Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloroplasts provides synergistically enhanced protection against various abiotic stresses
Abstract
Plants synthesize compatible solutes such as glycinebetaine (GB) in response to abiotic stresses. To evaluate the synergistic and protective effect of GB, transgenic potato plants expressing superoxide dismutase (SOD) and ascorbate peroxidase (APX) targeting to chloroplasts (referred to as SSA plants) were retransformed with a bacterial choline oxidase (codA) gene to synthesize GB in chloroplast in naturally occurring non-accumulator potato plants (including SSA) under the control of the stress-inducible SWPA2 promoter (referred to as SSAC plants). GB accumulation resulted in enhanced protection of these SSAC plants and lower levels of H2O2 compared with SSA and non-transgenic (NT) plants after methyl viologen (MV)-mediated oxidative stress. Additionally, SSAC plants demonstrated synergistically enhanced tolerance to salt and drought stresses at the whole-plant level. GB accumulation in SSAC plants helped to maintain higher activities of SOD, APX and catalase following oxidative, salt and drought stress treatments than is observed in SSA and NT plants. Conclusively, GB accumulation in SSAC plants along with overexpression of antioxidant genes rendered the plants tolerant to multiple environmental stresses in a synergistic fashion.
Abbreviations-
-
- APX
-
- ascorbate peroxidase
-
- CAT
-
- catalase
-
- codA
-
- choline oxidase
-
- GB
-
- glycinebetaine
-
- HPLC
-
- high-performance liquid chromatography
-
- NT
-
- non-transgenic
-
- MS
-
- Murashige and Skoog
-
- MV
-
- methyl viologen
-
- ROS
-
- reactive oxygen species
-
- RT-PCR
-
- reverse transcription-polymerase chain reaction
-
- RWC
-
- relative water content
-
- SOD
-
- superoxide dismutase
Introduction
Abiotic stresses such as salinity, drought and extremes of temperature are critical factors limiting crop yields worldwide, reducing crop yields by up to 80%. Today, approximately 20% of the world's cultivated land is salt-affected, and this problem is increasing every year because of poor soil-management practices (Munns 2005). Drought is also an important factor in reduced crop productivity. Oxidative stress caused by reactive oxygen species (ROS), which include the superoxide anion radical (O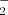 ), hydroxyl radical (OH−) and hydrogen peroxide (H2O2), has also been implicated during diverse environmental stresses such as wounding, senescence, ozone, temperature extremes, drought, salt and heavy metals in plants (Asada 1999, Foyer et al. 1994).
), hydroxyl radical (OH−) and hydrogen peroxide (H2O2), has also been implicated during diverse environmental stresses such as wounding, senescence, ozone, temperature extremes, drought, salt and heavy metals in plants (Asada 1999, Foyer et al. 1994).
Plants have developed various mechanisms to cope with the stresses imposed by harsh environments, such as modulating the expression of stress tolerance genes and synthesizing compatible solutes. Plants have well-known systems to scavenge for ROS produced in different stressful conditions, such as activation of the antioxidant enzymes superoxide dismutase (SOD) and ascorbate peroxidase (APX) (Allen et al. 1997, Kwon et al. 2002). Previous studies showed that simultaneous expression of antioxidant genes provided enhanced protection compared with single gene expression in transgenic plants (Allen et al. 1997, Kwon et al. 2002, Zhou et al. 2008). Additionally, glycinebetaine (GB), along with others, is an important and frequently documented compatible solute that plays vital roles in osmotic adjustment, stabilization of complex proteins and membranes in vivo and protection of enzyme activities (Chen and Murata 2002a,b, 2008, Sakamoto and Murata 2001, Yang et al. 2007, 2008). Several distinct species such as sugerbeet, spinach and wheat are natural accumulators of GB, while others such as Arabidopsis, rice, tomato and potato are non-accumulators (Jones and Storey 1981). Various studies have shown that overexpression of GB-synthesizing enzymes in GB non-accumulating plants resulted not only in GB accumulation but also in enhanced tolerance against various environmental stresses such as salt, drought and extremes temperature (Ahmad et al. 2008, Mohanty et al. 2002, Park et al. 2004, Sakamoto et al. 1998).
Potato (Solanum tuberosum L.) is one of the major food crops in many world regions, ranking fourth after wheat, maize and rice (Newell et al. 1991). Compared with other crops, potato is considered to be both drought- and salt-sensitive: even a short cycle of drought can cause a significant reduction in tuber yield (Van Loon 1981). Increasing salinity can also significantly reduce the total and average yields of different potato cultivars; the presence of 50 mM NaCl in liquid MS (Murashige and Skoog) media can result in 50% reduction in the growth of potato plants (Hmida-Sayari et al. 2005). To enhance the abiotic stress tolerance of potato plants, various genes such as barley oxalate oxidase, nucleoside diphosphate kinase 2 and tehalose-6-phosphate synthase have been utilized in the development of transgenic potato plants with enhanced tolerance to oxidative, salt and drought stresses (Stiller et al. 2008, Tang et al. 2008, Turhan 2005).
Abiotic stress tolerance is a multigenic trait; tolerance cannot be increased substantially by introducing any single gene (Ashraf 2004). There are reports showing that transgenic plants expressing multiple genes have enhanced protection against various environmental stresses compared with plants expressing single genes (Allen et al. 1997, Kwon et al. 2002, Tang et al. 2006, Zhou et al. 2008). Previously, it has been reported that exogenous application of GB resulted in an increase in the activity of various antioxidant enzymes, such as SOD, APX and catalase (CAT), during stress conditions (Banu et al. 2009, Sairam et al. 2002). Additionally, in vivo synthesis of GB by overexpression of GB-synthesizing enzymes in GB non-accumulator plants has resulted in induced and stabilized activities of antioxidant enzymes, which subsequently resulted in enhanced protection for the transgenic plants against various environmental stresses (Park et al. 2007, Yang et al. 2007, 2008). Therefore, speculation about the synergistic effects of compatible solutes such as GB on stabilizing enzymes and membranes in stressful conditions needs to be addressed.
