ROS in biotic interactions
Abstract
Production of reactive oxygen species (ROS) is a hallmark of successful recognition of infection and activation of plant defenses. ROS play multifaceted signaling functions mediating the establishment of multiple responses and can act as local toxins. Controversy surrounds the origin of these ROS. Several enzymatic mechanisms, among them a plasma membrane NADPH oxidase and cell wall peroxidases, can be responsible for the ROS detected in the apoplast. However, high levels of ROS from metabolic origins and/or from downregulation of ROS-scavenging systems can also accumulate in different compartments of the plant cell. This compartmentalization could contribute to the specific functions attributed to ROS. Additionally, ROS interact with other signals and phytohormones, which could explain the variety of different scenarios where ROS signaling plays an important part. Interestingly, pathogens have developed ways to alter ROS accumulation or signaling to modify plant defenses. Although ROS have been mainly associated with pathogen attack, ROS are also detected in other biotic interactions including beneficial symbiotic interactions with bacteria or mycorrhiza, suggesting that ROS production is a common feature of different biotic interactions. Here, we present a comprehensive review describing the newer views in ROS signaling and function during biotic stress.
Abbreviations-
-
- ABA
-
- abscisic acid
-
- ET
-
- ethylene
-
- ETI
-
- effector-triggered immunity
-
- HR
-
- hypersensitive response
-
- JA
-
- jasmonic acid
-
- NO
-
- nitric oxide
-
- OGs
-
- oligogalacturonides
-
- PAMPs
-
- pathogen associated molecular patterns
-
- PTI
-
- PAMP-triggered immunity
-
- ROS
-
- reactive oxygen species
-
- SA
-
- salicylic acid
-
- SAR
-
- systemic acquired resistance
-
- TMV
-
- Tobacco Mosaic Virus
Introduction
A rapid and transient production of reactive oxygen species (ROS), termed ‘oxidative burst' is a hallmark of successful recognition of plant pathogens (Lamb and Dixon 1997, Torres et al. 2006). This production of ROS is typically apoplastic (Levine et al. 1994) and biphasic, with a first unspecific, transitory phase that usually takes place within minutes of the interaction with the pathogen, and a second sustained phase that occurs hours after pathogen attack and that is usually associated with the establishment of the defenses and the hypersensitive response (HR; Grant and Loake 2000, Piedras et al. 1998). Although superoxide anion ( ) is the proximal product generated, the more stable hydrogen peroxide (H2O2) species is detected in many studies. However, other reactive species such as singlet oxygen or hydroxyl radical can be produced (Foyer and Noctor 2005).
) is the proximal product generated, the more stable hydrogen peroxide (H2O2) species is detected in many studies. However, other reactive species such as singlet oxygen or hydroxyl radical can be produced (Foyer and Noctor 2005).
Plants activate several barriers of defense against the attack of pathogens (Jones and Dangl 2006). The first line of defense is triggered by the recognition of invariant microbial epitopes known as pathogen associated molecular patterns (PAMPs) for recognition of potential pathogens in the innate immune system of both plants and animals. Examples of these PAMPs are conserved cell surface structures like flagellin, lipopolysaccharides or peptidoglycanes from gram-negative bacteria or fungal cell wall components like glucan or chitosan. These PAMPs are recognized by distinct cell surface pattern recognition receptors to activate basal or innate immune responses, what is termed PAMP-triggered immunity (PTI). Pathogens use effectors to block this PTI leading to virulence, the effector being called in this case a virulence factor, and the pathogen virulent. However, some plant cultivars have evolved specific surveillance proteins, the Resistance (R) proteins to recognize these effectors (called then avirulence – avr – factors and the pathogens being avirulent), mounting a second line of defense called effector-triggered immunity (ETI). ETI is stronger and usually displays an HR with cell death associated to the pathogen infection (Jones and Dangl 2006).
ROS production is detected invariably during the activation of both PTI and ETI (Fig. 1). PAMPs from gram-negative bacteria like flg22 (a peptide derived from flagellin), harpin or lipopolysaccharides or from fungi like chitin (a major component of the fungal cell walls) or fungal polygalacturonases (that degrade pectin from the plant cell wall into oligogaracturonides) can all induce a transient but robust production of ROS (Felix et al. 1999, Galletti et al. 2008, Krause and Durner 2004, Kaku et al. 2006). However, recognition of pathogen avirulence factors from bacterial, fungal or viral pathogens by the corresponding R gene product also induces a strong oxidative burst (Doke and Ohashi 1988, Piedras et al. 1998, Torres et al. 2002). Moreover, a significant oxidative burst was recorded when tomato carrying the resistant gene Mi-1 recognizes the root knot nematode (Melillo et al. 2006) and in response to insects (Fester and Hause 2005, Martinez de Ilarduya et al. 2003, Maffei et al. 2006, Santos et al. 2001). Thus, although ROS are traditionally associated with plant defense responses to bacteria and fungi, ROS are produced in a great variety of interactions between plants and other organisms.
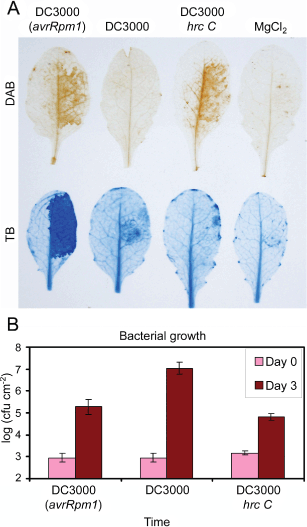
ROS production is associated with induction of the defenses in response to Pseudomonas syringae pv tomato in Arabidopsis Col-0: DC3000 (avrRpm1) induces ROS and HR associated with ETI after recognition of avrRpm1; DC3000 hrc C induces ROS associated to PTI. (A) Diaminobenzidine stain for H2O2 (top) and trypan blue stain for cell death (bottom) different times after injection of 107 cfu of different strains of P. syringae: DC3000 (avrRpm1) 8 h; DC3000 12 h; DC3000 hrc C 12 h; MgCl2 control 12 h. (B) Bacterial growth 3 days after injection of 105 cfu of different strains of P. syringae.
