Effectiveness of low dose immunotherapy in the treatment of canine atopic dermatitis: a prospective, double-blinded, clinical study
Information in the text was presented as a free communication at the Fifth World Congress of Veterinary Dermatology, Vienna 2004.
SC was supported by the British Small Animal Veterinary Association's Petsavers.
Abstract
Abstract There are anecdotal reports of increased effectiveness of allergen-specific immunotherapy (ASIT) in dogs with doses of vaccine lower than that recommended by the manufacturers. However, no controlled studies have been carried out. The aim of this prospective, double-blinded study was to evaluate whether induction and maintenance with low dose (LD) ASIT resulted in a different success rate compared with the standard dose (SD). Twenty-seven dogs with confirmed atopic dermatitis were allocated by block randomization to two groups. One group (n = 13) received SD ASIT; the other group (n = 14) received LD ASIT (1/10 of the SD) following the same frequency protocol. Cases were graded at 0, 3, 6 and 9 months for clinical signs using a modified canine atopic dermatitis extent and severity index (mCADESI) and for pruritus using a 0–5 descriptor scale. There were no significant differences between the groups in the pruritus and mCADESI scores (P > 0.155) at the end of the study, and the changes in pruritus (P > 0.920) and mCADESI (P > 0.296) scores from the beginning to the end of the study were similar in both groups. Pruritus scores in both groups did not change during the study (P > 0.052). However, significant reductions in mCADESI scores were seen in both groups (P < 0.032). Six dogs achieved a final pruritus score of 0, six achieved a reduction in pruritus score and 15 did not improve or worsened. There was, therefore, no evidence that LD ASIT is more effective than the standard protocol.
INTRODUCTION
Canine atopic dermatitis (AD) has been recently defined as a genetically predisposed, inflammatory and pruritic allergic skin disease with characteristic clinical features, and is most commonly associated with immunoglobulins of the E class (IgE) to environmental allergens.1 AD is a very common pruritic skin disease, believed to affect approximately 10% of the canine population.2,3 The diagnosis of AD is based on a combination of epidemiological, clinical and diagnostic findings and on ruling out other conditions that may present with similar clinical signs.4–6 Diagnostic tests such as intradermal testing (IDT) or allergen-specific IgE serology (ASIgES) are commonly used to select the relevant allergens for allergen-specific immunotherapy (ASIT).6
Allergen-specific immunotherapy (ASIT) is defined as the practice of administering gradually increasing quantities of an allergen extract to an allergic subject to ameliorate the symptoms associated with subsequent exposure to the causative allergen, and represents one of the main treatments for canine AD.1,7 The effectiveness of ASIT has recently been reviewed by Griffin and Hillier.7 When effectiveness is defined as at least 50% improvement of the clinical signs after more than 4 months, it appears to be between 50 and 100% effective according to numerous published studies8–18 and meeting abstracts.19–22 However, different inclusion criteria, diagnostic tests, type of allergens (aqueous vs. alum-precipitated) and concentrations, administration protocols and assessment criteria have been used in different studies, making the comparison of results extremely difficult.7 Two recent retrospective studies defining effectiveness as control of clinical signs of AD without requiring administration of antipruritic drugs have reported good results in approximately 20% of dogs, with aqueous or alum-precipitated ASIT.23,24 A small number of open studies have also described the use of higher or lower doses of ASIT, either from the beginning of the treatment or in the maintenance stage only, and all protocols seemed to be effective.25,27
The present study was designed to test the hypothesis that induction and maintenance with a lower dose of ASIT would result in a different success rate compared to induction and maintenance following the standard protocol. Since all dogs were treated with ASIT, albeit with different doses, effectiveness of ASIT as a treatment for canine AD was also evaluated. The term ‘effectiveness’ has been chosen because it implies assessing a treatment in the field, while ‘efficacy’ refers to evaluating a treatment under ideal conditions.
MATERIALS AND METHODS
The study was designed as a double-blinded, prospective, randomized, clinical trial. All dogs entered the study with the owners’ informed consent.
Inclusion criteria
Dogs with clinical signs and laboratory and other diagnostic tests consistent with previously reported diagnostic criteria for AD4,5 were included. Ectoparasitic diseases were ruled out based on negative results of multiple skin scrapings, coat brushings and serological testing (IgG Enzyme-linked immunosorbent assay [ELISA] for Sarcoptes scabiei) where necessary. All dogs underwent a food trial of 6 weeks’ duration using a novel protein home-cooked or commercial diet with subsequent provocative testing before starting ASIT. All dogs had positive results on either IDT performed according to a published protocol28 or ASIgES (Fc∈ R1α-based ELISA, Heska®, Fribourg, Switzerland).
