Efficacy of pymetrozine against Myzus persicae and in reducing potato virus Y transmission on tobacco plants
Abstract
Forty-four parthenogenetic lineages of Myzus persicae s.l. (Sulzer) from tobacco crops and peach orchards located in various regions of Greece were examined to determine their response to the insecticide pymetrozine using leaf-dip bio-assays. The results show that the aphid has not developed resistance, as all lineages exhibited resistance factors bellow 6.0. In transmission experiments of potato virus Y (PVY) using a lineage of the tobacco-adapted subspecies M. persicae nicotianae Blackman on tobacco plants, one foliar application with pymetrozine provided adequate protection for 7 days. Pymetrozine significantly reduced both virus acquisition and inoculation compared with the untreated control and the reduction was comparable to a mineral oil application. These results are discussed in terms of the advantage of incorporating pymetrozine as a compound of pest management strategies against M. persicae s.l. and for control of non-persistent viruses, especially in crops such as tobacco because of the high selection pressure from neonicotinoids resulting in potential of resistance developing in aphid populations.
Introduction
The green peach aphid Myzus persicae sensus lato (Sulzer) (Hemiptera: Aphididae) is a worldwide insect pest causing serious direct and indirect damages by vectoring viruses (Blackman and Eastop 2000). It is considered to be one of the most important virus vectors transmitting over 200 plant-viruses. Myzus persicae exhibits a typical holocyclic (cyclical parthenogenetic) life cycle, a sexual generation laying over-wintering eggs on peach, Prunus persica L. (Rosaceae), through autumn and winter alternates with a succession of parthenogenetic (all-female) generations through spring on peach and summer on various herbaceous crops. Some aphid genotypes have completely or partially lost the sexual generation and thus overwinter anholocyclically on various weeds or winter crops, either reproducing exclusively by parthenogenesis or contributing few males (androcyclic genotypes) and/or few mating females (intermediate genotypes) to the sexual phase (Blackman 1971; Margaritopoulos et al. 2002). Therefore, M. persicae populations on summer crops will be established by a mixture of emigrants developed on peach trees, weeds, or winter crops.
The control of Myzus persicae worldwide almost exclusively involves the use of chemical insecticides belonging to various chemical classes. However, such reliance on insecticides for over four decades has resulted in populations developing resistance. Multiple mechanisms of insecticide resistance are exploited by aphids. One mechanism involves overproduction of two closely related carboxylesterases (E4 and FE4) that sequester or hydrolyse organophosphate and carbamate based insecticides and, to some extent, pyrethroid based insecticides (Devonshire and Moores 1982; Field et al. 1988). Two other mechanisms involve insensitive target sites. The first is a modified acetylcholinesterase (MACE) that confers resistance to dimethyl carbamates, pirimicarb and triazamate (Moores et al. 1994). The second is a mutation in the voltage-gated sodium channel protein (kdr) conferring resistance to pyrethroid based insecticides (Martinez-Torres et al. 1999). Neonicotinoids is another class of insecticides used for controlling M. persicae. Currently, there is no evidence that neonicotinoids do not control aphids when applied at recommended rates. However, low-level tolerance (generally <10-fold) (Nauen et al. 1996; Foster et al. 2003) and in a few cases higher resistance factors (Nauen and Elbert 1997; Margaritopoulos et al. 2007b) have been reported. Because of insecticide resistance problems in carbamate, organophosphate and pyrethroid based insecticides or the risk of resistance development to the neonicotinoids, compounds with a novel mode of action may reduce the potential for resistance and assist pest management programs. Pymetrozine, a pyridine azomethine, has specific activity against phloem-feeding insects including aphids, planthoppers and whiteflies, and is an anti-feedant (Flückiger et al. 1992; Allemann et al. 1995; Nicholson et al. 1996). It has been demonstrated that pymetrozine causes immediate and irreversible feeding cessation of aphids resulting in death within days due to starvation (Kayser et al. 1994; Harrewijn and Kayser 1997).
