Insecticidal activities of essential oil of Callistemon viminalis applied as fumigant and powder against two bruchids
Abstract
The fumigant and contact toxicity of essential oil (EO) extracted from the leaves of Callistemon viminalis and its aromatized clay powder (ACP) was evaluated against adults of Acanthoscelides obtectus and Callosobruchus maculatus (Coleoptera: Bruchidae). The results obtained for fumigation assays showed that C. maculatus seems to be more susceptible (LC50 = 0.019 μl/cm3) to the vapours of the essential oil than A. obtectus (LC50 = 0.011 μl/cm3) after 12 h exposure. On the other hand, A. obtectus seems to be more susceptible (LD50 = 0.133 μl/g) to the essential oil applied by contact on grains than C. maculatus (LD50 = 0.170 μl/g) after 2 days exposure. The ACP was also very toxic towards the adults of A. obtectus (LD50 = 0.100 μl/g) and C. maculatus (LD50 = 0.098 μl/g) by contact on grains. At the doses of 0.133 μl/g and 0.266 μl/g, mortalities caused by ACP on grains were higher than those caused by the same dose of EO against the two bruchids. It is also established that both the EO and the ACP caused higher inhibition of F1 progeny production of A. obtectus than that of C. maculatus. The loss of insecticidal activity of the two materials in the course of time has been observed; however, the toxicity of the ACP was more persistent than that of the oil in the course of time when applied on grains. These results suggest that EO from the leaves of C. viminalis can be used as fumigant agent against A. obtectus and C. maculatus. In addition, it could be advisable to use an adsorbent mineral material as carrier of this EO for the prolongation of its insecticidal activity in the course of time.
Introduction
During storage, foods are currently destroyed by insects and other pests. In Cameroon, these insects of stored grains are mostly Sitophilus zeamais and S. oryzae (Coloptera: Curculionidae), Acanthoscelides obtectus and Callosobruchus maculatus (Coleoptera: Bruchidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) (Ngamo et al. 2001). For example, it has been estimated that the common pulse weevil Callosobruchus maculatus (F.) alone caused an annual losses of 24% of stored pulses in Nigeria (Caswell 1968); the main pest of stored common beans is Acanthoscelides obtectus (Says) which starts infestation in the field in dry pods and continues in stored common beans to cause both quantitative and qualitative damage to grain (Kumar 1991). Protection of agricultural stored products against insect pests is of utmost importance to secure a continuous and safe food supply all over the world. Conventional treatments have been used for this purpose, but nowadays, other ecologically sound methods based on the use of natural compounds are needed for an integrated approach to pest management. Many plants and minerals have been widely used in the past to protect stored products against damage by insect infestation (Golob and Webley 1980). Nowadays, the plants are tested in the laboratories in the form of powder, vegetable oil, essential oil (EO), aqueous and organic extracts (Boeke et al. 2004).
There are several scientific reports that describe various biological effects of EO such as insecticidal properties, repellence, fumigant activity and antifeedants, antifungal and bactericidal properties (Buchbauer 2000; Lahlou 2004). Oils may affect some biological parameters of insects such as growth rate, life span and the reproduction (Boeke et al. 2004). Mineral powders and sand have also been used successfully to control insect populations in stored products (Subramanyam et al. 1994). Finely powdery clay is recognized by some traditional societies for its ability to control insect populations (Ramaswamy et al. 1995).
The use of such powders, aromatized with EO has a twofold advantage because of the combined effects of mechanism action, blocking the insect’s articulation, filling intergranular spaces at high dosages, and chemical action, acting primarily on granular cells (Ramaswamy et al. 1995).
Callistemon viminalis belonging to Myrtaceae family is an ornamental plant that is found in several areas with the exception of localities extremely cold and dry. It is also found along the streets and in the botanical gardens (Anonymous, 1992). In Cameroon and particularly in the town of Dschang (West Cameroon), it is found along major roads and near homes. The EO from leaves of C. viminalis presented anthelmenthic effects while the aqueous extracts of flowers and leaves have antibacterial effects against gram-positive bacteria (Srivastava et al. 2003). In our continuous search for new strategies to protect stored grains against insect infestation by using biopesticides (Tapondjou et al. 2003, 2005; Ndomo et al. 2008), the EO from the leaves of C. viminalis was evaluated for its insecticidal activities against A. obtectus and C. maculatus in bean and cowpea grains respectively.
