Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging
Abstract
The objectives of the present work were to investigate whether loss of sunflower (Helianthus annuus L.) seed viability was affected by the embryo moisture content (MC) during seed pretreatment at 35°C, and was related to changes in energy metabolism and in the antioxidant defence system. Non-dormant seeds were equilibrated at MC of the embryonic axis ranging from 0.037 to 0.605 g H2O g−1 dry matter (DM) for 1 day at 15°C, and they were then placed at 35°C for various durations up to 14 days before the germination assays at 15°C. As expected, the higher the MC, the faster was seed deterioration. There existed a negative linear relationship between the time taken for germination to drop to 50% (P50) and the embryonic axis MC ranging from 0.108 and 0.438 g H2O g−1 DM. In dry seeds, adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate represented 6.3, 14.8 and 70.9% of the adenylate pool, respectively, and the energy charge (EC) was very low (0.14). ATP and ADP levels and EC increased sharply during the first day of equilibrium of seeds at a MC above 0.158 g H2O g−1 DM. Subsequent controlled deterioration at 35°C resulted in a decrease in the adenylate pool, and consequently in ATP level. The higher the energy metabolism during ageing, the lower was seed viability. Loss of seed viability was associated with an accumulation of H2O2, and then of malondialdehyde (MDA) suggesting that lipid peroxidation was not the only cause of seed deterioration. When there was a sublinear relationship between H2O2 content in the embryonic axis and seed viability, MDA accumulation only occurred when 50% of the seed population died within 7 days, i.e. when MC was higher than 0.248 g H2O g−1 DM. Ageing was associated with a decrease in the activity of superoxide dismutase, catalase and glutathione reductase, the main enzymes involved in cell detoxification. The involvement of seed MC, as key factor of ageing is discussed with regards to energy metabolism and the regulation of active oxygen species accumulation.
Abbreviations –
-
- ADP
-
- adenosine diphosphate
-
- AMP
-
- adenosine monophosphate
-
- AOS
-
- active oxygen species
-
- ATP
-
- adenosine triphosphate
-
- CAT
-
- catalase
-
- EC
-
- energy charge
-
- FW
-
- fresh weight
-
- GR
-
- glutathione reductase
-
- MC
-
- moisture content
-
- MDA
-
- malondialdehyde
-
- PCD
-
- programmed cell death
-
- SD
-
- standard deviation
-
- SOD
-
- superoxide dismutase
Introduction
Sensitivity of seeds to high temperatures is strongly dependent on their water content, loss of viability being faster with increasing moisture content (MC) (McDonald 1999, Priestley 1986, Roberts and Ellis 1989). The rate of loss of viability in orthodox seeds (i.e. seeds which are desiccation tolerant) is a positive function of water content. However, the application of the viability equation originally developed for barley (Ellis and Roberts 1980a, 1980b) has a lower limit, which coincides with a critical MC between 2 and 6% fresh weight (FW) basis, and an upper one for water content ranging from 15 to 28% FW, depending on whether the seeds are oily or non-oily (Roberts and Ellis 1989). In sunflower, for example, Ellis et al. (1988) have found a lower limit to the range of MCs within which the viability equation applies at around 2% FW.
Seed deterioration is associated with various cellular, metabolic and chemical alterations including loss of membrane integrity, reduced energy metabolism, impairment of RNA and protein synthesis, and DNA degradation (Cheah and Osborne 1978, Edler et al. 1987, McDonald 1999, Priestley 1986, Vazquez-Ramos et al. 1988). Although the exact mechanisms of loss of viability are by no means elucidated, free radicals and lipid peroxidation, and the ability of the tissues to scavenge radicals are considered to be the major causes of seed deterioration (Bailly 2004, Hendry 1993). Lipid peroxidation has been demonstrated to be involved in seed ageing in soybean (Sung 1996), Norway maple (Pukacka 1991), peanut (Sung and Jeng 1994) and sunflower (Bailly et al. 1996, Gidrol et al. 1989), but it does not seem to play a key role in ageing in other species such as pigeonpea (Kalpana and Madhava Rao 1996), wheat (Girard and Le Meste 1992) and maize (Linn and Pearce 1990). In sunflower seeds, loss of viability during accelerated ageing is associated with an accumulation of malondialdehyde (MDA), suggesting that seed deterioration is associated with lipid peroxidation related to a decrease in the efficiency of the antioxidant defence system (Bailly et al. 1996, Torres et al. 1997). In addition, the work of Gidrol et al. (1989) suggests that lipid reserves undergo peroxidation serving as a detoxifying trap thus protecting the membranes.
