NO synthesis and signaling in plants – where do we stand?
Abstract
Over the past 20 years, nitric oxide (NO) research has generated a lot of interest in various aspects of plant biology. It is now clear that NO plays a role in a wide range of physiological processes in plants. However, in spite of the significant progress that has been made in understanding NO biosynthesis and signaling in planta, several crucial questions remain unanswered. Here we highlight several challenges in NO plant research by summarizing the latest knowledge of NO synthesis and by focusing on the potential NO source(s) and players involved. Our goal is also to provide an overview of how our understanding of NO signaling has been enhanced by the identification of array of genes and proteins regulated by NO.
Abbreviations-
-
- ABA
-
- abscisic acid
-
- CA
-
- carbonic anhydrase
-
- cGTPases
-
- circularly permuted GTPases
-
- GC
-
- guanylate cyclase
-
- GSNO
-
- S-nitrosoglutathione
-
- GSNOR
-
- GSNO reductase
-
- MEP
-
- methylerythritol phosphate
-
- NOS
-
- NO synthase
-
- NPR1
-
- non-expressor of PR-1
-
- NR
-
- nitrate reductase
-
- PR-1
-
- pathogenesis-related-1
-
- PrxIIE
-
- peroxiredoxin II E
-
- PTMs
-
- post-translational modifications
-
- ROS
-
- reactive oxygen species
-
- SA
-
- salicylic acid
-
- SNO
-
- S-nitrosothiol
Introduction
Nitric oxide (NO) is a small, gaseous molecule involved in a wide array of functions in diverse organisms. In mammals, NO participates in the maintenance of vascular tone, neuronal signaling and host response to infection (Schmidt and Walter 1994). In bacteria, NO is not only an intermediate of denitrification but is also involved in pathogenicity or defense against reactive oxygen species (ROS) (Sudhamsu and Crane 2009). The discovery that NO is also produced in plants upon exposure to either bacterial or viral pathogens stimulated a growing interest in plant NO research (Delledonne et al. 1998, Durner et al. 1998). It is now increasingly apparent that NO plays important roles in multiple plant processes (Wilson et al. 2008). NO is implicated in plant growth and development from germination to fruit ripening and flowering (Beligni and Lamattina 2000, He et al. 2004, Villarreal et al. 2009). NO is also generated in response to abiotic stressors, such as drought and salt (Neill et al. 2008). During biotic stress, NO is produced in response to both biotrophic and necrotrophic pathogens, as well as viruses, and it orchestrates a wide range of responses from defense gene regulation to defense hormone production and the hypersensitive response development (Asai and Yoshioka 2009, Delledonne et al. 1998, Durner et al. 1998).
NO was first characterized as a biological product of nitrite reduction by denitrifying bacteria. In the late 1980s, NO synthase (NOS) enzymes were isolated from mammals, and it was shown that animals have evolved to oxidize arginine into NO. Interestingly, some bacteria also contain the oxygenase domain of NOS (Sudhamsu and Crane 2009). More recently, it has become clear that nitrite reduction to NO also occurs in mammals, specifically in hypoxic conditions when arginine oxidation becomes less efficient (Gladwin et al. 2005). While there is a growing amount of data demonstrating the importance of NO in plants, our understanding of its biosynthesis and signaling pathways has lagged behind that in animals. Here we compile evidence from the plant science literature that argues for the presence of both nitrite reduction and arginine-dependent NO-formation pathways. However, the identity of the players and the importance of each biosynthetic pathway as a function of the physiological process remain a matter of debate.
Regardless of the pathway through which NO is synthesized, it is now clear that NO regulates a large number of genes in plants. The mechanisms underlying these NO responses continue to receive a great deal of attention. In animals, NO signaling is mediated either through the second messenger cGMP or through interaction with reactive species such as metals, ROS or thiols. Upon binding, NO activates the soluble guanylate cyclase leading to the conversion of GTP to cGMP. Plants seem to accumulate cGMP in NO-producing situations (Donaldson et al. 2004, Durner et al. 1998); nevertheless, an NO-sensitive guanylate cyclase (GC) has not yet been found. NO can also directly react in plants to inactivate metal centers of proteins (Clark et al. 2000, Navarre et al. 2000). A body of evidence also reveals the importance of post-translational modifications (PTMs) in NO signaling. The reversible coupling of an NO moiety to a reactive cysteine thiol [leading to S-nitrosothiol (SNO)], a process called S-nitrosylation, represents one critical PTM; nitration of tyrosine residues (leading to 3-nitrotyrosine) is another (Leitner et al. 2009).
