Persistence of Alarm-Call Behaviour in the Absence of Predators: A Comparison Between Wild and Captive-Born Meerkats (Suricata Suricatta)
Abstract
Performing correct anti-predator behaviour is crucial for prey to survive. But, are such abilities lost in species or populations living in predator-free environments? How individuals respond to the loss of predators has been shown to depend on factors such as the degree to which anti-predator behaviour relies on experience, the type of cues evoking the behaviour, the cost of expressing the behaviour and the number of generations under which relaxed selection has taken place. Here we investigated whether captive-born populations of meerkats (Suricata suricatta) used the same repertoire of alarm calls previously documented in wild populations and whether captive animals, as wild ones, could recognize potential predators through olfactory cues. We found that all alarm calls that have been documented in the wild also occurred in captivity and were given in broadly similar contexts. Furthermore, without prior experience of odours from predators, captive meerkats seemed to distinguish between faeces of potential predators (carnivores) and non-predators (herbivores). Despite slight structural differences, the alarm calls given in response to the faeces largely resembled those recorded in similar contexts in the wild. These results from captive populations suggest that direct, physical interaction with predators is not necessary for meerkats to perform correct anti-predator behaviour in terms of alarm-call usage and olfactory predator recognition. Such behaviour may have been retained in captivity because relatively little experience seems necessary for correct performance in the wild and/or because of the recency of relaxed selection on these populations.
Introduction
Predation is a major selective force leading to numerous behavioural and morphological adaptations in prey (Lima & Dill 1990). Many species, for example, produce alarm calls to warn conspecifics of impending danger (Klump & Shalter 1984). Several mammalian studies have shown that young individuals need to learn about alarm calls and that the amount of exposure to these calls can affect the speed of such learning (e.g. Hauser 1988; Mateo 1996; Ramakrishnan & Coss 2000; Hanson & Coss 2001; McCowan et al. 2001; Hollén & Manser 2006; Hollén, L. I., Clutton-Brock, T. & Manser, M. B., unpubl. data). Few studies have, however, considered whether regular encounters with predators are necessary to maintain appropriate anti-predator behaviours within populations (but see Brown et al. 1992; Fichtel & Hammerschmidt 2003; Fichtel & van Schaik 2006; Coss et al. 2007). Moreover, in several species, alarm calls are known to provide far more information than a simple warning. They may, for example, indicate the type of predator and/or the urgency of the threat (reviewed in Macedonia & Evans 1993; Manser 2001; Coss et al. 2007). It remains unclear whether a lack of relevant experience leads to elimination of alarm-call behaviour, some alteration in the subtleties of sophisticated systems or has no discernible effect at all.
Species which have become isolated from predators, either on islands or in captivity, provide powerful opportunities to investigate the importance of predator experience on alarm-call behaviour. Although such isolation may reduce the selection pressure, anti-predator behaviour is not inevitably lost (e.g. Coss 1991, 1999; Blumstein et al. 2000; Blumstein & Daniel 2002). How animals respond to isolation from predators can, for example, depend on the degree to which anti-predator behaviour relies on experience (Coss 1999; Blumstein 2002), the cost of performing such behaviour (Magurran 1999; Berger et al. 2001; Blumstein et al. 2006) and the type of cues evoking the behaviour (Blumstein et al. 2000). Experience-independent behaviour, behaviour with low production costs and behaviour evoked by cues with convergent features may be more likely to persist in predator-isolated environments. However, the persistence of behaviour will also depend on the number of generations under which the relaxed selection has taken place (Coss 1999). Because of the complexity of the genetic–epigenetic processes leading to the expression of predator recognition and appropriate anti-predator behaviour, a few generations of relaxed selection will not alter any innate perceptual properties.
Meerkats (Suricata suricatta) provide an ideal opportunity to investigate the importance of predator experience on the maintenance of alarm-call behaviour because they are found in numerous zoos and their anti-predator behaviour has been extensively studied in the wild (Manser 2001; Manser et al. 2001; Hollén & Manser 2006, 2007; Hollén, L. I., Clutton-Brock, T. & Manser, M. B., unpubl. data). They are cooperatively breeding mongooses which naturally inhabit arid regions of southern Africa, where they are preyed on by a variety of raptors, mammals and snakes (Clutton-Brock et al. 1999a,b). They exhibit a sophisticated alarm-call system, consisting of calls given only in response to specific predator types (for example, raptors) and calls that are unrelated to a single predator type (for example, moving animals) (Manser 2001). Additionally, the acoustic structure of predator-specific calls simultaneously encodes information about the signaller’s perception of response urgency: calls given on spotting a close predator (termed high urgency) are structurally different from those given to the same predator encountered at intermediate (medium urgency) and far (low urgency) distances (Manser 2001). Calls of different urgency do not fall into discrete categories, but rather grade from a harmonic to a noisy structure as the level of urgency increases (Manser 2001).