However, the situation is more complicated when protection against non-persistent virus transmission by M. persicae on crops is considered. In non-persistent transmission manner, the acquisition and inoculation periods are very short (seconds to minutes) and there is no latent period (Fereres and Collar 2001). An ideal insecticide should kill aphids before they are able to accomplish the transmission process, which is rather difficult for non-persistent viruses taking also into account that many aphid vectors do not colonize the crop and they come in contact with the insecticides for a very limited period. Therefore, efficient control of aphid populations in the field with chemical insecticides may not inhibit virus transmission (Ferro et al. 1980; Maelzer 1986). Some studies reported that pyrethroid based insecticides with a quick knockdown could be used to control non-persistent viruses (Gibson et al. 1982; Perring and Farrar 1993) but this has not been finally adopted. An alternative strategy against non-persistent viruses, especially in high value crops has been the use of light mineral oils. Bradley et al. (1962) first discovered that mineral oils inhibit transmission of non-persistent viruses by aphids and since then various studies have demonstrated that light oils either alone or in combination with insecticides prevent or reduce the spread of non-persistent viruses (Ferro et al. 1980; Gibson and Rice 1986; Perring et al. 1999). Although the mechanism is not fully exploited it seems that mineral oils interfere with the retention of viral particles in the aphid stylets (Qiu and Pirone 1989; Wang and Pirone 1996); however, behavioural changes induced by the mineral oils may also be involved (Simons et al. 1977; Fereres and Collar 2001). Because pymetrozine has anti-feedant properties, it has also been used against aphid-transmitted viruses and significantly reduced transmission of the semi-persistent cauliflower mosaic virus (CaMV) on turnip, Brassica campestris L. (Brassicaceae) (Bedford et al. 1998); the non-persistent Potato virus Y (PVY) (Harrewijn and Piron 1994); and the persistent potato leaf roll virus (PLRV) (Harrewijn and Piron 1994; Mowry 2005) on potato, Solanum tuberosum L. (Solanaceae).
In Greece, M. persicae s.l. is one of the most important insect pests of peach and tobacco Nicotiana tabacum L. (Solanaceae). In addition to the direct damage caused by the tobacco-feeding subspecies Myzus persicae nicotianae Blackman (tobacco aphid) tobacco crop is susceptible to non-persistent viruses such as PVY, alfalfa mosaic virus (AMV) and cucumber mosaic virus (CMV) (Chatzivassiliou et al. 2004). Insecticides belonging to different chemical classes such as organophosphates, carbamates, pyrethroids and neonicotinoids have been used against M. persicae s.l. with variable success. In high value crops a common practice for the control of non-persistent viruses is the use of light mineral oils alone or in combination with chemical insecticides. Pymetrozine is used on tobacco and other crops as an alternative to the aforementioned insecticides against M. persicae s.l. or in some cases as the primary insecticide. As pymetrozine has been used in Greece for more than a decade, it is essential to determine the status of resistance in M. persicae s.l. populations throughout Greece in order to evaluate its potential use in pest control or resistance management programs. Furthermore, there is no information whether pymetrozine may reduce spread of non-persistent viruses transmitted by M. persicae nicotianae on tobacco. Therefore, the objectives of the present study was to determine whether M. persicae s.l. has developed resistance in pymetrozine and to evaluate the effects of pymetrozine in PVY transmission on tobacco plants by the tobacco aphid M. persicae nicotianae. As such we report on leaf-disc bio-assays comparing the response of M. persicae s.l. parthenogenetic lineages from various regions of Greece to pymetrozine, and PVY transmission on tobacco plants by a M. persicae nicotianae lineage comparing the effectiveness of pymetrozine to a mineral oil.
Materials and methods
Myzus persicae parthenogenetic lineages used in bio-assays
Forty-four M. persicae s.l. parthenogenetic lineages were used in the bio-assay experiments with pymetrozine. Forty-two lineages derived from aphids collected from tobacco and peach in several regions of Greece during 2005–2006 (fig. 1). One lineage was collected near Ljubljana, Slovenia from potato in 2006 and a laboratory M. persicae strain (US1L), which does not have the three known resistance mechanisms, were also used. US1L was collected in England and it has been reared in the laboratory since 1974. A sample of this strain was kindly provided by Rothamsted-Research for the purposes of the present study. Sampling included aphid lineages that have different life cycle category and exhibit variable genetic properties. Both green and red colour lineages of M. persicae were included in the study and before experimentation their life cycle category was determined according to Margaritopoulos et al. (2002) (table 1). In addition, sampling focused on both subspecies of M. persicae. Aphid populations on peach trees in eastern central regions belong to the subspecies M. persicae persicae, while those from tobacco plants as well as from peach trees in the north belong to the subspecies M. persicae nicotianae (Margaritopoulos et al. 2007a). Aphid lineages were maintained on Chinese cabbage, Brassica chinensis Juslen (Brassicaceae) leaves in Blackman boxes (Blackman 1971) for several generations at 17 ± 0.5°C and a photoperiod of L16:D8. Then the lineages were mass reared on potted radish Raphanus sativus L. (Brassicaceae) plants covered with plastic cages to produce an adequate number of individuals for the bio-assay experiments. An opening at the top of the cages sealed with aphid-proof fine muslin allowed adequate ventilation.