There are several excellent and recent reviews of NO production and signaling in plants (Besson-Bard et al. 2008b, Wilson et al. 2008). Here we present the most up-to-date evidence showing that two major pathways for NO production, one reductive and one oxidative, are still very much in consideration even though the identity of particular NO-producing enzymes is unknown (Fig. 1). We also provide an update of the understanding of NO signaling in plants, focusing particularly on gene regulation and NO-mediated protein PTMs (Fig. 2).
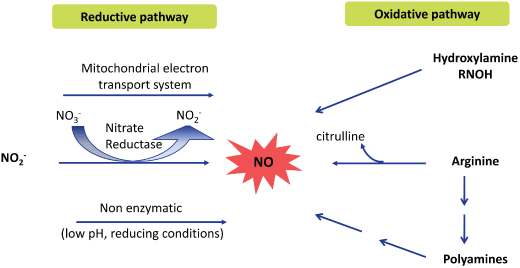
Two major routes of NO formation in plants. Evidence for the existence of both a reductive and an oxidative pathway for NO synthesis in plants has been obtained. The electron needed to reduce nitrite (NO ) to NO can be provided by the mitochondrial electron transport system, by NAD(P)H in nitrate reductase or in an acidic reducing environment. There is also evidence, though mostly indirect, arguing for the existence of an oxidative way to make NO in plants. Several substrates are proposed such as arginine, polyamines and hydroxylamines but no enzyme has been identified thus far.
) to NO can be provided by the mitochondrial electron transport system, by NAD(P)H in nitrate reductase or in an acidic reducing environment. There is also evidence, though mostly indirect, arguing for the existence of an oxidative way to make NO in plants. Several substrates are proposed such as arginine, polyamines and hydroxylamines but no enzyme has been identified thus far.
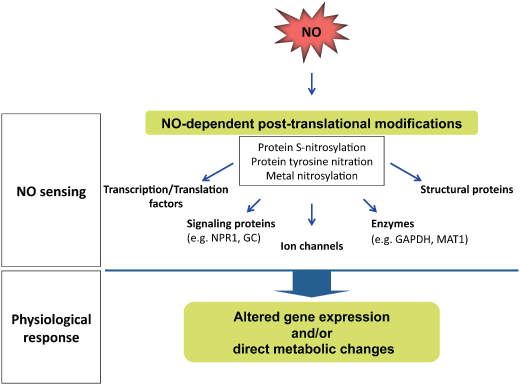
Illustration of the NO signaling. The signaling function of NO requires its sensitive detection. NO sensing is based on post-translational protein modifications, including protein S-nitrosylation, protein tyrosine nitration and metal nitrosylation. These NO-dependent modifications induce a physiological response because of the alteration of gene expression and/or direct metabolic changes.
NO biosynthesis in plants
Nitrite as a source of NO
Nitrate reductase
The primary function of nitrate reductase (NR) is the reduction of nitrate to nitrite in an NAD(P)H-dependent manner (Crawford 1995). In vitro experiments have shown that NR is also capable of reducing nitrite to NO (Dean and Harper 1988, Rockel et al. 2002, Yamasaki and Sakihama 2000). The reduction efficiency is low (∼1% of NR activity); it is more pronounced in a low-oxygen environment and requires nitrite levels to be in excess of the natural substrate nitrate (Rockel et al. 2002). Approximately 20 studies provide evidence supporting NR's role in NO synthesis in planta. For example, it has been demonstrated that NR is responsible for NO production during stomatal closure (Bright et al. 2006, Desikan et al. 2002, Neill et al. 2008), in response to defense elicitors (Shi and Li 2008, Srivastava et al. 2009, Wu et al. 2009), in response to abiotic stress (Sang et al. 2008) and during developmental processes such as flowering (Seligman et al. 2008) or lateral root induction (Kolbert et al. 2008). The evidence for NR's role in NO production in planta is both pharmacological and genetic. Addition of nitrite to plant extracts induces NO formation that coincides with NR activity (Rockel et al. 2002, Sakihama et al. 2002). NR inhibitors like tungstate impair stomatal closure induced by abscisic acid (ABA) in Arabidopsis or by a fungal elicitor in pea (Bright et al. 2006, Srivastava et al. 2009). Furthermore, the NR inhibitors sodium azide and potassium cyanide inhibit NO production in planta (Liu et al. 2007, Sang et al. 2008, Wu et al. 2009). However, these effects alone cannot rule out the involvement of other heme proteins, as these compounds are general heme ligands.