Oxidative reactions are largely responsible for ageing of dry seeds (Hendry 1993), but they are dependent on the seed water content. It is likely that they are involved in deterioration processes when MC is very low, i.e. in region 1 of the sorption isotherm. Enzymatic oxidation, such as that of lipoxygenase, may be facilitated in region 2 of the sorption isotherm, and may contribute to cellular damage observed at these MCs (Vertucci and Farrant 1995, Vertucci and Leopold 1987). At water contents higher than 0.25 g H2O g−1 dry matter (DM), mitochondrial respiration is estimated to occur (Vertucci and Farrant 1995), and to be one of the major sources of production of active oxygen species (AOS). Electron leakage from the transport chain generates thus superoxide, and subsequently H2O2, that is directly proportional to respiratory activity (Staniek and Nohl 2000). On the other hand, alteration in respiratory pathway and energy metabolism in aged seeds have been demonstrated by various authors (Corbineau et al. 2002, Gidrol et al. 1988, McDonald 1999). Effect of ageing on adenylate pool must also be considered with regards to the recent findings dealing with the involvement of oxidative stress on mitochondrial respiration and programmed cell death (PCD) (Jones 2000, Lam et al. 2001). Although Boubriak et al. (2000) demonstrated that different DNA fragmentation patterns occurred at different water contents, suggesting that DNAase and nuclease activity are activated at various levels of embryo hydration, there are no clear data that report that PCD is involved in loss of viability during seed storage.
The objectives of the present work were to investigate how deterioration of sunflower (Helianthus annuus L.) seeds was affected by their water content during controlled deterioration at 35°C, and whether it was related to the energy metabolism and the antioxidant defence system in the embryonic axis.
Materials and methods
Plant material
Experiments were carried out with sunflower (H. annuus L.) seeds harvested in 2003 and received from Monsanto-France (Peyrehorade, France). Seeds with a mean MC of 0.043 g H2O g−1 DM were stored dry in paper bags at 20°C and 75% relative humidity for at least 3–4 months before the experiments started, to completely break their dormancy (Corbineau et al. 1990). After such a storage, they germinated easily at 15°C, a suboptimal temperature for this species (Corbineau et al. 1990).
Germination assays and seed viability
Germination assays were performed at 15°C in darkness, in four replicates of 25 whole achenes placed in 9-cm diameter Petri dishes on a layer of cotton wool moistened with deionised water. A seed was scored as germinated when the radicle had pierced the envelopes (seed coat + pericarp). Germination counts were made daily for 7 days. Results presented correspond to the means of the germination percentages obtained after 7 days in four replicates ± standard deviation (sd).
Topographical tetrazolium testing was also used to verify that seeds, which did not germinate at 15°C, were dead. For this purpose, seeds were cut longitudinally and were incubated in 1% solution of 2,3,5-triphenyl tetrazolium chloride (Moore 1973) for 6–8 h at 25°C in darkness. Seeds of the embryo which exhibited no overall carmine red staining were scored as non-viable.
Water content and water potential determination
Ten whole seeds, isolated embryonic axes or cotyledons were weighed and then dried by oven drying at 105°C for 48 h for determination of the DM and calculation of water content. Water content was calculated on a DM basis. Results are expressed as g H2O g−1 DM and correspond to the means of the values obtained in three replicates of 10 whole seeds or 10 organs ± sd. Table 1 shows the respective water contents of embryonic axes and cotyledons after seed equilibrium at various water contents ranging from 0.043 to 0.488 g H2O g−1 DM.
| Water content (g H2O g−1 DM) of whole seeds | Water potential (−MPa) of whole seeds | Water content (g H2O g−1 DM) of | |
|---|---|---|---|
| Embryonic axes | Cotyledons | ||
| 0.043 ± 0.007 | 123.5 ± 5.5 | 0.037 ± 0.003 | 0.042 ± 0.003 |
| 0.084 ± 0.003 | 22.6 ± 1.3 | 0.108 ± 0.011 | 0.085 ± 0.005 |
| 0.146 ± 0.006 | 7.8 ± 0.6 | 0.158 ± 0.003 | 0.137 ± 0.008 |
| 0.213 ± 0.012 | 4.2 ± 0.4 | 0.248 ± 0.011 | 0.216 ± 0.007 |
| 0.299 ± 0.012 | 3.4 ± 0.5 | 0.352 ± 0.006 | 0.298 ± 0.011 |
| 0.375 ± 0.007 | 2.5 ± 0.2 | 0.438 ± 0.004 | 0.395 ± 0.009 |
| 0.488 ± 0.013 | 1.0 ± 0.1 | 0.605 ± 0.011 | 0.528 ± 0.010 |
Water potentials were measured using a WP4-T Dewpoint potentiometer (Decagon devices, Inc., Pullman, WA). Results are expressed as MPa and correspond to the means of three measurements carried out with 50 seeds each ± sd.
Ageing treatment
Seeds (about 20 g) were placed in tightly closed 250-ml flasks with different amounts of water to obtain various seed water contents ranging from 0.043 (control seeds) to 0.488 g H2O g−1 DM. Water equilibrium was carried out by rotating the flasks for 24 h at 20°C, on a roller with a rotating rate of 60 rotations min−1. Ageing treatment was performed by transferring the flasks at 35°C for 14 days. After various durations at 35°C, seeds were dried at 20°C for 1 day before the germination assays at 15°C and biochemical measurements (MDA and H2O2 contents and enzyme activities). No visible germination occurred during the ageing treatment.
The half-viability period (P50) was determined graphically as the time taken for germination to drop to 50%.