The goal of this study was to assess the effect of applying EO from the leaves of this plant as a fumigant and applying by contact the aromatized clay powder (ACP) with the same oil to protect bean and cowpea grains against A. obtectus and C. maculatus infestation respectively. The evaluation of the kinetic loss of efficacy of the EO and its ACP against A. obtectus and C. maculatus adults in the course of time was also performed.
Materials and Methods
Plant materials
The leaves of C. viminalis were collected in November 2005 in Dschang city (altitude of about 1420 m, 5°26 latitude North and 10°26 longitude East) located in the Menoua division of the Western highlands of Cameroon. The identity of the plant was confirmed in the Plant Biology Department of the University of Dschang. The plant materials were air dried at room temperature (23 ± 1°C) for 3 days before being submitted to hydrodistillation using Clevenger apparatus type for 6 h. EO collected was dried over anhydrous sodium sulphate, filtered and weighed yielding 0.89% (W/W) of pale yellow oil.
Chemical analysis of the oil was achieved by GC-MS on a HP 5890 II gas chromatograph coupled to a HP 5972 mass selective spectrometer using a DB wax fused silica capillary column. Its chemical constituents were identified as: 1.8-Cineole (58.49%), 3-Carene (8.61%), α-terpinol (7.83%), limonene (7.01%), β-linalool (1%), β-pinene (0.93%), 4-terpinenol (0.79%), 2-methylpropylisobutyrate (0.44%), α-pinene (0.38%), ocimenol (0.18%), eugenol (0.17%), Isoamylacetate (0.12%) and ocimene (0.81%).
Insects
Adults of A. obtectus and C. maculatus were cultured on dried whole bean and cowpea grains respectively according to the method used by Tapondjou et al. (2005). Cultures were then maintained in 5 l glass jars held in a controlled temperature chamber at 27 ± 2°C, relative humidity of 75 ± 5% and photoperiod of LD 12 : 12 h (light : dark) (Tapondjou et al. 2005). To assure high oviposition, 20 days after infestation of the glass jars with adult insects, all of them (alive and dead) were sieved out to enable from the 30th day emergence of new individuals that could be classified as same-age progeny. Unsexed adults of 1-day old were used for toxicity tests.
Preparation of aromatized clay powder
The mineral material used was fine white clay of smectitic nature and montmorillionitte type, named Sabga. This clay, sampled at Bambili in the locality of Bamenda (North-West Division of Cameroon), was initially investigated by Tonle (2004) for its chemical and mineralogical composition. The powder was obtained by crushing the dry clay in a mortar (Coors USA 60316) and by sieving through a mesh size of 106 μm (Arthor. H. Thomas).
Preliminary tests were carried out to choose the non-toxic quantity of clay powder to insects and which must be able to remain in powder form but not paste after admixture with tested volumes of EO. Four different samples of ACP were prepared by mixing separately 2, 4, 8 and 16 μl of EO with 0.05 g of clay powder. The mixtures were manually stirred for 5 min to ensure homogenous spread of the EO over the clay powder.
Biological tests
Experimental conditions
Fumigation, contact toxicity and persistence of the EO and its ACP were carried out in glass jars of 270 cm3 volume placed in a chamber conditioned at a photoperiod of LD 12 : 12 h (light : dark) at 27 ± 2°C and 75 ± 5% r.h.
Insecticidal efficiency of EO towards adults of A. obtectusandC. maculatus
To test the efficiency of EO towards the insects, contact toxicity on grains was evaluated as earlier described by Tapondjou et al. (2003). The contact toxicity of EO against adults of A. obtectus and C. maculatus were carried out in glass jars containing bean and cowpea grains, respectively. Test solutions were obtained by diluting 2, 4, 8 and 16 μl of EO of C. viminalis in 1 ml of acetone. Sixty grams samples of grains contained in 270 cm3 glass jars were mixed with each of the previous test solutions by tumbling for 5 min to ensure homogenous spread of the material over the surface of the grains. In the control jars the grains were treated only with acetone (1 ml) and all the jars were manually stirred for 5 min and kept open during 15 min to allow the complete evaporation of solvent. The grains were then infested with 1 day old unsexed adult insects (25 per jar) and each jar was covered with fine cloth porous maintained with rubber bands. Each treatment was replicated four times. Mortality counts were made daily up to 4 days.