Genetic evidence using NR knockout mutants or silenced plants also supports a role for NR in NO production, as these plants fail to accumulate NO or otherwise mediate NO effects upon elicitation (Bright et al. 2006, Desikan et al. 2002, Seligman et al. 2008, Shi and Li 2008, Yamamoto-Katou et al. 2006). More specifically, one of the two Arabidopsis NR isoforms, NIA1, is the functional NO-producing enzyme involved in ABA-induced stomatal closure (Ribeiro et al. 2009). However, results involving nia mutants should be interpreted with caution. While the lower NO content observed in these mutants could be attributed to the loss of the NO-producing enzyme, it could also correspond to more extensively altered metabolism. In this light, it is not surprising that the Arabidopsis mutant nia1nia2 showed not only a lower nitrite content but also a lower amino acid content, particularly arginine (Modolo et al. 2006). Arginine supplementation did not rescue the nia1nia2 phenotype, contrary to nitrite addition, suggesting that the observed impairment in NO production and resistance to pathogens observed in nia mutants are more likely related to their lower nitrite levels (Oliveira et al. 2009). The production of NO upon nitrite addition to nr/nia mutants suggests that NR is not the only player in nitrite-derived NO formation in planta.
Other nitrite-dependent NO sources
A plasma membrane-bound nitrite:NO reductase, distinct from the plasma membrane NR, has been shown to convert nitrite to NO in tobacco (Stohr and Stremlau 2006). Mitochondrial electron transport-dependent reductase is also capable of reducing nitrite to NO (Planchet et al. 2005). However, these latter routes seem to be localized in the roots of higher plants, where the oxygen tension is low (Gupta et al. 2005). Lastly, non-enzymatic reduction of nitrite is also possible but it requires an acidic pH, thus restricting this reaction to the apoplast of seeds and roots (Bethke et al. 2004).
Arginine-dependent NO formation
Even though there is a large body of evidence supporting the importance of NR and nitrite in NO production in plants, at least twice as many publications in the past 20 years have reported the existence of an NOS-like enzyme, raising the possibility that another NO-formation route exists. Thus, as more plant genomes became available, it was surprising that no proteins or plant genes with homology to the mammalian NOSs have been found.
Most of the studies suggesting the existence of arginine-dependent NO production in plants are based on the inhibition of either NO formation or a physiological NO-associated response by arginine analogs with modified guanidinium unsuitable for oxidation to NO. Initially, application of a mammalian NOS inhibitor was shown to compromise plant resistance to pathogens, defense gene induction and hypersensitive response; this correlated with a decrease in NO formation (Delledonne et al. 1998, Durner et al. 1998). Since then, several studies using NOS inhibitors have further supported the existence of an arginine-dependent pathway for NO formation during plant development (Corpas et al. 2006, Wang et al. 2009a), in response to abiotic stresses, such as cadmium exposure (Besson-Bard et al. 2009, De Michele et al. 2009, Rodriguez-Serrano et al. 2006), and in response to pathogens or elicitors (Asai and Yoshioka 2009, Besson-Bard et al. 2008a). The presence of an NOS-like activity that converts arginine to citrulline further argues that plants contain an NOS-like enzyme that produces NO via a mechanism similar to the one in animals (Corpas et al. 2006, Delledonne et al. 1998, Durner et al. 1998, He et al. 2007). However, the recent demonstration that Arabidopsis extracts contain an unrelated, arginine-dependent activity that produces arginosuccinate indicates that citrulline-based NOS activity assays may be misinterpreted if citrulline production is not directly verified (Tischner et al. 2007). Ideally, a direct correlation between arginine and NO would provide an additional proof of the existence of a plant NOS. NO measurement from arginine incubation with plant extracts provides more credibility to the search for a plant NOS-like enzyme (Chaki et al. 2009, Corpas et al. 2006, Delledonne et al. 1998).
