Diel-feeding activity in early summer of racer goby Neogobius gymnotrachelus (Gobiidae): a new invader in the Baltic basin
Summary
The first recording of the Ponto-Caspian racer goby in Poland was during 1995 in the River Bug (River Vistula system). Within 5 years, the species had spread to the downstream section of the Vistula. One of the potential impacts of invasive species on native fauna is competition for food. Therefore, the diel patterns in diet composition and gut fullness coefficient (FC) of racer goby were examined at one study site in the Włocławski Reservoir (lower River Vistula), during May 2003. An average of 20 individuals were examined each 4 h over one 24-h period (125 fish in total). The proportion of main food items and diet width did not differ among three size-groups (43–59, 60–79 and 80–97 mm total length), and the relative biomass ratio of main food categories did not differ over the diel cycle. Amphipods constituted 11–70% of total gut content biomass and were found on average in 84% of analysed alimentary tracts. The second prey types were chironomid larvae (16–63% of total food biomass; frequency occurrence: 61–91%), and to a lesser extent chironomid pupa, ceratopogonid larvae, oligochaets, dipteran imagines and copepods, with fish larvae found in the gut of eight gobies. Gut fullness coefficient (FC) differed significantly over the 24-h period, with the highest value at night and in early morning. In conclusion, racer goby forages mainly on benthos and has a nocturnal-feeding activity. No significant diet overlap was found between racer goby and native percids, i.e. Eurasian perch Perca fluviatilis and ruffe Gymnocephalus cernuus.
Introduction
Knowledge of the environmental biology of non-native species is essential to evaluate their potential invasiveness and impacts on native biota. One of the most likely interactions with native species is food competition (Holčík, 1991). However, particularly successful non-natives species have not necessarily exploited similar resources as native species and as such have avoided competition (Brown, 1989). ‘Perfect invaders’ are often characterized by a wide dietary spectrum, which inevitably facilitates the invasion (Ehrlich, 1989). Of the European fin fishes, Gobiidae are often associated with recent invasion events (Copp et al., 2005a). Of these, round goby Neogobius melanostomus, monkey goby N. fluviatilis and racer goby N. gymnotrachelus have all invaded Polish waters, and they are currently expanding their ranges (Kostrzewa and Grabowski, 2002, 2003).
In its native range, the racer goby inhabits the brackish lagoons of the Black and the Caspian Seas and lower courses of their rivers: Danube and its larger tributaries, the Dniester drainage, Boh (known also as Southern Bug or Eastern Bug), Dnieper and Don (Pinchuk et al., 2003; Copp et al., 2005a). In 1995, racer goby was found in the middle section of the River Bug, also known as Western Bug (Danilkiewicz, 1996), and the species spread rapidly downstream (Danilkiewicz, 1998). In 2000, racer goby appeared in our samples from the Włoclawski Reservoir, a river regulation structure in the lower River Vistula (Kostrzewa and Grabowski, 2001), where it has formed a vivid, abundant and self-sustaining population (Kostrzewa and Grabowski, 2003). At present, our unpublished records for racer goby include the middle and lower sections of the River Vistula, down to the vicinity of Toruń (53°02′N, 18°37′E).
There is limited information about the diet of racer goby outside its native range (Kakareko et al., 2003, 2005; Kostrzewa and Grabowski, 2003), and the diel-feeding activity of racer goby has not been investigated until 2003 (present study and Kakareko et al., 2005). The main objectives of the present study were to describe the diet composition of racer goby in newly colonized areas (lower section) of the River Vistula, and determine diel-feeding patterns with regard to size-related differences. Because diel sampling provides much material within a short period of time, we limited our study to a single 24-h cycle within a period of characteristic early summer climate. A study elsewhere has been shown that diel patterns are consistent during relatively stable climatic periods within a given season (Copp et al., 2005b). Additionally, racer goby diet composition was compared with that of native percid species, Eurasian perch (Perca fluviatilis) and ruffe (Gymnocephalus cernuus), potential competitors of racer goby in Włocławski Reservoir. We discuss our results in light of a similar preliminary diel study carried out during late-September 2003 on monkey and racer goby diet in the main channel of the River Vistula downstream of Włocławski Reservoir (Kakareko et al., 2005).
