New indolicidin analogues with potent antibacterial activity*
A preliminary account of this work was presented at the 18th American Peptide Symposium, Boston, MA, 2003 and will be published in the proceedings.
Abstract
Abstract: Indolicidin is a 13-residue antimicrobial peptide amide, ILPWKWPWWPWRR-NH2, isolated from the cytoplasmic granules of bovine neutrophils. Indolicidin is active against a wide range of microorganisms and has also been shown to be haemolytic and cytotoxic towards erythrocytes and human T lymphocytes. The aim of the present paper is two-fold. First, we examine the importance of tryptophan in the antibacterial activity of indolicidin. We prepared five peptide analogues with the format ILPXKXPXXPXRR-NH2 in which Trp-residues 4,6,8,9,11 were replaced in all positions with X = a single non-natural building block; N-substituted glycine residue or nonproteinogenic amino acid. The analogues were tested for antibacterial activity against both Staphylococcus aureus American type culture collection (ATCC) 25923 and Escherichia coli ATCC 25922. We found that tryptophan is not essential in the antibacterial activity of indolicidin, and even more active analogues were obtained by replacing tryptophan with non-natural aromatic amino acids. Using this knowledge, we then investigated a new principle for improving the antibacterial activity of small peptides. Our approach involves changing the hydrophobicity of the peptide by modifying the N-terminus with a hydrophobic non-natural building block. We prepared 22 analogues of indolicidin and [Phe4,6,8,9,11] indolicidin, 11 of each, carrying a hydrophobic non-natural building block attached to the N-terminus. Several active antibacterial analogues were identified. Finally, the cytotoxicity of the analogues against sheep erythrocytes was assessed in a haemolytic activity assay. The results presented here suggest that modified analogues of antibacterial peptides, containing non-natural building blocks, are promising lead structures for developing future therapeutics.
Abbreviations:
-
- ACTH
-
- adrenocorticotropic hormone
-
- ATCC
-
- American type culture collection
-
- Boc
-
- tert-butyloxycarbonyl
-
- BSA
-
- bovine serum albumin
-
- But
-
- tert-butyl
-
- CD
-
- circular dichroism
-
- CFU
-
- colony-forming units
-
- DCM
-
- dichloromethane
-
- DIEA
-
- N,N′-diisopropylethylamine
-
- DIPCI
-
- N,N′-diisopropylcarbodiimide
-
- DMF
-
- N,N′-dimethylformamide
-
- DMSO
-
- dimethyl sulfoxide
-
- Fmoc
-
- 9-fluorenylmethyloxycarbonyl
-
- HATU
-
- N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridino-1-ylmethylene]-N-methyl methanaminium hexafluorophosphate N-oxide
-
- HOBt
-
- 1-hydroxybenzotriazole
-
- LC-MS
-
- liquid chromatography-mass spectrometry
-
- MALDI-TOF-MS
-
- matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
-
- MHB
-
- Mueller-Hinton broth
-
- MIC
-
- minimum inhibitory concentration
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
-
- NMP
-
- N-methyl-2-pyrrolidone
-
- Pbf
-
- 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulphonyl
-
- PBS
-
- phosphate-buffered saline
-
- RP-HPLC
-
- reversed-phase high-performance liquid chromatography
-
- TBTU
-
- N-[(1H-benzotriazol-1-yl) (dimethylaminomethylamino)methylene]-N-methylmethanaminium tetrafluoro-borate N-oxide
-
- TFA
-
- trifluoroacetic acid
-
- TFE
-
- trifluoroethanol
-
- TIS
-
- triisopropylsilane
-
- Trt
-
- triphenylmethyl. All amino acids are of the l-configuration and all solvent ratios and percentages are volume/volume unless stated otherwise
Introduction
Antimicrobial peptides are produced by animals, plants, insects and bacteria and are an important part of innate immunity (1,2). Numerous studies have demonstrated their key role in the first line of defence against invading pathogens (3–5). Antimicrobial peptides are active against a wide range of pathogenic microorganisms (6), including viruses, Gram-positive and Gram-negative bacteria, protozoa, yeasts, and fungi, as well as cancer cells. Currently, more than 800 naturally occurring antimicrobial peptides are known (7). For recent reviews see Zasloff (8), Lohner (9), Andreu (10), Hancock (11), Van't Hoff (12) and Dutton (13).
Antimicrobial peptides are very diverse in length, overall charge and conformation, but a large majority of them are cationic and amphipathic (14,15). They are generally short, 10–40 amino acids in length, and can be arbitrarily categorized according to their secondary structure as: α-helices, β-sheet, extended helices and loops.
The majority of antimicrobial peptides are toxic to bacteria but not erythrocytes (16). This selectivity is due to the difference in organization of bacterial membranes and membranes of multicellular organisms (17). The lipid bilayer of bacteria consists of a hydrophobic core and a hydrophilic outer region composed of negatively charged phospholipid head groups. The outer lipid bilayer region of erythrocytes is zwitterionic with the negatively charged phospholipid groups facing the cytoplasm. Generally speaking, antimicrobial peptides are believed to disrupt the bacterial membrane (18–21). However, the exact mechanism by which antimicrobial peptides kill bacteria is not yet clearly understood (22,23).
Indolicidin, ILPWKWPWWPWRR-NH2, was first isolated from cytoplasmic granules of bovine neutrophils (24). This peptide belongs to the cathelicidin family (25) of antimicrobial peptides and its amino acid content is unique in the sense that it contains five tryptophans, representing 38% of the total amino acid content. This is the highest content of Trp of any known naturally occurring peptide. The peptide is active against Gram-positive and Gram-negative bacteria (26), protozoa (27), fungi (28) and HIV-1 (29). However, indolicidin has also been shown to be toxic towards human T lymphocytes and to lyse erythrocytes (30). Indolicidin does not adopt an α-helix or β-sheet structure when bound to membranes but rather an extended structure (31).
Sequence modification and structure–activity studies of indolicidin have been reported (32–35). Uchida and Shindo (32) prepared deletion analogues of indolicidin, lacking only the amide function, which displayed less inhibitory effect against Gram-positive and Gram-negative bacteria than indolicidin itself. In addition, fragment 1–11 showed no activity, indicating that the C-terminus, especially Arg-Arg-NH2 is important for activity. Fragments 8–13 and 6–13, still showed significant activity against the tested bacteria.
The antibacterial activity of several analogues of indolicidin were reported by Staubitz et al. (33). These studies included Ala-scan, retro-, inverso- and retro-inverso analogues. The single Ala-replacement analogues that had the lowest activity were, [Ala5]- and [Ala12]-indolicidin, indicating the importance of the position. The retro,- inverso- and retro-inverso analogues essentially displayed the same activity as the parent indolicidin. This is in agreement with the general belief that the mechanism of antimicrobial peptides is not receptor based. Ösapay and co-workers (36) identified an artefact obtained using standard cleavage conditions. They synthesized this by-product containing a single Trp-Trp cross-link. This analogue was more stable to digestion with trypsin and chymotrypsin than the parent peptide and displayed antibacterial activity in the same range as indolicidin.