Plant NOS but not AtNOS1
The search for a plant NOS has been complicated and the identity of this protein remains elusive. Initially, a variant form of the P protein of the glycine decarboxylase complex was described as a potential plant NOS but when this activity was found to be non-reproducible and unreliable, the paper was retracted (Chandok et al. 2003). In 2003, Crawford et al. identified a putative Arabidopsis NOS gene, AtNOS1, through characterization of an Arabidopsis mutant that was defective in NO accumulation in roots (Guo et al. 2003). AtNOS1 encodes a protein with sequence similarity to a hypothetical snail NOS, or NOS partner, that cross-reacts with mammalian NOS antibodies. Several groups have now shown independently that this protein is not an NOS per se but might be associated with NO accumulation (Crawford et al. 2006, Moreau et al. 2008, Zemojtel et al. 2006a). For this latter reason, AtNOS1 has been renamed NO-associated protein 1 (AtNOA1).
AtNOA1 belongs to a family of circularly permuted GTPases (cGTPases) and has been shown to hydrolyze GTP to GDP (Moreau et al. 2008). All members of the bacterial cGTPase family contain RNA/ribosome binding domains (Anand et al. 2009, Daigle and Brown 2004, Matsuo et al. 2006, Saveanu et al. 2001). Because the physiological defect of atnoa1 mutant is complemented by overexpression of the bacterial closest homolog YqeH (43.6% similarity), AtNOA1 also might function by binding RNA/ribosomes (Sudhamsu et al. 2008).
The relationship between NO accumulation and AtNOA1 function is currently unclear. Atnoa1 displays lower NO accumulation under several stress conditions (Bright et al. 2006, He et al. 2004, Zeidler et al. 2004, Zottini et al. 2007) but accumulates as much NO as wild-type plants in other situations (Arnaud et al. 2006, Besson-Bard et al. 2009, Bright et al. 2006, Kolbert et al. 2008, Shi and Li 2008, Shi et al. 2009, Tun et al. 2008). In addition, the atnoa1 mutant was identified in a screen for resistance to fosmidomycin, an inhibitor of the isoprenoid biosynthetic pathway, where it was designated rif1. The rif1/atnoa1 mutant displayed a chloroplastic protein synthesis defect and elevated expression of methylerythritol phosphate (MEP) pathway enzymes (Flores-Perez et al. 2008). Exogenous NO application partially rescued the pale yellowish phenotype of atnoa1/rif1, but did not alter the other physiological traits associated with this mutant (e.g. chloroplastic protein synthesis, MEP pathway regulation). As the main argument in favor of AtNOA1 being involved in NO production is based on the ability of an NO donor to complement the mutant phenotype, this incomplete chemical complementation might indicate that the connection between NO and AtNOA1 is indirect. Whereas the localization of the protein is unclear, the two possible locations chloroplastic and mitochondrial that have been reported so far (Flores-Perez et al. 2008, Guo and Crawford 2005) are both associated with electron transfers, which if not properly controlled can lead to ROS production. Based on structural and complementation data, it was proposed that a defect in organellar function in atnoa1, caused by disruption of ribosome/RNA binding, could lead to a high content of NO-scavenging ROS thus lowering NO bioavailability (Moreau et al. 2008, Sudhamsu et al. 2008).