Study area, material and methods
The study site was located on the northern bank of the Włocławski Reservoir (19°31′E; 52°36′N), lower section of the River Vistula (Baltic basin, Poland). On 29 May 2003 (sunrise: 3.24 hours; sunset: 19.45 hours), beginning at 12.00 hours, samples were collected every 4 h (six excursions in total: 12.00, 16.00 and 20.00 hours; midnight, 04.00 and 08.00 hours) at a depth of 0.5–1 m, along 150 m transect parallel to the coastline, using a 5 m long seine net (mesh size 1 mm). Numbers of individual collected from each sampling occasion (i.e. at 12.00, 16.00 and 20.00 hours; midnight, 04.00 and 08.00) were 23, 14, 28, 16, 22 and 22, respectively. In total, 125 fish were anaesthetized and preserved in 4% formaldehyde. Five specimens had empty alimentary tracts and were not considered further.
In the laboratory, the fish were then measured for total length (TL; to the nearest 1 mm), weighed (with 0.01 g accuracy) and dissected. Based on the length–frequency distribution of examined individuals, which ranged from 43 to 97 mm TL (mean TL = 61.54 mm, SE = 1.10 mm), fish were divided into three size-groups to assess ontogenetic changes in diet composition: small (43–59 mm TL), intermediate (60–79 mm TL) and large (80–97 mm TL). The gut contents were removed, weighed (to 0.0001 g accuracy) and examined under stereomicroscope to identify prey categories. The fishes were re-weighed to obtain eviscerated weight. The frequency of occurrence (defined as proportion of fish containing given prey category: %F) and percentage of biomass (weight of given prey category in relation to total weight of gut content: %B) were quantified for each prey type (Hyslop, 1980). Dietary width was calculated as a Simpson diversity index (Krebs, 1978; Keast, 1985): D = 1 − Σpi2, where pi2 is the proportion of different prey in the diet. The mean values and their standard errors were estimated with the jack-knife procedure (Magurran, 1988) using prey category biomass, rather than the number of individuals, as biomass seemed to be a better measure of prey importance. The jack-knife procedure estimates the normally distributed pseudo-values of the statistic and thus permits the use of confidence limits and parametric tests on the transformed data (Magurran, 1988). One-way anova was used to test for differences fish size classes in the dietary breadth. Gut FC, used to investigate diel-feeding periodicity, was calculated as the ratio of gut content wet weight to eviscerated fish wet weight. One-way anova and post hoc Fisher Least Significant Difference (LSD) tests were used to test for differences in gut fullness among samples collected over the diel cycle. Kendall's coefficient of concordance was used to compare diel diet composition of the diel cycle as well as to compare relative biomass of main food categories in the three defined size-groups (Zar, 1984).
The native percids, Eurasian perch and ruffe, were caught in seine nets during sampling for gobies. As they were observed to forage exclusively during dusk at the sampling site, all fish were captured at 20.00 hours only were used for the comparison of food composition. Altogether, 28 individuals of racer goby (minimum–maximum TL: 48–97 mm, mean TL = 64.04 mm, SE = 2.56), 25 Eurasian perch (minimum–maximum in TL: 93–107 mm, mean TL = 103.00 mm, SE = 1.35) and 17 ruffe (minimum–maximum in TL: 77–132 mm, mean TL = 92.94 mm, SE = 3.04) were included for the analysis. Dietary overlap between each pair of fish species was calculated using Schoener's index (Wallace, 1981): α = 1–0.5 [Σn=1 (pij − pik)], where pij is the proportion of the ith resource used by species j, and pik is the proportion of the ith resources used by species k; overlap values exceeding 0.5 were regarded as high or biologically significant.
Results
A total of 24 food types, representing seven categories (amphipods, dipteran larvae, dipteran pupae, terrestrial arthropods, fish, detritus and others) were observed in the diet of racer goby (Table 1). The proportion of main food categories did not differ among three size-groups of fish (Kendall's W = 0.7196; d.f. = 8.3; P < 0.05), and the primary prey was amphipods and chironomid larvae. The mean (jack-knife) values of diet width (mean and SE) for small (mean = 0.7475, SE = 0.0232), intermediate (mean = 0.8183, SE = 0.0268) and large (mean = 0.7658, SE = 0.0813) gobies did not vary significantly (anova, P > 0.50). No significant differences in diet were found between size classes, and the overall (i.e. all samples pooled) relative biomass ratio of main food categories was not found to differ (Kendall's W = 0.6949; d.f. = 7.6; P < 0.001) over the diel cycle (Fig. 1).
