a complete hypural fused dorsally to the basis of hypural 3, which reinforced the interpretation that hypural 3 is associated with a regressive hypural. Within the ural centrum itself (U2), a vertical internal line was present in most analyzed fish (Fig. 2d).
The previously described vertebral centra then undergo one more fusion, between the compound centrum [PU1+U1] and U2 (Fig. 2e). This last fusion develops over a long period of time and was observed in all specimens from 8.6 to 12.0 mm.
The adjacent vertebral bodies PU2 and PU3 are the last vertebral bodies to form in the vertebral column (around 6.4 mm). We have registered that 23% of the analyzed individuals had, at least, one extra spine either in PU2 and/or PU3 (Fig. 2f).
Other vertebral fusions, whether in the caudal or trunk regions, were not detected in any of the fishes examined.
Discussion
To establish the zebrafish caudal skeleton as a model for studying the process of vertebral fusion it is fundamental to characterize all elements involved, in particular to determine whether or not vertebrae that form the caudal complex are the product of fusion.
Therefore we have determined the identity of all elements in the zebrafish caudal skeleton known to form the urostyle. In addition we have analyzed the adjacent vertebrae, preural 2 and 3, in order to determine whether or not they are a product of fusion and can therefore be used as a negative control for fusion.
Considering the presence of three transformed haemal arches (parhypural and hypurals 1 and 2) and the frequent presence of three rudimentary neural spines, the complex PU1+U1 is reinterpreted as being originally composed of three, and not two, vertebral centra as described by Bird and Mabee (2003). We have determined that PU1 is actually formed by PU1, associated with the parhypural, and by an extra ural, associated with hypural 1. Although no physical separation was ever observed within these centra we will rename it as PU1+. In addition we have observed cases where PU1+ and U1 mineralize separately in time or as two distinct elements, supporting the idea of two pre-existing vertebral centra.
While [PU1++U1] was already considered a compound centrum (Bird and Mabee, 2003), zebrafish U2 was never considered to be a product of fusion. We have observed several specimens showing an extra cartilaginous structure on the ventral side of hypural 3 or hypural 3 forming with a broadened base. This suggests that U2 is associated with a regressive hypural, in addition to its haemal arch, hypural 3. Therefore we will rename the latter into Hy3+. Together with the evidence of a vertical internal line in ural 2, we have concluded that U2 is a fusion product that we have renamed as U2+.
Finally, in order to form the urostyle as a stable internal support for the caudal fin (Gosline, 1997; Bird and Mabee, 2003) all zebrafish undergo one last fusion, between the compound centrum [PU1++U1] and U2+. Thus the urostyle is now considered to be the fusion product of five vertebral bodies, and not three as previously described (Bird and Mabee, 2003). This last fusion process is relatively slow, allowing for a direct monitoring in live fish and constituting a potential tool for the molecular characterization of the fusion process.
In the part of the spine immediately anterior to the urostyle, we have observed a relatively high percentage of fusion, either in PU2 and/or PU3. Even though it was clear that the vertebrae supporting the caudal fin fuse during development, the predisposition of adjacent vertebrae (PU2 and PU3) to fuse was not yet known. The supernumerary neural or haemal spines connected to preural vertebral bodies, suggest an early fusion process in this region of the vertebral column, since there is no morphological evidence in early ontogeny of individual centra. These findings are in agreement with previous records in catostomids, where preurals are generally more likely to undergo fusion (Eastman, 1979) than anterior vertebrae. The fact that in several teleosts a complete unification of the centra with the accessory spine occurs, suggests that the process takes place in early life, before the centra are fully formed as single units (Barrington, 1937; Eastman, 1979), thus eliminating the possibility of detecting internal morphological evidence of fusion.
In conclusion, the caudal centra in zebrafish, which include preural and ural centra, have a clear predisposition to fuse as part of normal development. This is in line with the assumption that evolution tends to reduce the number of elements. Indeed, the evolution of the complex of caudal vertebrae in modern teleosts has been based on progressive reduction of elements from an original complex of rather numerous independent bones (Gosline, 1961; Eastman, 1979). In addition, the high variation of fusion status and incomplete fusion characters among individuals cannot be considered to be a malformation. Likewise, in salmonids, the trend is to reduce ural centra to two elements, as in most teleosts; a primitive polyural character present in some individuals is not considered an abnormality (Arratia and Schultze, 1992).
The characters that we have described above make the zebrafish caudal skeleton a valuable model to study the mechanisms involved in normal vertebral fusion. The fact that all individuals in our sample of zebrafish larvae undergo fusion between [PU1++U1] and U2+, and that this process is relatively slow, allows for a thorough characterization of the fusion process and the molecular and cellular events underlying it. In addition, the reported absence of pathological vertebral fusion in the anterior parts of the spine in zebrafish (Ferreri et al.,2000), confirmed by our observations, allows the use of trunk vertebrae (with the exception of PU2 and PU3) as negative control for fusion. The latter provides us with an adequate system to manipulate factors in order to induce fusion and confirm possible candidate genes involved in this process. Thus, zebrafish provides us with a reliable tool for a molecular dissection of the fusion process and for obtaining relevant information towards understanding why vertebrae can fuse or persist as individual skeletal elements.
Acknowledgements
For assistance in sample collection we thank G. Dionisio, J. Cardeira da Silva and R. Peres dos Santos. For the fruitful discussion and comments we thank M. Willems. A. Bensimon-Brito is the recipient of a FCT PhD fellowship SFRH/BD/40573/2007 and A. Huysseune acknowledges a grant from FWO 3G.0040.08.