Many are the functions postulated for ROS produced in response to pathogens (Fig. 2). Early work suggested that the oxidative burst could have a direct effect on pathogen or the defenses because of its reactivity. ROS could directly kill the pathogen, especially in the case of the more reactive species like hydroxyl radicals (Chen and Schopfer 1999). ROS could also contribute to the establishment of physical barriers at the large papillae that are formed at the site of interaction of many pathogens by cross linking of cell wall glycoproteins (Bradley et al. 1992) or via oxidative cross linking of precursors during the localized biosynthesis of lignin and suberin polymers (Huckelhoven 2007). However, evidence suggests that ROS also have a signaling function mediating defense gene activation and establishment of additional defenses, by redox control of transcription factors or by interaction with other signaling components like phosphorylation cascades (Kovtun et al. 2000, Mou et al. 2003). ROS can generate lipid derivatives by non-enzymatic oxygenation that can produce membrane damage or function as signaling molecules like cyclic oxylipins of the jasmonate type (Montillet et al. 2005). ROS can also mediate the generation of phytoalexins and secondary metabolites that arrest pathogen growth (Thoma et al. 2003). But ROS are most distinctively associated with the HR, a localized response at the site of pathogen attack that displays programmed cell death and that could contribute to limit the spread of the pathogens or be a source of signals for establishment of further defenses (Mur et al. 2008). Thus, many are the functions accounted to ROS in response to pathogens.

Pathogen recognition leads to ROS production that has different functions associated to activation of plant defenses. Thin arrows depict signaling events that point to ROS production both in the apoplast and inside the plant cell. Double-head arrow indicates the cross talk between ROS in these compartments. Thick arrows point to the functions of these ROS in relation to activation of plant defenses.
The plasticity of ROS generation/accumulation and its tight compartmentalization could explain the variety of events mediated by these reactive molecules (Torres et al. 2006). Another feature of ROS signaling is its interaction with other signals and plant hormones. ROS form complex regulatory circuits with calcium signaling and phosphorylation cascades. Many regulatory functions of ROS in plant defenses have been associated with hormones like salicylic acid (SA) and nitric oxide (NO) (Torres et al. 2006). However, new interplays with additional signal cascades and hormones are emerging. The aim of this review is to offer an ample view about the flexibility of function that ROS are accounted for in plant defense to pathogens, a view that may also be extrapolated to other biotic interactions where ROS are also detected.
Sources of ROS in plant defense
The NADPH oxidase, also known as the Respiratory Burst Oxidase has been proposed as the source for this apoplastic oxidative burst in most plant–pathogen interactions (Torres and Dangl 2005). This enzyme was initially described in mammalian phagocytes as a multicomponent complex that mediates microbial killing, where gp91phox is the enzymatic subunit of the oxidase that transfers electrons to molecular oxygen to generate  (Lambeth 2004). However, the ROS produced are not directly responsible for the killing. The data suggest that ROS mediate pH changes and ion fluxes leading to the activation of specific proteases that do the microbial killing in phagocytes (Segal 2008). A family of NADPH oxidases (NOXs) exist in animals with specific functions ranging from controlling immunity, cell proliferation to thyroid hormone biosynthesis (Lambeth 2004). Plant NADPH oxidases, called Respiratory burst oxydase homologs (Rboh), have been described in many species (Torres and Dangl 2005). The Rboh protein localizes to the plasma membrane consistent with its function in producing the apoplastic oxidative burst (Keller et al. 1998, Kobayashi et al. 2006, Sagi and Fluhr 2001). As in animals, the plant Rboh genes form a large gene family suggesting specific functions for these oxidases. Interestingly, the plant Rboh proteins possess an N-terminal extension similar to some NOX5 and DUOX oxidases that function in animal immunity (Torres and Dangl 2005). This extension, which is absent in gp91phox from the phagocytes, contains EF-hands calcium binding motifs suggesting that the regulation of these oxidases is different from the phagocytic NADPH oxidase. Thus, most regulatory components of the NADPH from phagocytes will be absent in plants, with the exception of Rac/Rop GTPases that have been shown to mediate ROS production in the plant defense response as does Rac2 in the human phagocytes (Bokoch and Diebold 2002, Kawasaki et al. 1999).
(Lambeth 2004). However, the ROS produced are not directly responsible for the killing. The data suggest that ROS mediate pH changes and ion fluxes leading to the activation of specific proteases that do the microbial killing in phagocytes (Segal 2008). A family of NADPH oxidases (NOXs) exist in animals with specific functions ranging from controlling immunity, cell proliferation to thyroid hormone biosynthesis (Lambeth 2004). Plant NADPH oxidases, called Respiratory burst oxydase homologs (Rboh), have been described in many species (Torres and Dangl 2005). The Rboh protein localizes to the plasma membrane consistent with its function in producing the apoplastic oxidative burst (Keller et al. 1998, Kobayashi et al. 2006, Sagi and Fluhr 2001). As in animals, the plant Rboh genes form a large gene family suggesting specific functions for these oxidases. Interestingly, the plant Rboh proteins possess an N-terminal extension similar to some NOX5 and DUOX oxidases that function in animal immunity (Torres and Dangl 2005). This extension, which is absent in gp91phox from the phagocytes, contains EF-hands calcium binding motifs suggesting that the regulation of these oxidases is different from the phagocytic NADPH oxidase. Thus, most regulatory components of the NADPH from phagocytes will be absent in plants, with the exception of Rac/Rop GTPases that have been shown to mediate ROS production in the plant defense response as does Rac2 in the human phagocytes (Bokoch and Diebold 2002, Kawasaki et al. 1999).