Adenosine phosphate assays
The energy metabolism was characterised in the axes only. Adenosine phosphates were extracted according to Olempska-Beer and Bautz-Freeze (1984) from three axes excised from seeds aged for 7 days. Adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate (AMP) contents of the extracts were measured using the bioluminescence method with a pico-ATP biophotometer (Jobin et Yvon, France), as described by Corbineau et al. (2002) and Saglio et al. (1979). The results obtained are expressed as nmol ATP, ADP or AMP mg DM−1 and are the means of values obtained with five to seven extracts ± sd.
The ATP/ADP ratio and the energy charge [EC = (ATP + 0.5 ADP)/(ATP + ADP + AMP)] were also calculated.
Hydrogen peroxide and MDA contents
H2O2 and MDA contents were determined in embryonic axes isolated from seeds after water equilibrium, i.e. before ageing, and from seeds aged for 7 days at 35°C at different water contents.
H2O2 contents were measured according to the method of O’Kane et al. (1996). Ten axes were ground in a mortar and homogenized with 4 ml of perchloric acid (0.2 N). After 15 min of centrifugation at 13 000 g at 4°C, the resulting supernatant was neutralized to pH 7.5 with 4 N KOH and then centrifuged at 1000 g for 3 min at the same temperature. The supernatant was immediately used for spectrophotometric determination of H2O2 at 590 nm using a peroxidase-based assay. The reaction mixture contained 12 mM 3-dimethylaminobenzoic acid in 0.375 M phosphate buffer (pH 6.5), 1.3 mM 3-methyl-2-benzothiazolidone hydrazone, 20 μl (0.25 units) horseradish peroxidase (Sigma, St. Louis, MO) and 50 μl of the supernatant for a total volume of 1.5 ml. The reaction started with the addition of peroxidase. Increase in absorbance at 590 nm was monitored after 5 min at 25°C and compared with the absorbance obtained with known amounts of H2O2.
Lipid peroxidation was evaluated by measuring MDA content from 10 isolated embryonic axes, according to Heath and Parker (1968) as described by Bailly et al. (1996). Axes were ground in 5 ml of deionised water and homogenized with an equal volume of 0.5% (w/v) 2-thiobarbituric acid in 20% (w/v) trichloroacetic acid. The homogenate was incubated at 95°C for 30 min. The supernatant was used for MDA determination.
Results correspond to the means of three measurements carried out with two different extracts ± sd. They are also expressed as percentages of the values obtained in control unaged seeds, i.e. after water equilibrium.
Enzyme extraction and assays
Ten embryonic axes isolated from seeds after water equilibrium, i.e. before ageing, and from seeds aged for 7 days at 35°C at different water contents, were ground and homogenized in a mortar in 3.5 ml of potassium phosphate buffer (0.1 M, pH 7.8) containing 2 mM dithiothreitol, 0.1 mM ethylenediaminetetraacetic acid, 1.25 mM polyethylene glycol-4000 and 20% polyvinylpolypyrrolidone. The homogenate was then centrifuged at 15 000 g for 15 min. The resulting supernatant was desalted on a PD 10 column (Amersham Biosciences, Sweden) and used for enzyme assays. All steps of the extraction procedure were carried out at 1–4°C.
Superoxide dismutase (SOD, EC 1.15.11), glutathione reductase (GR, EC 1.6.4.2) and catalase (CAT, EC 1.11.16) activities were determined at 25°C according to the method of Bailly et al. (1996). The results are expressed as units mg−1 protein (SOD), nmol of nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidized min−1 mg−1 protein (GR) and nmol H2O2 decomposed min−1 mg−1 protein (CAT).
The results presented correspond to the means ± sd of the values obtained with two different extracts and three measurements per extract (i.e. six measurements). The enzyme activities are also expressed as percentages of the activities measured in control unaged seeds, i.e. after water equilibrium.
Protein contents of the extracts were determined using the Bio-Rad (München, Germany) protein assay kit with bovine serum albumin as calibration standard.
Results
Effects of seed water content during treatment at 35°C on subsequent germination
Fig. 1 shows the effects of duration of controlled deterioration at 35°C of seeds previously equilibrated at various water contents ranging from 0.043 to 0.488 g H2O g−1 DM, i.e. corresponding to axis water contents ranging from 0.037 to 0.605 g H2O g−1 DM (Table 1), on subsequent germination percentages obtained after 7 days at 15°C. Almost all untreated seeds (91%) germinated within 7 days at 15°C. Treatment of seeds at 35°C markedly reduced the final percentages of germination, and the longer the treatment and the higher the seed water content, the stronger was the reduction of seed viability. At a seed water content as low as 0.043 g H2O g−1 DM, about 15% of the seed population died within 2 weeks. Increase in seed water content up to 0.084 g H2O g−1 DM resulted in a strong deterioration of seeds, and the half-viability period (P50), i.e. the time taken for germination to drop to 50%, was only around 8.5 days (Fig. 1). P50 decreased with increasing water content, and fell down to 4.5 days when seed water content was around 0.375 g H2O g−1 DM. The tetrazolium test showed that almost all seeds, which did not germinate were dead.