AtNOA1's homologs might also participate in proper regulation of NO formation in other organisms. In plants, NO accumulation depends on the presence of an AtNOA1 homolog in Nicotiana benthamiana and ortholog in rice (Kato et al. 2008, Qiao et al. 2009). In mice, mAtNOA1 (23.2% similarity) is localized in mitochondria and, as such, seems to regulate mitochondrial NO production and tyrosine nitration, a hallmark of concomitant NO and ROS production (Parihar et al. 2008a,b, Zemojtel et al. 2006b). A human homolog, associated with the inner mitochondrial membrane, was shown to interact with complex I and to be a positive regulator of apoptosis (Tang et al. 2009). Finally, a close homolog in diatoms, PtNOA (44% similarity), is located in chloroplasts, contributes to increased NO production and sensitizes the diatom to stress-inducing aldehydes when overexpressed (Vardi et al. 2008).
Other sources for NO formation
Several studies report that polyamines like spermine and spermidine trigger NO production in planta (Gaupels et al. 2008). Because this response is so rapid, there may be a direct correlation between polyamines and NO (Tun et al. 2006). The discovery that hydroxylamines (R-NHOH) can be oxidized to NO by superoxide- or hydrogen peroxide-generating systems, as well as by tobacco cells, has led to the recent proposal of another oxidative pathway for NO synthesis (Rumer et al. 2009). However, the efficiency of this oxidative process is low and the existence of hydroxylamines in plants has not been confirmed. Thus, further validation of this putative pathway will be required.
NO signaling
Gene regulation
NO regulates the expression of numerous genes in animal cells via several different mechanisms, including by directly interacting with receptors upstream in signaling cascades, by modulating the activity of transcription factors such as NF-κB (Lander et al. 1993) or by influencing mRNA stability and translation (Wang et al. 2008). In plants, large-scale gene expression analyses have identified many genes whose expression is also regulated by NO (Grun et al. 2006). Treating Arabidopsis plants or cell cultures with NO gas or NO donors was shown to modulate the expression of as many as 342 genes (Ahlfors et al. 2009, Besson-Bard et al. 2009, Huang et al. 2002, Parani et al. 2004, Polverari et al. 2003). A significantly lower number of NO-regulated genes were identified in Arabidopsis roots, where NO primarily represses gene expression (Badri et al. 2008). NO has also been shown to regulate several genes in tobacco (Zago et al. 2006). The conclusion from these studies is that NO-responsive genes are, for the most part, stress-related and serve a variety of functions ranging from plant defense, oxidative stress responses and hormone interplay to metabolism and development. Our understanding of the NO signaling network has benefited from studies combining not only NO-responsive gene analyses but also other treatments. For example, NO-regulated genes in Arabidopsis are modulated by ozone treatment, but no genes were specifically altered by the combined treatment (Ahlfors et al. 2009). These authors proposed, therefore, that NO and ROS signaling involve common pathways. A similar conclusion was reached based on the analysis of NO and H2O2-responsive genes in tobacco (Zago et al. 2006). Analysis of microarray results for common transcription factor-binding sites has further identified eight sites, among which are several stress-related candidates, that are overrepresented in the promoter regions of NO-modulated genes (Palmieri et al. 2008).
NO-dependant PTMs
Two principles underlie NO signaling (1) NO may act through the activation of GC, which produces the second messenger cGMP or (2) NO may act through PTM of proteins, either by S-nitrosylation of cysteine residues of redox-sensitive proteins or via tyrosine nitration. Because no NO-sensitive GC has been found in plants to date, most of the NO signaling appears to be manifested through PTM of proteins, particularly S-nitrosylation.
S-nitrosylation of cysteine residues
The majority of all NO-affected proteins seem to be regulated by S-nitrosylation, which occurs by oxygen-dependent chemical reactions or by the transfer of NO from a nitrosothiol to a sulfhydryl group of a cysteine residue (trans-nitrosylation). In animals and/or bacteria, NO-mediated S-nitrosylation has been shown to regulate enzymatic activities, ion channels and transcription factor activity. Furthermore, S-nitrosylation can modify cellular signaling by influencing potential protein–protein interactions (Hess et al. 2005).
Research into the role of S-nitrosylation in plants was sparked by the identification of putative candidates for this NO-dependent redox modification. More than 100 Arabidopsis proteins that are targets for S-nitrosylation have been identified from cell culture extracts treated with the NO donor S-nitrosoglutathione (GSNO) or from NO-treated plants (Lindermayr et al. 2005). Additionally, 16 S-nitrosylated proteins have been identified in Arabidopsis undergoing a hypersensitive response to pathogens (Romero-Puertas et al. 2008).