The identification of mutant/antisense lines in Rboh that show elimination of extracellular ROS led to identification of NADPH oxidase as the main source of ROS in response to successful recognition of the pathogen, both in PTI and ETI. Arabidopsis AtrbohD and AtrbohF are responsible for nearly all ROS produced in response to avirulent bacteria and oomycete pathogens (Torres et al. 2002), as well as in response to PAMP recognition (Zhang et al. 2007). Additionally, antisense strategies showed that tobacco NtrbohD is the enzyme responsible for ROS produced in response to the Phytophthora elicitor cryptogein, whereas Nicotiana benthamiana NbrbohA and NbrbohB generate ROS in response to the infection by Phytophthora infestans (Simon-Plas et al. 2002, Yoshioka et al. 2003).
Alternative enzymes like diamine oxidases and especially cell wall peroxidises have also drawn attention as contributors to the apoplastic ROS oxidative burst (Allan and Fluhr 1997, Bolwell et al. 1998, Yoda et al. 2009). Two recent reports showed genetic evidence, by using antisense technologies, for the implication of cell wall peroxidases in ROS production in plant defense (Bindschedler et al. 2006, Choi et al. 2007). Comparative studies with specific inhibitors showed that peroxidases or NAPDH oxidase could mediate the oxidative burst in different plant–pathogen interactions (Bolwell et al. 1998), suggesting that the enzymatic origin of these ROS could vary depending on the particular plant–pathogen interaction under study.
Although the primary oxidative burst following pathogen recognition occurs in the apoplasts, ROS produced inside the plant cell may also function in defense. High levels of ROS are produced as by-products of metabolic processes that can affect cellular homeostasis. In particular, light-driven production of ROS inside the plant occurs by uncoupling or inhibition of photosynthesis and photorespiration associated with chloroplast and peroxisomes (Karpinski et al. 2003). This ROS has been associated with some cellular responses, particularly to HR cell death (Mur et al. 2008). Additionally, mitochondria can be a source of ROS associated with alteration in respiration (Vidal et al. 2007). Interestingly, a study reporting real time H2O2 documented that the elicitor cryptogein-induced ROS in BY-2 tobacco cells more rapidly in internal compartments than in the apoplast (Ashtamker et al. 2007). The signal developed first in the nuclear region, cytoplasm and endoplasmic reticulum and subsequently was detected in the cell periphery, suggesting that apoplastic ROS from enzymatic origin may serve to amplify preceding ROS signals from inside the plant cell. The Arabidopsis fad7fad8 mutant deficient in the synthesis of trienoic fatty acids in the chloroplast shows reduced ROS accumulation in response to avirulent Pseudomonas, indicating cross talk between chloroplastic signals and apoplastic ROS production by NADPH oxidases (Yaeno et al. 2004). Interestingly, plasma membrane AtrbohD also regulates the levels of cytosolic ascorbate peroxidase APX1 that modulates ROS concentrations in the chloroplast from light stress, suggesting cross talk between pools of ROS in the apoplast and in the chloroplast (Davletova et al. 2005). Thus, although there is a complex spatial compartmentalization for ROS accumulation in response to pathogens, an interplay between different pools of ROS may modulate different responses.
Downregulation of scavenging/antioxidant systems and/or activities may also contribute to increase ROS accumulation (Mittler et al. 2004). Superoxide dismutases, catalases, ascorbate peroxidases and the mitochondrial alternative oxidase are among the enzymes that cope with this excess of ROS. These are complemented with antioxidants like ascorbate, glutathione and tocopherol among others (Foyer and Noctor 2005, Mittler et al. 2004). All these elements exhibit specific compartment location. Changes in the level of antioxidants and detoxifying enzymes are reported in fact in response to many pathogens that led to increase of ROS and activation of the defenses (Klessig et al. 2000). Reduction of the antioxidant machinery led to enhanced HR and defense to pathogens (Dat et al. 2003, Mittler et al. 1999). Conversely, suppression or delay of ROS synthesis in parallel with accumulation of metabolites that protect against ROS is a dominant feature of the metabolic changes occurring during early phases of disease establishment in the susceptible interaction between the rice blast fungus Magnaporthe grisea and its hosts (Parker et al. 2009). Thus, apart from restricting the ROS dependent damage, scavenging systems and detoxifying enzymes can fine tune ROS in specific cell compartments that can be exploited for redox modification of some proteins and signaling.
Function of ROS in plant defense
ROS were proposed to orchestrate the establishment of plant defenses and HR following successful pathogen recognition (Lamb and Dixon 1997). Genetic proof for the function of the Rboh-NADPH oxidases in response to pathogens came from the analysis of mutant and antisense lines (Simon-Plas et al. 2002; Torres et al. 2002; Yoshioka et al. 2003). However, elimination of ROS in these lines produced various effects on pathogen resistance and the HR. In Arabidopsis, the double mutant atrbohD atrbohF displayed reduced HR in response to avirulent bacteria but no effect on pathogen growth (Torres et al. 2002). However, the same line exhibited enhanced cell death and resistance to a weakly virulent strain of the oomycete Hyaloperonospora arabidopsidis (Torres et al. 2002). However, Nbrboh-silenced N. benthamiana plants showed reduced HR and enhanced susceptibility to infection with the avirulent oomycete P. infestans (Yoshioka et al. 2003). Also in basal defense following PAMP recognition, the Arabidopsis atrbohD mutant displayed impairment of ROS production and significant reduction of callose deposition associated with resistance after flg22 treatment (Zhang et al. 2007). By contrast, although oligogalacturonides derived from the action of fungal polygarlacturonases induce an oxidative burst that is dependent on AtrbohD, the defense response against the ascomycete Botrytis cinerea is unaffected in this mutant (Galletti et al. 2008). Taken all together, these data suggest that although the Rboh-NADPH oxidases are responsible for the extracellular ROS in response to many different pathogens, the effect on disease resistance and cell death varies depending on the plant–pathogen interaction. This indicates that ROS produced by this enzyme might serve different signaling functions, sometimes having opposed effects.