Sampling sites in Greece. Northern Greece, 1: Meliki (peach, tobacco), 2: Katerini (peach, tobacco), 3: Kozani (peach, tobacco); Eastern Central Greece, 4: Volos (peach); Central Greece, 5: Karditsa (tobacco); Southern Greece, 6: Amfiklia (tobacco), 7: Naphplion (tobacco).
| Lineages | Locality | Region | Host | Colour | LC | N | n | 4 days post-treatment | 7 days post-treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | LC50 | (95% CL) | χ 2 | RF | Slope | LC50 | (95% CL) | χ 2 | RF | ||||||||
| 06MelT39 | Meliki | NGR | Tobacco | Green | Hol | 487 | 6 | 2.90 | 7.67 | (6.27–9.37) | 7.2 | 5.4 | 2.97 | 5.73 | (4.81–6.81) | 12.9* | 6.0 |
| 06KozT06 | Kozani | NGR | Tobacco | Red | An | 507 | 5 | 3.73 | 7.29 | (6.02–8.82) | 7.3 | 5.1 | 4.11 | 4.86 | (4.13–5.72) | 3.6 | 5.1 |
| 06KozT09 | Kozani | NGR | Tobacco | Red | An | 347 | 5 | 3.14 | 6.94 | (4.84–9.94) | 3.8 | 4.9 | 3.84 | 4.57 | (3.38–6.18) | 7.7 | 4.8 |
| 06KatT77 | Katerini | NGR | Tobacco | Red | An | 333 | 6 | 3.62 | 6.88 | (5.04–9.39) | 7.7 | 4.8 | 2.61 | 4.04 | (3.16–5.18) | 10.6* | 4.2 |
| 05KarT55 | Karditsa | CGR | Tobacco | Green | Hol | 508 | 5 | 3.73 | 6.65 | (5.19–8.52) | 11.3* | 4.7 | 3.80 | 4.57 | (3.79–4.57) | 7.2 | 4.8 |
| 06MelT92 | Meliki | NGR | Tobacco | Red | An | 444 | 5 | 2.65 | 5.29 | (4.24–6.60) | 5.6 | 3.7 | 3.56 | 4.66 | (3.83–5.67) | 5.0 | 4.9 |
| 06MelT03 | Meliki | NGR | Tobacco | Red | An | 457 | 5 | 2.42 | 4.96 | (4.18–5.89) | 3.7 | 3.5 | 3.42 | 3.18 | (2.69–3.76) | 6.3 | 3.3 |
| 06LehP14 | Volos | ECGR | Peach | Green | Hol | 476 | 5 | 2.40 | 4.90 | (4.14–5.80) | 0.7 | 3.4 | 3.31 | 2.79 | (2.42–3.21) | 3.8 | 2.9 |
| 06KatT61 | Katerini | NGR | Tobacco | Red | Hol | 314 | 5 | 3.10 | 4.46 | (3.45–5.77) | 4.8 | 3.1 | 3.33 | 3.67 | (2.86–4.73) | 7.1 | 3.8 |
| 06KozP21 | Kozani | NGR | Peach | Green | Hol | 400 | 5 | 3.13 | 4.45 | (3.56–5.57) | 7.4 | 3.1 | 3.63 | 2.52 | (2.14–2.96) | 4.6 | 2.6 |
| 06VelP09 | Volos | ECGR | Peach | Green | Hol | 492 | 5 | 3.11 | 4.40 | (3.65–5.31) | 1.2 | 3.1 | 3.12 | 3.54 | (2.95–4.24) | 0.4 | 3.7 |
| 06MelT33 | Meliki | NGR | Tobacco | Red | An | 437 | 5 | 2.89 | 4.12 | (3.43–4.95) | 1.0 | 2.9 | 4.11 | 2.34 | (1.98–2.78) | 1.8 | 2.4 |
| 06MelT57 | Meliki | NGR | Tobacco | Green | Hol | 442 | 5 | 2.69 | 4.19 | (3.48–5.04) | 2.0 | 2.9 | 3.38 | 3.04 | (2.59–3.58) | 4.6 | 3.2 |
| 05KarT61 | Karditsa | CGR | Tobacco | Red | An | 409 | 5 | 3.27 | 4.03 | (3.24–5.02) | 6.0 | 2.8 | 4.01 | 1.05 | (0.91–1.21) | 1.6 | 1.1 |
| 05KarT06 | Karditsa | CGR | Tobacco | Red | An | 422 | 5 | 3.44 | 3.88 | (3.13–4.83) | 7.4 | 2.7 | 3.78 | 1.47 | (1.27–1.69) | 13.6* | 1.5 |
| 06KozP13 | Kozani | NGR | Peach | Green | Hol | 411 | 5 | 3.07 | 3.83 | (3.14–4.67) | 7.7 | 2.7 | 3.47 | 2.18 | (1.86–2.56) | 6.5 | 2.3 |
| 06MelT01 | Meliki | NGR | Tobacco | Red | An | 467 | 5 | 3.31 | 3.72 | (3.13–4.43) | 5.2 | 2.6 | 3.13 | 2.75 | (2.35–3.22) | 3.2 | 2.9 |
| 06KozT28 | Kozani | NGR | Tobacco | Red | An | 400 | 5 | 3.23 | 3.52 | (2.88–4.30) | 5.0 | 2.5 | 3.71 | 2.34 | (1.96–2.81) | 8.4 | 2.4 |
| 06MelP12 | Meliki | NGR | Peach | Green | Hol | 405 | 5 | 3.11 | 3.53 | (2.93–4.25) | 2.5 | 2.5 | 3.53 | 2.13 | (1.82–4.00) | 5.7 | 2.2 |
