Filamentous fungi found on foundress queens of leaf-cutting ants (Hymenoptera: Formicidae)
Abstract
Queens of the leaf-cutting ant species Atta laevigata and Atta capiguara were collected soon after their mating flight and maintained in the laboratory until death. Ant corpses showing signs of contamination by insect pathogenic fungi were selected for fungal identification. Filamentous fungi such as Beauveria bassiana and Paecilomyces lilacinus actively sporulated in the ant’s corpses. This is the first report of the latter fungus on reproductives of leaf-cutting ants. The fact that queens may acquire filamentous fungi including saprophytic and potential insect pathogens after their mating event is especially interesting regarding the impacts of such microbes on the establishment of a new nest.
Introduction
Leaf-cutting ants (Hymenoptera: Formicidae: tribe Attini) maintain a complex mutualism with a basidiomycetous fungus cultivated as food (Schultz and Brady 2008). The fungus grows on plant substrate brought by workers to underground nests and accumulated as the fungus garden. In mature nests, once a year, winged females (gynes) leave their parental colony for the mating flight carrying with them a small pellet of the fungus garden in their mouth parts as the incipient culture for their new nest (Weber 1972).
Besides the cultivated fungus, gynes transport additional microbes including actinomycetes, filamentous fungi and yeasts in the fungal pellet or on their cuticles (Currie et al. 1999; Pagnocca et al. 2008). Reports of filamentous fungi found on workers of leaf-cutting ants are common and mostly concern insect pathogenic fungi occurring on corpses of these ants (Kermarrec et al. 1986; Schmid-Hempel 1998 see references within; Hughes and Boomsma 2004). On the other hand, few studies have focussed on the diversity of filamentous fungi found on gynes and queens of leaf-cutting ants. For example, Alves and Sosa Gomez (1983) isolated the well-known insect pathogens Beauveria bassiana and Metarhizium anisopliae from queens of Atta sexdens rubropilosa in Brazil. Also, Carrión et al. (1996) isolated Aspergillus flavus, B. bassiana and M. anisopliae var. anisopliae from queens of Atta mexicana in Mexico. López et al. (1999) isolated the latter fungus from dead queens of Atta cephalotes in Colombia. Recently, Hughes et al. (2009) described a novel fungal disease on naturally infected queens of Acromyrmex octospinosus and Atta colombica. Fungi in the genus Ophiocordyceps have the potential to establish infections in at least three genera within the tribe Attini.
To establish if ants are in close contact with filamentous fungi including insect pathogens during the first steps of colony foundation, the aim of this work was to systematically profile the possible insect pathogenic microfungi associated with queens of leaf-cutting ants collected soon after their mating flight.
Material and Methods
On September 2005, after a light rain in a pasture near the city of Botucatu, São Paulo State, Brazil (GPS locality: S22°50.579′, W48°26.131′), alates of Atta capiguara and Atta laevigata were observed leaving the colonies located in this area. Within 1 hour after their mating flight (i.e. when ants lost their wings and started digging the nest entrance), 100 mated females were randomly picked up from the ground. By the time of collection, we could not distinguish from which parental colony a particular queen came from and because of the morphological similarity between queens no identification was possible at the species level. Ants were maintained in the laboratory for several weeks without nutrition in UV-sterilized plastic containers (250 ml of volume). All containers were filled with plaster in the bottom to keep the humidity high to provide optimum conditions for fungal growth. Ants were inspected daily and once an ant died its corpses were observed under a stereomicroscope for any signs of infection by entomopathogenic fungi (i.e. growth of fungi from the inside of the ant).
Fungal mycelium or spores were streaked on potato dextrose agar plates supplemented with 150 μg/ml of tetracycline. All plates were incubated at 25°C for 7–14 days in the dark. Identification was carried out using morphological markers (Domsch et al. 1980) or by sequencing the ribosomal DNA large subunit gene (LSU) or the internal transcribed spacers (ITS) following the protocol of Pagnocca et al. (2008). Sequences were compared with others available at NCBI – GenBank. All cultures were stored at −80°C in glycerol 10% and deposited at Center for the Study of Social Insects. Key fungal isolates were deposited at The Brazilian Collection of Microorganisms from the Environment and Industry – CBMAI.