In our previous study, we developed oxidative stress-tolerant transgenic potato plants (cv. ‘Superior’) by simultaneous expression of chloroplast-targeted CuZnSOD and APX genes (referred to as SSA plants) (Tang et al. 2006) under the control of an oxidative stress-inducible sweetpotato peroxidase (SWPA2) promoter (Kim et al. 2003). To evaluate the possible synergistic effects of GB, we retransformed SSA plants with the GB-synthesizing choline oxidase (codA) gene targeted to chloroplasts under the control of the SWPA2 promoter (referred to as SSAC plants). Introduction of the codA gene in SSA plants resulted in GB synthesis and helped to maintain higher antioxidative enzyme activities in oxidative, salt and drought stress conditions. Simultaneous synthesis of GB along with the overexpression of CuZnSOD and APX also rendered transgenic plants synergistically tolerant to various abiotic stresses including oxidative, salt and drought stresses. To the best of our knowledge, this is one of the very few reports adopting a gene stacking technique to develop crop plants such as potato for use in marginal soil conditions.
Materials and methods
Plant material and plasmid construction
Sterile transgenic potato plants (cv. Superior) expressing chloroplast-targeted CuZnSOD and APX genes under the control of the SWPA2 promoter (Tang et al. 2006) were used in transformation experiments. The plants were propagated via subculturing of shoot tips and stem nodal sections every 3–4 weeks on MS medium containing 3% sucrose (Murashige and Skoog 1962). The plant expression vector pScodA (Ahmad et al. 2008) containing a chloroplast-targeted codA gene under the control of the SWPA2 promoter was mobilized in the AGL0 strain of Agrobacterium and utilized for transformation.
Plant transformation and regeneration
For potato transformation, Agrobacterium harboring the pScodA vector was grown for 48 h at 28°C in 5 ml of yeast extract peptone medium (containing rifampicin 100 mg l−1). The transformation was carried out as described by Ahmad et al. (2008). Briefly, stem internodes without axillary buds were cut into 5- to 10-mm-long sections. The explants were precultured for 2 days on MS basal medium containing 0.2 mg l−1 2, 4-D. These explants were inoculated with Agrobacterium (concentration 1 × 106 cells ml−1) for 10 min and cocultured for 2 more days in the dark on preculture medium (MS basal medium containing 0.2 mg l−1 2, 4-D). After coculture, explants were blot dried on sterile tissue papers and shifted to regeneration medium (MS medium containing 0.01 mg l−1 naphthalene acetate (NAA), 0.1 mg l−1 GA3 and 2 mg l−1 zeatin) supplemented with 400 mg l−1 cefotaxime and 1 mg l−1 bialaphos. The first shoot regeneration was acquired after 1 month and was transferred to rooting medium (MS medium containing 3% sucrose, 400 mg l−1 cefotaxime and 1 mg l−1 bialaphos).
Methyl viologen treatment and tolerance assay
A leaf disc assay for oxidative stress tolerance was conducted as described by Kwon et al. (2002). In brief, leaf discs were punched from the fifth to sixth leaf from the top of 5- to 7-week-old plants and floated on solution containing 5 or 10 µM methyl viologen (MV) in 0.4% sorbitol. After incubation in the dark for 12 h , leaf discs were illuminated in continuous light (150 µmol photons m−2 s−1) at 25°C. Ion leakage of the solution was assessed using an ion conductivity meter (Model 455C, Isteck Co., Seoul, Korea). The H2O2 content was assessed with xylenol orange, in which H2O2 is reduced by ferrous ions in an acidic solution that forms a ferric product—xylenol orange complex, which was detected at 560 nm (Bindschedler et al. 2001). H2O2 measurements were expressed as relative values.
Salt stress treatment and tolerance assay
For the salt stress analysis at the whole-plant level, transgenic SSA, SSAC and non-transgenic (NT) plantlets were allowed to grow in a growth chamber with a 16/8 h photoperiod at 25°C and in 100 µmol photons m−2 s−1 light conditions. After 6 weeks of growth, plants under similar height and health conditions were selected for NaCl treatment. A volume of 200 mM NaCl solution was supplied via irrigation through the trays underneath the pots on alternate days for 2 weeks. Afterward, the plants were irrigated only with water for 2 weeks in order to allow the plants to recover. The fresh and dry weights of salt-treated plants were determined and compared with the plants grown under normal conditions. After harvesting and measuring the fresh weight, the plant samples were dried for 48 h at 80°C in order to measure the dry weight. Photosynthetic activity was recorded via chlorophyll fluorescence determinations of photochemical yield (Fv/Fm), which represented the maximum quantum yield of photosystem II, using a portable chlorophyll fluorescence meter (Handy PEA, Hansatech, England) after 30 min of dark adaptation. Chlorophyll content after salt stress was measured by a portable chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan) from intact fully expanded fifth leaves from the top of individual plants.
Drought stress treatment and tolerance assay
Plantlets rooted on MS medium were transferred to pots filled with equal quantities of soil and then grown in a growth chamber for 4 weeks under similar conditions as described earlier for the salt stress treatment. Drought stress was imposed by withholding the water supply to the plants, which were maintained at 25°C under light conditions of 100 µmol photons m−2 s−1. Before withholding the water supply, the plants were irrigated with similar quantities of water through trays placed underneath the pots for 1 week. After 2 weeks of water withholding, the plants were again irrigated and permitted to recover from the drought conditions. Fresh and dry weight gain was measured after 1 week of recovery, as described in the preceding section. The degree of drought stress was assessed by the relative water contents (RWCs) of leaves after 12 and 14 days of water withholding. The RWCs% were estimated as described by Ma et al. (2006). RWC% was measured from the first fully expanded leaf from the top.