In this study, we investigate whether meerkats from European zoos produce alarm calls in response to natural visual cues and experimentally presented olfactory cues (faeces). We use these two cue types to assess what type of predatory experience might be important for the maintenance of alarm-call behaviour: captive meerkats are likely to have no experience of predatory olfactory cues, but might have encountered some visual predatory threats (albeit from different species to those usually seen in the wild). We assess whether the repertoire of calls found in the wild is present in captivity and whether the acoustic structure of alarm calls produced by captive meerkats, and the context in which they are given, matches that for wild individuals.
Methods
Study Sites and Populations
Between Aug. 2004 and Dec. 2005, we studied six captive populations of meerkats living in zoos in Switzerland (Basel), Germany (Cologne, Karlsruhe, Hannover and Osnabrück) and Ireland (Dublin). All individuals present in these populations were born in captivity and the number of generations of captive living ranged from one to five. Groups had access to both outdoor (range: 30–480 m2, mean = 178 m2) and indoor (range: 1–40 m2, mean = 19 m2) enclosures. All outdoor enclosures had a clear view of the sky. The substrate in the outdoor enclosures composed a mix of sand and mud, and the meerkats could therefore dig natural burrows and holes themselves. Additional structures such as hollow tree trunks and termite mounds were also present, providing ecologically natural shelters. The outdoor enclosures were directly alongside walking paths for visitors, but obscured by glass or stone walls (at least 1 m in height). Dogs were not allowed in any of the zoos, but in Cologne zoo a keeper once walked past with a dog. Some of the groups were close to other carnivore enclosures (20–30 m), whereas others were more than 100 m away and out of visual contact. Group size varied between six and 16 individuals, which is within the natural range (Clutton-Brock et al. 1999a). Except for one zoo, where individuals were distinctly marked with hair dye, individual identification was not feasible. All individuals from which we collected data were of adult age (>12 mo, Clutton-Brock et al. 1999b).
Alarm-call behaviour in wild meerkats was studied at the Kuruman River Reserve in the South African part of the Kalahari Desert (26°58′S, 21°49′E) (study site details provided in Clutton-Brock et al. 1999a). At this study site, a range of five to 13 (varying between years) wild but habituated (close observation within 1 m) groups, varying in size from three to 50 individuals, have been followed since 1995. Each animal was marked for individual identification with hair dye or hair cuts applied to their fur unobtrusively during basking at the morning sleeping burrow. The exact age and life histories of all individuals except a few immigrant males were known because they had been monitored since birth. Although their alarm-call system has been described in detail elsewhere (Manser 1998, 2001), Table 1 provides an overview of the common call types and the contexts in which they are given.
| Call type | Urgency | Context |
|---|---|---|
| Specific | ||
| Low aerial | Low | Raptorsa far away (>500 m) |
| Medium aerial | Medium | Raptorsa at medium distances (100–500 m) |
| High aerial | High | Raptors nearby (<100 m) |
| Low terrestrial | Low | Herbivores/ground predators far away (>200 m) |
| Medium terrestrial | Medium | Herbivores/ground predators at medium distances (20–200 m) |
| High terrestrial | High | Herbivores/ground predators nearby (<20 m) |
| Low recruitment | Low | Deposits such as faeces or hair samples of predators or foreign meerkats |
| High recruitment | High | Snakes/deposits of predators (seldom to deposits of foreign meerkats) |
| General | ||
| Alert | Low | Non-dangerous birds nearby, raptors far away, terrestrial animals |
| Moving animal | Low/high | Animals moving (raptors, mammals, non-dangerous birds, foreign meerkats) |
| Bark | High | Perched raptors (<500 m), raptors circling above, ground predators very nearby |
| Panic | High | Sudden movements in close proximity |
- aOccasionally also elicited by vultures.