Fumigation of adult insects with EO
Knowing that by contact toxicity the EO can act both by contact and/or fumigation, the fumigant effect of the EO was checked through to evaluation of the toxicity of its vapours to adult’s stage of each of the two insects. As such, airtight glass jars of 270 cm3 volume with screwed metallic cap were used as exposure chambers. Twenty unsexed adult insects of 1-day old were introduced in each jar. A small piece of filter paper (Whatman No. 1) of 4 cm2 was attached using a cotton wire hung in the half height of the undersurface cap to serve as an oil diffuser on which varying volumes (2, 4, 8 and 16 μl) of EO were applied. After diffusion of EO, the supposed concentrations occurred were 0.007, 0.015, 0.029 and 0.059 μl/cm3 respectively; these concentrations were obtained by dividing each quantity of essential oil by the inner volume of the glass jar (270 cm3). Control treatment consisted of an identical apparatus but without EO. Four replications were carried out for each set of treatment. The number of dead insects was counted after 6, 12, 18 and 24 h. Knocked-down adults were regarded as alive if they showed continued movement of their appendages.
Insecticidal efficiency of ACP towards adults of A. obtectusandC. maculatus
Each sample of ACP previously prepared was introduced into a glass jar containing 60 g of grains and the jars were manually stirred so that all the grains were uniformly coated, thus leading to the following doses of 0.033, 0.066, 0.133 and 0.266 μl/g (volume of EO per quantity of grains), respectively. In the control jar, the grains were treated with non-ACP. Twenty-five 1-day-old adult insects of mixed sex were introduced into each jar. Four replications were carried out for each dose. Each jar was covered with fine cloth porous maintained using rubber bands of fastener. The number of dead individuals was counted daily up to 4 days.
Effect of EO and ACP on F1 progeny production
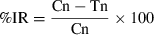
Where Cn is the number of newly emerged insects in the untreated (control) jar and Tn the number of insects in the treated jar.
Evaluation of the persistence of the biological activity of EO towards adults of A. obtectusandC. maculatusin the course of time
Two doses were used to evaluate the persistence of the activity of the EO on grains towards the two insects: the dose having induced the highest mortality of insects in each case (0.266 μl/g of grain) and the dose corresponding to the LD50 value in each case (0.133 μl/g of grain for A. obtectus and 0.170 μl/g of grain for C. maculatus). The volume of oil corresponding to each of these doses (16 and 8 μl for A. obtectus and, 16 and 10.5 μl for C. maculatus) was diluted in 1 ml of acetone and introduced into a glass jar containing 60 g of grains. In the control jar the grains were treated only with acetone (1 ml) and all the jars were manually stirred for 5 min and kept open during 20 min to allow the complete evaporation of solvent. Thereafter, all the jars were covered with a piece of fine cloth porous maintained using rubber band to allow the ventilation. Thirty-six jars were prepared for each of the three treatments (two doses and one control) and divided into nine groups of four jars each. For each treatment the four jars of the nine different groups were gradually infested by 25 one-day-old adult insects after 0, 6, 12, 18, 24, 30, 36, 42, 48 h and the dead insects in each group were counted 1 day after infestation.
Evaluation of the persistence of the activity of ACP towards adults of A. obtectusand C. maculatusin the course of time
The same conditions as previously described for the EO were used to carry out this experiment except that the previous volumes of oil (8 and 16 μl for A. obtectus and 10.5 and 16 μl for C. maculatus) were directly mixed with 0.05 g of clay powder and the control was made with clay powder only (0.05 g).
Data analysis
All the data concerning mortality were corrected by using Abbott’s formula (Abbott 1925). Data obtained from each dose–response bioassay were subjected to probit analysis in which probit-transformed mortality was regressed against log-transformed dose; LC50/LD50 values were generated (Finney 1971). One-way analysis of variance was performed to compare the effect of dose tested for each exposure period. Means were separated using a subsequent Waller–Duncan and t Student (Steel and Torrie 1980). All these data analysis were performed using SPSS 2000 program.