Western blot analysis
Leaf discs treated with 10 µM MV solution for 72 h were used to detect the codA protein in selected SSAC lines. Primarily, western blots were performed as described by Sakamoto et al. (1998). Briefly, proteins were extracted in 20 mM sodium phosphate buffer (pH 7.0) by centrifuging the leaf homogenate at 12 000 g for 10 min to remove cell debris and insoluble fractions. Protein samples (40 µg) were subjected to electrophoresis on an 8% polyacrylamide gel containing 10% sodium dodecyl sulfate. Following electrophoresis, proteins were blotted onto polyvinyl difluoride membranes (Immobilin-N; Millipore, Bedford, MA). The resultant membrane was allowed to react with an antiserum raised against codA, as described previously (Deshnium et al. 1997).
GB analysis
GB was extracted and quantified as previously described by Park et al. (2004) and Ahmad et al. (2008). In brief, leaves frozen in liquid nitrogen were powdered with a ceramic mortar and pestle. This powder (2 g) was then suspended in 2 ml of ice-cold methanol:chloroform:water (60:25:15) and an equal volume of distilled water was added to the tubes. The resultant homogenate was shaken gently for 10 min and then centrifuged for 10 min at 570 g at room temperature. The upper methanol–water phase was transferred to clean tubes. The extracts were freeze-dried by speed vacuum and dissolved in distilled water (2 ml). These extracts were treated with the strong anion exchange resin, AG 1-X8 (Bio-Rad, Hercules, CA), as described by Bessieres et al. (1999). Micro Bio-spin chromatography columns (Bio-Rad) were packed with AG 1-X8 resin via the addition of 1 ml of resin slurry and were centrifuged at 1000 g at room temperature. Afterward, 1 ml of crude GB extract was loaded into the resin bed and centrifuged for 3 min at 1000 g. The resin was washed with 0.5 ml of distilled water and mixed with the previous flow-through fraction. GB was measured via high-performance liquid chromatography (HPLC) (SPD-10A VP, Shimadzu, Tokyo, Japan), as previously described by Bessieres et al. (1999). In brief, purified GB was detected on an Apollo C18 column (250 × 4.6 mm) under isocratic conditions with 13 mM sodium 1-heptanesulphonate and 5 mM Na2SO4 solution (pH 3.7) as a mobile phase. The GB peak was monitored using a Ultraviolet detector at 200 nm and quantification was conducted via comparison of the peak surface areas with those obtained using a pure GB standard (Sigma, St Louis, MO).
RT-PCR analysis
For expression analysis of foreign genes, total RNA was extracted using the cetyltrimethylammonium bromide method (Kim and Hamada 2005) and treated extensively with RNase-free DNase I in order to remove any contaminating genomic DNA. Two micrograms of RNA was utilized for reverse transcription in accordance with the manufacturer's instructions (Reverse Transcription System, Promega, Madison, WI). Gene-specific primers used for PCR reactions were as follows: the CuZnSOD primer set (5′-ATGGTGAAGGCTGAAGCTGTTCTT-3′, 5′-CTATCCTCGCAAACCAATACCG-3′), the APX primer set (5′-ATGGGAAAATCTTACCCAACTGTTA-3′, 5′-TTA GGCTTCAGCAAATCCAAGCTC-3′) and the codA gene primer set (5′-GCTGCTGGAATCGGGATA-3′, 5′- TGGG CTTATCGCGGAAGT-3′) were used to amplify products for CuZnSOD, APX and codA, respectively, from cDNA. The total synthesized cDNA was also used to amplify the actin gene as an internal standard using actin gene-specific primers (5′-TGGACTCTGGTGATGGTGTC-3′, 5′-CCTCCAATCCAAACACTGTA-3′).
Analysis of SOD, APX and CAT activity
Potato leaves were homogenized on ice with a mortar in a 0.1 M potassium phosphate buffer (pH 7). The homogenate was centrifuged at 12 000 g for 15 min at 4°C. The supernatant was used immediately for enzyme assays. Protein concentration was determined according to the Bradford (1976) method using BioRad's protein assay reagents and bovine serum albumin as a standard. SOD activity was measured according to the method of McCord and Fridovich (1969) using xanthine, xanthine oxidase and cytochrome c. One unit of SOD was defined as the amount of enzyme that inhibits the rate of ferricytochrome c reduction by 50%. APX activity was assayed according to the method described by Nakano and Asada (1981) using ascorbic acid as a substrate. The oxidation of ascorbate was initiated by H2O2, and the decrease at 290 nm for 1 min 30 s was monitored. One unit of APX was defined as the amount of enzyme oxidizing 1 µM of ascorbate per minute. CAT activity was assayed according to the method described by Aebi (1984). The activity was determined by the decrease at 240 nm for 1 min because of H2O2 consumption.
Statistical analysis
Data were statistically analyzed with Duncan's multiple range tests using Statistical Package for the Social Sciences (spss 12) and Student's t-test using Microsoft Excel 2003. The significance refers to statistical significance at the P≤ 0.05 level.
Results
Generation of transgenic potato plants and line selection for further characterization
To evaluate the synergistic effects of multiple genes on stress tolerance in transgenic plants, we retransformed SSA transgenic potato plants (Fig. 1A) by using pScodA that harbored a chloroplast-targeted codA gene under the control of the SWPA2 promoter in an expression vector (Fig. 1B). In order to target codA to chloroplasts, a transit peptide (TP) from the small subunit of tobacco Rubisco was utilized (Hayashi et al. 1997). The putative transgenic potato plants were selected on MS medium containing bialaphos. Fifteen transgenic lines were obtained, and the codA gene integration was verified primarily via PCR of genomic DNA; the expected amplification profiles were acquired from all transgenic lines (data not shown). The transgenic lines harboring the codA, CuZnSOD and APX genes under the control of the SWPA2 promoter were referred to as ‘SSAC’ plants. All 15 of the transgenic lines were grown in a growth chamber for 6 weeks and were utilized to evaluate enhanced tolerance against MV-mediated oxidative stress using leaf discs. MV is a non-selective herbicide that produces massive bursts of ROS, which upon overaccumulation disrupt the membrane stability and lead to cell death (Bowler et al. 1991). Leaf discs were punched from the fifth to sixth leaves from the shoot tip and floated on a 0.4% sorbitol solution containing 5 µM MV (data not shown). Ion leakage was assessed over a time course; among these 15 lines, 2 transgenic lines with the highest observed tolerance (referred to as SSAC1 and SSAC2 plants) were selected for further characterization.