| Food categories | Size class of fish | |||||
|---|---|---|---|---|---|---|
| Small | Intermediate | Large | ||||
| %B | %F | %B | %F | %B | %F | |
| Amphipods (57.6) | ||||||
| Pontogammarus robustoides | 36.0 | 55.0 | 33.4 | 56.5 | 42.0 | 14.3 |
| Dikerogammarus haemobaphes | 2.9 | 8.3 | 2.8 | 4.4 | 16.7 | 21.4 |
| Chelicorophium curvispinum | 3.7 | 16.7 | 8.4 | 30.4 | 2.4 | 28.6 |
| Gammaridea (not determined) | 2.1 | 8.3 | 13.7 | 23.9 | 16.5 | 50.0 |
| Dipteran larvae (1.3) | ||||||
| Chironomidae | 39.1 | 78.3 | 21.2 | 76.1 | 12.0 | 71.4 |
| Ceratopogonidae | 1.5 | 5.0 | 1.1 | 6.5 | ||
| Other Diptera | 1.2 | 3.3 | 0.1 | 6.5 | ||
| Dipteran pupae (5.3) | ||||||
| Chironomidae | 2.7 | 8.3 | 5.1 | 17.4 | 6.0 | 28.6 |
| Other Diptera | 1.5 | 2.2 | ||||
| Terrestrial arthropods (1.8) | ||||||
| Chironomidae imagines | 0.8 | 6.5 | ||||
| Other dipteran imagines | 0.3 | 1.7 | 0.7 | 2.2 | 1.7 | 7.1 |
| Orthoptera | 0.1 | 2.2 | ||||
| Thysanoptera | 0.1 | 3.3 | 0.6 | 2.2 | ||
| Araneida | 0.6 | 1.7 | 0.1 | 4.4 | ||
| Others (2.5) | ||||||
| Copepoda | 1.4 | 11.7 | 0.1 | 6.5 | ||
| Ostracoda | 0.1 | 5.0 | 0.1 | 10.9 | ||
| Oligochaeta | 0.6 | 1.1 | 8.7 | 0.2 | 7.1 | |
| Gastropoda | 0.7 | 1.7 | 0.4 | 2.2 | ||
| Hemipteroidea | 0.2 | 1.7 | 0.2 | 2.2 | ||
| Zygoptera larvae | 0.5 | 2.2 | ||||
| Coleoptera larvae | 0.1 | 2.2 | ||||
| Ephemeroptera larvae | 0.6 | 4.4 | ||||
| Fish (2.3) | ||||||
| Larvae | 4.5 | 10.0 | 0.6 | 2.2 | 0.5 | 7.1 |
| Eggs | 0.8 | 4.4 | 0.4 | 7.1 | ||
| Detritus (3.8) | 2.5 | 13.3 | 6.0 | 23.9 | 1.8 | 21.4 |
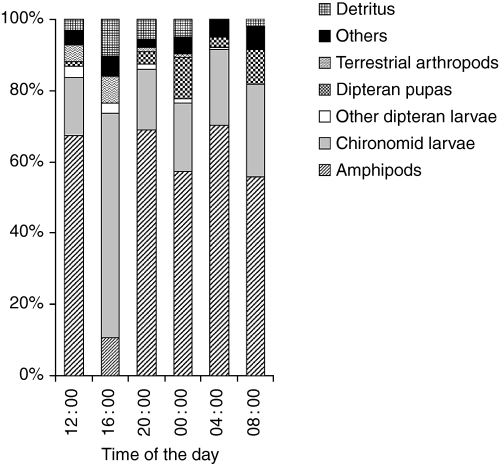
The relative biomass ratio of main food categories taken by racer goby in Włocławski Reservoir (River Vistula, Poland) over a 24-h period on 29 May 2003, pooled for all fish size-groups (n = 120)
Amphipods constituted a small proportion of total gut content biomass at 16.00 hours but a major proportion at 04.00 hours (Fig. 1) and were found in a high proportion of fish (Fig. 2). Among amphipods, Pontogammarus robustoides was the most abundant species (Table 1) followed by Chironomidae larvae, which represented a higher proportion of total food biomass at 16.00 hours than at 12.00 hours (Fig. 1) but occurred more frequently at 08.00 hours than at 16.00 hours. Fish larvae were recorded in the gut of eight of 120 fishes examined (Fig. 2), with six of predatory fish being <60 mm TL (Table 1). These compositional variations were not reflected in gut fullness, which differed between night and day (F = 4.162, d.f. = 5, 114, P = 0.0016; Fig. 3), with night/early morning samples (midnight, 04.00 and 08.00 hours) having significantly (Fisher PLSD at 95%) higher values than those during daytime/evening (12.00, 16.00 and 20.00 hours).