Results
Insecticidal efficiency of EO towards adults of A. obtectus and C. maculatus
Figure 1a, b show the percentage mortalities of A. obtectus and C. maculatus respectively in grains treated with different doses of the EO of C. viminalis after different intervals of times. It appears that there was a dose-dependant increase in mortality of adult insects treated with the EO; the LD50 values after 2 days exposure were 0.133 μl/g and 0.170 μl/g towards A. obtectus and C. maculatus respectively. In addition, there was a significant difference (P < 0.05) between the mortalities induced by the highest doses (0.133 and 0.266 μl/g of grain) and the lowest ones (0.033 and 0.066 μl/g of grain) after 4 days exposure. As shown by the LD50 values and by general observation of the two figures A. obtectus seems to be more susceptible to the EO of C. viminalis than C. maculatus.
(a) Effect of different doses of EO of Callistemon viminalis on mortality of Acanthoscelides obtectus adults. (b) Effect of different doses of EO of C. viminalis on mortality of Callosobruchus maculatus adults.
Fumigation of adult insects with EO
After 12 h of fumigation, mortality of adult insects in each case was found to increase as the EO concentration increased (table 1). At the highest concentration (0.059 μl/cm3), 83.7% mortality was recorded with A. obtectus, 100% with C. maculatus and 0% in the control. However, after 6 h the EO exhibit quick knock-down activity in all the concentrations except in the control test. Moreover, the data mentioned in table 1 show that after 12 h exposure C. maculatus is more susceptible to the vapours of the EO of C. viminalis than A. obtectus. This observation was confirmed with the LC50 values calculated after 12 h exposure which were 0.019 and 0.011 μl/cm3 towards A. obtectus and C. maculatus, respectively.
| Exposure time (h) | Insects | Concentration (μl/cm3 surface of jar) | ||||
|---|---|---|---|---|---|---|
| 0.000 | 0.007 | 0.015 | 0.029 | 0.059 | ||
| 6 | Acanthoscelides obtectus | 0.0 ± 0.0a | 2.5 ± 2.8a | 2.5 ± 2.8a | 35.0 ± 16.8b | 58.7 ± 6.3c |
| Callosobruchus maculatus | 0.0 ± 0.0a | 5.0 ± 0.0a | 21.3 ± 4.7b | 68.7 ± 2.5c | 88.7 ± 13.1d | |
| 12 | A. obtectus | 0.0 ± 0.0a | 21.3 ± 8.5b | 37.5 ± 8.6c | 63.7 ± 13.1d | 83.7 ± 6.3e |
| C. maculatus | 0.0 ± 0.0a | 25.0 ± 12.2b | 72.5 ± 9.6c | 92.5 ± 2.8d | 100.0 ± 0.0d | |
| 18 | A. obtectus | 0.0 ± 0.0a | 37.5 ± 10.4b | 68.7 ± 16.0c | 100.0 ± 0.0d | 100.0 ± 0.0d |
| C. maculatus | 0.0 ± 0.0a | 32.5 ± 8.6b | 91.3 ± 6.3c | 98.7 ± 2.5d | 100.0 ± 0.0d | |
| 24 | A. obtectus | 0.0 ± 0.0a | 51.3 ± 22.8b | 81.3 ± 4.8c | 100.0 ± 0.0d | 100.0 ± 0.0d |
| C. maculatus | 0.0 ± 0.0a | 41.3 ± 12.5b | 97.5 ± 2.8c | 100.0 ± 0.0c | 100.0 ± 0.0c | |
- Mortalities(%) (Mc ± SD) in any line followed by the same alphabetical letter are not significantly different (P > 0.05) at Waller-Duncan test.
- Mc, corrected mortality; SD, standard deviation.
Insecticidal efficiency of ACP towards adults of A. obtectus and C. maculatus
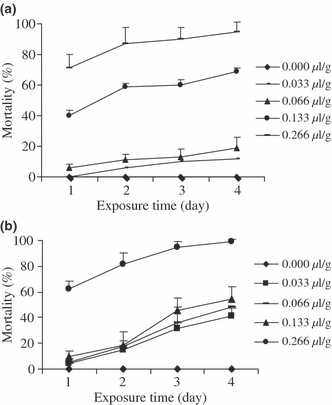
As shown on fig. 2a, b, the effect of the ACP based on the mixture of clay powder with the different volume of EO of C. viminalis on grains was dose-dependant and no mortality was recorded in the control jars after 4 days exposure. There was significant difference (P < 0.05) between mortalities induced by the doses of 0.066, 0.133 and 0.266 μl/g against A. obtectus during the 4 days. In the case of C. maculatus, from the second day exposure all the mortalities recorded in glass jars (treated and control) show a significant difference (P < 0.05) between each others. The LD50 values of ACP calculated after 2 days exposure were 0.100 and 0.098 μl/g towards A. obtectus and C. maculatus, respectively.