| 06KozP22 | Kozani | NGR | Peach | Green | Hol | 278 | 5 | 2.95 | 3.41 | (2.79–4.17) | 1.8 | 2.4 | 3.66 | 2.13 | (1.81–2.51) | 1.2 | 2.2 |
| 06NauT27 | Naphplion | SGR | Tobacco | Green | An | 386 | 5 | 2.93 | 3.40 | (2.86–4.03) | 1.6 | 2.4 | 4.08 | 1.86 | (1.60–2.17) | 7.5 | 1.9 |
| 06KozP10 | Kozani | NGR | Peach | Green | Hol | 387 | 5 | 3.17 | 3.10 | (2.60–3.69) | 8.0* | 2.2 | 3.45 | 1.55 | (1.31–1.83) | 2.5 | 1.6 |
| 05AmfT22 | Amphiklia | SGR | Tobacco | Green | An | 386 | 5 | 3.75 | 3.04 | (2.39–3.86) | 4.2 | 2.1 | 3.84 | 1.70 | (1.40–2.06) | 3.2 | 1.8 |
| 06MelP64 | Meliki | NGR | Peach | Green | Hol | 415 | 5 | 3.73 | 2.97 | (2.38–3.70) | 0.6 | 2.1 | 4.02 | 1.67 | (1.37–2.03) | 1.7 | 1.7 |
| 06KatT22 | Katerini | NGR | Tobacco | Red | Hol | 410 | 5 | 3.74 | 2.81 | (2.37–3.33) | 1.4 | 2.0 | 3.93 | 1.93 | (1.67–2.24) | 4.1 | 2.0 |
| 06LehP08 | Volos | ECGR | Peach | Green | Hol | 440 | 5 | 3.64 | 2.87 | (2.36–3.50) | 4.2 | 2.0 | 3.96 | 1.74 | (1.45–2.09) | 7.5 | 1.8 |
| 06MelT18 | Meliki | NGR | Tobacco | Red | An | 432 | 5 | 3.45 | 2.92 | (2.43–3.50) | 9.6* | 2.0 | 3.62 | 1.95 | (1.67–2.27) | 10.7* | 2.0 |
| 06MelT75 | Meliki | NGR | Tobacco | Red | Hol | 430 | 5 | 3.15 | 2.82 | (2.40–3.32) | 2.8 | 2.0 | 3.55 | 2.19 | (1.84–2.62) | 5.0 | 2.3 |
| 06NauT52 | Naphplion | SGR | Tobacco | Red | An | 428 | 5 | 3.39 | 2.89 | (2.42–3.46) | 8.5 | 2.0 | 3.56 | 1.59 | (1.34–1.87) | 3.2 | 1.7 |
| US1L | – | UK | – | Green | An | 409 | 5 | 3.24 | 2.89 | (2.43–3.44) | 4.5 | 2.0 | 3.69 | 1.79 | (1.52–2.11) | 1.0 | 1.9 |
| 06KarT05 | Karditsa | CGR | Tobacco | Red | An | 456 | 5 | 3.01 | 2.58 | (2.22–3.00) | 9.2* | 1.8 | 3.01 | 2.09 | (1.79–2.44) | 4.9 | 2.2 |
| 06KozT45 | Kozani | NGR | Tobacco | Red | An | 417 | 5 | 2.78 | 2.62 | (2.30–2.30) | 4.0 | 1.8 | 4.08 | 1.85 | (1.61–2.14) | 4.3 | 1.9 |
| 06KatT59 | Katerini | NGR | Tobacco | Red | An | 421 | 5 | 3.69 | 2.41 | (2.02–2.88) | 3.2 | 1.7 | 3.89 | 1.61 | (1.38–1.86) | 1.5 | 1.7 |
| 06LehP31 | Volos | CGR | Peach | Green | Hol | 265 | 5 | 3.78 | 2.41 | (1.88–3.10) | 0.4 | 1.7 | 4.15 | 1.37 | (1.09–1.71) | 1.1 | 1.4 |
| 06MelT17 | Meliki | NGR | Tobacco | Red | An | 418 | 5 | 3.21 | 2.26 | (1.95–2.61) | 5.8 | 1.6 | 3.84 | 1.48 | (1.27–1.73) | 4.4 | 1.5 |
| 06VelP40 | Volos | CGR | Peach | Green | Hol | 385 | 5 | 3.57 | 2.35 | (1.97–2.79) | 1.3 | 1.6 | 3.72 | 1.62 | (1.38–1.89) | 1.6 | 1.7 |
| 06MelP43 | Meliki | NGR | Peach | Green | Hol | 434 | 5 | 3.45 | 2.14 | (1.83–2.51) | 2.5 | 1.5 | 3.81 | 1.12 | (0.96–1.30) | 5.1 | 1.2 |
| 06KatP20 | Katerini | NGR | Peach | Green | Hol | 422 | 5 | 3.74 | 1.89 | (1.59–2.24) | 7.9 | 1.3 | 3.89 | 1.43 | (1.23–1.65) | 5.9 | 1.5 |
| 06KozT52 | Kozani | NGR | Tobacco | Red | An | 391 | 5 | 3.79 | 1.92 | (1.63–2.25) | 5.3 | 1.3 | 4.12 | 1.51 | (1.31–1.74) | 8.4 | 1.6 |
| 06LehP01 | Volos | CGR | Peach | Green | Hol | 371 | 5 | 3.69 | 1.83 | (1.55–2.16) | 0.1 | 1.3 | 4.34 | 1.23 | (1.05–1.44) | 3.9 | 1.3 |
| 06MelT73 | Meliki | NGR | Tobacco | Red | An | 443 | 5 | 3.86 | 1.92 | (1.62–2.28) | 2.9 | 1.3 | 4.20 | 0.97 | (0.83–1.15) | 7.2 | 1.0 |
| 06NauT05 | Naphplion | SGR | Tobacco | Green | An | 399 | 5 | 3.96 | 1.74 | (1.45–2.09) | 10.1* | 1.2 | 4.37 | 0.96 | (0.81–1.15) | 6.4 | 1.0 |