Alarm-Call Usage
We spent 2–3 d collecting data in each zoo. To determine whether captive meerkats used the same repertoire of alarm calls as those described for wild meerkats and whether the calls were used in similar contexts, we recorded alarm calls produced by captive meerkats in response to natural sightings on an ad libitum basis. We also recorded calls given during faecal presentations (see below). All alarm calls were recorded (44.1 kHz sampling frequency; 16-bit PCM-WAV) at a distance of 2–4 m from the caller using a Sennheiser directional microphone (ME66/K6 with a MZW66 pro windscreen; frequency response 40–20 000 Hz ± 2.5 dB; Sennheiser, Old Lyme, CT, USA) connected to a Marantz PMD-670 solid-state recorder (D&M Holding, Inc., Kanagawa, Japan). The stimuli eliciting natural alarm calls were spoken onto the recordings. For comparison, we used alarm calls from wild meerkats recorded (at a distance of 1–2 m from the caller) between 2003 and 2005 using a Sennheiser directional microphone (ME66/K6) connected to a Sony digital audio tape recorder DAT-TCD D100 (Sony Corporation, Tokyo, Japan) or a Marantz PMD-670 solid-state recorder. All calls were uploaded to a PC (sampling frequency: 44.1 kHz; resolution: 16 bit). Because of the lower sound quality of calls recorded in captivity (due to high levels of background noise and disturbances), we compared and classified calls visually, using Cool Edit 2000 (Syntrillium, Phoenix, AZ, USA).
Presentation of Olfactory Cues
To test whether captive meerkats responded to olfactory cues from predators, and to compare the calls given in such circumstances with those produced in the wild, we presented captive groups with faeces from carnivores (potential predators) and herbivores (non-predators/control). Because certain species of carnivores and herbivores were present in some zoos but not others, we had to use faeces from different species. For the carnivore category, we used faeces from African lions (Panthera leo), Siberian tigers (Panthera tigris), snow leopards (Unica unica) and cheetahs (Acinonyx jubatus). For the herbivore category, we used faeces from impalas (Aepyeros melampus), common duikers (Sylvicapra grimmia), scimitar horned oryx (Oryx dammah) and alpakas (Lama pacos). Wild meerkats could encounter faeces from lions, cheetahs, impalas and duikers, whereas faeces from the other species will never be encountered naturally. The captive populations we studied were unlikely to have encountered any of these faeces prior to the experiments.
Faeces from some species were presented in more than one zoo, but the meerkat group in each zoo received only one sample of carnivore faeces and one sample of herbivore faeces. Samples were kept in a freezer (−20°C) and defrosted shortly before use. Faeces were presented in the outdoor enclosures on removable trays or sticks (replaced between presentations) placed on the ground. Because access to enclosures was limited, herbivore and carnivore faeces were presented on the same day. However, at least 2 h was left between presentations and faeces were removed immediately after testing (when animals showed no further interest). Although a randomized design is usually preferred to minimize the chance of order effects, we decided to present herbivore faeces before carnivore faeces because the latter typically elicited a strong response, which might have influenced subsequent reactions.
We recorded the behavioural responses with a Sony digital video camera DCR-TRV50E (Sony Corporation, Tokyo, Japan) and analysed the video tapes using a frame-by-frame analysis (12.5 frames per second) in Microsoft Windows Movie Maker version 5.1. We determined the total time a group spent inspecting the faeces, measured as the time from when the first individual started sniffing the faeces to the last individual leaving. Because individuals repeatedly returned to sniff the faeces after an initial inspection, we defined the end of a response as the time when 1 min had passed without any animal returning. We also extracted the total length of alarm-call bouts produced by the group (time from the first call to the end of the last call). We used the group response because identity of individuals could not always be determined from the video recordings.
We had sufficient high-quality recordings of the most common call type (low-urgency recruitment, Fig. 1) produced in response to the faeces to analyse and compare acoustically with those produced during similar circumstances in the wild (Ncalls captive = Ncalls wild = 10). The analysed exemplars were obtained from five of the six zoos. We only included calls (from captivity and the wild) that were recorded from different individuals. We first conducted a fast Fourier transformation (1024-point FFT) of all calls using AVISOFT-SASLab pro 4.38 (R. Specht, Berlin, Germany). We used a frequency range of 11.025 kHz (frequency resolution 28 Hz) and time resolution of 1.45 ms (98.43% overlap). The resulting frequency–time spectra were analysed with LMA 2005 (developed by K. Hammerschmidt), a software tool that extracts a set of call parameters from acoustic signals (Schrader & Hammerschmidt 1997). Eight acoustic parameters were included in the analysis (see Table 2 for a list and description of parameters). We included parameters describing: (1) the fundamental frequency and its first harmonic; (2) the distribution of spectral energy measured as the first and second quartiles of the distribution of frequency amplitudes in the spectrum; (3) the peak frequency (the frequency with the highest amplitude in a time segment); (4) the call duration; and (5) the amplitude ratio between the fundamental frequency and the first harmonic.