Structure–activity studies have shown that the haemolytic activity of indolicidin is closely related with the tryptophan residues of the peptide (37). An indolicidin analogue in which the five tryptophan residues were replaced with phenylalanine, [Phe4,6,8,9,11] indolicidin, displays an antibacterial activity in the range of indolicidin, but is significantly less haemolytic and less hydrophobic (38). Similarly, potent indolicidin analogues with low-haemolytic activity, including [Lys4,6,8,9,11] indolicidin, were reported by Kolodkin et al. (39).
The aforementioned examples suggest that synthetic indolicidin analogues could lead to new antimicrobial peptides with promising characteristics, and prompted us to investigate alternative analogues of indolicidin. Replacing natural amino acids with non-natural building blocks in peptides has previously proven to be a successful strategy (40). An example is the N-substituted glycine building block (peptoid), which displays antibacterial activity (41–43). Alternatively, the use of nonproteinogenic amino acids has proven useful by resisting proteolysis (44).
In the present paper, we first address the importance of tryptophan in the antibacterial activity of indolicidin. We synthesized five peptide analogues with the format ILPXKXPXXPXRR-NH2 in which Trp-residues 4,6,8,9,11 were substituted with X = a single type N-substituted glycine residue or nonproteinogenic amino acid, termed non-natural building blocks. The synthetic indolicidin analogues were tested for antibacterial activity against Staphylococcus aureus and Escherichia coli.
Secondly, we investigate a new principle for improving the antibacterial activity of small peptides by modifying the N-terminus with a non-natural building block. We synthesized 22 analogues of indolicidin and [Phe4,6,8,9,11] indolicidin, with non-natural building blocks coupled to the N-terminus. The analogues were purified and tested for antibacterial activity. Finally, the cytotoxicity of the indolicidin derivatives on mammalian cells was assessed using a haemolytic activity assay.
Materials and Methods
HPLC
Analytical high-performance liquid chromatography (HPLC) was performed using a Waters C18 reverse-phase column (Delta-Pak 100 Å, 15 μm; Millipore, Billerica, MA, USA) on a Waters 600E system equipped with Millennium software. Samples were chromatographed at a flow-rate of 1.5 mL/min starting with 0.1% aqueous trifluoroacetic acid (TFA) (buffer A) for 10 min and increasing over 45 min to 0.1% TFA in CH3CN/H2O (9 : 1) (buffer B), detection at 220 nm.
Preparative HPLC was carried out on a Waters C18 reverse-phase column (SymmetryPrepM, 7 μm; Millipore, Billerica, MA, USA). Samples were initially chromatographed at a flow-rate of 1 mL/min starting with buffer A/buffer B (9 : 1) for 10 min and increasing over 60 min to buffer A/buffer B (4 : 6) and finally to buffer B over an additional 8 min. Flow-rate was 4 mL/min, detection at 220 nm.
MALDI-TOF-MS and LC-MS
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was carried out on a VG Tof Spec E Fisons instrument (Fisons Instruments, Beverly, MA, USA), using α-cyano-p-hydroxycinnamic acid as matrix. Substance P and adrenocorticotropic hormone (ACTH) were used as calibrants.
Liquid chromatography-mass spectrometry (LC-MS) was carried out on a Bruker Esquire Mass Spectrometer (Bruker Daltonics, Billerica, MA, USA). The LC part consisted of a HP 1100 equipped with a Vydac C18 column (cat no. 238MS215) and a diode array detector. Samples were chromatographed at a flow-rate of 0.25 mL/min starting with 0.1% aqueous TFA (buffer A) and increasing over 25 min to 0.1% TFA in CH3CN/H2O (4 : 1) (buffer B), finally increasing to buffer B over 10 min, detection at 220 nm.
Amino acid analysis
Amino acid analysis was performed on a Waters PicoTag (45) analyzer (Waters, Milford, MA, USA), after samples were hydrolyzed with 6 m aqueous HCl and 0.1% phenol at 110 °C. The concentration of each antibacterial solution was determined by including a standard, α-aminobutanoic acid.
Materials
Sterile 96-well polypropylene plate was from COSTAR, Corning Incorporated (Corning, NY, USA); sterile 96-well polystyrene microtiter plates was from Nunc (Roskilde, Denmark); Mueller-Hinton broth was from Fluka (Buchs, Schwitzerland). Fresh sheep blood in Alsever's solution was obtained from Statens Serum Institute (Copenhagen, Denmark).
Chemicals
TentaGel S RAM resin (loading 0.23 meq/g) from RAPP Polymers (Tübingen, Germany), 1-hydroxybenzotriazole (HOBt) and protected amino acids were purchased from PerSeptive Biosystems (Hamburg, Germany), Novabiochem (Läufelfingen, Switzerland), and Bachem (Bubendorf, Switzerland), piperidine, anhydrous ampicillin, triisopropylsilane (TIS), N,N′-diisopropylcarbodiimide (DIPCI), dehydroabiethylamine, 4-(aminomethyl)pyridine, 1-naphthalenemethylamine, 5-chloro-o-anisidine, aminomethylcyclohexane, 4-aminodiphenylamine, benzylamine, isobutylamine, sec-butylamine, 9-fluorenylmethyloxycarbonyl (Fmoc)-3-(1-naphthyl)-Ala and Fmoc-3-(2-naphthyl)-Ala were obtained from Fluka, Fmoc-3,3-diphenylalanine was purchased from, Synthetech (Albany, NY, USA), α-aminobutanoic acid, 2,2-diphenylethylamine and 1-naphthalenemethylamine were from Aldrich (Steinheim, Germany), bovine serum albumin (BSA) and Triton X-100, ACTH and substance P were obtained from Sigma-Aldrich (St Louis, MO, USA), TFA from Merck (Schuchardt, Germany), dichloromethane (DCM) was from Riedel de Haën (Seelze, Germany). All starting chemicals were used without further purification.
Peptide synthesis
Synthesis of ILPWKWPWWPWRR-NH2 and ILPFKFPFFPFRR-NH2
Compounds 1 and 2 Indolicidin and [Phe4,6,8,9,11] indolicidin were synthesized on an Applied Biosystems 433A Peptide Synthesizer (Applied Biosystems, Foster City, CA, USA) using TentaGel S RAM resin (400 mg, 0.23 meq/g) and the Fmoc strategy (46). The following side chain protection for amino acid derivatives were used: 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl (Pbf) for Arg; tert-butyloxycarbonyl (Boc) for Trp and Lys. Protected amino acids were coupled in five-fold excess employing a Fastmoc protocol with N-[(1H-benzotriazol-1-yl) (dimethylaminomethylamino)methylene]-N-methylmethanaminium tetrafluoro-borate N-oxide (TBTU)/HOBt/N,N′-diisopropylethylamine (DIEA) (1 : 1 : 1.5) activation, and N-methyl-2-pyrrolidone (NMP) as solvent. Deprotection of the Fmoc group was effected with 20% piperidine in NMP.