(a) Effect of different doses of ACP on mortality of Acanthoscelides obtectus adults. (b) Effect of different doses of ACP on mortality of Callosobruchus maculatus adults.
Comparison of the effects of the EO and its ACP against A. obtectus and C. maculatus
The effects of the EO and its ACP against adults of A. obtectus and C. maculatus compared at the same dose and exposure time are shown in tables 2 and 3. In the two cases, it arises that during all the 4 days exposure, mortalities induced by ACP at the doses of 0.133 μl/g and 0.266 μl/g were in general significantly higher than those caused by the same doses of EO applied directly.
| Exposure time (day) | Product applied on grain | Dose (μl/g of grains) | ||||
|---|---|---|---|---|---|---|
| 0.000 | 0.033 | 0.066 | 0.133 | 0.266 | ||
| 1 | EO | 0.0 ± 0.0a | 0.0 ± 0.0a | 6.0 ± 2.3a | 40.0 ± 3.3a | 71.0 ± 8.8a |
| ACP | 0.0 ± 0.0a | 0.0 ± 0.0a | 5.3 ± 2.3a | 52.0 ± 6.9b | 93.3 ± 4.6b | |
| 2 | EO | 0.0 ± 0.0a | 6.0 ± 2.3a | 11.0 ± 3.8a | 59.0 ± 2.0a | 87.0 ± 10.5a |
| ACP | 0.0 ± 0.0a | 6.6 ± 2.3a | 8.0 ± 4.0a | 70.6 ± 12.2a | 96.0 ± 6.0a | |
| 3 | EO | 0.0 ± 0.0a | 10.0 ± 4.0a | 13.0 ± 5.0a | 60.0 ± 3.3a | 90.0 ± 7.6a |
| ACP | 0.0 ± 0.0a | 17.3 ± 6.1a | 18.6 ± 4.6a | 81.3 ± 8.1b | 100.0 ± 0.0a | |
| 4 | EO | 0,0 ± 0,0a | 12.0 ± 3.3a | 19.0 ± 6.8a | 69.0 ± 2.0a | 95.0 ± 6.0a |
| ACP | 0.0 ± 0.0a | 20.0 ± 8.0a | 28.0 ± 10.6a | 88.0 ± 8.8b | 100.0 ± 0.0a | |
- Mortalities(%) (Mc ± SD) followed by the same alphabetical letter in a column or for the same concentration are not significantly different (P > 0.05) at Student test.
- Mc, corrected mortality; SD, standard deviation; EO, Essential Oil of Callistemon viminalis (μl/g of grains). ACP, Aromatized Clay Powder (μl/g of grains).
| Exposure time (day) | Product applied on grain | Dose (μl/g of grains) | ||||
|---|---|---|---|---|---|---|
| 0.000 | 0.033 | 0.066 | 0.133 | 0.266 | ||
| 1 | EO | 0.0 ± 0.0a | 4.0 ± 0.0a | 5.3 ± 2.3a | 9.3 ± 4.6b | 62.7 ± 6.1a |
| ACP | 0.0 ± 0.0a | 4.0 ± 0.0a | 9.3 ± 6.1a | 22.7 ± 46a | 85.3 ± 10.1b | |
| 2 | EO | 0.0 ± 0.0a | 14.7 ± 2.3b | 17.3 ± 4.6a | 18.7 ± 10.1a | 81.3 ± 9.2a |
| ACP | 0.0 ± 0.0a | 9.3 ± 2.3a | 14.7 ± 2.3a | 25.3 ± 4.6a | 98.7 ± 2.3b | |
| 3 | EO | 0.0 ± 0.0a | 32.0 ± 4.0b | 36.0 ± 12.0a | 45.3 ± 10.1a | 94.7 ± 4.6a |
| ACP | 0.0 ± 0.0a | 13.3 ± 2.3a | 25.3 ± 6.1a | 36.0 ± 12.0a | 100.0 ± 0.0a | |
| 4 | EO | 0.0 ± 0.0a | 41.3 ± 6.1b | 48.0 ± 0.0a | 54.0 ± 10.0a | 98.7 ± 2.3a |
| ACP | 0.0 ± 0.0a | 26.7 ± 4.6a | 49.3 ± 10.1a | 68.0 ± 10.6a | 100.0 ± 0.0a | |
- Mortalities(%) (Mc ± SD) followed by the same alphabetical letter in a column or for the same concentration are not significantly different (P > 0.05) at Student test.