| 06Slov05 | Ljubljana | SLO | Potato | Green | An | 220 | 5 | 4.06 | 1.65 | (1.27–2.15) | 3.8 | 1.2 | 4.05 | 0.96 | (0.76–1.22) | 11.1* | 1.0 |
| 06LehP68 | Volos | CGR | Peach | Green | Hol | 419 | 5 | 3.87 | 1.43 | (1.24–1.65) | 10.2* | 1.0 | 3.80 | 1.16 | (1.01–1.34) | 6.8 | 1.2 |
- NGR, northern Greece; CGR, central Greece; ECGR, eastern central Greece; SGR, southern Greece; SLO, Slovenia; LC, life cycle category; Hol, holocyclic; An, anholocyclic.
- n = number of concentrations (excluding control) used by Siply Probit software for the regression. Six to seven concentrations were performed in each bio-assay replicated three times.
- N = Total number of aphids used in the bio-assays.
- RF = resistance factor.
- *Chi-square values are significant (P < 0.05).
Leaf-dip assays with pymetrozine
For each bio-assay, young leaves of tobacco variety S79 (oriental type) were dipped in 6–7 aqueous solutions of pymetrozine (Plenum 50WG, Syngenta Hellas) ranging from 0.547 to 35 mg/l and placed in Blackman boxes (control leaves were dipped in water only). In each box, 6–7 wingless adult females were allowed to reproduce for 1 day and 20–30 first-instar nymphs were retained. Nymphal mortality was recorded after 4 and 7 days. At the fourth day, the leaves were replaced with untreated fresh ones. Three replicates were used for each concentration including control. During the experiments all aphids were retained at 17 ± 0.5°C and a photoperiod of L16:D8.
PVY transmission assays
In this experiment, the effect of pymetrozine in transmission of PVY from and to tobacco plants by a red M. persicae nicotianae lineage (05KarT06) was determined. In addition to pymetrozine there was a light mineral oil treatment and untreated control. A PVYN isolate obtained from tobacco in northern Greece was used the experiments. The Virginia type tobacco variety VE9 was used as both a virus source and test plants. A different tobacco variety was used from the leaf-dip assays for reasons related to availability of tobacco plants. Virus source tobacco plants were used 3–4 weeks post-inoculation. All virus source plants and test plants were kept in wooden cages covered with aphid-proof netting in different compartments room at 20–23°C and a photoperiod of L16:D8. The tobacco plants had at least four expanded leaves and were approximately 15–20 cm tall when used for the transmission assays. The red lineage of M. persicae nicotianae, collected from tobacco in central Greece and retained on Chinese cabbage leaves in Blackman boxes at 17 ± 0.5°C and a photoperiod of L16:D8, was used in all transmission tests. The aphids were mass-reared on potted radish plants covered with plastic cages as described above.
Young (<24 h old) wingless adult females were obtained from the clonal culture and placed in glass vials for 30 min starving period. Then, batches of aphids were transferred to the upper side of a leaf on a virus source plant (two source plants were used in each transmission assay and treatment) for 3-min acquisition access period and then transferred onto the test plants (three aphids per plant) and left for 10-min inoculation access period. After the inoculation period was elapsed, the aphids were removed from the test plants and killed.