Some of the variety of signaling functions attributed to the oxidative burst may be explained by functional overlap of the different pools of ROS produced in response to pathogens. For instance, there is an evidence of functional overlap between the Rboh proteins, because, in Arabidopsis, the phenotypes of the individual atrbohD and atrbohF mutants are accentuated in the double mutant atrbohD atrobhF (Torres et al. 2002). ROS produced by other enzymes like peroxidases may also contribute to these responses. Trangenic Arabidopsis with an antisense construct with a type III peroxidase from French bean (FBP1) were impaired in ROS production that led to increased susceptibility to both virulent and avirulent pathogens, and a reduction of the HR (Bindschedler et al. 2006). Also, virus induced gene silencing (VIGS) of CaPO2, a novel extracellular peroxidase from pepper, compromised ROS production and disease resistance, whereas its overexpression led to the increased levels of ROS and systemic acquired resistance (SAR) in Arabidopsis. This outlines the importance of cell wall peroxidases on the regulatory functions of ROS, and that different pools of ROS produced by different enzymatic mechanisms may produce the activation of different responses to pathogen attack.
Interestingly, both Arabidopsis NADPH oxidase mutants and cell wall peroxidase antisense lines exhibited an absence of ROS production in response to the same avirulent bacterial pathogen Pseudomonas syringae carrying avrRpm1 (Bindschedler et al. 2006, Torres et al. 2002). Also, a study using luciferase imaging detecting ROS generation showed that the oxidative burst in response to avirulent bacteria was compromised by the inhibitors of both enzymes (Grant et al. 2000b). These observations raise the possibility that both enzymatic mechanisms work together to mediate ROS production. Given that the peroxidase antisense lines displayed enhanced susceptibility to pathogens, it has been proposed that cell wall peroxidases could be the initial origin of apoplastic ROS and that the NADPH oxidase may amplify the initial ROS signal (Bindschedler et al. 2006). This is in agreement with AtrbohD being transcriptionally upregulated by H2O2 (Desikan et al. 1998). However, killing by the phagocytic NADPH oxidase appears to be produced indirectly through the activation of ion fluxes and pH change (Segal 2008). Plant Rboh could also act in the apoplast by initiating an ROS cascade to induce alkalinization and the activation of peroxidases (Bolwell et al. 2002). Thus, different enzymatic mechanisms contribute to the apoplastic oxidative burst, and these pools of ROS from different origins could in some cases work together in a complex self-amplifying signaling network in response to the pathogen.
ROS could contribute to the activation of plant defenses by inducing changes in gene expression (Kotchoni and Gachomo 2006, Levine et al. 1994). The rapidity of its production and the potential for H2O2 to freely diffuse across membranes suggested that ROS could exert this function either directly through redox regulation of transcription factors or indirectly by interacting with other signaling components like phosphorylation cascades (Kovtun et al. 2000, Mou et al. 2003). Moreover, ROS, in association with SA, were proposed to mediate the establishment of SAR (Durrant and Dong 2004). Interestingly, changes in redox status trigger oligomerization and movement to the nucleus of NPR1, a master regulator of SA-dependent transcriptional responses. Once in the nucleus, NPR1 interacts with TGA transcription factors to induce defense gene expression (Despres et al. 2003, Mou et al. 2003). Microbursts of ROS and microHRs were observed in presumably uninfected systemic tissues parallel to the activation of SAR, and these responses were blocked with an inhibitor of the NADPH oxidase (Alvarez et al. 1998). However, a recent study showed that SAR response in pepper plants was also accompanied by a systemic microburst and microlesions that correlated with the systemic expression of defense-related genes and that these responses were compromised in the pepper VIGS plants with the extracellular peroxidase CaPO2 (Choi et al. 2007). All these suggest that apoplastic ROS produced by NADPH oxidases and cell wall peroxidases could mediate the activation of systemic defenses. Additionally, a recent study shows that ROS produced by AtrbohD mediate a rapid, long-distance, cell-to-cell propagating signal in response to diverse stimuli, suggesting that ROS could have a general role in mediating the communication over long distances not only in response to pathogens but also in response to pests, wounding and other stress conditions (Miller et al. 2009).
New roles for ROS have been documented in the context of defense response. For example, ROS could mediate signaling by modulating vesicle trafficking. In mammalian phagocytes, specific granules are a site for assembly and activation of the NADPH oxidase (Segal 2008). In parsley-powdery mildew interaction, ROS were visualized in vesicles, suggesting a delivery of ROS, among other factors, to the site where they exert their action (Collins et al. 2003). Recently, the Phytophthora elicitor cryptogein has been shown to stimulate clathrin-mediated endocytosis in tobacco BY-2 cells and that this process required ROS production by NtrbohD (Leborgne-Castel et al. 2008). Additionally, ligand-induced endocytosis of FLS2, the LRR receptor that interacts with flagellin, has been reported (Robatzek et al. 2006), and inhibitors of ROS production in response to flagellin prevented FLS2 endocytosis (Serrano et al. 2007). Thus, ROS function is related to vesicle trafficking, for delivery of ROS signals at specific location or for the endocytosis of specific receptors during defense activation or for desensitizing and recycling immune receptors.