Schematic representation of expression vectors used for potato transformation. (A) Schematic representation of the pSSA expression vector. SWPA2 pro, sweetpotato peroxidase promoter; TEV, tobacco etch virus 50-untranslated region; TP, chloroplast-targeted transit peptide; CuZnSOD, cassava copper–zinc superoxide dismutase cDNA; NOS ter, nopaline synthesis terminator; APX, pea ascorbate peroxidase cDNA. (B) Schematic representation of the T-DNA regions of pScodA used for potato transformation. SWPA2 pro, sweetpotato peroxidase promoter; TP, chloroplast-targeted transit peptide; codA, bacterial choline oxidase cDNA; NOS ter, nopaline synthesis terminator.
Enhanced tolerance of transgenic plants to MV-mediated oxidative stress
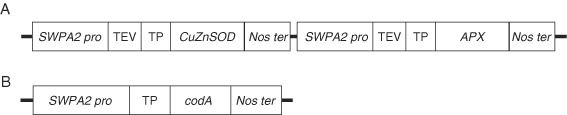
To evaluate the synergistically enhanced tolerance of SSAC transgenic plants over SSA and NT plants, leaf discs from same-age plants were prepared and treated with 10 µM MV solutions. The differential visible damage on the leaf discs demonstrates the enhanced tolerance of SSAC plants as compared with SSA and NT plants (Fig. 2A). After 72 h of MV treatment, ion leakage of SSAC1 and SSAC2 plants was 42 and 37%, respectively, while it was 63% in SSA plants and 90% in NT plants (Fig. 2B). H2O2 is produced in the plants during stressful conditions; if it remains unscavenged, it can cause severe damage to the plants. We evaluated the relative H2O2 content of SSAC, SSA and NT plants after 10 µM MV treatment for 72 h. SSAC plants showed lower H2O2 content than NT and SSA plants. The order of relative H2O2 content was NT > SSA > SSAC (Fig. 2C).
Effect of MV-mediated oxidative stress treatment in NT, transgenic SSA and SSAC plants. (A) Differential visible damage on leaf discs after 72 h of 10 µM MV treatment. (B) Relative ion leakage of leaf discs treated with 10 µM MV and incubated at 25°C and a light intensity of 150 µmol photons m−2 s−1. Ion leakage was measured after 0, 24, 48 and 72 h of MV treatment. Relative ion leakage were calculated using 100% to represent values obtained after autoclaving. (C) H2O2 contents in the leaf discs of NT, SSA and SSAC plants treated with 10 µM MV. Leaf discs were treated with MV, and H2O2 content was determined at 0, 48 and 72 h of treatment. NT, non-transgenic plants; SSA, transgenic plants expressing CuZnSOD and APX in chloroplasts; SSAC1 and SSAC2, independent transgenic lines expressing codA, CuZnSOD and APX in chloroplasts. Data are expressed as the mean ±sd of three replicates. Bars carrying the same letter are not significantly different (P = 0.05) according to Duncan's multiple range test.
Enhanced tolerance of SSAC potato to oxidative stress by an increase in GB content and antioxidant enzyme activities
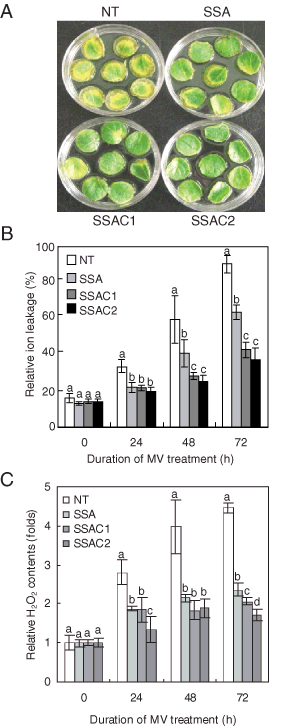
To understand the tolerance mechanism that is active in SSAC plants following MV-mediated oxidative stress, we performed reverse transcription-polymerase chain reaction (RT-PCR) to determine time-course changes in the gene expression of the introduced CuZnSOD, APX and codA genes following the 10 µM MV treatments. Transcripts of chimeric genes were not detected in NT plants, while SSA and SSAC plants did show transcripts of chimeric genes (Fig. 3A). Additionally, SSAC plants maintained higher expression levels of the CuZnSOD and APX genes than did SSA transgenic plants. To further confirm the expressions of codA protein in SSAC plants, we performed western blot analysis. Leaf discs of NT, SSA and SSAC plants were treated with a 10 µM MV solution for 72 h, and then proteins were extracted and used for western blot analysis, which revealed that the SSAC1 and SSAC2 lines accumulated a 64-kDa protein corresponding to codA, while no such protein was detected in NT and SSA plants (Fig. 3B). In order to determine whether the expression of the codA gene resulted in GB synthesis in the transgenic SSAC plants, GB content was analyzed quantitatively with HPLC. Generally, higher MV concentrations are required for the imposition of stress at the whole-plant level, and thus transgenic and NT plants were subjected to 400 µM MV prior to sampling in order to induce the expression of the GB-synthesizing codA gene. The potato plant is naturally a non-accumulator of GB; we could not find GB in NT plants, whereas transgenic SSAC plants accumulated GB. The GB content in SSAC1 and SSAC2 plants was 1.26 and 1.65 µmol g−1 FW after 72 h of MV treatment, respectively, while under non-stressed conditions, the GB content in SSAC1 and SSAC2 plants was 0.26 and 0.35 µmol g−1 FW, respectively (Fig. 3C).