Two separate trials were conducted. In the first (day 0 assessment), there were five treatments: CC = both virus source and test plants untreated (control, sprayed only with water); CΟ = virus source plant untreated and test plant sprayed with mineral oil; CP = virus source plant untreated and test plant sprayed with pymetrozine; ΟC = virus source plant sprayed with mineral oil and test plant untreated; and ΡC = source plant sprayed with pymetrozine and test plant untreated. With this experimental design it was possible to infer about the effects of products on both PVY acquisition (OC and OP) and inoculation (CO and CP). Each product was applied as foliar spray to the point of “run off”. This trial were carried out 6 h post-treatment. To determine the duration of the protection against PVY transmission provided by pymetrozine and the mineral oil a second trial was conducted. The treatments were similar but transmission assays were performed 7 days post-treatment (day 7 assessment). In both, trials plants were sprayed with pymetrozine (Plenum 50 WG, Syngenta Hellas) and light mineral oil (BIOLID 80 EW, SIPCAM Hellas) at a rate of 0.15 g a.i./l and 5 g a.i./l, respectively. For each treatment, 15 tobacco test plants were used and the assays were replicated three times.
After transmission assays the test plants were retained in the cages described previously for approximately 20 days for PVY evaluation. An enzyme-linked immunosorbent assay (ELISA) procedure using a polyclonal antibody (Agdia Incorporated, Elkhart, IN, USA) was used to assess PVY infection.
Statistical analyses
LC50 values were calculated by probit analysis using Simple Probit 1.21 (Pisces Conservation Ltd, Lymington, UK). Simple Probit uses the maximum likelihood method of Finney (1966) to undertake the probit analysis. All three replications were used to calculate LC50 values. Resistance factors (RF) were calculated for each aphid lineage based on the formula LC50 of a lineage/LC50 of the most susceptible lineage. Mean LC50 values among Greek regions (northern Greece, tobacco; northern Greece, peach, eastern-central Greece, peach; southern and central Greece, tobacco) were compared using anova with ‘region’ and ‘observation’ (the two observations on mortality at 4 and 7 days post-treatment) as the main effects.
The effects of pymetrozine and the light mineral oil on PVY transmission by the M. persicae nicotianae lineage were analysed using anova with ‘treatment’ (oil, pymetrozine and control) and ‘time’ (day 0 and day 7 assessment) as the main effects. The arcsine transformed percentages of transmission were used in anova while non-transformed data are presented.
The above parametric statistics were followed as deviations from normality were not detected using the Kolmogorov–Smirnov test. Data were analysed using the spss statistical package version 10 (SPSS Inc., Chicago, IL, USA).
Results
Leaf-dip assays with pymetrozine
The aphid material used in the leaf-dip assays consisted of parthenogenetic lineages with different genetic properties. Red and green lineages and sexual and asexual genotypes were included as well as both subspecies of M. persicae (M. persicae: ECGR – peach; M. persicae nicotianae: CGR, SGR, NGR – tobacco and NGR – peach) (table 1). The LC50 values for pymetrozine, 4 days post-treatment ranged from 1.43 to 7.67 mg/l with a maximum RF of 5.4. Corresponding values 7 days post-treatment were 0.96 to 5.73 mg/l and RF of 6.0. A few cases of heterogeneity (significant chi-square test) were detected which may be attributed to environmental factors, such as variation of leaf quality, and less to variation between aphid individuals as the lineages used were clonal. The genetic traits (colour, life cycle category) of the aphid lineages or the geographical origin had non-significant effects on RF values (table 1). There was a significant difference between the post-treatment observations, with the mean LC50 value significantly lower 7 days (2.34 ± 0.18) than 4 days post-treatment (3.63 ± 0.25) (d.f.1,76, F = 15.96, P < 0.001). This shows that additional aphids died beyond 3 days when aphids were transferred to untreated leaves 4 days after exposure to pymetrozine. This enhanced mortality was observed in all the aphid lineages (table 1). There were significant differences among regions with the highest mean LC50 value observed in tobacco lineages from northern Greece (fig. 2) (d.f.3,76, F = 3.56, P < 0.018). The interaction between ‘region’ and ‘observation’ was not significant (d.f.3,76, F = 0.14, P < 0.269).
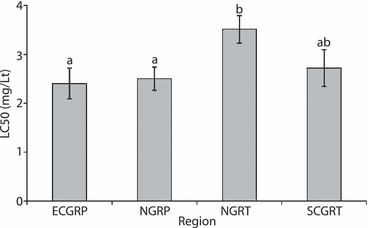
Mean LC50 values (mg a.i./l, bars denote SE.) of pymetrozine for Myzus persicae lineages from various regions of Greece (pooled data for 4 and 7 days post-treatment). ECGRP = eastern central Greece – peach, NGRP = northern Greece – peach, NGRT = northern Greece – tobacco, SCGRT = southern and central Greece (pooled data) – tobacco. Means followed by a different letter are significantly different from each other based on a Tukey test at P < 0.05.