Role of ROS in the HR
A correlation exists between the apoplastic oxidative burst and the HR. Evidences suggest that this is a genetically regulated and active process that has similarities with the animal apoptosis (Lam 2004). Genetics studies have implicated contributions of ROS coming from plasma membrane and cell wall enzymes to the HR. In most plant pathogen responses, ROS act as positive regulators of the HR (Bindschedler et al. 2006, Choi et al. 2007, Torres et al. 2002, Yoshioka et al. 2003). However, depending on the system, the ROS produced by the same source could also negatively regulate the spread or control of cell death. For example, the atrbohD atrbohF double mutants display reduced HR in response to avirulent bacteria, but enhanced cell death in response to an oomycete or in the spreading cell death initiated by an avirulent pathogen in the lesion mimic mutant lsd1 (Torres et al. 2002, 2005). Also, an oxidative burst dependent on AtrbohD limited the spread of cell death induced by Phytophthora in the rph1 mutant (Belhaj et al. 2009). Additionally, ROS accumulation and the HR are uncoupled in some plant–pathogen interactions (Glazener et al. 1996, Yano et al. 1999). Thus, although the common thought is that the oxidative burst mediates the HR, alternatives exist where ROS do not regulate or even are antagonistic to pathogen-induced cell death.
Intracellular ROS can also have an effect on the HR directly by elevated toxicity of the ROS produced or indirectly by the release of prodeath signals that mediate further responses. Particularly, in the case of plant chloroplast and peroxisomes, different ROS can be produced by anomalies in the photosynthetic machinery that play a crucial part in the HR (Asada 2006). This is shown in the ubiquitous requirement for light for the pathogen-induced HR to progress (Goodman and Novacky 1994, Tang et al. 1999). Also non-enzymatic dependent lipid peroxidation by H2O2 was shown to play a key role in cell death execution during the HR occurring in the light, whereas enzymatic lipid peroxidation occurs predominantly in the dark (Montillet et al. 2005). Further support for the role of the chloroplast in the regulation of the HR comes from the light dependence of some lesion mimic mutants that display lesions reminiscent of the HR associated with ROS production (Brodersen et al. 2002, Jabs et al. 1996, Muhlenbock et al. 2008). Indication that ROS generated in the chloroplasts are essential for the HR is also shown in a recent study with tobacco plants expressing a cyanobacterial flavodoxin targeted to chloroplasts that resulted in inhibition of ROS production and a reduction of the localized cell death (Zurbriggen et al. 2009). Respiration in the mitochondria can also be a source of ROS signals that could affect the HR. In animals, release of cytochrome c from the mitochondria is a key step that precedes activation of caspase leading to apoptosis (Martinou et al. 2000). Similarly, release of cytochrome c was observed in response to the rise of mitochondrial ROS in Arabidopsis cells in response to the elicitor harpin (Krause and Durner 2004). Also, mitochondrial respiratory routes and antioxidants systems like the alternative oxidase were shown to contribute to the regulation of the HR induced by harpin (Vidal et al. 2007). Thus in addition to the requirement for apoplastic oxidative burst, high levels of ROS from intercellular organelles can have a dramatic effect on the HR. In addition, pathogen alteration of the complex network of scavenging/detoxifying systems that operates in different cellular compartments can alter dramatically ROS homeostasis in these organelles and influence the HR (Livaja et al. 2008, Mittler et al. 2004). Some of these changes could be mediated by SA, which can downregulate the expression of some key detoxifying enzymes, promoting increased ROS levels and cell death (Klessig et al. 2000, Yang et al. 2004). Accordingly, early work showed that plants with reduction of catalase and ascorbate peroxidase activities resulted in plants that are hyperresponsive to pathogens (Mittler et al. 1999). Also, excess light in plants with downregulation of scavenging systems leads to an ectopic oxidative burst and to cell death reminiscent of the HR (Dat et al. 2003). All these examples illustrate the requirement for a tight control of the ROS signals coming from different compartments to regulate the HR and that these different compartmentalized pools of ROS could have specific independent functions but could also show an interplay that can produce the amplification of the signals.
Regulation and signaling
Early work on cell suspension cultures with specific inhibitors pointed out to calcium signaling and phosphorylation as regulatory elements of the oxidative bursts (Jabs et al. 1997, Piedras et al. 1998). Many elicitors and plant pathogens induce phosphorylation cascades associated to ROS production (Asai et al. 2002, Samuel et al. 2005). ROS signals seem to work both downstream and upstream of these phosphorylation cascades. For example, plants overexpressing the constitutive active MAPK kinase MKK4 exhibited ROS production associated with a strong HR-like response (Ren et al. 2002, Takahashi et al. 2007). But, H2O2 regulates MEKK1 and MEKK3 activity and protein stability in a proteasome dependent manner, suggesting that these MAPKKK function in integrating ROS homeostasis in pathogen signaling among other responses (Doczi et al. 2007, Nakagami et al. 2006). MAPK3 and MAPK6, downstream targets of MKK4 that mediate PTI and ETI (Menke et al. 2004), may work upstream of AtrbohD-dependent ROS in response to flagellin (Zhang et al. 2007). But, H2O2 can also trigger the activation of MAPK3 and MAPK6 cascade (Kovtun et al. 2000) through Oxidative Signal-Inducible 1 kinase in Arabidopsis (Rentel et al. 2004). Thus, both ROS and MAPK cascade may form an activation circuit to integrate a cross talk of regulatory signals that mediate several responses.