Following synthesis, the peptide-resin was washed with NMP, EtOH and ether and dried in vacuo. The resin (50 mg) was treated with TFA/H2O/TIS (95 : 2.5 : 2.5, 2 mL) for 2 h, filtered, and washed with TFA/H2O (95 : 5, 2 mL). TFA was removed by evaporation and the product precipitated in ether. The product was washed with ether (3 × 2 mL), dried and lyophilized twice from 50% aqueous CH3CN.
Synthesis of indolicidin analogues-containing tryptophan replacements
Synthesis of indolicidin analogues with substitution of Trp with non-natural building blocks was accomplished manually, in a syringe equipped with a filter by a Fmoc stepwise solid-phase peptide synthesis procedure, and using a TentaGel S RAM resin (200 mg, 0.23 meq/g).
Synthesis of indolicidin analogues-containing tryptophan → naphthylalanine replacements
Compounds 3–4 Amino acids (4 eq) were coupled using DIPCI (4 eq) and HOBt (4 eq) for 30 min. The resin was washed, drained and a 1-h recoupling was performed. Following synthesis, the peptide resins were washed, dried and cleaved as described for compounds 1 and 2.
Synthesis of indolicidin analogues-containing tryptophan → peptoid replacements
Compounds 5–7 Amino acids Ile1, Leu2, Arg12 and Arg13 (4 eq) were coupled using DIPCI (4 eq) and HOBt (4 eq) for 30 min. The resin was washed, drained and a 1-h recoupling was performed. Amino acids Pro3, Lys5, Pro7, Pro10 (5 eq) were coupled using N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridino-1-yl-methylene]-N-methyl methanaminium hexafluorophosphate N-oxide (HATU) (5 eq) and DIEA (10 eq) in NMP for 2 h, followed by a 1 h recoupling step. The peptoid monomers replacing Trp4,6,8,9,11 were coupled as described below for compounds 8–21. Following synthesis the peptide-resins were washed, dried and cleaved as described for compounds 1 and 2.
Synthesis of indolicidin analogues modified at the N-terminus
Compounds 8–21 Following overnight swelling in NMP, the protected peptide-resin (50 mg) was bromoacetylated by adding 0.6 m bromoacetic acid (10 eq) and 3.2 m DIPCI (12.8 eq) both in NMP. The support was agitated for 30 min, drained and another portion of bromoacetic acid/DIPCI was added for yet another 30 min. After washing with NMP the side chain was introduced by nucleophilic substitution of the halide with a primary amine (40 eq) and agitated for 2 h. The resin was washed, dried, cleaved, lyophilized as described above.
Compounds 22–29 After overnight swelling of the resin in NMP, the non-natural building block (4 eq) was coupled to the peptide-resin (50 mg), using DIPCI (4 eq) and HOBt (4 eq) for 30 min. The resin was drained and the building block recoupled for 1 h. The resin was then washed with NMP, deprotected with 20% piperidine in NMP [except the resin with Lys(Fmoc)], and washed with NMP, EtOH and ether. Next, the resin was dried, cleaved, lyophilized as described above.
Purification and characterization of peptides
Following synthesis, the products were purified by preparative HPLC, lyophilized and the masses verified by MALDI-TOF-MS and/or LC-MS as described in the general section (see figure 2 for a representative HPLC and LC-MS chromatogram). Stock solutions of the peptides were dissolved in 1% dimethyl sulfoxide (DMSO) to a concentration of approximately 1 mg/mL. The exact concentration was determined by amino acid analysis. These stock solutions were used for antibacterial and haemolytic activity studies.
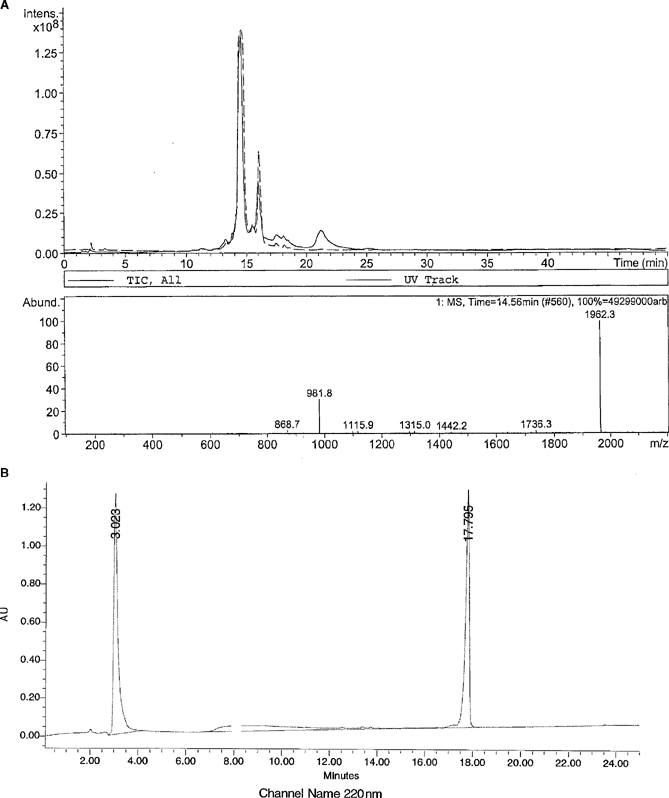
Panel A: crude liquid chromatography-mass spectrometry (LC-MS) of [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin. Panel B: analytical high-performance liquid chromatography (HPLC) of purified [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin.
Antibacterial activity
Strains used for determining antibacterial activity included the two American Type Culture Collection (ATCC) strains E. coli ATCC 25922 and S. aureus ATCC 25923.
The minimum inhibitory concentration (MIC) of each peptide was determined using a broth micro-dilution assay modified from the method of Hancock (47). Further dilutions of the stock solution to 10 times the required test concentration were made to reach a final concentration of 0.2% BSA and 0.01% acetic acid. Serial two-fold dilutions of the peptides were made in 0.2% BSA and 0.01% acetic acid in sterile 96-well polypropylene microtiter plates. To each well was added 100 μL of the test bacteria in Mueller-Hinton broth to a final concentration of 2 × 105 colony-forming units (CFU)/mL and 100 μL of the peptide in the different concentrations. Following the addition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (48), (10 μL, 3 mm), the MIC of each peptide was read as the lowest concentration of peptide that inhibited visible growth of the bacteria after 24 h incubation at 37 °C. All MIC determinations were performed in duplicate, and are the average of three independent determinations. Indolicidin and ampicillin were used as controls.