ASIT treatment groups and patient randomization
An alum-precipitated vaccine (Artuvetrin® Therapy, or Artuvetrin® Therapy Forte, Artu, Holland), containing a mixture of allergens specific for each dog and formulated based on the results of IDT or ASIgES, was used in all cases. This vaccine is widely used in Europe and its use has previously been reported.9 The number of allergens in each vial varied. Artuvetrin® Therapy contained one to four allergens, with a final concentration of 10–2.5% v/v for each allergen. Artuvetrin® Therapy Forte contained five to eight allergens, with a final concentration of 5–2.5% v/v for each allergen. The manufacturer does not provide any further information regarding allergen concentration in these vaccines.
The dogs were allocated to two treatment groups by block randomization. The control group (standard dose group, SD group) consisted of dogs receiving ASIT following the standard protocol as indicated by the manufacturer (Table 1). The experimental group (low dose group, LD group) consisted of dogs treated with ASIT but receiving 0.1 mL only of the vaccine at the same standard intervals (Table 1). Dogs with multiple sensitivities requiring two different vaccines (vial 1 and vial 2) were treated as follows: dogs on the LD schedule received 0.1 mL of each vaccine and dogs on the SD schedule received 1 mL of each vaccine. A staff member of the Dermatology Unit or a veterinary nurse (nonblinded investigators) administered the ASIT injections. Both the main investigator and the owner were unaware of the dose of ASIT administered.
| Low dose | Standard dose | ||
|---|---|---|---|
| Week | Dose | Week | Dose |
| 0 | 0.1 mL | 0 | 0.2 mL |
| 2 | 0.1 mL | 2 | 0.4 mL |
| 4 | 0.1 mL | 4 | 0.6 mL |
| 6 | 0.1 mL | 6 | 0.8 mL |
| 9 | 0.1 mL | 9 | 1.0 mL |
| 12 | 0.1 mL | 12 | 1.0 mL |
| 16 | 0.1 mL | 16 | 1.0 mL |
| Every 4 weeks | 0.1 mL | Every 4 weeks | 1.0 mL |
Concurrent treatments
Ectoparasite control measures with selamectin (Stronghold® Spot-on, Pfizer Animal Health, Sandwich, Kent, UK), twice at 1-month intervals before and subsequently monthly during the study, were instituted in all dogs regardless of negative results of diagnostic tests and to prevent flea or other infestations during the study. Secondary infections (superficial pyoderma, Malassezia dermatitis and Malassezia/bacterial otitis externa) were treated, if present, before and throughout the study. Glucocorticoid therapy was allowed in severely pruritic dogs, with oral prednisolone at the lowest possible anti-inflammatory dose (0.5–0.75 mg kg−1 daily). Glucocorticoid administration was discontinued 6 months into the study and was not allowed over the last 3 months in order to obtain objective final clinical scores.
Exclusion criteria
Dogs were withdrawn from the study if there was unacceptable discomfort in the opinion of the owner or the veterinary surgeon, unacceptable adverse effects of immunotherapy or poor owner compliance. Dogs were withdrawn if they required oral prednisolone to control their symptoms during the last 3 months of the study.
Clinical assessment
Dogs were examined and scored for both cutaneous lesions and pruritus on the day ASIT was started (day 0), and at three (day 90), six (day 180) and nine (day 270) months afterwards. Dogs were scored for clinical lesions following a modification of the Canine Atopic Dermatitis Extent and Severity Index (CADESI).29 Dogs were scored for erythema, hyperpigmentation, lichenification, papular/pustular eruption and greasy seborrhoea on 36 different body sites. The lesions were scored on a scale of 0–3 (0: none, 1: mild, 2: moderate, 3: severe). A scoring system for pruritus with descriptors based on modifications of normal behaviour of the dog was specifically developed for the present study (Table 2). The pruritus score was carried out by the owners at each examination.
| Score | Descriptor |
|---|---|
| Grade 0 | Normal dog: the dog does not itch more than before the disease began. |
| Grade 1 | Occasional episodes of itching (small increase in itch compared with before the disease began). |
| Grade 2 | More frequent episodes of itching, but the itching stops when the dog is sleeping, eating, playing or exercising, or is otherwise distracted. |
| Grade 3 | Regular episodes of itching are seen when the dog is awake. The dog occasionally wakes up because of itching, but the itching stops when the dog is eating, playing, exercising, or is otherwise distracted. |
| Grade 4 | Prolonged episodes of itching are seen when the dog is awake. The dog regularly wakes up because of itching, or itches in its sleep. The itching can also be seen when the dog is eating, playing, or exercising, or is otherwise distracted. |
| Grade 5 | Almost continuous itching, which does not stop when the dog is distracted, even in the consulting room (the dog needs to be physically restrained from itching). |
Overall effectiveness of ASIT
Complete response to ASIT was defined as reaching a pruritus score of 0 at the end of the study. Partial response to ASIT was defined as achieving a lower pruritus score on day 270 but not reaching a score of 0. No response to ASIT was defined as having the same or a higher pruritus score at the end of the study compared to day 0.