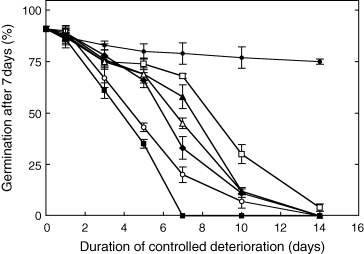
Effects of duration of treatment at 35°C of seeds previously equilibrated at water contents of 0.043 (•), 0.084 (□), 0.146 (▴), 0.213 (▵), 0.299 (♦), 0.375 (○) and 0.488 (▪) on subsequent germination percentages obtained after 7 days at 15°C. Means of four replicates ± standard deviation (sd). Where no bars are shown, the spread of sd is less than the size of symbols.
There existed a linear relationship between P50 and seed water content ranging from 0.084 to 0.375 g H2O g−1 DM, and between P50 and water content of the embryonic axes ranging from 0.108 to 0.438 g H2O g−1 DM (Fig. 2).
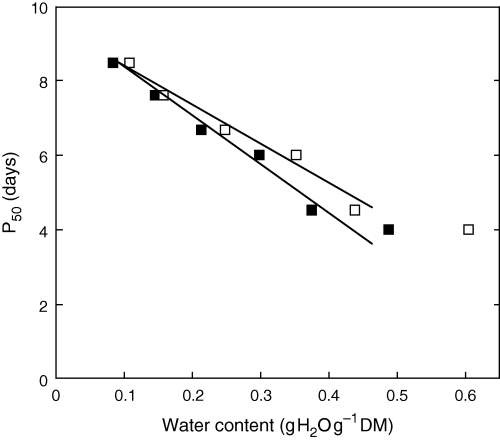
Relationships between the half-viability period (P50) and water content of whole seeds (▪) and embryonic axes (□). Means of four replicates. Values of P50 were determined graphically from Fig. 1.
Energy metabolism
Table 2 shows the changes in the adenylate pool (Σ) and in adenylic nucleotide levels after 1 day of water equilibrium at 20°C and after a subsequent 7-day-controlled deterioration at 35°C. In dry untreated seeds, the ATP, ADP and AMP contents in the axis were 18.2, 42.7 and 227.3 nmol g−1 DM, respectively, representing 6.3, 14.8 and 78.9% of the adenylate pool. Seed imbibition resulted in a progressive increase in Σ from 288.2 to 422.4 nmol g−1 DM when water content of the axis enlarged from 0.037 to 0.605 g H2O g−1 DM. This increase in Σ was associated with an increase in ATP, whereas AMP content continuously decreased. After 1 day of imbibition at the highest water content (0.605 g H2O g−1 DM), ATP and AMP contents were 297.6 and 25.9 nmol g−1 DW, representing 70.1 and 6.1% of Σ, respectively. As for ADP level, it increased to 125.5 nmol g−1 DM (i.e. 37.6% of Σ) when axis water content reached 0.158 g H2O g−1 DM, it decreased then slightly and remained at about 25% of Σ at higher water contents. As a consequence of these changes in ATP, ADP and AMP levels during imbibition, the EC in the axis, which was very low (0.14) in dry seeds, increased with water content, reaching a value of 0.76 at 0.352 g H2O g−1 DM, and then remained close to this value.
| Water content (g H2O g−1 DM) | Seed treatment | ∑ | ATP | ADP | AMP | EC |
|---|---|---|---|---|---|---|
| 0.037 | Before ageing | 288.2 ± 20.5 | 18.2 ± 1.9 | 42.7 ± 3.5 | 227.3 ± 14.0 | 0.14 ± 0.01 |
| 0.108 | 306.9 ± 20.5 | 37.9 ± 2.9 | 41.7 ± 3.2 | 227.3 ± 17.2 | 0.19 ± 0.01 | |
| 0.158 | 333.7 ± 16.2 | 84.1 ± 1.7 | 125.5 ± 7.0 | 124.1 ± 7.8 | 0.44 ± 0.01 | |
| 0.248 | 343.5 ± 32.1 | 157.3 ± 12.6 | 103.6 ± 13.8 | 82.6 ± 7.5 | 0.61 ± 0.01 | |
| 0.352 | 341.7 ± 19.5 | 223.5 ± 14.9 | 73.1 ± 2.3 | 45.1 ± 3.8 | 0.76 ± 0.01 | |
| 0.438 | 384.4 ± 20.1 | 237.9 ± 18.3 | 111.1 ± 8.6 | 35.4 ± 4.7 | 0.77 ± 0.01 | |
| 0.605 | 422.4 ± 18.5 | 297.6 ± 14.3 | 98.9 ± 8.9 | 25.9 ± 8.2 | 0.82 ± 0.01 | |
| 0.037 | After ageing | 269.8 ± 15.1 | 16.5 ± 1.2 | 39.9 ± 5.1 | 213.4 ± 19.3 | 0.14 ± 0.01 |
| 0.108 | 260.1 ± 11.2 | 40.8 ± 2.6 | 47.1 ± 4.6 | 172.2 ± 18.3 | 0.25 ± 0.01 | |
| 0.158 | 254.3 ± 12.2 | 57.5 ± 5.2 | 60.5 ± 7.1 | 136.3 ± 11.1 | 0.35 ± 0.01 | |
| 0.248 | 261.1 ± 20.2 | 96.4 ± 10.3 | 97.5 ± 9.5 | 67.2 ± 5.6 | 0.56 ± 0.01 | |
| 0.352 | 251.0 ± 16.7 | 106.1 ± 9.4 | 82.5 ± 8.8 | 62.5 ± 5.8 | 0.59 ± 0.01 | |
| 0.438 | 233.6 ± 16.5 | 117.4 ± 10.4 | 66.3 ± 5.1 | 49.9 ± 5.1 | 0.65 ± 0.01 | |