Haemolytic activity study
Fresh sheep blood in Alsever's solution was washed three times (1157 g) with a cold solution of 0.15 m phosphate-buffered saline (PBS). The erythrocytes were diluted to a final concentration of 0.5% in PBS. To each well of a polypropylene microtiter plate was added 75 μL of sheep erythrocytes in PBS and 75 μL of peptide solution (100 μm). The microtiter plates were allowed to incubate at 37 °C for 1 h and centrifuged 10 min at 4000 rpm. The supernatant (60 μL) of each well was then transferred to a polystyrene microtiter plate and the absorbance was read at 414 nm on an enzyme-linked immunosorbent assay (ELISA)-reader. PBS and 0.1% Triton X-100 were used as references. Melittin was used as positive control.
The haemolysis percentage was calculated as follows (49): [(Apeptide − APBS)/(ATriton − APBS)] × 100.
All haemolysis determinations were performed in duplicate and are the average of three independent determinations.
CD spectroscopy
Circular dichroism (CD) spectra were recorded at 25 °C on a Jasco J10 spectropolarimeter (Jasco, Tokyo, Japan). Peptides were dissolved to a final concentration of 50 μm in either 10 mm phosphate buffer pH 7.0 or 10 mm phosphate buffer pH 7.0 containing 50% trifluoroethanol (TFE). Scans between 280 and 195 nm were made in a 0.1 cm cell. Following baseline correction, the observed ellipticity θ (mdeg) was converted to the mean residue ellipticity [Θ] (deg cm2/dmol), using the relationship =100θ/(l c n), where l is the path length, c is the molar concentration and n the number of residues in the peptide.
Results and Discussion
Indolicidin is a small hydrophobic 13-residue antimicrobial peptide amide isolated from bovine neutrophils, which exhibits activity against a wide range of microorganisms. However, it is also toxic to eukaryotic cells. Indolicidin is special in the sense that it contains five tryptophans of a total of 13 amino acids. This is the highest content of tryptophan of any known naturally occurring peptide or protein.
Synthesis
Studying the role of tryptophan in the antibacterial activity of indolicidin
To investigate the role of tryptophan in the antibacterial activity of indolicidin, we prepared various peptide analogues, ILPXKXPXXPXRR-NH2 in which Trp-residues 4,6,8,9,11 were replaced in all positions by a single non-natural building block: X; 3-(1-naphthyl)-Ala 3, or 3-(2-naphthyl)-Ala 4, N-isobutyl-Gly 5; N-(sec-butyl)-Gly 6; N-benzyl-Gly 7 (structures are shown in Fig. 1). The chosen building blocks were selected to represent different size, aromaticity and hydrophobicity. Furthermore, the insertion of non-natural building blocks was anticipated to enhance the stability towards proteolysis (50). Synthesis of peptoid-peptide hybrids, compounds 5–7, proved to be more difficult than anticipated. The coupling of an Fmoc-protected amino acid onto an N-terminus peptoid building block was especially challenging. In our initial attempt all amino acids were coupled using standard DIPCI/HOBt chemistry. However, this resulted in a significant amount of deletion products, including des Pro3, des Lys5, des Pro7, and des Pro10 (results not shown). For coupling of an Fmoc-protected amino acid onto an N-terminus peptoid building block, we found that HATU (5 eq) and DIEA (10 eq) in NMP for 2 h, followed by a recoupling, gave satisfactory results.
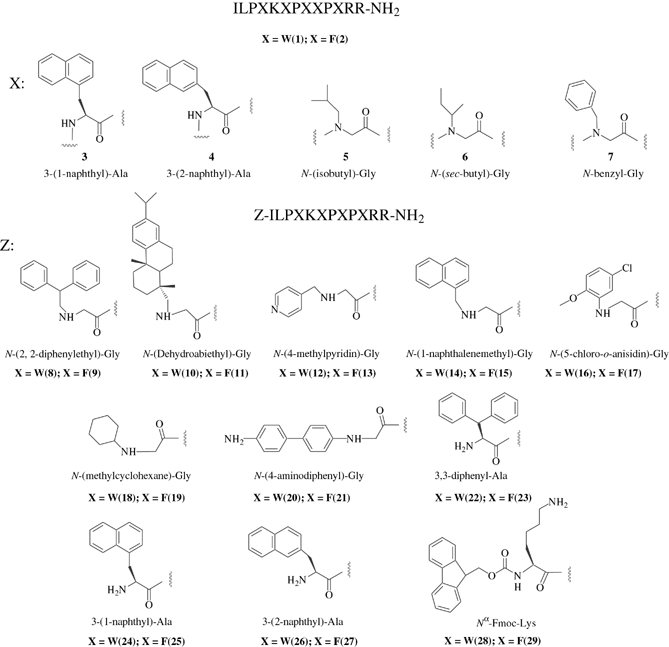
Indolicidin analogues containing tryptophan → non-natural building block replacements and N-derivatized indolicidin analogues used in this study.
Synthesis of non-natural building block-ILPFKFPFFPFRR-NH2 and the corresponding indolicidin derivatives
The analogue [Phe4,6,8,9,11] indolicidin compound 2, displays lower antibacterial activity than indolicidin, but is significantly less haemolytic (38), making this analogue an excellent lead structure.
We synthesized a series of peptides in which all Trp-residues were replaced by phenylalanine and the N-terminus capped by either a peptoid or nonproteinogenic amino acid. These analogues are written in the general format: non-natural building block-ILPFKFPFFPFRR-NH2, odd compounds 9–29 (Fig. 1). Nearly all of the building blocks used to cap the N-terminus are hydrophobic aromatic compounds. We anticipated that acylating the N-terminus with an aromatic building block would result in analogues that were hydrophobic enough to display higher antibacterial activity than [Phe4,6,8,9,11] indolicidin, but not hydrophobic enough to display significant haemolytic activity. Finally, for comparison we synthesized the corresponding indolicidin analogues: non-natural building block-ILPWKWPWWPWRR-NH2, even numbers 8–28 (Fig. 1). As above, these analogues of indolicidin have the N-terminus capped by a non-natural building block.
Most of the non-natural building blocks which were attached to the N-terminus of ILPWKWPWWPWRR-NH2 or ILPFKFPFFPFRR-NH2, gave a clean crude product following cleavage and lyophilization.