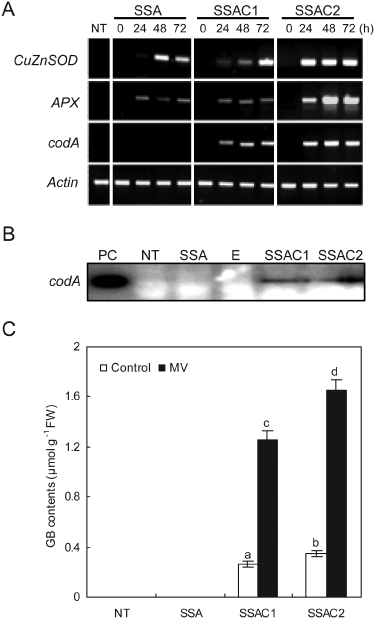
Analysis of RT-PCR, western blot and GB content in NT and transgenic (SSA and SSAC) plants under MV treatments. (A) RT-PCR analysis of CuZnSOD, APX and codA gene expression in NT, SSA and SSAC plants subjected to 10 µM MV. Gene for actin was utilized as an internal control. (B) Western blot analysis of two selected SSAC lines with enhanced tolerance to various abiotic stresses expressing stable codA gene integration and posttranscriptional processes in the transgenic plants following MV treatment. PC, positive control; E, empty lane. (C) GB content in SSAC transgenic potato. GB was extracted from non-treated and MV-treated plants. Samples were collected after 72 h of MV treatment. Data are expressed as the mean ±sd of three replicates. Bars carrying the same letter are not significantly different (P = 0.05) according to Duncan's multiple range test.
We also investigated the enzyme activities of SOD, APX and CAT in the total protein isolated from leaf discs of NT and transgenic plants over time following the 10 µM MV treatments. SSAC transgenic plants maintained higher SOD and APX activities during MV treatments than did SSA and NT plants (Fig. 4A, B). We also determined the CAT activity in the leaves of SSAC, SSA and NT plants after MV treatment. The CAT activity of SSAC plants was higher than SSA and NT plants during MV treatment (Fig. 4C).
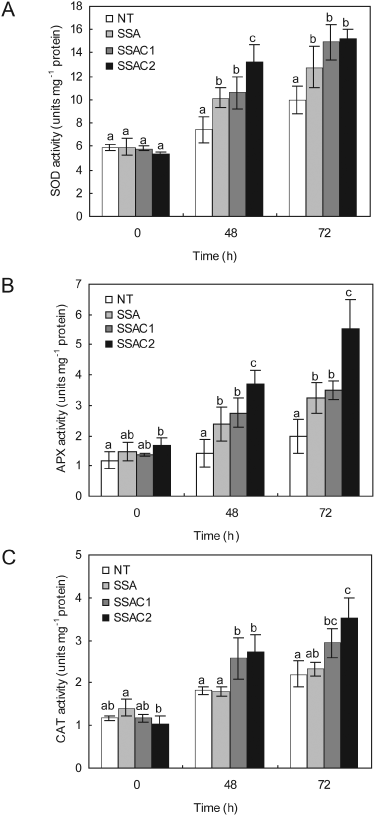
Analysis of antioxidant enzymes activities after MV treatment. (A) Changes of specific SOD activities after MV treatments. (B) Changes of specific APX activities after MV treatments. (C) Changes of specific CAT activities after MV treatments. Data are expressed as the mean ±sd of three replicates. Bars carrying the same letter are not significantly different (P = 0.05) according to Duncan's multiple range test.
Enhanced tolerance of transgenic plants to higher levels of salinity
We evaluated transgenic (SSA and SSAC) and NT plants against salt stress at the whole-plant level. After 2 weeks of salt (200 mM NaCl) administration, plants were supplied with water only. Changes in photosynthetic activities induced by salt stress were determined by measuring fluorescence parameters (Fv/Fm). Salt stress affected the photosynthetic machinery of all transgenic and NT plants; however, the transgenic lines maintained significantly higher levels of photosynthetic activity than NT plants (Fig. 5A, B). A continuous decrease was noticed in the Fv/Fm value of plants treated with salt until the end. The Fv/Fm value of NT plants was decreased by 76% after 4 weeks of stress treatment, while SSA plants exhibited a 38% decrease in photosynthetic activity. On the other hand, GB-synthesizing SSAC plants showed significantly higher Fv/Fm values than NT and SSA plants. The photosynthetic activity of SSAC1 and SSAC2 plants was attenuated by 26 and 20%, respectively. We also measured the changes in the chlorophyll content of NT, SSA and SSAC plants after salt stress (Fig. 5C). The chlorophyll content of NT plants started to decrease earlier than that of SSA and SSAC plants. NT plants exhibited an 80% decrease in chlorophyll content, while SSA plants showed a 43% reduction in chlorophyll content. Contrary to NT and SSA plants, the chlorophyll content of SSAC1 and SSC2 plants was decreased by 23 and 18%, respectively. In addition, the SSAC transgenic lines 1 and 2 exhibited higher levels of tolerance in the presence of 200 mM NaCl, as seen by assessments of plant dry weight (Fig. 5D).