| 0.605 | 220.4 ± 9.8 | 136.4 ± 11.7 | 63.7 ± 7.4 | 20.3 ± 1.6 | 0.77 ± 0.01 |
Subsequent controlled deterioration at 35°C resulted in a significant decrease in adenylate pool (Table 2), which enlarged with increasing seed water content. Σ remained at about 75% of its initial value before ageing when ageing was performed at water contents ranging from 0.158 to 0.352 g H2O g−1 DM, but represented only 51.9% of this value when water content was 0.605 g H2O g−1 DM. ATP content, expressed as % of its initial value before ageing, strongly decreased when water content during ageing was higher than 0.158 g H2O g−1 DM. It was only around 45–49% of its initial value when water content was higher than 0.352 g H2O g−1 DM. However, ATP level in axes of aged seeds remained high when MC during controlled deterioration was higher than 0.158 g g−1 DM. It increased with increasing water content, representing from 6 to 61.9% of Σ when water content raised from 0.037 to 0.605 g H2O g−1 DM. Increase in ATP content was associated with a decrease in AMP content, whereas ADP increased with increasing water content until 0.248 g H2O g−1 DM, and then decreased.
These changes in adenylic nucleotide contents resulted in an increase in EC with increasing water content, but EC was only slightly reduced after ageing (Table 2). The higher the energy metabolism during ageing, the lower was the seed viability (Table 2 and Fig. 1).
H2O2 and MDA contents
In axes of untreated seeds, H2O2 and MDA contents were 1.5 and 35.6 μmol g−1 DM, respectively. During the first day of imbibition at 20°C, they both progressively increased with increasing water content, and almost doubled when water content reached 0.605 g H2O g−1 DM (data not shown).
Seven days of controlled deterioration at 35°C resulted in H2O2 and MDA accumulation. H2O2 content, expressed as % of its initial value before ageing, increased from 125.2 to 144.3% when seed MC during ageing rose from 0.043 to 0.488 g H2O g−1 DM (Fig. 3). A sublinear relationship between water content of the embryonic axes and H2O2 content existed. In contrast, MDA content did not markedly enlarge until water content reached 0.248 g H2O g−1 DM, and then it increased reaching 282% after 7 days of ageing at a seed MC of 0.605 g H2O g−1 DM.
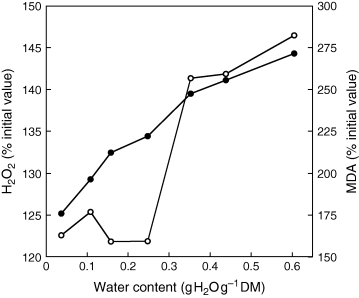
Effects of embryonic axis water content during 7 days of ageing at 35°C on H2O2 (•) and malondialdehyde content (○) in the embryonic axes, expressed as % of the initial values before ageing. Means of six replicates.
Fig. 4 shows that a linear relationship existed between H2O2 content and seed viability, which was not the case for MDA.
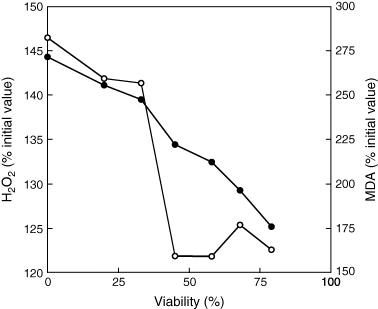
Relationship between H2O2 (•) and malondialdehyde (MDA) contents (○) in embryonic axes after 7 days of controlled deterioration at 35°C and seed viability. Means of four replicates (seed viability) and of six measurements (H2O2 and MDA contents).
Antioxidant defence system
Table 3 summarizes the results obtained regarding the changes in SOD, CAT and GR activities in embryonic axes during the first day of equilibrium of seeds at various water contents. A sharp increase in the activities of the three enzymes was observed with increasing water content. Water uptake resulted in a significant improvement of enzyme activities as soon as water content reached 0.158 g H2O g−1 DM.