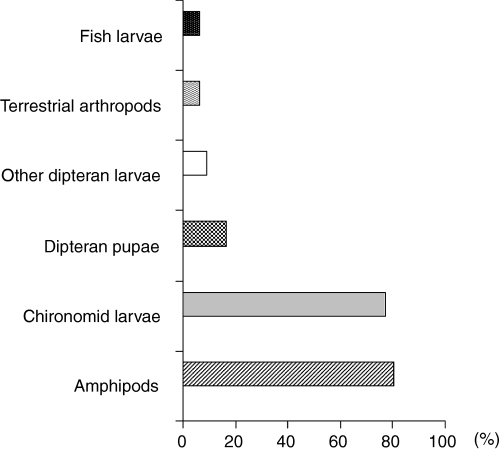
Frequency of occurrence (%F) of main prey items in the gut of racer goby from Włocławski Reservoir (River Vistula, Poland) over a 24-h period on 29 May 2003, pooled for all 120 fish individuals
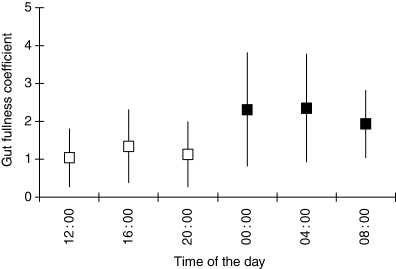
The differences in the gut fullness coefficient (mean ± SD) over a 24-h period (29 May 2003) in Włocławski Reservoir (River Vistula, Poland), pooled for all fish size-groups (n = 120). The coefficients marked in the same box colours (black and white) are not significantly different (Fisher PLSD at 95%)
Despite some common dietary components amongst racer goby and the native species during the main foraging period of the 24-h cycle, i.e. 20.00 hours (Table 2), there was little dietary overlap (Schoener's index) between racer goby and Eurasian perch (α = 0.26) and between racer goby and ruffe (α = 0.13). Whereas, considerable overlap was observed between the two native species, Eurasian perch and ruffe (α = 0.67); dipteran larvae, pupae and imagines were important food items at dusk to both species (Table 2), followed by amphipods, especially P. robustoides in Eurasian perch) and exclusively Chelicorophium curvispinum in ruffe. Indeed, amphipods were one food type also taken by racer goby at dusk, with few dipterans taken (Table 2).
| Food categories | Fish species | ||
|---|---|---|---|
| Racer goby | Perch | Ruffe | |
| Amphipods | |||
| Pontogammarus robustoides | 11.7 | 22.4 | |
| Dikerogammarus haemobaphes | 2.8 | 1.7 | |
| Chelicorophium curvispinum | 0.1 | 0.4 | 7.2 |
| Gammaridea (non-determined) | 44.7 | ||
| Dipteran larvae | |||
| Chironomidae | 1.3 | 16.0 | 36.6 |
| Ceratopogonidae | 9.7 | 0.2 | 0.7 |
| Other Diptera | 2.0 | 0.2 | |
| Dipteran pupae | |||
| Chironomidae | 1.1 | 51.1 | 45.3 |
| Other Diptea | 1.6 | ||
| Terrestrial arthropods | |||
| Chironomidae imagines | 1.2 | 4.8 | 7.4 |
| Trichoptera | 0.9 | ||
| Araneida | 0.4 | ||
| Others | |||
| Copepoda | 0.2 | ||
| Ostracoda | 5.2 | ||
| Fish | |||
| Larvae | 0.2 | ||
| Detritus | |||
| Animal | 0.4 | 3.4 | |
| Plants | 0.3 | 1.8 | |
Discussion
Within its native range, such as in Lake Razelm (Danube estuarine complex), the diet of racer goby is often reported to include chironomid larvae, small crustaceans (i.e. amphipods, mysids, cumaceans) as well as Ostracods, cyclopoid copepods, amphipods and mysids (Pinchuk et al., 2003). In more saline habitats, such as the northern Caspian Sea (Tsikhon-Lukanina, 1959), crustaceans (corophiids and amphipods) constituted almost 70% of the gut content, but polychaets were also significant (21%). A similar diet composition was observed in the Dniester estuary (Strautman, 1972), although the proportion of food categories changed seasonally, i.e. in summer, young-of-the-year gobies were readily available and were observed in the diet. Fish larvae and juveniles, as well as molluscs are often reported to be eaten by racer goby, but in Włocławski Reservoir we found these prey to occur infrequently (Fig. 3), and mainly in small racer goby (Table 1).