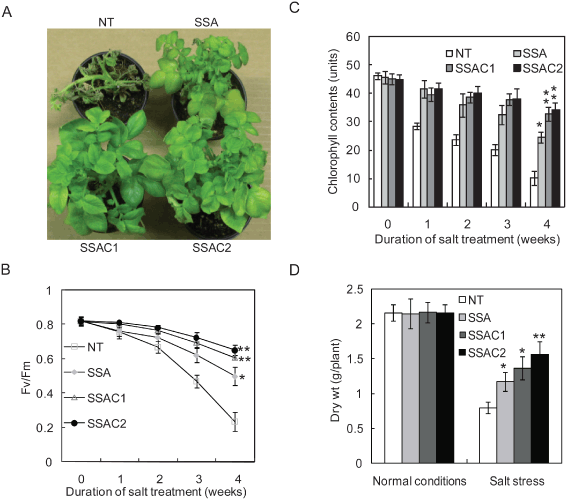
Effect of salt stress (200 mM NaCl) on 6-week-old NT and transgenic (SSA, SSAC) plants grown in soil. Salt was applied for 2 weeks on alternate days, and over the next 2 weeks, recovery was allowed via the application of water only for 2 weeks. (A) Visible damage appeared on plants after salt stress. Pictures were taken after 2 weeks of recovery. (B) Effects of salt stress on photoinhibition measured by Fv/Fm values, where 0 means normal growing conditions. (C) Effect of salt stress on chlorophyll content. (D) Dry weight of NT, SSA and SSAC plants after salt stress (200 mM) in greenhouse conditions compared with the dry weight gained in normal conditions. Data are expressed as the mean ±sd of five replicates. Bars labeled with asterisk show significant differences between NT, SSA and SSAC plants by t-test at P = 0.05.
Enhanced drought stress tolerance of transgenic plants
To evaluate the enhanced tolerance of transgenic plants against a multitude of stresses, we subjected the plants to drought stress. Four-week-old soil-grown plants were used to impose drought stress by withholding the water supply for 2 weeks. Severe wilting of NT plants was noticed along with SSA plants, while SSAC plants showed less wilting compared with NT and SSA plants. Plants were rewatered after 2 weeks of drought stress and allowed to grow for 1 more week to recover. NT plants could not recover from severe drought stress, while SSA and SSAC plants recovered successfully; however, the pace of recovery was slow in SSA plants (Fig. 6A). RWC gives a good indication of the turgidity maintained by plants. We determined the RWC of drought-stressed plants after 12 and 14 days of water withholding (Fig. 6B). Drastic water loss was observed in the NT plants after 14 days of drought stress: the RWC was only 34% in NT plants, while SSA plants maintained 52%. Contrary to both, SSAC1 and SSAC2 plants maintained significantly higher RWC: 68 and 73%, respectively. We also determined the vegetative biomass of drought-stressed plants and compared it with the biomass obtained in normal growth conditions (Fig. 6C). The dry weight of drought-stressed NT plants was adversely affected. There was a 77 and 66% decrease in dry weight gain of NT and SSA plants, respectively. Compared with NT and SSA plants, SSAC1 and SSAC2 plants maintained better growth, and there was significantly less loss (47–42%) in biomass accumulation, respectively.
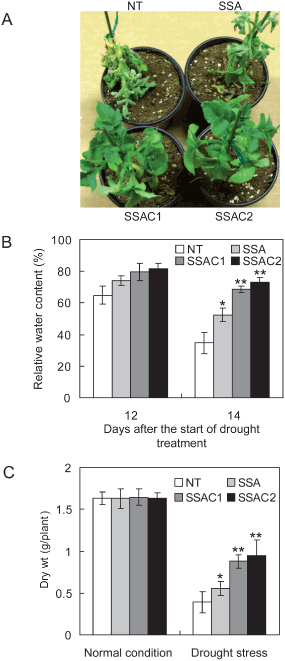
Drought stress analysis of NT and transgenic (SSA and SSAC) plants. (A) Visible damage appeared on plants after drought stress. Pictures were taken after 2 weeks of water withholding. (B) RWC of NT, SSA and SSAC plants after 12 and 14 days of water withholding. The RWC was measured from the first fully expanded leaf from the shoot tip. (C) Dry weight of NT, SSA and SSAC plants after drought stress. Dry weights were measured after 7 days of recovery and compared with the dry weight gained in normal conditions. Data are expressed as the mean ±sd of five replicates. Bars labeled with asterisk show significant differences between NT, SSA and SSAC plants by t-test at P = 0.05.
Endogenous GB accumulation stabilizes gene expression and enzymatic activities during salt and drought stress
GB accumulation can stabilize endogenous gene exressions and protects the proteisn during stressful conditions. To evaluate the GB accumulation, CuZnSOD, APX and codA genes' expression and enzymatic activities of SOD, APX during salt and drought stress, 6-week-old NT, SSA and SSAC plants were used. The GB content in SSAC1 and SSAC2 plants after salt treatment was found to be 1.28 and 1.77 µmol g−1 FW, respectively, whereas the GB content after 1 week of drought stress was 1.25 and 1.74, respectively (Fig. 8A, B). We used RT-PCR analysis to reveal the changes in expression levels of chimeric genes in transgenic and NT plants treated with 200 mM NaCl or drought stress. Transcripts of chimeric genes were not detected in NT plants, while SSA and SSAC plants showed transcripts of chimeric genes. Particularly, SSAC2 plants maintained the highest expression levels of the CuZnSOD and APX genes relative to SSAC1 and SSA transgenic plants under stress conditions (Fig. 7). SSAC transgenic plants also maintained higher SOD enzyme activities during salt and drought stresses than SSA and NT plants did (Fig. 8C, D). Similarly, SSAC plants also showed higher APX and CAT activities than SSA and NT plants after salt and drought stress treatments (Fig. 8E–H).