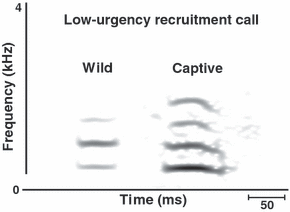
Examples of low-urgency recruitment calls produced by wild and captive meerkats in response to olfactory predatory cues.
| Parameter | Description |
|---|---|
| df1med | Median frequency of the fundamental frequency, across all time segments (Hz) |
| df2med | Median frequency of the first harmonic, across all time segments (Hz) |
| q1med | Median frequency of the first quartile of distribution of frequency amplitudes, across all time segments (Hz) |
| q2med | Median frequency of the second quartile of distribution of frequency amplitudes, across all time segments (Hz) |
| pfmed | Median peak frequency across all time segments (Hz) |
| pftrfak | Slope of the linear trend of the peak frequency (global modulation) |
| ampratio1 | Amplitude ratio between fundamental frequency and first harmonic |
| duration | Duration (ms) |
Statistical Analysis
Because of differences in the amount of time spent observing each captive population (due to factors such as bad weather, disturbance and limited access), we were unable to record alarm calls in a standardized way across all zoos. We therefore present the data on alarm-call usage qualitatively. All statistical analyses of acoustic differences and responses to olfactory cues were conducted in R for Microsoft Windows version 2.4.1 (R Development Core Team 2006; URL: http://www.r-project.org), using the software packages ‘MASS’ (Venables & Ripley 2002) and ‘ipred’ (Peters & Hothorn 2004). Sample size was too low to evaluate statistically the differences in the length of calling in response to faeces but results are presented descriptively.
For the analysis of acoustic differences between captive and wild populations, we first used a multivariate analysis of variance including all of the eight measured call parameters. Significant parameters were then entered in a discriminant function analysis (DFA) to determine classification probabilities of alarm calls produced in captivity and in the wild. DFA identifies linear combinations of predictor variables that best characterize the differences among groups and assigns each call to its appropriate group (correct assignment) or to another group (incorrect assignment). For external validation, we used a 10-fold cross-validation procedure in which the data were randomized and partitioned into 10 folds. In each of 10 turns, nine of the folds were used to establish the model and the remaining fold was used to estimate the model’s validity. Because of questions about the use of unbalanced designs in DFA and because sample sizes of calls from captive meerkats were relatively low compared with those from wild animals, we randomly chose equal-sized subsets of calls from wild meerkats to minimize a possible bias in our results. We calculated assignment probabilities expected by chance using a bootstrap approach. Taking into account the initial sample sizes in the actual data, random numbers were assigned to each call class. Chance probabilities from 1000 repeats are presented with ±1 SE. On average, assignment probabilities equal to or greater than that obtained in the DFAs were generated by chance in less than 1% of all bootstrap repeats.
Results
Alarm-Call Usage
The amount of calling differed between captive populations, but alarm calling was observed in all six groups. Combining results from all zoos, we found that the alarm-call repertoire present in wild meerkats (Table 1) was also present in captivity. The most reliable contexts that elicited alarm calls in captivity were the sightings of airplanes, helicopters, zeppelins and non-dangerous birds, such as crows (Corvus corone). Airplanes, helicopters and zeppelins were typically far away (>500 m), whereas birds commonly flew past nearby (<50 m). Many of the alarm calls given in response to these stimuli had the same general structure as the alert calls given by wild meerkats in similar situations, where they seem simply to alert other group members in relatively low-urgency situations (Manser 2001). Some of the calls produced in response to airplanes, helicopters and zeppelins were also similar to the medium-urgency aerial calls normally elicited by raptors in the wild. However, non-dangerous birds such as vultures (Torgos tracheliotus and Gyps africanus) occasionally elicit such calls in the wild (Hollén, L. I. & Manser, M. B., personal observations). Zeppelins in particular also elicited noisy bark calls, which in the wild are produced in high-urgency situations.