| Water content (g H2O g−1 DM) | Activity of | ||
|---|---|---|---|
| SOD (units mg−1 protein) | CAT (nmol H2O2 min−1 mg−1 protein) | GR (nmol NADPH min−1 mg−1 protein) | |
| 0.037 | 12.27 ± 1.56 | 10.97 ± 0.55 | 15.96 ± 0.24 |
| 0.108 | 12.38 ± 0.19 | 13.97 ± 0.57 | 17.17 ± 1.31 |
| 0.158 | 13.59 ± 0.54 | 14.33 ± 1.65 | 17.92 ± 2.21 |
| 0.248 | 14.34 ± 0.58 | 14.52 ± 0.35 | 19.12 ± 1.07 |
| 0.352 | 15.68 ± 0.60 | 13.40 ± 0.35 | 21.78 ± 0.63 |
| 0.438 | 15.74 ± 0.92 | 14.02 ± 0.63 | 23.18 ± 0.81 |
| 0.605 | 17.65 ± 0.86 | 19.96 ± 0.70 | 25.80 ± 0.56 |
Seed treatment at 35°C for 7 days resulted in a decrease in antioxidant defence system (Fig. 5). SOD, CAT and GR activities remained at around 75% of their initial values when water content was 0.037 g H2O g−1 DM. The higher the MC during controlled deterioration, the lower were the enzyme activities, expressed as % of the initial values before ageing (Fig. 5). However, GR and SOD activities deteriorated faster than that of CAT. For example, after 7 days of ageing at seed water content of 0.213 g H2O g−1 DM, (i.e. embryonic axis water content of 0.248 g H2O g−1 DM), CAT, SOD and GR activities were 62.6, 47.7 and 42.2% of the initial values before ageing, respectively. Fig. 6 shows the relationship between the three enzyme activities and seed viability. The higher the activities of CAT, SOD and GR, the higher was the seed viability.
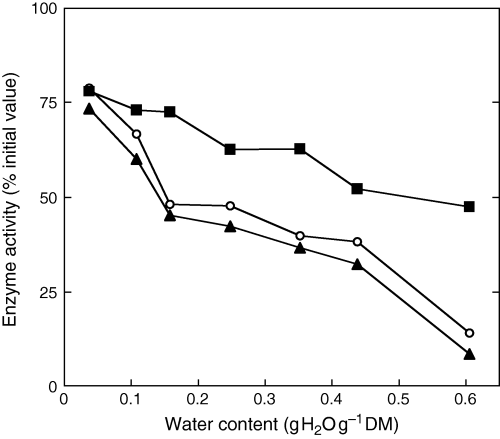
Effects of embryonic axis water content during 7 days of ageing at 35°C on superoxide dismutase (○), catalase (▪) and glutathione reductase (▴) activities in embryonic axes, expressed as % of the initial values before ageing. Means of six replicates.
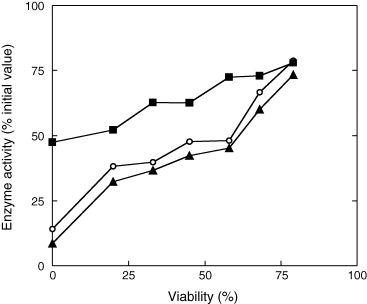
Relationship between superoxide dismutase (SOD) (○), catalase (CAT) (▪) and glutathione reductase (GR) activities (▴) in embryonic axes after 7 days of controlled deterioration at 35°C and seed viability. Means of four replicates (seed viability) and of six measurements (SOD, CAT and GR activities).
Discussion and conclusion
Besides temperature, MC is a key factor of seed deterioration during storage, and both factors have been integrated in viability equations to predict seed lifespan (Ellis and Roberts 1980a, 1980b). Water plays an important role in the regulation of metabolic reactions through enzyme activities and cytoplasmic viscosity because it acts as plasticizer of glasses (Walters 1998). In sunflower seeds, a slight increase in MC had a pronounced inhibitory effect on seed survival at 35°C (Fig. 1). At a seed MC as low as 0.043 g H20 g−1 DM (i.e. at the very low water potential of −123.5 MPa) (Table 1), seed viability only decreased to some extent after 2 weeks at 35°C, whereas 50% of the seed population died after 9 days at this temperature when ageing was carried out at a seed MC of 0.084 g H2O g−1 DM (i.e. at a water potential of −22.6 MPa) (1, 2). Further increase in MC resulted in a faster deterioration rate, but it must be emphasized that increase of seed mortality with increasing MC was not related to a homogeneous water repartition within the seed tissues (Table 1). Axis MC was always higher than that of cotyledons (Table 1). The higher water content in the axis might lead to a faster deterioration rate during ageing, which would explain the rapid loss of seed viability, because completion of the germination process requires elongation of this organ. Similar observations have been made by Pukacka and Ratajczak (2005) during loss of viability of Fagus sylvatica seeds during storage. Sorption isotherms obtained with axes or cotyledons isolated from sunflower seeds (data not shown) and with whole seeds (Vertucci and Ross 1990) indicate that at 0.084 g H2O g−1 DM, the lowest seed MC used in the present study after adding water, water belonged to the water-binding zone 3 of the sorption curves, i.e. a region where water is weakly bound and enzymatic systems are functional (Vertucci and Farrant 1995). Moreover, sunflower seeds are oily seeds in which tissues, including the axis, are filled with large oil bodies excluding water. Therefore, in the non-lipid fraction of the cells, MC probably reached much higher values than those determined experimentally and shown in Table 1. This water status explains why several metabolic events, such as increase in adenylate pool and synthesis of ATP (Table 2), activation of the antioxidant defence system (Table 3) and production of H2O2 (Fig. 3), occurred in an apparently seed MC as low as 0.084 g H2O g−1 DM. For example, respiration is known to be possible when MC is higher than 0.25 g H2O g−1 DM (Vertucci and Farrant 1995), a critical value, which is much higher than that determined in sunflower embryonic axis (i.e. 0.108–0.158 g H2O g−1 DM) for inducing ATP synthesis (Table 2). ATP content in axes doubled when MC reached 0.108 g H2O g−1 DM, and EC reached 0.44 at 0.158 g H2O g−1 DM, suggesting that, despite an apparently low MC, embryonic axis tissues contain fully hydrated domains, which allow metabolic activities. Equilibrium of seeds to higher MC, as it has been carried out in the frame of this study, provides more free water in the tissues, thus enhancing the reaction rates including those involved in seed deterioration. This might explain the linear relationship between P50 and embryonic axis MC ranging from 0.108 to 0.438 g H2O g−1 DM (Fig. 2), the upper MC corresponding to the optimal metabolism.