Antibacterial activity of ILPXKXPXXPXRR-NH2
Indolicidin and its 28 analogues were purified by preparative reversed-phase (RP)-HPLC, the molecular masses were verified by MALDI-TOF MS and tested for antibacterial activity against E. coli ATCC 25922 and S. aureus ATCC 25923. The results are shown in Table 1. Generally, indolicidin analogues with the format ILPXKXPXXPXRR-NH2, compounds 3–7 (Fig. 1), were more active against S. aureus than E. coli. The most active of the indolicidin analogues containing tryptophan → naphthylalanine substitutions was compound 4, [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin. This analogue was more active against E. coli and S. aureus than the parent indolicidin, with MIC values of 2.3 μg/mL (1.2 μm) against S. aureus and 4.6 μg/mL (2.3 μm) against E. coli. However, the related derivative [(3-(1-naphthyl)-Ala)4,6,8,9,11] 3 displayed the same activity as compound 4 against S. aureus, but was less active against E. coli with a MIC value of 9.3 μg/mL (4.6 μm). This may be explained by an unfavourable steric interaction with the membrane. We postulate that the 2-naphthyl ring is pushed further away from the extended peptide backbone when compared with the 1-naphthyl derivative, and enables the 2-naphthyl derivative to penetrate deeper into the outer membrane of E. coli. The aromatic side chain of both naphthyl derivatives is of similar size to that of tryptophan but possess no hydrogen-bonding ability. The finding that [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin is more active than indolicidin indicates that hydrogen bonding ability of tryptophan is not essential for antibacterial activity. This is in agreement with Haug and Svendsen (51) who investigated the role of tryptophan in the antibacterial activity of a 15-residue bovine lactoferricin peptide, FKCRRWQWRMKKLGA. They synthesized analogues in which both Trp were replaced with Phe, 2-(benzothien-3-yl)-Ala, 3-(1-naphthyl)-Ala, or 3-(2-naphthyl)-Ala, the latter being the most active.
| Sequence | Massa | Staphylococcus aureus b | Escherichia coli b | Haemolysis (%)c |
|---|---|---|---|---|
| 1 ILPWKWPWWPWRR-NH2 | 1906.31 (1907.2) | 3.8 (2.0) | 7.7 (4.0) | 19 |
| 2 ILPFKFPFFPFRR-NH2 | 1711.13 (1711.8) | 23.0 (13.4) | 23.0 (13.4) | 4 |
| 3 [(3-(1-naphthyl)-Ala)4,6,8,9,11] indolicidin | 1961.40 (1962.3) | 2.3 (1.2) | 9.3 (4.6) | 100 |
| 4 [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin | 1961.40 (1962.3) | 2.3 (1.2) | 4.6 (2.3) | 100 |
| 5 [(N-(isobutyl)-Gly)4,6,8,9,11] indolicidin | 1541.02 (1541.3) | >64 | >64 | ND |
| 6 [(N-(sec-butyl)-Gly)4,6,8,9,11] indolicidin | 1541.02 (1541.2) | >64 | >64 | 4 |
| 7 [(N-benzyl)-Gly4,6,8,9,11] indolicidin | 1711.1 (1712.1) | 35.8 (20.9) | 35.8 (20.9) | ND |
| 8 N-(2,2-diphenylethyl)-Gly-ILPWKWPWWPWRR-NH2 | 2143.61 (2144.1) | 4.1 (1.9) | 32.6 (15.2) | 56 |
| 9 N-(2,2-diphenylethyl)-Gly-ILPFKFPFFPFRR-NH2 | 1948.43 (1949.1) | 1.8 (0.9) | 7.0 (3.6) | 100 |
| 10 N-(dehydroabiethyl)-Gly-ILPWKWPWWPWRR-NH2 | 2231.80 (2232.8) | 7.7 (3.5) | 7.7 (3.5) | 100 |
| 11 N-(dehydroabiethyl)-Gly-ILPFKFPFFPFRR-NH2 | 2036.62 (2037.5) | 5.4 (2.7) | 10.9 (5.4) | 68 |
| 12 N-(4-methylpyridine)-Gly-ILPWKWPWWPWRR-NH2 | 2054.47 (2055.3) | 5.4 (2.6) | 21.4 (10.4) | 11 |
| 13 N-(4-methylpyridine)-Gly-ILPFKFPFFPFRR-NH2 | 1859.29 (1860.0) | 32.3 (17.4) | 32.3 (17.4) | 4 |
| 14 N-(1-naphthalenemethyl)-Gly-ILPWKWPWWPWRR-NH2 | 2103.55 (2104.1) | 2.6 (1.2) | 20.5 (9.7) | 84 |
| 15 N-(1-naphthalenemethyl)-Gly-ILPFKFPFFPFRR-NH2 | 1908.36 (1909.1) | 1.4 (0.7) | 5.7 (3.0) | 100 |
| 16 N-(5-chloro-o-anisidin)-Gly-ILPWKWPWWPWRR-NH2 | 2103.93 (2104.1) | 56.3 (26.8) | 56.3 (26.8) | 67 |
| 17 N-(5-chloro-o-anisidin)-Gly-ILPFKFPFFPFRR-NH2 | 1908.75 (1909.1) | 8.6 (4.5) | 34.6 (18.1) | 99 |
| 18 N-(methylcyclohexane)-Gly-ILPWKWPWWPWRR-NH2 | 2059.53 (2060.3) | 1.6 (0.8) | 6.5 (3.2) | 100 |
| 19 N-(methylcyclohexane)-Gly-ILPFKFPFFPFRR-NH2 | 1864.35 (1865.1) | 3.6 (1.9) | 14.4 (7.7) | 18 |
| 20 N-(4-aminodiphenyl)-Gly-ILPWKWPWWPWRR-NH2 | 2130.57 (2131.1) | 1.9 (0.9) | 7.7 (3.6) | 34 |
| 21 N-(4-aminodiphenyl)-Gly-ILPFKFPFFPFRR-NH2 | 1935.39 (1936.2) | 3.9 (2.0) | 15.7 (8.1) | 92 |
| 22 3,3-diphenyl-Ala-ILPWKWPWWPWRR-NH2 | 2129.58 (2130.1) | 2.6 (1.2) | 5.3 (2.5) | 52 |
| 23 3,3-diphenyl-Ala-ILPFKFPFFPFRR-NH2 | 1934.40 (1935.1) | 2.8 (1.4) | 5.6 (2.9) | 100 |
| 24 3-(1-naphthyl)-Ala-ILPWKWPWWPWRR-NH2 | 2103.55 (2104.1) | 3.0 (1.4) | 24.3 (11.6) | 60 |
| 25 3-(1-naphthyl)-Ala-ILPFKFPFFPFRR-NH2 | 1908.36 (1908.8) | 2.6 (1.4) | 5.2 (2.7) | 68 |
| 26 3-(2-naphthyl)-Ala-ILPWKWPWWPWRR-NH2 | 2103.55 (2104.1) | 2.5 (1.2) | 19.6 (9.3) | 39 |
| 27 3-(2-naphthyl)-Ala-ILPFKFPFFPFRR-NH2 | 1908.36 (1909.1) | 2.4 (1.3) | 9.4 (4.9) | 75 |
| 28 (Fmoc)-Lys-ILPWKWPWWPWRR-NH2 | 2256.73 (2258.0) | 0.5 (0.2) | 3.8 (1.7) | 98 |
| 29 (Fmoc)-Lys-ILPFKFPFFPFRR-NH2 | 2061.54 (2062.3) | 2.5 (1.2) | 2.5 (1.2) | 100 |
- a. Calculated and found mass in parentheses.