Alarm calls other than alert calls or medium-urgency aerial calls were relatively uncommon in captivity. Calls similar to low-, medium- and high-urgency terrestrial calls produced in the wild were only heard in one zoo, when a keeper walked past the enclosure with a dog less than 10 m away. All the meerkats showed an intense response and continued giving alarm calls for at least 15 min after the dog disappeared. Wild meerkats encountering dogs at such distances show similar strong responses (Hollén, L. I. & Manser, M. B., personal observations). Like wild meerkats, captive animals switched from low- and medium-urgency calls to high-urgency calls (including bark calls) as the dog came closer. The dog also elicited calls similar to the moving-animal call produced in response to moving dangerous or non-dangerous animals in the wild. Moving-animal calls were also commonly given in response to keepers bringing food, visitors walking past and reflections of their own mirror images. In response to sudden disturbances, such as rapid movements, captive meerkats produced calls very similar to the panic calls produced in such situations in the wild. As in wild meerkats (Manser 2001), this call typically caused others to seek shelter. Finally, the majority of alarm calls produced in response to faecal presentations resembled the low-urgency recruitment calls (Fig. 1) elicited in response to olfactory cues in the wild. Some of these calls, however, looked more similar to medium-urgency terrestrial calls given in the wild. In contrast to wild meerkats, captive animals very rarely produced high-urgency recruitment calls.
Olfactory Predator Recognition
In all six captive populations, carnivore faeces were inspected for a significantly longer duration than for herbivore faeces (carnivore: 124 ± 61 s; herbivore: 20 ± 19 s; Wilcoxon test: V5 = 21, p = 0.03, Fig. 2a). Carnivore faeces elicited recruitment calling in all six presentations compared with four of six in response to herbivore faeces, and carnivore faeces elicited much longer bouts of calling than herbivore faeces, which typically elicited only one or two calls (carnivore: 179 ± 96 s, N = 4, because of low sound quality we could not measure the length of the bouts in two of the cases; herbivore: 21 ± 11 s, N = 4, Fig. 2b).
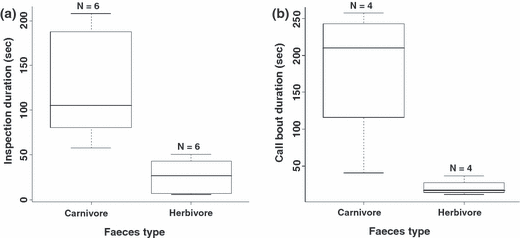
The time that captive meerkats spent (a) inspecting stimuli and (b) calling in response to presentations of carnivore and herbivore faeces. Because of low sample size, a statistical analysis was conducted only on inspection time. Sample sizes reflect the number of groups.
Despite looking spectrographically similar, low-urgency recruitment calls produced by captive meerkats in response to faecal presentations differed in their acoustic structure from those given in similar contexts in the wild. The analysis of variance revealed statistically significant differences for five of the eight measured call parameters. These variables were the: (i) duration of the calls (F1,18 = 7.98, p = 0.01); (ii) median fundamental frequency (F1,18 = 10.24, p = 0.005); (iii) median frequency of the first harmonic (F1,18 = 9.40, p = 0.007); (iv) median frequency of the second quartile of distribution of frequency amplitudes (F1,18 = 5.36, p = 0.03); and (v) amplitude ratio between the fundamental frequency and its first harmonic (F1,18 = 38.98, p < 0.001). Captive meerkats produced longer calls with a higher fundamental frequency and first harmonic, more energy located at lower frequencies and a higher amplitude ratio than wild individuals.
With the five significant parameters, calls showed a 100% correct classification (classified as ‘wild’ or ‘captive’) before and after cross-validation, compared with the 50 ± 5% expected by chance (Fig. 3). Call duration and amplitude ratios were the most discriminating parameters. Because some of the calls from captive individuals looked spectrographically similar to medium-urgency terrestrial calls given in the wild, we included a set of these calls (N = 10) in the DFA to see how they classified. This yielded, after cross-validation, a correct assignment of 87% (93% before); higher than the 33 ± 3% expected by chance (Fig. 3). Calls given in captivity were longer than both call types given in the wild, but fundamental frequency, frequency amplitude and amplitude ratio values were closer to those of medium-urgency terrestrial calls than low-urgency recruitment calls recorded in the wild.