Lipid peroxidation and oxidative stress have been widely indicated as the major causes of deterioration of oilseeds during ageing (Bailly 2004, Hendry 1993, McDonald 1999, Priestley 1986). In sunflower seeds, it has already been demonstrated that loss of seed vigour, and then seed viability during accelerated ageing is associated with a decrease in the enzymatic antioxidant potential of the cells, thus leading to lipid peroxidation (Bailly et al. 1996, 1998, De Paula et al. 1996, Torres et al. 1997). Depletion of antioxidant enzyme activities was also observed during seed ageing in soybean (Murthy et al. 2002), cotton (Goel et al. 2003) and beech (Pukacka and Ratajczak 2005). The results presented here show that loss of sunflower seed viability during controlled deterioration was also related to an impairment of the enzymatic antioxidant system, i.e. CAT, SOD and GR activities, in the embryonic axis (Fig. 5), which resulted in an accumulation of H2O2 and in lipid peroxidation dependent on the MC (Fig. 3). In particular, H2O2 content in the embryonic axis was nicely correlated with seed viability, whereas severe lipid peroxidation appeared later, i.e. when 50% of seeds were already dead (Fig. 4). Previous data (Bailly et al. 1996, Gidrol et al. 1989) obtained with sunflower seeds have shown that accelerated ageing at 45°C and 100% relative humidity (RH) (i.e. at seed MC of about 0.30 g H2O g−1 DM), induces both loss of seed viability and accumulation of MDA. However, such an ageing does not result in a significant change in electrolyte leakage. Lack of such a relationship between lipid peroxidation and electrolyte leakage was explained by the peroxidation of lipid reserves serving as a detoxification and thus protecting the membranes (Corbineau et al. 2002, Gidrol et al. 1989).
A short treatment of hydration, i.e. 1 day of hydration at 20°C before ageing, resulted in a marked increase in SOD, CAT and GR activities (Table 3). This effect of hydration has already been shown during osmopriming of sunflower seeds (Bailly et al. 2000), but it appeared to be insufficient to prevent H2O2 accumulation and lipid peroxidation during subsequent treatment at 35°C (Fig. 3). Seven days of ageing decreased SOD, CAT and GR activities, but this effect depended on the enzyme, SOD and GR being more sensitive to controlled deterioration than CAT at any MC (Fig. 5). In contrast, Bailly et al. (1996) demonstrated in sunflower seeds that CAT activity was more sensitive to accelerated ageing (45°C and 100% RH) than that of GR, SOD being almost not changed. This different sensitivity of the antioxidant defence system to ageing might result from the ageing conditions and/or from the genotypes used.
Although loss of seed viability probably results from an impairment of the efficiency of the cell antioxidant defence system, several mechanisms seem to be involved in mortality as a function of seed MC. During seed ageing at 35°C, accumulation of H2O2 led without any doubt to reserve lipid peroxidation, as shown by the sharp increase in MDA content, but only when embryonic axis MC exceeded 0.248 g H2O g−1 DM (Fig. 3). This suggests that sunflower seed mortality at relatively low MC, i.e. below a whole seed MC of 0.213 g H2O g−1 DM (Table 1), cannot be explained by peroxidative damage against lipids. Bailly et al. (1996) and Gidrol et al. (1989) have proposed that lipid peroxidation is the major cause of sunflower seed deterioration, but these studies were carried out using accelerated ageing. We clearly demonstrate, here, that in milder conditions of deterioration (i.e. lower MC and temperature), oilseed deterioration may also occur without lipid peroxidation. This might explain why the lipid peroxidation model has often been subject to debate, because the occurrence of these reactions strongly depend on seed MC (McDonald 1999), and that the various experiments of controlled deterioration and accelerated ageing dealing with lipid oxidation were carried out in a wide range of MC. Lipoperoxidation can indeed be prevented when MC is between 0.06 and 0.14 g H2O g−1 DM, but favoured at very low MC (McDonald 1999). Below the critical seed MC of 0.213 g H2O g−1 DM, cellular damage can be attributed to accumulation of hydrogen peroxide. This compound or its derivatives such as the highly toxic hydroxyl radical, are known to react with other molecules than lipids, such as proteins and nucleic acids, causing oxidative attacks to theses molecules (Beckman and Ames 1997). Enzymes can be easily inactivated by reactive oxygen species when amino acids essential or close to the active sites are degraded. Hydrogen peroxide reacts with thiol groups and has been shown to lead to inactivation of some enzymes such as, for example, those of the Calvin cycle (Charles and Halliwell 1980), and to damage transport proteins, receptors and ion channels and thus leading to extensive cellular dysfunction (Halliwell and Gutteridge 1999). In particular, it cannot be excluded that H2O2 participates to the inactivation of the antioxidant enzymes, as proposed by Bailly (2004), which can lead to a loss of the detoxifying potential necessary for the completion of germination. In addition, H2O2 can also alter mitochondria functioning through the disruption of the electron transfer chain, as discussed below.