- b. Minimum inhibitory concentration (MIC) value in μg/mL and μm in parentheses.
- c. Percentage haemolysis of the indolicidin derivatives (50 μm) against sheep erythrocytes.
- ND, not determined.
The most active of the indolicidin analogues containing tryptophan → N-glycine derivative replacements, compounds 5–7, was compound 7, [(N-benzyl-Gly)4,6,8,9,11], with a MIC value of 35.8 μg/mL (20.9 μm) against both S. aureus and E. coli. The [(N-benzyl-Gly)4,6,8,9,11] derivative showed some activity but was still eight times less active than indolicidin. The corresponding [(N-isobutyl)-Gly)4,6,8,9,11] and [(N-(sec-butyl)-Gly)4,6,8,9,11] derivatives 5 and 6 did not display any significant antibacterial activity. These data show that all-tryptophan replacements with an aliphatic peptoid side chain result in loss of antimicrobial activity. Similar findings were reported by Staubitz et al. (33), while this work was in progress. They found that Trp4,6,8,9,11 replacement with the aliphatic amino acids Leu and Ile, ILPLKLPLLPLRR-NH2 and ILPIKIPIIPIRR-NH2, resulted in an approximately 63-fold reduction of antibacterial activity, against S. aureus Newman and E. coli DH5α, while generating only a fourfold reduction in haemolytic activity.
Antibacterial activity of non-natural building block-ILPFKFPFFPFRR-NH2
The work presented here clearly shows that it is possible to modify an antibacterial peptide to display higher activity by attaching a hydrophobic non-natural building block to the N-terminus of the peptide. Nearly all analogues of the format non-natural building block-ILPFKFPFFPFRR-NH2, odd compounds 9–29 (Fig. 1), displayed an antibacterial activity of 16 μg/mL or lower against S. aureus and 23 μg/mL or lower against E. coli (Table 1). The results are significantly better than the parent compound [Phe4,6,8,9,11] indolicidin which showed MIC values of 23 μg/mL (13.4 μm) against both S. aureus and E. coli. Previously reported (33) MIC values of this analogue are 32 μg/mL against S. aureus Newman and 64 μg/mL against E. coli DH5α. Subbalakshmi et al. reported (37) an activity of 2–4 μg/mL against S. aureus ATCC 8530 and 25–30 μg/mL against E. coli W160 37.
In addition, we found that of 11 [Phe4,6,8,9,11] indolicidin derivatives, 9, 15, 23, 25, 29, showed more than a three-fold improvement in the MIC-value against S. aureus and E. coli, when compared with ILPFKFPFFPFRR-NH2.
The most active compounds against S. aureus were the [Phe4,6,8,9,11] indolicidin analogues containing N-(2,2-diphenylethyl)-Gly (9, 1.8 μg/mL; 0.9 μm), N-(1-naphthalenemethyl)-Gly (15, 1.4 μg/mL; 0.7 μm), 3,3-diphenyl-Ala (23, 2.8 μg/mL; 1.4 μm), 3-(1-naphthyl)-Ala (25, 2.6 μg/mL; 1.4 μm) and 3-(2-naphthyl)-Ala (27, 2.4 μg/mL; 1.3 μm). These compounds were also active against E. coli, but to a lesser extent: N-(2,2-diphenylethyl)-Gly (9, 7.0 μg/mL; 3.6 μm), N-(1-naphthalenemethylamine)-Gly (15, 5.7 μg/mL; 3.0 μm), 3,3-diphenyl-Ala (23, 5.6 μg/mL; 2.9 μm), 3-(1-naphthyl)-Ala (25, 5.2 μg/mL; 2.7 μm) and 3-(2-naphthyl)-Ala (27, 9.4 μg/mL; 4.9 μm).
Moreover, we found (α-Fmoc-ɛ-Lys)-ILPFKFPFFPFRR-NH2, 29, to be very active against both bacterial strains used in this study. The analogue displayed MIC values of 1.2 μm (2.5 μg/mL) against both S. aureus and E. coli. The activity against E. coli is especially interesting, as Houghten and co-workers (52) identified four series of Fmoc N-derivatized antimicrobial tetrapeptides, (α-Fmoc-ɛ-Lys)WfI-NH2, (α-Fmoc-ɛ-Lys)WKW-NH2, (α-Fmoc-ɛ-Lys)WYr-NH2, and (α-Fmoc-ɛ-Lys)cir-NH2, which were active against Gram-positive and Gram-negative bacteria. The MIC values were in the range of 4–8 μg/mL and 32–62 μg/mL for S. aureus and E. coli, respectively. In addition, the tetrapeptides also displayed activity against methicillin-resistant S. aureus, in the range 32–124 μg/mL. Dehydroabiethylamine, compound 11, showed MIC values of 5.4 μg/mL, (2.7 μm) against S. aureus and 10.9 μg/mL (5.4 μm) against E. coli. This is an interesting result since, Goodson et al. (42) found that dehydroabiethylamine, incorporated in peptoid trimers or by itself is active against Gram-positive but not Gram-negative bacteria.
The fact that both the Fmoc Nα-derivatized compound 29 and the N-(dehydroabiethyl)-Gly-[Phe4,6,8,9,11] indolicidin, compound 11, are significantly more active against E. coli, than the previously reported Fmoc-Nα-tetrapeptides (52) and peptoid trimers (42), indicate that the additional length, and positive charges of the indolicidin analogue are important for the antibacterial activity.
There seems to be a general trend that the antibacterial activity of the [Phe4,6,8,9,11] indolicidin derivatives correlates with the aromaticity of the non-natural building block, the most active being Fmoc-α-Lys, N-(1-naphthalenemethyl)-Gly, N-(2,2-diphenylethyl)-Gly. However, an exception is the non-aromatic compound, N-(methylcyclohexane)-Gly 19, which was active against both bacterial strains, with MIC values of 3.6 μg/mL (1.9 μm) and 14.4 μg/mL (7.7 μm) against S. aureus and E. coli, respectively.
To explain our results, we speculate that the derivatized hydrophobic N-terminus of these analogues interact more favourably with the membrane, enabling the N-capped analogue to disrupt the membrane to a greater extent than the parent peptide. However, in order to clarify this, further studies of peptide to membrane interactions and orientation within the lipid environment are needed, e.g. attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (53).
Our findings that attachment of non-natural building blocks to the N-terminus of indolicidin and [Phe4,6,8,9,11] indolicidin result in conjugates with potent antibacterial activity, are in agreement with a study published recently by Avrahami and Shai (54). They reported that the antimicrobial peptide magainin 2, could be modified with lipophilic acids, such as undecanoic acid and palmitic acid, to display antifungal activity. Furthermore, using CD spectroscopy, tryptophan fluorescence and analytical ultracentrifugation, the authors showed that these modifications result in well defined changes in hydrophobicity, secondary structure and self-association. However, Staubitz and co-workers (33) reported that the acetylated analogue of indolicidin showed a two-fold reduction in biological activity against the tested pathogenic factor rendering strain.