Analyses of knock-out mutants that are impaired for GSNO metabolism have further demonstrated the importance of nitrosothiols in plant disease resistance, thermotolerance and plant growth (Chaki et al. 2009, Feechan et al. 2005, Lee et al. 2008). GSNO is thought to be a form of NO storage and transport. GSNO reductase (GSNOR) is able to breakdown GSNO and thus regulates the cellular level of nitrosothiols (Liu et al. 2001). Consequently, plants lacking GSNOR activity contain higher levels of nitrate and nitroso species. Interestingly, these plants are compromised for acclimating to heat, fail to grow on nutrient plates and exhibit increased reproductive shoots and reduced fertility (Lee et al. 2008). Furthermore, GSNOR knock-out plants display compromised plant defense responses, while plants with enhanced GSNOR activity are more resistant to virulent microbial pathogens (Feechan et al. 2005). GSNOR activity in these plants directly correlates with the accumulation of salicylic acid (SA), a critical signal for disease resistance, and the expression of a defense-associated gene, pathogenesis-related-1 (PR-1), following pathogen infection. Thus, GSNOR appears to be a positive regulator of the SA network and thereby influences disease resistance. In contrast with this result, Rusterucci et al. reported that Arabidopsis with reduced GSNOR expression due to antisense technology showed enhanced basal resistance against Hyaloperonospora arabidopsidis; this correlated with higher levels of intracellular SNOs and constitutive activation of PR-1 (Rusterucci et al. 2007). Furthermore, systemic acquired resistance was impaired in plants overexpressing GSNOR and enhanced in the antisense plants. While the disparity between the results of these two studies has yet to be resolved, these findings reinforce the physiological importance of fine-tuning SNO levels in plants and indicate that S-nitrosylation is as an important redox-dependent regulation mechanism in plants as in animals.
In addition to this more general survey about the importance of SNO in plants, several detailed analyses of S-nitrosylation of specific proteins have been published, including studies on hemoglobin 1, S-adenosylmethionine synthetase, metacaspase, Rubisco, non-expressor of PR-1 (NPR1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), peroxiredoxin II E (PrxIIE) and the SA-binding protein 3 (Abat et al. 2008, Belenghi et al. 2007, Grun et al. 2006, Holtgrese et al. 2008, Lindermayr et al. 2006, Perazzolli et al. 2004, Romero-Puertas et al. 2007, Tada et al. 2008, Wang et al. 2009b).
Although GAPDH is part of the glycolytic pathway, it also fulfils important regulatory functions. In the animal system, S-nitrosylated GAPDH stabilizes an E3 ubiquitin ligase (Siah1). This complex is translocated into the nucleus, where Siah1 degrades nuclear proteins which leads to apoptosis (Hara et al. 2005). Similar events–including S-nitrosylation and nuclear translocation–have also been observed for cytosolic plant GAPDH, suggesting that S-nitrosylation of this enzyme could also play a role in cell death regulation (Holtgrefe et al. 2008).
The activity of PrxIIE is also regulated by S-nitrosylation (Romero-Puertas et al. 2007). Plant PrxIIE is able to degrade peroxynitrite, which is the major toxic reactive nitrogen species in cells and is responsible for lipid oxidation and tyrosine nitration. S-nitrosylation of PrxIIE inhibits its peroxynitrite detoxification activity, resulting in increased peroxynitrite-dependent formation of nitrotyrosine residues. Thus, NO regulates the effect of its own reactive species through S-nitrosylation of crucial components of the antioxidant defense system that function as common triggers for ROS- and NO-mediated signaling events.
Another very interesting example of the regulatory function of NO is S-nitrosylation of AtSABP3 (Wang et al. 2009b). AtSABP3 exhibits SA binding and carbonic anhydrase (CA) activities, and S-nitrosylation of AtSABP3 at Cys280 suppresses both. Interestingly, only the CA activity is required for establishing disease resistance, suggesting that inhibition of CA activity could contribute to a negative feedback loop that modulates plant defense responses.