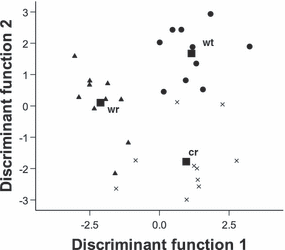
Classification results from the discriminant function analysis on low-urgency recruitment calls produced in response to carnivore faeces in captivity (cr, Ncalls = 10) and hair samples of the African wildcat in the wild (wr, Ncalls = 10). Medium-urgency terrestrial calls produced by wild meerkats in response to mammalian predators were also included (wt, Ncalls = 10).
Discussion
All alarm calls that have been documented in wild meerkats (Manser 1998, 2001) were produced by captive meerkats on one or several occasions. This suggests that captive meerkats exhibit the same vocal repertoire of alarm calls as wild meerkats. That the amount of calling differed between populations may simply reflect differences in the time spent observing each population or variation in the presence of disturbances. Captive meerkats not only produced alarm calls, but produced them in contexts resembling those in the wild. Although calls often elicited by raptors in the wild were regularly evoked by stimuli such as airplanes, this may not be surprising given the presumably lesser likelihood of encountering real threats. Besides, wild meerkats occasionally alarm to airplanes (Manser, M. B., personal observation). Our observations are similar to those on some non-human primates, where captive populations use the same or very similar alarm-call types as wild populations (Fichtel & van Schaik 2006; Coss et al. 2007), but occasionally alarm to harmless stimuli (Brown et al. 1992).
There are a number of explanations to why alarm calling could have been retained in captive meerkats. First, it is possible that the presence of some predatory stimuli may be sufficient for call production to persist. Although we never observed encounters with potential predators, except one dog, it is possible that raptors such as hawks (Accipiter sp.) and buzzards (Buteo sp.) fly past, or feral cats (Felis catus) or foxes (Vulpes vulpes) prowl the zoos. Moreover, some of the zoos had visual access to other carnivore enclosures. Perceiving such stimuli, or even harmless stimuli with features broadly similar to predators, could preserve the functionality of neural activity involved in and necessary for appropriate anti-predator behaviour (Coss 1991). Nevertheless, the lack of experience and few environmental challenges in captivity may have resulted in an elevated excitability obviously when encountering, for example, zeppelins far away (see also Coss 1991). Second, in many species, including non-human primates (reviewed in Seyfarth & Cheney 1997) and meerkats (Hollén & Manser 2007), the ability to produce calls seems largely innate. Behaviours which are essentially independent of experience may change slowly following the loss of predators (Coss 1999; Blumstein et al. 2000; Blumstein 2002). However, a long period of evolutionary time is also likely to be necessary to change innate predispositions. Given that our study populations belonged to, at maximum, the fifth generation of the wild-caught founders, the recency of relaxed selection on these populations might provide a more plausible explanation for the retention of the alarm-call repertoire.
The results from our faecal presentations suggest that captive meerkats growing up in a relatively predator-free environment can still recognize and respond adaptively to odours signalling the presence of potential predators, in a similar fashion to wild individuals (Manser 2001). Captive meerkats inspected carnivore faeces for a longer time than faeces from non-predatory herbivores, and carnivore faeces also elicited longer bouts of alarm calling than did herbivore faeces. These responses are similar to those observed in response to olfactory cues in the wild: hair samples of one of their main predators, the African wildcat (Felis lybica), typically elicit long bouts of calling and recruitment of the rest of the group, whereas hair samples of the non-dangerous Cape ground squirrel (Xerus inauris) are, if at all, inspected only briefly and do not elicit any calling (Graw 2005). That captive adult meerkats, which are unlikely to have had experience with odours of predators, recognize and respond to them, suggests that such recognition is relatively innate. Given the convergent features of carnivore faeces (sulphurous compounds produced after digesting meat; Nolte et al. 1994), meerkats might have been selected to recognize such cues independent of experience. Experience-independent odour recognition has been shown in some species (e.g. Ward et al. 1997; Barreto & Macdonald 1999; Coss 1999; Monclús et al. 2005), but in other species, predator-naïve individuals seem to modify their behaviour in response to olfactory cues through learning (Mathis et al. 1996; Berger et al. 2001; Blumstein et al. 2002).