Antibacterial activity of non-natural building block-ILPWKWPWWPWRR-NH2
The majority of the non-natural building block-ILPWKWPWWPWRR-NH2 compounds (even compounds 8–28, Fig. 1), displayed an antibacterial activity of 10 μg/mL or lower against S. aureus and 32 μg/mL or lower against E. coli. Eight of 11 indolicidin derivatives, 8, 14, 18, 20, 22, 24, 26, 28 displayed an improved MIC towards S. aureus and five compounds out of 11, 10, 18, 20, 22, 28, showed higher activity towards Gram-negative bacteria, when compared with indolicidin. The most active derivatives against both bacterial strains used in this study were: Fmoc-α-Lys (28, S. aureus: 0.5 μg/mL, 0.2 μm; E. coli: 3.8 μg/mL, 1.7 μm), and N-(cyclohexylethyl)-Gly (18, S. aureus: 1.6 μg/mL, 0.8 μm; E. coli: 6.5 μg/mL, 3.2 μm). This is in the same range as indolicidin which in our study displayed MIC values of 3.8 μg/mL (2.0 μm) and 7.7 μg/mL (4.0 μm) against S. aureus and E. coli, respectively. The only indolicidin derivative showing higher MIC values against the tested bacterial strains was the derivative containing, 5-chloro-o-anisidine, 16, which in both cases displayed a MIC value of 56.3 μg/mL (26.8 μm). This derivative has both lipophilic and polar properties, and the chlorine atom and methoxy group clearly does not favour hydrophobic interaction between the N-terminus of the peptide and the bacterial membrane.
Antibacterial activity of non-natural building block-ILPFKFPFFPFRR-NH2 vs. non-natural building block -ILPWKWPWWPWRR-NH2
The activity against S. aureus and E. coli of non-natural building block-ILPFKFPFFPFRR-NH2 compounds vs. the corresponding indolicidin derivatives may be summarized as follows: four of the [Phe4,6,8,9,11] derivatives, 9, 11, 15, 17 were more active towards S. aureus than the corresponding indolicidin compounds, three displayed nearly the same activity (22 and 23, 24 and 25, 26 and 27) and four indolicidin compounds were more active (12, 18, 20, 28), than the [Phe4,6,8,9,11] derivatives. Six of the [Phe4,6,8,9,11] derivatives 9, 15, 17, 25, 27, 29, were more active against E. coli than the corresponding indolicidin compounds. Two derivatives 22, 23 showed a difference <0.2 μg/mL. Four indolicidin compounds displayed higher activity against E. coli than the corresponding [Phe4,6,8,9,11] derivative 10, 12, 18, 20. In three cases, 4-aminomethylpyridine 12, aminomethylcyclohexane 18, and 4-aminodiphenylamine 20, we found that the indolicidin-derivative was more active than the corresponding [Phe4,6,8,9,11] compound, against both strains. Similarly, three [Phe4,6,8,9,11] derivatives displayed the highest activity against both strains: 2,2-diphenylamine (9), 1-naphthalenemethylamine 15, 5-chloro-o-anisidine 17. The fact that the indolicidin and [Phe4,6,8,9,11] derivatives are more active against S. aureus than E. coli, is in agreement with other studies of antimicrobial peptides (8–13). The reason for this is probably related to the fact that Gram-positive bacterial membranes, e.g. S. aureus, consist of a single bilayer membrane and a peptidoglycan-teichoic acid network, which gives the bacteria an overall negatively charged surface. On the contrary, Gram-negative bacteria, e.g. E. coli, consist of an inner and outer membrane with an intervening layer of peptidoglycan in the periplasmic space. The outer membrane consists mainly of lipopolysaccharides, resulting in a negatively charged surface (55).
Haemolytic activity study
The cytotoxicity of the indolicidin derivatives towards mammalian cells was assessed by measuring the percentage haemolysis against sheep erythrocytes at a peptide concentration of 50 μm. This concentration was found to be suitable, since most of the compounds turned out to be equally or more haemolytic than indolicidin. Zero haemolysis and 100% haemolysis were determined by using PBS and 0.1% Triton X-100, respectively. We used the strongly cytotoxic bee venom melittin as positive control. As shown in Table 1, indolicidin and [Phe4,6,8,9,11] indolicidin display a haemolysis percentage of 19% and 4%, respectively. Subbalakshmi and co-workers (37) tested haemolysis of rat erythrocytes and found a haemolysis percentage at 50 μm of 95% and 20%, respectively. Ahmad and co-workers (56) reported the haemolysis percentage of indolicidin towards human red blood cells to be 50% at 50 μm. Clearly, the haemolytic activity of the same peptide may be very different towards various mammalian erythrocytes (57).
Most of the indolicidin analogues turned out to be haemolytic in our assay. The two [(3-(naphthyl)-Ala)4,6,8,9,11] indolicidin derivatives, 3 and 4, lyse sheep erythrocytes completely at 50 μm. Three of the N-capped indolicidin derivatives, 10, 18, 28 showed 100% haemolysis and five derivatives, 8, 14, 16, 22, 24, displayed an activity between 50 and 92%. Two analogues, 20 and 26, were in the range of 34% and 39%, respectively. Only one indolicidin derivative, 12, showed a haemolytic activity below the parent compound, with 11% haemolysis.
The [Phe4,6,8,9,11] indolicidin compounds, 9, 15, 17, 23, 29 showed a haemolytic activity of approximately 100%. Four [Phe4,6,8,9,11] indolicidin derivatives 11, 21, 25 and 27 displayed an activity between 50 and 92%. Only two [Phe4,6,8,9,11] indolicidin derivatives, 13 and 19, showed a haemolytic activity below indolicidin. Compound 19, N-(methylcyclohexane)-Gly-[Phe4,6,8,9,11] indolicidin, is the most interesting of the [Phe4,6,8,9,11] derivatives with haemolytic activity of 18%, which is better than indolicidin although higher than [Phe4,6,8,9,11] indolicidin. Yet, we were able to improve the MIC-value from 23.0 μg/mL against both strains to 3.6 and 14.7 μg/mL, against S. aureus and E. coli, respectively.
The data presented here suggest that the increased haemolytic activity of the modified peptides correlate with the hydrophobicity of the building blocks used to modify the lead structures. It is certainly possible to improve the MIC-value of indolicidin and [Phe4,6,8,9,11] indolicidin by all-tryptophan replacements or modifying the N-terminus, but difficult to dissociate the haemolytic activity from the antibacterial activity in the resulting compound.