- Mc, Corrected Mortality; SD, standard deviation; EO: Essential Oil of Callistemon viminalis (μl/g of grains); ACP: Aromatized Clay Powder (μl/g of grains).
F1 progeny Production
The EO of C. viminalis and its ACP effectively have an effect on the first progeny production as shown in table 4. The percentage of inhibition of adults of A. obtectus and C. maculatus at F1 increased with the dose of the EO and the ACP. The dose of 0.133 μl/g caused 100% and 68.7% inhibition of F1 progeny of A. obtectus and C. maculatus respectively. It appears from table 4 that every dose of EO or ACP applied on grains induced higher inhibition of F1 progeny production of A. obtectus compared with that of C. maculatus. Moreover, significant differences (P < 0.05) were observed in the numbers of F1 progeny produced in grains treated with EO compared with those obtained with its ACP.
| Dose (μl/g of grain) | Acanthoscelides obtectus | Callosobruchus maculatus | |||
|---|---|---|---|---|---|
| Number of emerged insects | Percentage of inhibition of adults of Acanthoscelides obtectus at F1 | Number of emerged insects | Percentage of inhibition of adults of Callosobruchus maculatus at F1 | ||
| Control | EO | 16.0 ± 6.0a | 0 | 249.0 ± 18.2a | 0 |
| ACP | 7.0 ± 3.0a | 0 | 147.0 ± 2.9b | 0 | |
| 0.033 | EO | 6.0 ± 1.0a | 62.5 | 209.0 ± 13.9a | 16.1 |
| ACP | 2.0 ± 1.0b | 71.4 | 81.0 ± 2.9b | 44.9 | |
| 0.066 | EO | 4.0 ± 2.0a | 75 | 191.0 ± 21.3a | 23.3 |
| ACP | 1.0 ± 0.0b | 85.7 | 53.0 ± 24.9b | 64 | |
| 0.133 | EO | 2.0 ± 1.0a | 87.5 | 103.0 ± 6.9a | 58.6 |
| ACP | 0.0 ± 0.0b | 100 | 46.0 ± 1.8b | 68.7 | |
| 0.266 | EO | 0.0 ± 0.0a | 100 | 0.0 ± 0.0a | 100 |
| ACP | 0.0 ± 0.0a | 100 | 0.0 ± 00a | 100 | |
- Means (N ± SD) followed by the same alphabetical letter in a column and for the same concentration are not significantly different (P > 0.05) at Student test.
- N, Average number of emerged insects; SD, standard deviation; EO, Essential Oil of Callistemon viminalis (μl/g of grains); ACP, Aromatized Clay Powder (μl/g of grains).
Toxicity and persistence of the activity of EO and its ACP towards adults of A. obtectus and C. maculatus in the course of time
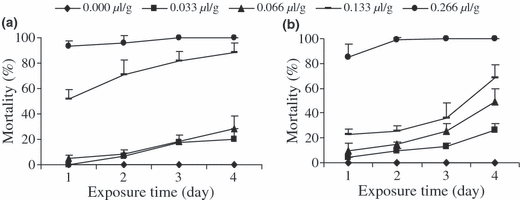
Globally, the toxicity of EO and ACP decrease in the course of time (fig. 3). Nevertheless, the ACP remained more toxic and its effect more persistent compared with EO. For example at the dose of 0.266 μl/g, the EO completely loosed its activity after 36 h while the ACP loosed it after 48 h exposure against adults of A. obtectus; the situation is similar with C. maculatus (fig. 4).
Evolution of Acanthoscelides obtectus mortalities exposed to different doses of EO and ACP of Callistemon viminalis according to exposure time before infestation.
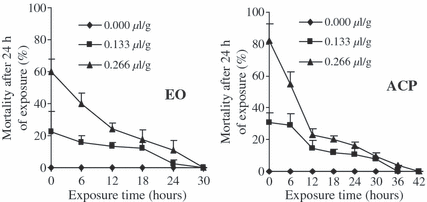
Evolution of Callosobruchus maculatus mortalities exposed to different doses of EO and ACP of Callistemon viminalis according to exposure time before infestation.