The identification of the plant NADPH oxidase-Rboh allowed the identification of an important link between phosphorylation and ROS production in this enzyme. Yoshioka et al. (2003) reported that MAPK-induced cell death in N. benthamiana may be mediated by ROS produced by NbrbohB and that the same MAPK cascade acts to increase NbrbohB levels. This suggests that transcriptional activation of the Rboh is one of the functions of MAPK cascades in ROS production. The Rboh proteins have an N-terminal extension absent in mammalian phagocytic NADPH oxidase (but present in NOX5 and DUOX proteins). Interestingly, the use of a quantitative phosphoproteomics approach pointed out that two phosphorylation sites at the N-terminal of AtrbohD, that are strongly induced in response to flg22, are required for ROS production by this enzyme (Nuhse et al. 2007). This suggests a different regulation of the activation of the plant NADPH oxidases.
Calcium metabolism is also intimately related to ROS signaling. Increase in cytosolic Ca2+ is also one of the fastest responses upon pathogen infection and the use of specific inhibitors showed that Ca2+ influx is required for ROS production after elicitation (Blume et al. 2000, Grant et al. 2000a). Ca2+ was shown to activate an Rboh protein in vitro (Sagi and Fluhr 2001), and all plant Rboh proteins contain two EF-hands in their N-terminal region that may account for this Ca2+ regulation (Torres and Dangl 2005). However, ROS appear to be required to prime Ca2+ influx after elicitation (Levine et al. 1996). In abscisic acid (ABA)-induced stomata closure and plant cell growth, Rboh-dependent ROS mediate the activation of Ca2+ (Foreman et al. 2003, Kwak et al. 2003). Therefore, Ca2+ fluxes could function both upstream and downstream of ROS production, indicating a complex spatiotemporal Ca2+ regulation of these signaling networks. Interestingly, calcium dependent protein kinases were shown to phosphorylate two additional sites in the N-terminal region of potato StrbohB and be required for ROS production in N. benthamiana leaves (Kobayashi et al. 2007). This is the indication of cross talk between calcium and phosphorylation in the regulation of ROS production by the NADPH oxidases.
In mammalian phagocytes, Rac2 interacts with some of the cytosolic regulatory components of the oxidase driving the assembly and its activation (Bokoch and Diebold 2002). In plants, the other cytosolic components of this oxidase are absent (Torres and Dangl 2005), but the function of Rac/Rop proteins is maintained (Hassanain et al. 2000). Several studies showed that Rac/Rop proteins are positive regulators of ROS production and disease resistance. By using dominant active and dominant negative constructs, rice Osrac1 was shown to function in activating ROS production and promoting HR together with enhanced resistance to M. grisea and bacteria blight Xanthomonas oryzae (Kawasaki et al. 1999, Ono et al. 2001). Also, the same dominant negative form of Osrac1 showed decreased ROS production and Tobacco Mosaic Virus (TMV) resistance in tobacco plants carrying the N resistance gene (Moeder et al. 2005). However, other Rac/Rop proteins were shown to be negative regulators of ROS production and defense response (Morel et al. 2004, Schultheiss et al. 2003). Thus, Rac/Rop proteins may act as both positive and negative regulators of ROS production and disease resistance. A recent study showed that the rice Rac proteins interact directly with the N-terminal extension present in all plant Rboh, suggesting a direct regulation of the activity of the oxidase by Rac and further suggesting that this regulation might be modulated by calcium (Wong et al. 2007). A multigene gene family exists for both Rboh and Rac proteins, and two hybrid studies showed that most Rac proteins were able to interact with more than one N-terminal Rboh (Wong et al. 2007). These data suggest a complex combinatorial regulation of ROS production, where plant Rac GTPases may act as a molecular switch for regulating the NADPH oxidases. Interestingly, OsRac1 downregulates the expression of a rice metallothionein gene that scavenges ROS, thus potentiating ROS accumulation and function as signals in resistance response (Wong et al. 2004). Thus, OsRac1 is a key redox regulator that plays a dual role as an inducer of ROS production and a suppressor of ROS scavenging.
A strong interplay between ROS and other signaling molecules and phytohormones exists in HR and defense response, which could explain the variety of signaling roles that ROS play in different scenarios. SA is a plant signaling molecule involved in defense responses to biotrophic pathogens, local and systemic (Durrant and Dong 2004). ROS were proposed to synergize in a signal amplification loop with SA to drive the HR and the establishment of systemic defenses (Draper 1997). Hydrogen peroxide and pathogen drive SA accumulation and subsequent increases in SA enhanced ROS production (Leon et al. 1995, Shirasu et al. 1997). SA accumulation can also downregulate ROS-scavenging systems, contributing indirectly to increased overall ROS levels following pathogen recognition (Klessig et al. 2000, Yang et al. 2004). However, ROS and SA have been shown to antagonize each other's action in the negative regulation of cell death spread (Torres et al. 2005), indicating that ROS can mediate different functions in different cellular contexts and in relation to other regulatory signals. ROS functions have also been related to NO, another reactive oxygen derivative produced following pathogen recognition. A fine balance between NO and H2O2 (but not  ) might be required for the potentiation of the pathogen-induced cell death (Delledonne et al. 2001). Cytological studies showed that ROS and NO are associated with cell death adjacent to infected cells and that both signals modulate each other's accumulation (Tada et al. 2004, Zeier et al. 2004). Interestingly, a recent study indicates cross talk at the level of MAPK cascades that control production of ROS and NO in response to elicitors (Asai et al. 2008). However, downregulation of ROS and NO production did not confer synergistic effects in the resistance to different fungal pathogens, indicating that the requirement for ROS and NO appears to be different for resistance to different pathogens (Asai et al. 2008). All these underscore the complexity of the signaling and the diversity of responses mediated by these regulatory molecules.