As previously shown for seeds of many species (Corbineau et al. 2002, Raymond et al. 1982, 1985), adenylic nucleotides were mainly in the monophosphate form (AMP) (78.9% of the adenylate pool) and EC was very low (0.14) in dry embryonic axes (Table 2). Imbibition of seeds at 20°C induced a sharp increase in ATP and ADP levels, resulting in a marked increase in EC, which reached 0.76 at 0.352 g H2O g−1 DM (Table 2), a value indicating that metabolism was almost normal (Pradet and Raymond 1982). This activation of energy metabolism was probably linked to an oxygen-dependent cyanide-sensitive respiratory pathway as shown by Raymond et al. (1985) in seeds of other species. Seven days of treatment at 35°C led to a marked depletion of the adenylate pool, inducing a decrease in ATP content when MC was higher than 0.158 g H2O g−1 DM. This depletion effect increased with increasing water content during ageing (Table 2). However, ATP content remained at about 45–50% of its initial value before ageing and EC was still high (0.65–0.77) when MC content of the embryonic axis was higher than 0.352 g H2O g−1 DM (Table 2), i.e. when almost no seed was able to germinate (Fig. 1). Therefore, in agreement with Corbineau et al. (2002) and Mazor et al. (1984), ATP level and EC cannot be considered as good indicators of germination ability. After 7 days of controlled deterioration at 35°C, the three adenine nucleotides were maintained in equilibrium. A decrease in ATP produced an increase in AMP, suggesting that the activity of adenylate kinase (EC 2.7.4.3) remained sufficiently high to maintain this equilibrium even when ATP regeneration systems were reduced. After 7 days of ageing at 0.605 g H2O g−1 DM, EC value was 0.77, and the value of the ATP/ADP ratio (2.1), which is a good marker of the degree of phosphorylation, indicates that mitochondrial function was not altered. Longer treatments would probably induce irreversible alteration in ATP synthesis, because of damage at the ultrastructural mitochondrial level as observed in embryos of aged seeds (Smith and Berjak 1995).
It is well known that mitochondria participate in the production of AOS, because under normal physiological conditions ca 1–2% of the oxygen used by the mitochondria is transformed into H2O2 (Kowaltowski and Vercesi 1999, Puntaluro et al. 1988). In aged sunflower seeds, there was a positive relationship between energy metabolism and H2O2 accumulation (Table 2 and Fig. 3). Tiwari et al. (2002) have shown that even a mild oxidative stress could increase respiratory electron transport and lead in turn to an overproduction of AOS, concomitant to a depletion of ATP, thereby amplifying oxidative stress. Depletion of ATP, which occurred in the axis during controlled deterioration at 35°C, is known to induce apoptosis in plants when it is coupled with calcium influx (Jones 2000, Lam et al. 2001). In plants, as in animals, mitochondria would integrate the oxidative stress signal, leading to a release of cytochrome c, which is considered to be a signal for the cytoplasm to execute a PCD (Jones 2000). We propose that the accumulation of H2O2 during seed ageing might be involved in the decrease in ATP content, two events which could trigger PCD and then loss of seed viability. Further evidence is nevertheless required to sustain for a role of PCD and mitochondria in loss of viability in sunflower, but this is to our knowledge the first time that a study establishes a direct link between H2O2 accumulation, energy metabolism and seed deterioration.
In conclusion, although our results give evidence of the pivotal role of antioxidant defence system in seed ageing, they demonstrate that lipid oxidation is probably not the sole event to be involved in loss of sunflower seed germinability. Deterioration of oily seeds should therefore not be regarded only as a consequence of lipid peroxidation, as it is often mentioned in the literature, because it might also be related to damaging events occurring in the non-lipid cellular fraction. Finally, our results suggest that H2O2-induced ATP depletion could trigger cytochrome c release, which in turn might lead to PCD and loss of viability. They also underline the key role of seed MC in the mechanisms involved in seed deterioration, in relation with the mitochondria functioning and, more importantly, the role of water status, i.e. its strength of association with other macromolecules, in triggering molecular events related to cell death.
Edited by M. J. Oliver