Discussion
In this study, the mortality of the insect species tested both by contact and by fumigation varied with the dose of EO or ACP and the insect species. High mortality rates and inhibition of F1 progeny production were recorded by contact in grains treated with EO for A. obtectus compared with C. maculatus. However, on grains treated with ACP, adults of A. obtectus and C. maculatus were very susceptible and their LD50 were much closed (0.100 and 0.098 μl/g, respectively). The analysis of the fumigation data showed that the EO vapours exhibit a strong toxic action against the adults of A. obtectus and C. maculatus. It has also been established that the ACP generally remained more toxic and its effect more persistent compared with EO applied directly. Emergence of adult insects from all control samples indicated that test insects were capable of effective oviposition within the different periods used for mortality studies and that prevention of progeny emergence was exclusively because of treatments. All these results indicated that EO from the leaves of C. viminalis is a source of biologically active vapours as earlier mentioned by Keita et al. (2000) for various other EO vapours. In fact, EOs may block respiration or disrupt the water balance of eggs and developing embryos (Messina and Renwick 1983), and consequently affect negatively the emergence of new adult insects.
The variation in the responses of the two bruchids could be attributed to the morphological and behavioural differences between them and any toxicity effect occurred in the present test could be attributed to the volatile compounds contained in the EO. Although, the mode of insecticidal action of EOs is not formally identified, that of certain constituents taken individually is recognized. The knock-down effect observed during the fumigation test could be attributed to limonene contained in this oil; indeed this component was recognized in its rapid provocation of knock-down and the dead of adults of Blattella germinica and Musca domestica (Karr and Coats 1988). Furthermore, 1,8-cineole and limonene present in the EO of C. viminalis are toxic by penetrating insect’s body through the respiratory system (fumigant effect), the cuticle (contact effect) or digestive system (ingestion effect) (Prates et al. 1998). In addition, in contact with insects, 1,8-cineole act by blocking the synthesis of juvenile hormones. It inhibits acetylcholinesterase by occupying the hydrophobic site of enzyme’s active centre (Obeng-Ofori et al. 1997). Moreover, the toxicity of the EO no matter how it is used couldn’t be attributed only to its major constituents, but generally these components act synergically.
The loss of insecticidal activity of EO and ACP in the course of time may be attributed to rapid evaporation and degradation of the chemicals (Obeng-Ofori et al. 1997). It was demonstrated that, oils with high content of hydrogenated compounds loss their activity quicker than those containing mainly oxygenated compounds (Huang and Ho 1998; Regnault-Roger et al. 2002). The speed of the oxidation of hydrogenated monoterpenes is greater for compounds as sabinene, 1,8 cineole and α-pinene; this oxidation leads to the reduction of the insecticidal efficiency of the oil (Kim et al. 2002). The high persistence of the ACP on grains could be explained by the fact that the clay powder has a high absorbent properties (Tonle 2004), and as such fixed the volatile constituents and gradually released them out in the course of time.
The results obtained suggest good potential for the use of the EO of C. viminalis as toxic agent against adults of A. obtectus and C. maculatus. However, this oil would be advised for the protection of bean than cowpea grains because of its higher toxicity towards adults of A. obtectus compared with C. maculatus. It will also be advisable to use mineral material such as clay as carrier of EO to provide a protection of food for long duration. The advantage of this formulation is that it can be prepared on site with local materials, providing farmers with a natural insecticide that is compatible with their culture. While oils used alone can effectively protect stored grains from insects, it can leave a persistent odour that can be unpleasant when eating the seeds (Pierrard 1986). The use of clay powders aromatized with EOs has a twofold advantage because of the combined effects of mechanism action, blocking the insect’s respiration, filling intergranular spaces at high doses, and chemical action, acting primarily on granular cells (Ramaswamy et al. 1995).
Acknowledgements
The authors would like to thank Dr Tonle K. Ignas (Department of Chemistry, University of Dschang, Cameroon) for provision of clay sample. Financial support was partially provided by the Belgian Cooperation to Development (CUD) through “Storeprotect” project. The authors thank the reviewers for their comments.