Results and Discussion
Within 1-month after collection, a total of 30 out of 100 queens died in the laboratory. Only 10 corpses showed the presence of putative entomopathogenic fungi (fungi growing from the inside of the ant) during the first 2 weeks of observation. From such corpses, 12 microfungal isolates were recovered. These included fungi such as, Acremonium sp. (n = 2 isolates), Fusarium oxysporum (n = 1), Fusarium solani (n = 1), Fusarium graminearum (n = 1), Paecilomyces lilacinus CBMAI 1013 (n = 3), and Trichoderma atroviride (n = 1) as well as the insect pathogenic fungi B. bassiana CBMAI 1014 (n = 3). All fungal isolates were identified by morphological characteristics except for F. oxysporum, P. lilacinus and T. artroviride that were also identified by DNA sequencing (GenBank accession No. and identity of closest hits: EU326223 – 99% and EU280133 – 99% for ITS sequences of F. oxysporum and T. artroviride, respectively; AB363751 – 99% for LSU rDNA sequence of P. lilacinus).
Sporulation of B. bassiana and P. lilacinus from ant corpses was particularly prolific (fig. 1), a general characteristic of insect pathogenic fungi (Hughes et al. 2004). This is the first report of P. lilacinus isolated from alates of leaf-cutting ants. Despite its saprophytic nature, the occurrence of this fungus is interesting because several isolates have been reported as insect pathogens causing diseases in different groups of insects (Domsch et al. 1980; Inglis and Tigano 2006). Similarly, some Acremonium and Fusarium species have the potential to cause disease in insects (Teetor-Barsch and Roberts 1983; Steenberg and Humber 1999). On the other hand, Trichoderma sp. is generally assumed to be an aggressive general pathogen of the symbiotic fungus of leaf-cutting ants (Currie and Stuart 2001).
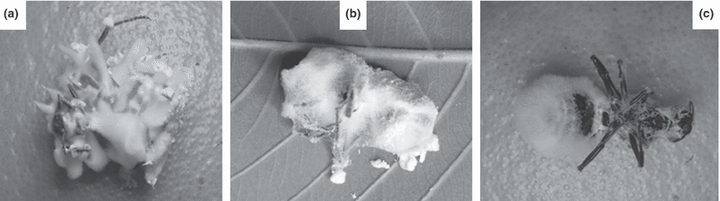
Filamentous fungi found on corpses of mated females of leaf-cutting ants. (a) Beauveria bassiana CBMAI 1014, (b) Paecilomyces lilacinus CBMAI 1013 and (c) Fusarium oxysporum.
Carrión et al. (1996) suggested that mated females of leaf-cutting ants may acquire filamentous fungi during the excavation of a new colony. This is especially possible because of the high prevalence of insect pathogenic fungi like M. anisopliae var. anisopliae in the soil surrounding the ant nests (Hughes et al. 2004). Also, Pagnocca et al. (2008) found fungi such as Fusarium sp. and Trichoderma sp. in gynes of A. capiguara and A. laevigata when leaving their parental nests for the mating flight, but no classical entomopathogenic fungi were isolated. Regardless of the fungal nature, it is clear that gynes and queens regularly come into contact with fungi before and after their mating flight.
On the other hand, we cannot unequivocally attribute the death of queens in this study to the fungi isolated because one cannot rule out that ants may have died for other reasons such as starvation. However, once present on the body of the ant, fungi may survive for a long period and potentially become a threat for the establishment of an incipient nest. For example, Autuori (1950) observed that 97.5% of founding Atta spp. queens died over a 1-year period. The author pointed out as the critical stage for colony establishment the period in which queens are closed in the claustral chamber (until 3 month-old). The causes of such high mortality were attributed to several factors including predation of queens by armadillos and nest flooding; but we suggest that filamentous fungi may be an additional source that contributes to the mortality of queens. For instance, Fowler (1992) observed insect pathogenic fungi (identified as B. bassiana) growing on dead Acromyrmex balzanii and Acromyrmex niger queens found in incipient nests. Also, during the nest establishment period queens are likely to be vulnerable because they cannot groom the dorsal part of their thoraxes, which may thus be a particular place where fungi may develop (Autuori 1942).
This scenario is particular interesting regarding the direct impacts that filamentous fungi could have on the fitness of the ant colonies. Previously, no study has systematically evaluated the incidence of fungal parasites on queens of leaf-cutting ants in field conditions and whether such fungi are responsible for preventing colony establishment. Future studies of this issue may inform direct applications for the biological control of these pest ants.
Acknowledgements
We would like to thank “CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico” (grants No. 301731/2005-7 and 476250/2008-0) and “FAPESP – Fundação de Amparo à Pesquisa do Estado de São Paulo” (grants No. 2007/50139-4 and 2008/54386-9) for financial support. We also thank “CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” for a postdoctoral research fellowship to A. Silva. Two anonymous reviewers are also acknowledged for their valuable comments on this manuscript.