) might be required for the potentiation of the pathogen-induced cell death (Delledonne et al. 2001). Cytological studies showed that ROS and NO are associated with cell death adjacent to infected cells and that both signals modulate each other's accumulation (Tada et al. 2004, Zeier et al. 2004). Interestingly, a recent study indicates cross talk at the level of MAPK cascades that control production of ROS and NO in response to elicitors (Asai et al. 2008). However, downregulation of ROS and NO production did not confer synergistic effects in the resistance to different fungal pathogens, indicating that the requirement for ROS and NO appears to be different for resistance to different pathogens (Asai et al. 2008). All these underscore the complexity of the signaling and the diversity of responses mediated by these regulatory molecules.
Alongside SA, JA (jasmonic acid) and ET (ethylene) are hormones usually associated with the induction of defenses depending on the pathogen lifestyle (Kunkel and Brooks 2002). ROS signaling has also been associated to these hormones in the regulation of necrotrophic pathogens and insects (Glazebrook 2005). ROS were proposed to be the central component of a self-amplifying loop that regulates the interaction balance between SA, JA and ET that mediate the response to ozone and possibly some defense and cell death processes (Bouchez et al. 2007, Overmyer et al. 2003). Moreover, ROS could be an important mediator in the interplay with other hormones. The DELLAs, transcription factors associated to growth and developmental processes by gibberellin, are also involved in defense response, probably by modulating JA and SA in relation to necrotrophic and biotrophic pathogens, respectively (Achard et al. 2008, Navarro et al. 2008). DELLAs could exert this function by modulating ROS production and scavenging (Achard et al. 2008). Also, ABA can modulate disease resistance by interfering with signals transduction pathways mediated by SA, JA and ET (Ton et al. 2009). ROS could also have an important part in this cross talk between hormones because ABA can also regulate ROS production in response to pathogens and in other contexts (Asselbergh et al. 2007, Kwak et al. 2003). Taken together, ROS signals could play a critical regulatory role in the interplay between different phytohormones that mediate pathogen responses, which could explain the variety of actions regulated by ROS in response to pathogens.
ROS from the pathogen side
In the constant coevolution between the pathogen and the host, pathogens have developed counterdefense mechanisms to evade host defenses. Many bacterial pathogens use effectors that block the recognition of the pathogen or the signal transduction pathway that leads to PTI, and many of them also block the oxidative burst. For example, the Pseudomonas effector AvrPtoB suppresses ROS production and further defenses after PAMP recognition from flg22 or chitin treatment (Gimenez-Ibanez et al. 2009). Also, HopAI1 directly dephosphorylates and inactivates Arabidopsis MAPK3 and MPK6 and consequently suppresses flg22 gene expression, apoplastic ROS production and callose deposition, resulting in increased disease susceptibility (Zhang et al. 2007). A more sophisticated mode of action is mediated by coronatine, a virulence factor from Pseudomonas that mimics the action of plant JA, a plant hormone that antagonizes SA function. Thus, coronatine suppresses SA-mediated defense responses (Kloek et al. 2001). Interestingly, coronatine induced effects on the photosynthetic machinery and ROS accumulation in the light as to modulate cell death (Ishiga et al. 2009). Thus, bacterial pathogens use several strategies to modify the ROS levels as a way to alter the defense in the host.
Fungal pathogens have also developed ways to sense and modify ROS accumulation in host plants. Defense suppressor 1 (DES1) is a novel pathogenicity gene from filamentous fungi Magnaporthe oryzae that regulates counterdefenses against host basal defense (Chi et al. 2009). DES1 is necessary for scavenging extracellular ROS within host cells through the modification of peroxidase activity, probably by controlling ferrous ions availability. This is in agreement with the fact that the distribution of iron within different cell compartments was linked to ROS production and activation of plant defenses (Liu et al. 2007). Another example is shown by the biotrophic fungus Ustilago maydis. This fungus has a bZIP transcription factor, Yap1, that functions as a redox sensor controlling H2O2 detoxification systems (Molina and Kahmann 2007). Yap1 is required for virulence and is responsible for preventing the accumulation of H2O2 produced by the plant NADPH oxidases in the vicinity of the hyphae during early stages of biotrophic growth, allowing the fungus to cope with early plant defenses. Also, deletion of CPTF1, a CREB like transcription factor in Claviceps purpurea that controls the expression of a fungal catalase, results in a mutant that induces a plant oxidative burst in rye (Nathues et al. 2004). Thus, the use of transcription factors to modify the host oxidative burst could be a general strategy for the pathogen to cope with early plant defences. There are further examples in the interactions with plant viruses. For example, the TMV coat protein diminishes ROS coming from photosystem-II in the chloroplast causing an enhancement of the symptoms associated to TMV infection (Lehto et al. 2003). These examples illustrate the variety of strategies that different pathogens use to overcome host defenses mediated by ROS.