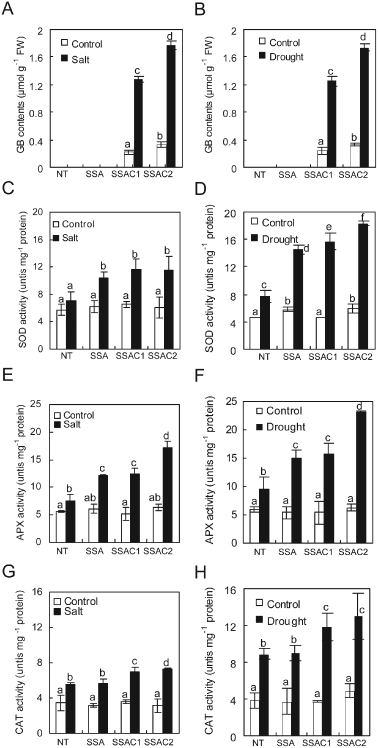
Analysis of GB contents and antioxidant enzyme activities after imposition of 200 mM salt and drought stress for 2 weeks. (A, B) GB content in SSAC transgenic potato. (C, D) SOD activity in SSAC transgenic potato. (E, F) APX activity in SSAC transgenic potato. (G, H) CAT activity in SSAC transgenic potato. Data are expressed as the mean ±sd of three replicates. Bars carrying the same letter are not significantly different (P = 0.05) according to Duncan's multiple range test.
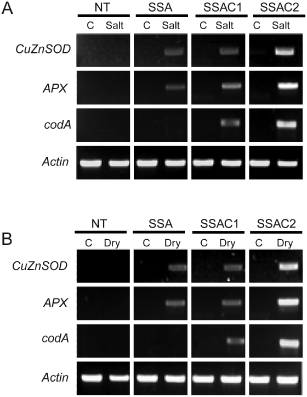
RT-PCR analysis of SOD, APX and codA gene expression in the leaves of NT and transgenic (SSA, SSAC) plants subjected to salt and drought stresses. (A) Expression patterns of the CuZnSOD, APX and codA genes in the leaves of NT, SSA and SSAC plants subjected to 200 mM NaCl. (B) Expression patterns of the CuZnSOD, APX and codA genes in the leaves of NT, SSA and SSAC plants subjected to drought stress. Actin was utilized as an internal control.
Discussion
Plants tolerate abiotic stresses, including oxidative stress, by modulating multiple genes and coordinating the action of various genes from different pathways. There is a speculation that multiple gene transfer can act additively or synergistically to enhance tolerance to abiotic stresses such as salt stress (Bohnert et al. 1996). However, there are few reports on the transformation of multiple genes into plants and their role in imparting stress tolerance (Zhou et al. 2008). To further evaluate the protective effects of GB on various enzyme activities, including the antioxidant enzymes, and to address the speculations about possible synergism between GB and other stress-related genes, we retransformed the transgenic potato plants ‘cv: Superior’ expressing the CuZnSOD and APX genes (SSA plants) in chloroplasts by introducing the codA gene for chloroplast-targeted codA under the control of the stress-inducible SWPA2 promoter (creating SSAC plants).
A total of 15 SSAC transgenic lines were screened via MV-mediated oxidative stress to obtain highly tolerant lines for further molecular and physiological characterization. Among the 15 transgenic lines, 2 transgenic lines with the least damage, referred to as SSAC1 and SSAC2, were selected (data not shown). Western blot analysis using antiserum raised against codA revealed the presence of a 64-kDa protein corresponding to codA (Fig. 3); this result shows stable translational and posttranslational processes in SSAC1 and SSAC2 plants. In our previous paper, the levels of SOD and APX in the chloroplast fraction of SSA potato plants were much higher than those in the total protein fraction, reflecting the chloroplast-targeted expression and stress-inducible promoter (Tang et al. 2006). Potato is naturally a non-accumulator of GB; we used HPLC to analyze the GB content in the SSAC plants. Both the SSAC1 and SSAC2 lines accumulated GB, but at variable quantities (Fig. 3). The GB content found in all transgenic studies in many plants, including Arabidopsis, tobacco, tomato and rice, remained much lower than that observed in the natural accumulators such as spinach (40 µmol g−1 FW). However, these lower levels of GB imparted appreciably high levels of tolerance in various transgenic plants against salt, drought, high temperature, chilling and freezing stresses (Ahmad et al. 2008, Mohanty et al. 2002, Park et al. 2004, Sakamoto et al. 1998). Moreover, it has been demonstrated that GB level as low as 0.09 µmol g−1 FW can impart chilling stress tolerance in tomato plants (Park et al. 2004) and 35 nmol g−1 FW GB was sufficient to increase salt stress tolerance in transgenic tobacco plants (Holmström et al. 2000).
Plants exposed to biotic and abiotic stresses accumulate more ROS, which can damage lipids, proteins, DNA and carbohydrates and can lead to cell death if not scavenged well in the proper amount of time. There are reports showing a positive effect of GB on the antioxidative system either as synthesized by plants or when applied exogenously (Ma et al. 2006, Park et al. 2007, Yang et al. 2008). Our results are consistent with previous reports showing synergistically enhanced tolerance of SSAC plants against MV-mediated oxidative stress as compared with SSA and NT plants (Fig. 2). To further understand the synergism, we measured the H2O2 content in leaf samples treated with MV. SSAC plants accumulated less H2O2 as compared with SSA and NT plants, demonstrating the protective effects of GB on the antioxidative system (Fig. 2). GB enhanced the effective scavenging of H2O2 by stabilizing the indigenous and chimeric antioxidant enzymes, as has been previously reported in numerous studies (Park et al. 2007).