CD spectroscopy
The CD spectra of compounds 1, 2, 4, 6, 18, and 19 in aqueous buffer and TFE/buffer are shown in Fig. 3A–F. The CD spectra of indolicidin and [Phe4,6,8,9,11] indolicidin (Fig. 3A,B) are similar to those previously reported (37,58). In aqueous buffer, indolicidin has a strong negative band at 200 nm. This is also present in TFE/buffer with additional weak positive bands at 230 nm. The curve intercepts the x-axis at approximately 225 nm. The CD spectra of [Phe4,6,8,9,11] indolicidin in aqueous buffer partly resemble that of indolicidin. In TFE/aqueous buffer, 2 has an additional negative band at 230 nm. The CD spectrum of compound 4 has a different pattern from other analogues. In aqueous buffer, 4 has a very weak negative band centred at 215 nm that does not intercept zero level of ellipticity. However, in the membrane-like environment TFE/buffer, 4 has a strong negative band at 213 nm, a strong positive band at 224 nm, followed by a weak negative band at 230 nm. This suggests that 4 changes conformation upon insertion into membranes and may account for its antibacterial activity. The spectral characteristics of 4 in TFE/buffer are neither consistent with an α-helix nor β-sheet conformation. Both have a strong positive band at approximately 200 nm and a negative band in the vicinity of 220 nm. In both solvent systems, compound 6 shows a negative band centred at approximately 200 nm with a weak positive band at 223 nm. These characteristics are also found in the reported CD spectra of Poly-l-Pro II helix (59). However, the curve of 6 intercepts with the x-axis at 214 nm, while a poly-l-Pro II helix intercepts at approximately 225 nm.
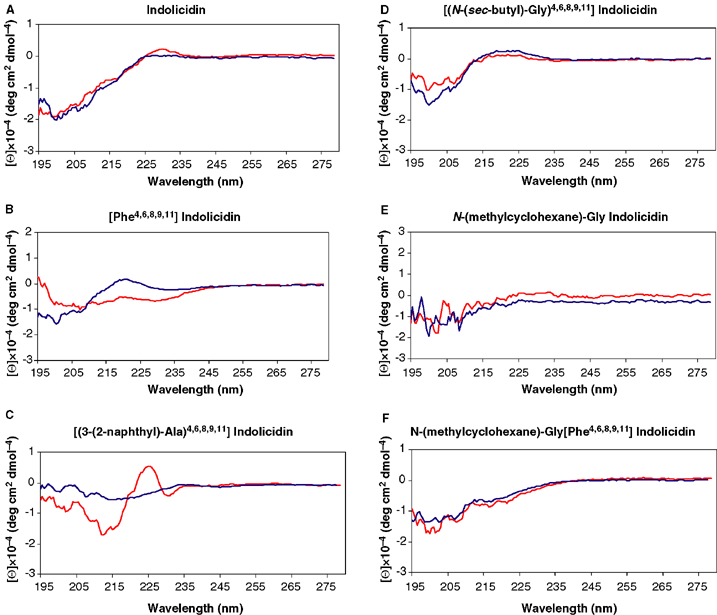
Circular dichroisms (CDs) of compounds of ILPWKWPWWPWRR-NH2 (A), ILPFKFPFFPFRR-NH2 (B), [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin (C), [(N-(sec-butyl)-Gly)4,6,8,9,11] indolicidin (D), N-(methylcyclohexane)-Gly-indolicidin (E), and N-(methylcyclohexane)-Gly-[Phe4,6,8,9,11] indolicidin (F) in phosphate buffer pH 7.0 (blue) and trifluoroethanol (TFE)/phosphate buffer pH 7.0 (red).
Compound 18 has, in both solvents, a very weak negative band around 205 nm as the only characteristic. The CD spectra of 19 in TFE/buffer has a negative band centred at 200 nm and intercepts zero level of ellipticity at 240 nm. This spectra has the shape of a random coil conformation, although it is displaced.
In agreement with previous reports (57), we were not able to correlate activity to structure using CD spectroscopy, since aromatic residues may absorb in the range between 195 and 250 nm. However, it appears that none of the investigated peptides have a defined secondary structure.
Conclusion
In the present paper, we demonstrated that tryptophan is not essential in the antibacterial activity of indolicidin, despite having five tryptophans of a total of 13 amino acids. We synthesized five peptide analogues with the format ILPXKXPXXPXRR-NH2 in which Trp-residues 4,6,8,9,11 were replaced by X = a single type N-substituted glycine residue or non-natural building block. The MIC for the indolicidin analogues was tested for antibacterial activity against S. aureus and E. coli. The most active analogue was [(3-(2-naphthyl)-Ala)4,6,8,9,11] indolicidin, which was more active against S. aureus and E. coli than the parent indolicidin, with MIC values of 2.3 μg/mL (1.2 μm) and 4.6 μg/mL (2.3 μm), respectively. In addition, we investigated a new principle for improving the antibacterial activity of small antimicrobial peptides. We synthesized 22 analogues of indolicidin carrying a non-natural building block attached to the N-terminus, and tested for antibacterial activity. Five of 11 [Phe4,6,8,9,11] indolicidin derivatives showed more than a three-fold improvement in the MIC-value against S. aureus and E. coli, when compared with ILPFKFPFFPFRR-NH2. Eight of 11 indolicidin derivatives displayed an improved MIC towards S. aureus and five compounds of 11 showed higher activity towards E. coli, when compared with indolicidin, although most of the indolicidin derivatives turned out to be haemolytic towards sheep erythrocytes. Therefore, we conclude that it is generally difficult, but not impossible, to dissociate the antibacterial activity from the haemolytic activity. Thus, we were able to improve the MIC-value of [Phe4,6,8,9,11] indolicidin from 23.0 μg/mL (13.4 μm) against both strains to 3.6 (1.9 μm) for S. aureus and 14.7 μg/mL (7.7 μm) for E. coli by modifying the N-terminus with N-(methylcyclohexane)-Gly. This resulted in a haemolytic activity increase from 4 to 18%.
We are pursuing related studies in which other parameters influencing antibacterial and haemolytic activity are investigated. These parameters include rearrangement/deletion of hydrophobic areas, amphipathicity and stereoisomerism.
In conclusion, our results suggest that modified analogues of antibacterial peptides, containing non-natural building blocks, are promising lead structures for developing future therapeutics.
Acknowledgments
Acknowledgements: Authors thank Ms Jette Petersen, Charlotte D. Sørensen and Ms Karen Jørgensen for skillful technical assistance. Furthermore, authors thank Professor Arne Holm for help in providing us with the financial support for this study and Dr Anne Gravesen for helpful discussions. Supported by the Danish Research Council grant number 9900234, Augustinus Foundation, Frimodt-Heineke Foundation, Familien Hede Nielsen Foundation and Simon Fougner Hartmanns Foundation, and Dana Lim are greatly acknowledged.