Necrotrophs also induce ROS accumulation in the host to trigger an active (programmed) cell death in the host that allows the fungus to access nutrients, contributing to survival and disease development (Govrin and Levine 2000, Govrin et al. 2006). It was shown that glucose/glucose oxidase infiltration (that generates H2O2) in Arabidopsis leaves increased susceptibility to B. cinerea and that an NADPH oxidase inhibitor prevented both ROS production and necrotic symptoms, suggesting that the ROS were NADPH oxidase dependent (Govrin and Levine 2000). Indeed, a recent study showed that the oxidative burst in response to oligogalacturonides (OGs) produced from the action of fungal oligogalacturonases on pectin from the plant cell wall is dependent on AtrbohD (Galletti et al. 2008). However, changes in gene expression and basal disease resistance to B. cinerea induced by OGs are not compromised in atrbohD, suggesting that OG-induction of defense responses effective against this fungus does not require AtrbohD. These contradictory results suggest that there are different kinds of ROS and/or cell death that might have different effects on the growth of necrotrophic fungi. Collectively, these data indicate that modification of the levels of ROS in the host might be a strategy that is extended among the pathogens to increase host susceptibility.
ROS in other biotic interactions
There are many similarities between the early response to infection by pathogenic and symbiotic organisms. ROS produced in early stages on symbiosis with bacteria and mycorrhizae are reminiscent of the pathogen-induced oxidative burst (Fester and Hause 2005, Santos et al. 2001). Superoxide and H2O2 are produced in alfalfa nodules responding to the infection with Sinorhizobium meliloti (Santos et al. 2001). This ROS production is consistent with the idea that symbiotic bacteria are initially perceived as invaders by the host plant. Accordingly, the symbionts have developed protecting mechanisms to counteract the plant defense response and particularly sustained ROS accumulation. For example several ROS-scavenging genes are activated for the proper Rhizobium–legume symbiosis to develop (Jamet et al. 2003). In addition, some bacterial surface polysaccharides are produced during early stages of invasion preventing incorporation of H2O2 inside the bacteria and allowing a deeper invasion of the plant tissues (D’Haeze et al. 2004). However, ROS may have important roles per se during the establishment of the symbiotic structures. For example, overexpression of a housekeeping catalase in S. meliloti led to an enlargement of infection threads that affect its optimal progression through the root hairs and plant cell layers producing a nodulation delayed phenotype (Mathis et al. 2005). This raises the hypothesis that H2O2 could be involved in biomechanics of infection thread growth possibly via cross linking and insolubilization of plant extensins of the extracellular matrix of legume tissues. Also, transient intracellular ROS accumulation has been detected inside the root hairs after treatment with Nod factors and linked to calcium signaling and cytoskeleton rearrangements (Cárdenas et al. 2008). Finally, a late phase of ROS is detected in these interactions in association to nodule and arbuscular branches senescence (Fester and Hause 2005, Puppo et al. 2005). Thus, sequential phases of ROS production/accumulation are observed during symbiotic interactions that could mediate different functions in early recognition, structural rearrangements and senescence.
Production of an oxidative burst is also observed in response to pest insects, both in response of sap-feeding (Martinez de Ilarduya et al. 2003) and chewing herbivores (Maffei et al. 2006). These ROS will be produced not only by the interaction with the insect but also by the mechanical wounding associated with feeding (Orozco-Cardenas and Ryan 1999). ROS could mediate the establishment of defensive mechanism, local and systemic, including the production of volatile products that could act not only against the insect but also against the subsequent invasion of microbial pathogens (Divol et al. 2005, Orozco-Cardenas et al. 2001). However, the responses by insect feeding are qualitatively and quantitatively different from mechanical wounding, most likely because of the role of herbivore oral secretions (Musser et al. 2006). Herbivore saliva has been proposed to have a dual role to induce plant defenses and also to protect the insect. Glucose oxidase in saliva catalyzes the generation of H2O2 that in this context may suppress induced defenses in plants (Musser et al. 2002). Thus, caterpillars with suppressed salivary glands induced reduced H2O2 levels in leaves that could mediate the downregulation of plant defenses, including lowering the amount of toxic nicotine released by Nicotiana tabacum (Musser et al. 2006). Additionally, herbivore insects induced expression of antioxidant genes in the midgut to be a protective response to ROS ingested during feeding or food processing (Mittapalli et al. 2007). Thus, ROS production is also observed in plant–insect interactions, where herbivore oral secretions have a preeminent protective role.
Fungi also have NADPH oxidase genes (Takemoto et al. 2007). Interestingly, these genes are more closely related to gp91phox, without the N-terminal extension present in the plant Rboh (Takemoto et al. 2007). These fungal NADPH oxidases mediate ROS production inside the fungi that sometimes has a function related to the host interaction. For example, the hemibiotrophic fungus M. grisea undergoes an oxidative burst by means of two NADPH oxidase enzymes, Nox1 and Nox2, that are necessary for the development of specialized structures called appressoria required for infection (Egan et al. 2007). However, this ROS is restricted to the infected host cell where it has a crucial developmental function and does not diffuse to the surrounding environment. An interesting scenario comes from studying the mutualistic symbiotic association between the fungus Epichloë festuca and ryegrass (Lolium perenne). The deletion of the NADPH oxidase noxA gene in the fungus changes the interaction of this biotrophic endophyte with its host from mutualistic to antagonistic (Tanaka et al. 2006). The most plausible scenario is that ROS produced by the NADPH oxidase regulate hyphal tip growth, thus restricting growth of the fungus and preventing excessive colonization, recognition by the host and activation of its defenses. Taken together, ROS are produced in a variety of different scenarios in plant interaction with different biotic agents. Their tight compartment regulation and the interplay with other signaling molecules may contribute to the variety of specific signaling functions attributed to ROS.
Acknowledgements
This work was supported by funding from the US National Science Foundation (IOS-0639964) to Jeffery L. Dangl, Grant BIO-2007-66806 from the Spanish MEC and from the International Reintegration Grant (2007) D/562971 from the European Union. I am indebted to Jeff Dangl, in whose laboratory the work that produced this review was begun and to both Jeff Dangl and Antonio Molina for critical reading of the manuscript.