Although the alarm calls produced during faecal presentations largely resembled the low-urgency recruitment calls produced in response to deposits such as faeces or hair samples of predators in the wild, there were some differences in their acoustic structure. Compared with wild individuals, captive meerkats produced longer calls with a higher fundamental frequency, higher amplitude ratio between the fundamental frequency and its first harmonic and more energy located at lower frequencies. Some of the values were closer to those of medium-urgency terrestrial calls produced in the wild. These differences could, however, be caused by factors other than a difference in the breadth of experience with predators. First, morphological size differences may be responsible for the increase in amplitude of the fundamental frequency relative to that of the first harmonic, the downward shift of the main energy to lower frequencies and increased call duration (Hsiao et al. 1994; Hammerschmidt et al. 2000, 2001). Second, a rise in fundamental frequency and an increase in call duration have been shown in humans (Banse & Scherer 1996; Scheiner et al. 2002) and non-human primates (Fichtel et al. 2001; Rendall 2003) during increased physiological arousal. An increase in arousal due to inexperience with predators has been shown in, for example, bonnet macaques (Macaca radiata) (Coss et al. 2007), but whether captive meerkats show a greater arousal, and whether this is due to a lack of experience, remains to be investigated. Finally, studies have shown that behaviour which can be robust in its general form can be manifested in a juvenile-like state due to developmental deprivation in captive animals (e.g. Bryan & Riesen 1989). Although all outdoor enclosures in this study were equipped with natural habitat structures similar to those in the wild and most of the enclosures were of reasonable sizes, slight deprivation might explain why captive individuals produced calls with a high fundamental frequency typical of young individuals (Hammerschmidt et al. 2000; Fischer et al. 2002).
It is also possible that other factors, such as differences in the evoking stimuli, the acoustic environment (background noise, wind speed, etc.) and/or genetics may have contributed to the acoustic differences in alarm calls produced in captivity and in the wild. For example, in contrast to the natural environment, zoos are typically very noisy and it has been shown in some bird species that amplitude and frequency parameters of song can be affected by the background noise level (Slabbekoorn & Smith 2002; Brumm 2004). Although genetic differences between populations can theoretically cause differences in acoustic structure (e.g. Macedonia & Taylor 1985), however, all meerkats (wild and captive), as far as we know, belonged to the same subspecies (Hollén, L.I., personal observation). Furthermore, the duration of captive rearing of our study populations renders it unlikely that selection has had sufficiently long time to act on these calls.
We conclude that captive meerkats with presumably little predator experience still exhibit the same alarm-call system as that observed in the wild, the contexts in which alarm calls are given largely resemble those in the wild and they seem able to recognize potential predators by means of olfactory cues. Future research is required to understand the exact mechanisms behind the persistence of such anti-predator behaviour in captive meerkats and to determine the exact cause of the structural differences found between alarm calls produced in captivity and in the wild. While our study shows that the basic alarm-call system persists in captivity, we cannot yet attribute the same degree of sophistication and accuracy of alarm calling as that described for wild meerkat populations. This may simply be a consequence of a limited sampling period or it may indicate that regular encounters with predators are needed to fine-tune a highly sophisticated innate alarm-call system.
Acknowledgements
We thank Thomas Jermann at Basel Zoo, Ute Magiera and Susanne Klomberg at Osnabrück Zoo, Clemens Becker at Karlsruhe Zoo, Klaus Müller-Schilling and Andreas Knieriem at Hannover Zoo, Paul ODonoghue, Robert Wiggins, Ron Willis and Eddie O Brien at Dublin Zoo, Waltraut Zimmerman and Alexandra Habicher at Cologne Zoo, and all animal keepers for providing valuable help. We are also grateful to Tim Clutton-Brock for access to the meerkat population of the Kalahari Meerkat Project; Family Hennie Kotze for allowing us to work on their land; Johan Du Toit and Martin Haupt at the Mammal Research Institute, University of Pretoria, for help with logistics; Kurt Hammerschmidt for allowing us to use his acoustic programme LMA 2005 and for valuable advice on how to use it; and Andrew Radford, Julia Fischer, Robert Magrath, Gabriel Beckers and two anonymous referees for valuable comments on the manuscript. The study on wild meerkats was conducted under licenses issued by the Northern Cape Conservation Service and the ethical committee of Pretoria University, South Africa. The faecal experiments in captivity were conducted after clearance from veterinarians in each zoo. This project was funded by a grant given to MBM from the Swiss National Science Foundations, SNF-Förderprofessur Nr 631-066129, and a grant given to LIH from the Swiss Academy of Sciences, SAS.