PVY transmission assays
There was no significant interaction between the ‘treatment’ and ‘time’ (df4, 20, F = 0.38, P < 0.82). In addition, there was no significant difference in the percent transmission of PVY by the M. persicae nicotianae lineage between the assays performed 6 h (day 0 assessment) and 7 days post-treatment (day 7 assessment) (d.f.1,20, F = 0.48, P < 0.498). Pymetrozine and mineral oil significantly reduced (d.f.4,20, F = 15.98, P < 0.001) both PVY acquisition (OC and OP treatments) and inoculation (CO and CP treatments) compared with the untreated control (CC treatment) (table 2). Acquisition and inoculation rates were lower in tobacco plants treated with the mineral oil than in those treated with pymetrozine although the difference was not significant (Tuckey test, P > 0.05). The above trends were apparent looking separately at the transmission rates in the day 0 and day 7 assessment (table 2).
| N | Treatments | |||||
|---|---|---|---|---|---|---|
| CC | CO | CP | OC | PC | ||
| A | 45 | 57.8 ± 8.0 | 13.3 ± 6.7 | 15.6 ± 5.9 | 15.6 ± 5.9 | 22.2 ± 2.2 |
| B | 45 | 55.6 ± 5.9 | 11.1 ± 4.4 | 24.4 ± 2.2 | 17.8 ± 4.4 | 26.7 ± 7.7 |
| Mean | 56.7 ± 4.5a | 12.2 ± 3.6b | 20.0 ± 3.4b | 16.7 ± 3.3b | 24.4 ± 3.7b | |
- Transmission assays were performed 6 h (A) and 7 days (B) post-treatment.
- Mean percentages followed by a different letter are significantly different based on a Tukey test at P < 0.05.
- N = number of plants tested in three replications.
- CC = source plant untreated - test plant untreated, CO = source plant untreated – test plant treated with oil, CP = source plant untreated – test plant treated with pymetrozine, OC = source plant treated with oil – test plant untreated, PC = source plant treated with pymetrozine – test plant untreated.
Discussion
Pymetrozine is an insecticide, which has been used extensively against plant sucking insects in various countries, including Greece, since the early 1990’s (Flückiger et al. 1992; Allemann et al. 1995; Nicholson et al. 1996; Fuog et al. 1998). It causes irreversible cessation of feeding by preventing stylet insertion into the plant vascular system or by terminating sap sucking, depending on the feeding state. Pymetrozine does not have a toxic effect but cibarial dilator muscles, which are innervated via the thoracic ganglion, are inhibited (Harrewijn and Kayser 1997). It is has been suggested that pymetrozine acts through a novel mechanism that is linked to the serotonin (monoamine neurotransmitter) signalling pathway (Kaufmann et al. 2004). As such, the known insecticide resistance mechanisms of M. persicae (carboxylesterase overproduction, kdr and MACE) are not effective on pymetrozine (Foster et al. 2002). The bio-assays reported in the present study revealed low RF values (<6.0), which could indicate natural variation among aphid lineages or a low-level tolerance for the few samples demonstrating RFs 5–6. Therefore, our results show that although pymetrozine has been used for more than a decade against aphids including M. persicae s.l. in Greece resistance has not developed. This is in accordance with field trials conducted in two main tobacco-growing areas in Greece that demonstrated successful control of M. persicae nicotianae by pymetrozine (Papadopoulou et al. 2004). Lack of resistance was not related to geographical origin or to genetic properties of the aphid lineages examined, such as colour and life cycle category, as both green and red colour lineages that were capable of sexual reproduction or reproduced exclusively parthenogenetically were included in the study. A previous study with M. persicae s.l. reported sensitivity to pymetrozine (Foster et al. 2002). The authors used aphid lineages from UK and other countries reporting a highest RF value of 6.7. Some regional differences in LC50 were found in the present study with the highest mean value observed in tobacco lineages from northern Greece. This might suggest a higher selective pressure compared with the other regions.