The diet of racer goby in Włocławski Reservoir varied between locations, with fish from the southern bank (Kostrzewa and Grabowski, 2003), taking mainly (77% of biomass) small molluscs (gastropods: Valvata sp., Bithynia tentaculata, Physa acuta, Potamopyrgus antipodarum and bivalves Pisidium sp., Sphaerium sp.), whereas Chironomidae and Gammaridae were taken infrequently. This underlines the racer goby's opportunistic foraging strategy, as small molluscs were a dominant fraction of available prey along the southern bank (Kostrzewa and Grabowski, 2003) and amphipods were virtually absent. Along the northern bank of Włocławski Reservoir (present study), amphipods comprised 11.4% of the macrozoobenthos, and the relatively large mollusc component consisted of very large specimens (Viviparus sp. and Dreissena polymorpha), which exceed the gap size of the racer goby we sampled (Grabowski and Grabowska, unpubl. data). All amphipods taken by racer goby in the Włocławski Reservoir (Table 1) are Ponto-Caspian species, which have completely replaced native amphipods fauna in the middle and lower Vistula (Jażdżewski et al., 2002). In separate studies of monkey and racer goby diet in Włocławski Reservoir (Kakareko et al., 2003, 2005), these species were found to feed mainly on Chironomidae larvae, Oligochaeta, Mollusca and amphipods, especially the latter in the case of racer goby. In the Vistula main channel (downstream of the reservoir), the diet in biomass of racer goby diet consisted almost entirely of amphipods and Chironomidae larvae (Kakareko et al., 2005) and, similar the present study in spring 2003 (Fig. 3), the dominance of these two prey types varied little over a 24-h cycle in autumn 2003 (Kakareko et al., 2005). The absence of significant changes in racer goby diet with increasing body size (see Results) is consistent with reports from the Dniester estuary (Strautman, 1972).
The racer goby appears to be a primarily nocturnal feeder, as gut fullness was greatest at night (Fig. 3), when racer goby migrate up into shallower waters and are easier to catch. The same habit was used by Eurasian perch, ruffe and young pikeperch Sander lucioperca, which where numerically important in night samples. However, dietary overlap between racer goby and the native species, during the main foraging period of the day (dusk), was minimal (Table 2; see Results); this suggests different foraging habits between racer goby and native fish species. The dominance of chironomid pupae and newly metamorphosed imagines in the diet of native percids (Table 2) indicates that they feed predominantly in the water column and just below the water's surface. Whereas, racer goby feeds mainly on the bottom, where benthic amphipods are most abundant. Benthic feeding by racer goby is evinced by ostracods, which were not found in native percids and probably were taken by racer goby accidentally whilst foraging on larger benthic invertebrates (Table 2). Thus, racer goby appears to avoid resource competition with native percids through spatial segregation during foraging periods in Włocławski Reservoir (Kakareko et al., 2003), where macrozoobenthos densities are sufficiently high (Żbikowski, 2000) that food resources are not limiting.
In conclusion, racer goby appears to display great plasticity and opportunism in its feeding habits, taking a variety of prey types and usually choosing the most abundant food organisms (Kostrzewa and Grabowski, 2003). Such an opportunistic feeding strategy is thought to be optimal for invasive species during the establishment of a population in a novel environment, where food base may be different from what it is in natural range (Kostrzewa and Grabowski, 2003). However, in the Włocławski Reservoir, establishment of racer goby appears to be facilitated by other invaders from its native range (Ricciardi, 2001), which in this case are the Ponto-Caspian amphipods that constitute an important part of racer goby diet (Table 1).
Acknowledgements
The present study was financed by a grant (project 3 P04F 056 23) from the State Committee for Scientific Research, as well as by internal grants from the University of Łódź. Authors thank P. Spychalski and M. Molski, for assistance in the fieldwork and M. Przybylski for help with the statistical analyses and comments to an earlier version of this manuscript.