NPR1 is a master positive regulator of SA-mediated defense genes, which are known to be redox-regulated (Fobert and Despres 2005). In uninfected cells, this protein is located in the cytoplasm as an oligomer stabilized by intermolecular disulfide bridges. SA-mediated activation of defense responses leads to changes in the cellular redox status resulting in the reduction of NPR1 to its active monomer form. In the monomeric form, NPR1 is translocated into the nucleus, where it interacts with the reduced form of the transcription factor TGA1 (Pieterse and Van Loon 2004). Surprisingly, S-nitrosylation of Cys156 of NPR1 by GSNO facilitates its oligomerization, maintaining protein homeostasis upon SA induction (Tada et al. 2008). These authors concluded that this highly specific modification of a distinct cysteine residue results in conformational changes to NPR1, which allows disulfide bridge formation.
Tyr nitration
Formation of 3-nitrotyrosine in proteins results from the substitution of a tyrosine hydrogen atom for a nitro group. In animals, this modification affects a small number of proteins and a few tyrosines within each protein (Souza et al. 2008). NO and superoxide are required for nitrotyrosine formation. They either rapidly react together to yield the oxidant and nitrating agent peroxynitrite or decay into NO2 and H2O2, respectively, and, together with heme peroxidases, mediate tyrosine oxidation and nitration (van der Vliet et al. 1997). The addition of the nitro group decreases tyrosine's pKa nearly 3 pH units and enhances tyrosine hydrophobicity. These changes can lead to either a loss of function, such as with GSNOR (Savvides et al. 2002) or a gain of function, as with cytochrome c (Cassina et al. 2000). The pKa shift also results in signal modulation, as it decreases the extent of tyrosine phosphorylation (Gow et al. 1996).
Tobacco nitrite reductase antisense plants were the first to display a high content of protein nitration (Morot-Gaudry-Talarmain et al. 2002). The non-symbiotic hemoglobins in Arabidopsis (AtGLB) are the only plant proteins identified as Tyr nitration targets. After treatment with nitrite, AtGLB can catalyze its own nitration and nitrate other proteins as well (Sakamoto et al. 2004). A growing body of evidence now shows that Tyr nitration occurs in plants following biotic or abiotic stresses (Chaki et al. 2009, Corpas et al. 2008, Romero-Puertas et al. 2007, Saito et al. 2006, Valderrama et al. 2007). The identity of the nitrated proteins is unknown, but the majority have molecular weights between 20 and 70 kDa. Plant–pathogen interactions are prone to the concomitant release of NO and ROS, thus creating conditions favorable for nitration. Interestingly, in the sunflower–mildew pathosystem, only the susceptible cultivar experiences an increase in protein nitration (Chaki et al. 2009). The authors thus propose that Tyr nitration constitutes a molecular marker for nitrosative stress in plants, as it does in animals. Recent evidence even suggests that Tyr nitration might be a reversible modification, thereby serving as a signaling mechanism (Souza et al. 2008).
Conclusion
Advances in NO plant research over the past 20 years have revealed the extent of NO involvement in plant physiological processes, and they have demonstrated that NO is as important in plants as in animals. Several challenges remain however. For example, the field will surely benefit from (1) the elucidation of an NOS-like enzyme and (2) a better understanding of the importance of the different players in NO production as a function of the plant organs, environmental conditions and physiological processes. The discovery of all the NO producers will also be a significant advance in this field and should help elucidate several aspects of NO signaling. Furthermore, a great deal of data has been generated on potential NO targets in planta. Nevertheless, the mechanisms of these interactions and the downstream events remain elusive. For example, which transcription factor does NO interact with to regulate the downstream gene networks and how? Is there an NO-sensitive GC leading to a cGMP-dependent regulatory pathway in plants? Which are the physiologically relevant targets of NO-dependent PTMs and how do these NO-induced protein modifications regulate their activity/function? Thus, much remains to be discovered in the plant NO field.
Acknowledgement
Studies of the authors summarized here were supported by a USA National Institutes of Health grant GM067011 to D. F. K.