Salt stress imposes different kinds of stress, including oxidative stresses. The accumulation of GB in plants—either naturally, when synthesized by genetic transformation, or when applied exogenously—rendered the plants tolerant to salt stress (Ahmad et al. 2008, Hayashi et al. 1997, Holmström et al. 2000, Mohanty et al. 2002, Waiditee et al. 2005). GB-synthesizing SSAC transgenic plants developed in this study were better in all aspects of growth (photosynthetic activity, chlorophyll content and dry weight gain) compared with SSA and NT plants under salt stress conditions (Fig. 5). It has been reported in many studies that GB synthesis in chloroplasts was more effective in imparting stress tolerance in transgenic plants as compared with plants in which GB was synthesized elsewhere (Park et al. 2007). We can assume that codA for GB synthesis was successfully targeted to the chloroplasts, because the same transit peptide was utilized as that reported by Hayashi et al. (1997) for Arabidopsis, Prasad et al. (2000) for Brassica and Mohanty et al. (2002) for rice. SSAC transgenic plants maintained higher values of Fv/Fm during the entire period of salt stress than SSA and NT plants. Chloroplasts are the major site of photooxidative reactions that abundantly produce ROS. In this study, GB synthesized in chloroplasts protected not only the photosynthetic machinery but also the antioxidant defense mechanisms of the plants. Nishiyama et al. (2006) presented evidence to suggest that the primary sites at which photodamage occurs are the oxygen-evolving complex and the D1 proteins. Thus, the inhibition of Photosystem II repair is the principal cause of poor photosynthetic activity occurring under stressful conditions. The protective effect conferred by GB has been shown to guard the machinery required for the degradation and synthesis of the D1 protein under stress exerted by high salt conditions (Ohnishi and Murata 2006). Demiral and Turkan (2004) reported enhanced activities of ROS scavenging enzymes in salt-stressed rice seedlings because of the exogenous application of GB.
GB is the most efficient osmoprotectant characterized to date (Felitsky et al. 2004). However, the low GB levels in transgenic plants indicate a role other than osmoprotection. It has been demonstrated in various studies that GB protects and stabilizes the macromolecules, enzyme activities and membrane stability in stressful conditions (Chen and Murata 2002a,b, Sakamoto and Murata 2001). In our previous study, GB-synthesizing transgenic potato plants showed enhanced tolerance to drought stress (Ahmad et al. 2008). GB-synthesizing transgenic maize was more tolerant to drought compared with wild-type plants (Quan et al. 2004), even though the GB levels were significantly lower than that of natural accumulators. Similarly, GB-synthesizing transgenic tobacco plants demonstrated enhanced tolerance to polyethyleneglycol-mediated osmotic stress (Shen et al. 2002). In the present study, GB-synthesizing SSAC transgenic potato plants were more tolerant to drought stress compared with SSA transgenic and NT plants (Fig. 6). We speculate that GB plays a similar role in SSAC plants as that observed in other GB-synthesizing transgenic plants.
Antioxidant enzymes and metabolites are reported to increase under various environmental stresses (Corpas et al. 1993) and comparatively higher activity has been reported in tolerant cultivars than in susceptible ones (Demiral and Turkan 2004, Hasegawa et al. 2000), suggesting that higher antioxidant enzyme activity has a role in imparting tolerance to these cultivars against environmental stresses. Various studies have demonstrated that GB has protected the structures of enzymes and enhanced their activity in various kinds of stresses. Yang et al. (2007) reported higher activities of antioxidative enzymes such as SOD, APX and CAT in GB-synthesizing transgenic tobacco plants under stressful conditions. Similar findings have been reported by other researchers demonstrating higher antioxidative enzyme activities in stress conditions, either by in vivo GB synthesis or by exogenous GB application (Ma et al. 2006, Park et al. 2007, Yang et al. 2008). In the current study, we found that, compared with NT and SSA plants, GB-synthesizing SSAC plants maintained higher activities of SOD, APX and CAT during oxidative, salt and drought stresses (4, 8). To further support the possible synergistic effects of GB on antioxidant enzymes, the expression levels of CuZnSOD and APX were determined after various stresses (3, 7). The higher expression levels of the CuZnSOD and APX genes in SSAC plants complement the results of the enzyme activities. However, expression levels of indigenous antioxidant enzymes need to be explored in future. Additionally, it has been reported that endogenous synthesis or exogenous application of GB can regulate various genes belonging to stress tolerance mechanisms (Chen and Murata 2008 and references there in). GB can protect Rubisco enzyme during salt stress by stabilizing its conformation (Nomura et al. 1998) and it can maintain membrane integrity and reduced lipid peroxidation during stress conditions (Chen et al. 2000). To further the discussion, we have already reported the transgenic potato plants expressing only codA gene referred to as ‘SC’ plants (Ahmad et al. 2008), in which, we showed that SC plant could tolerate 5 µM MV-mediated stress and enhanced tolerance to 150 mM NaCl stress. However, our SSAC plants efficiently tolerated 10 µM MV-mediated stress and depicted enhanced tolerance to 200 mM NaCl stress. In the context of this data, we can understand, to some extent, the synergistically enhanced tolerance of SSAC plants against various stresses. This speculation is further reinforced by the lower levels of H2O2 in SSAC plants after MV-mediated oxidative stress treatment (Fig. 2).
We also observed that the introduction of chimeric genes did not alter the phenotype of the plants in our green house experiments (5, 6). The dry weight gained by the NT and transgenic plants grown in normal conditions was similar to each other. Similarly, the photosynthetic activity (Fv/Fm) of NT and transgenic plants during normal growth conditions was identical. However, it will be interesting to evaluate the growth and production related parameters of transgenic plants in field conditions, which will be conducted in near future.
In conclusion, we successfully demonstrated the development and characterization of transgenic plants expressing three stress-protecting genes under the control of a stress-inducible promoter. The results of our study clearly show the hypothesized synergism of GB with other stress-protecting components in plants. Our results indicated increasingly higher levels of tolerance of SSAC plants against MV, salt and drought stresses in leaf discs as well as in the whole plant. However, comparative analysis of SSAC, SSA and NT plants in terms of yield and growth under field conditions remains to be conducted. We anticipate the evidence provided in this study will be very useful in engineering crop plants of diverse use, for growth in marginal soils, which may help to overcome the existing food security problems of the world. The strategy might also be employed by expressing tranegenes in specific tissues under the control of inducible promoters to harvest maximum benefits from this advanced technique for crop plant improvement.
Acknowledgements
This work was supported by grants from the Biogreen21 Program (20070301034015), Rural Development Administration, Korea; from the MEST/NRF to Environmental Biotechnology National Core Research Center (grant no. 20090091490), Korea; and from the Korea-China International Collaboration Project, NRF/MEST, and KRIBB initiative program.