The transmission experiments demonstrating that a single application of pymetrozine significantly reduced the transmission of PVY by a M. persicae nicotianae lineage and the reduction was comparable to that of a mineral oil. The insecticide affected both virus acquisition and inoculation. In a previous study, it was reported that pymetrozine affected PVY transmission onto potato by M. persicae primarily during the inoculation phase and much less during acquisition (Harrewijn and Piron 1994). This was attributed to the fact that pymetrozine did not affect the potential drops (cell punctures) during the first stylet penetration on the virus source plants and as a result did not effectively inhibit virus acquisition. In general, studies with aphids feeding on plants treated with pymetrozine showed that initial stylet penetration was not delayed and a number of cell punctures was produced. However, aphids took longer to reach the phloem and feeding time was significantly reduced whereas time to subsequent penetrations was significantly increased (Harrewijn and Piron 1994; Harrewijn and Kayser 1997). The reduction of virus acquisition in our study compared with the observations of Harrewijn and Piron (1994) might denote fewer cell punctures during acquisition phase. This may be attributed to different pymetrozine rates applied (100 vs. 150 mg/l), host plant (potato vs. tobacco) and aphid biotype used. In studies with a persistent virus, (potato leaf roll virus, PRLV) transmission rates by M. persicae was reduced because of a modest acquisition together with disruption of feeding during inoculation (Harrewijn and Piron 1994). In some cases, pymetrozine has been demonstrated to reduce PRLV transmission during both acquisition and inoculation (Mowry 2005). This insecticide significantly reduced the acquisition of CaMV, a semi-persistently transmitted virus, from turnip plants by M. persicae; whereas no viral transmission inhibition was recorded when the aphids were already viruliferous (Bedford et al. 1998). In general, pymetrozine seems to inhibit viral transmission by affecting either the acquisition and/or inoculation phase. The effects on the acquisition phase are important where virus may spread within a crop (secondary spread). However, in the case of primary spread of a virus from incoming viruliferous aphids, the inoculation phase is important. In tobacco crops, primary spread of PVY from adjacent infected potato crops and/or arable weeds is considered to be more important than secondary spread within the crop. In regards to PVY, Harrewijn and Piron (1994) and the present study demonstrated that pymetrozine provided protection against viruliferous aphids, therefore reducing its primary spread. Furthermore, the residual activity of pymetrozine is important. For example, in our study pymetrozine provided adequate protection even after 7 days following the application. Previous studies with CaMV (Bedford et al. 1998) and the whitefly transmitted viruses tomato yellow leaf curl virus (TYLCV) (Polston and Sherwood 2003) a single application of pymetrozine inhibited virus transmission for 7 days, but the effectiveness was significantly reduced after 11 days post-treatment. In our study, mineral oil provided comparable protection to that of pymetrozine against PVY and proved effective at reducing both acquisition and inoculation. Applications of mineral oil affects both acquisition and inoculation when oil is contacted by the aphids before or during either of these processes (Bradley 1963; Powell 1992). However, field experiments are needed to validate the results obtained in our study and compare the effectiveness of both products in reducing the spread of PVY and other non-persistent viruses in tobacco crops. In tobacco crops apart from M. persicae nicotianae, which is the only colonizer of the crop and efficient vector of non-persistent viruses (PVY, AMV and CMV), many non-colonizing aphid vectors are involved in their primary spread.
Based on the results, our study has shown that pymetrozine is effective against M. persicae s.l. populations throughout Greece with no evidence of resistance. Pymetrozine may be used as alternative to the neonicotinoids, the most effective insecticide class against this aphid in Greece, especially in crops such as tobacco where the selection pressure is high and cases of low-level resistance to imidacloprid have been reported (Margaritopoulos et al. 2007b). In Greece, during the last decade, control of the tobacco aphid has been based on the applications of imidacloprid or other neonicotinoids in setting water. Although this method minimizes side effects on beneficial insects, there is continuous selection for aphid-tolerant genotypes. Pymetrozine might help to reconsider this strategy where possible and can be proved a valuable tool in aphid control programs as a primary insecticide or in rotation strategies. Therefore, it might increase the life time of neonicotinoids. Pymetrozine, in crops such as tobacco, which are susceptible to non-persistent viruses (Chatzivassiliou et al. 2004) may reduce both primary and secondary virus spread. The use of neurotoxical insecticides may not provide any control of non-persistent viruses although in some cases prevent secondary virus spread within a crop (Broadbent et al. 1956; Maelzer 1986); however primary virus spread by immigrant winged aphids is more difficult to be avoided (Reagan et al. 1979; Jayasena and Randles 1985). Lastly, pymetrozine has been shown to be harmless to aphid natural enemies (Nicholson et al. 1996; Torres et al. 2003). Among the common aphicides, only pirimicarb, is considered to be selective and may be another insecticide to consider to protect natural enemies. However, in Greece, this insecticide is not effective against M. persicae s.l. because of the high levels of resistance present in aphid populations (Margaritopoulos et al. 2007b).
Conclusively, pymetrozine is an effective insecticide to control M. persicae s.l. populations throughout Greece, without any evidence for resistance, and it could be used as alternative to neonicotinoids in crops where rotation of insecticides with a different mode of action is of primary importance. Pymetrozine has the advantages of selectivity and that it can be used to reduce virus spread in crops which are susceptible to non-persistent viruses.
Acknowledgements
The authors thank Dr Kostas D. Zarpas, University of Thessaly, Greece for valuable comments on an early version of the manuscript and Dianna Cox, Rothamsted-Research, UK for providing the US1L laboratory strain of M. persicae. This study was supported by the research projects PYTHAGORAS II and PENED 03 funded by the Greek Ministry of Education and the Greek General Secretariat for Research and Technology, respectively.