Statistical analyses
Standard one-way analyses of variance using F-tests were carried out to investigate whether there were differences between the LD and SD groups at the beginning of the study in age, bodyweight, or interval between the onset of clinical signs suggestive of AD and the starting of ASIT. Fisher's exact test was performed to test the association between the two groups and sex and to test differences in the proportions of dogs withdrawn from each group in the course of the study.
To assess the change in pruritus and mCADESI scores, the end point was defined in two ways. Firstly, only those dogs that completed the study were considered (per protocol analysis). Secondly, those dogs that were withdrawn were added to the complete set, with their last value prior to withdrawal included as the end point (intention-to-treat analysis).30 In all the subsequent analyses, the tests were carried out on both per protocol and intention-to-treat sets. The results for both sets are indicated only when they differed. To evaluate whether the pruritus and mCADESI scores for each dose group separately had changed between the start and the end of the study, Wilcoxon signed rank tests for paired samples (Wp) were performed. Whether the change from beginning to end was different between the LD and SD groups was compared by taking the differences in the rank scores between the start and end and analysing the two groups by the Mann–Whitney test (MW). Spearman rank correlation (Sr) was used to assess the association for each dose group between the difference in scores between the beginning and end of the study and the weight, age and interval between the onset of clinical signs and the beginning of ASIT. In all cases, the relevant degrees of freedom are included as subscripts and P < 0.05 was taken to indicate significance. When more than one test was performed to answer a specific question, only one P-value (the biggest value if all the P-values were significant and the smallest if none was significant) is reported.
All the statistical analyses were performed using S-Plus® 2001 (Insightful Corp., Seattle, WA, USA).
RESULTS
Dogs
Twenty-nine dogs were enrolled in the study. Two dogs received the first immunotherapy injection and were lost to follow-up without any further clinical assessment. These dogs were excluded from the study too early to allow data evaluation, and the study was therefore carried out with 27 patients. There were 14 dogs in the LD group, and 13 in the SD group. Data concerning the signalment and the interval between the onset of clinical signs and the beginning of ASIT in the two groups are summarized in Table 3. Age in the LD group ranged between 1 and 5 years (median: 2.5) and bodyweight ranged between 8 and 36 kg (median: 28.5). The interval between the onset of clinical signs and the beginning of ASIT ranged between 5 and 63 months (median: 9.5). Age in the SD group ranged between 1.5 and 8 years (median: 3) while their bodyweights ranged between 7 and 65 kg (median: 27). The interval between the onset of clinical signs and the beginning of ASIT ranged between 8 and 39 months (median: 15). There was no significant difference between the two groups for age, bodyweight and interval between the onset of clinical signs and the beginning of ASIT (F1,25 < 3.369, P > 0.078). There was also no significant difference in the number of male and female dogs in the two groups (P = 0.120). No significant difference was found when the pruritus and mCADESI scores at the beginning of the study were compared (MW < 1.729, P > 0.083). The breed distribution was not analysed, as in some cases only one dog represented a certain breed, and one third of the dogs enrolled were Labrador retrievers and crosses.
| LD group | SD group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion number | Breed | Age | Sex | Weight | Interval* | Inclusion number | Breed | Age | Sex | Weight | Interval* |
| 2 | Labrador retriever | 3.5 | FS | 28 | 25 | 1 | English setter | 2 | M | 27 | 14 |
| 3 | German shepherd dog | 4 | M | 36 | 7 | 4 | German shepherd dog | 6 | FS | 25 | 17 |
| 6 | Boxer | 1 | M | 29 | 7 | 5 | West Highland white terrier | 2.5 | MN | 12 | 9 |
| 7 | West Highland white terrier | 2.5 | F | 8 | 9 | 8 | Dalmatian | 6 | FS | 27 | 39 |
| 10 | Staffordshire bull terrier | 1 | MN | 15 | 8 | 14 | Cavalier King Charles spaniel | 5 | FS | 10 | 22 |
| 12 | Labrador retriever | 2.5 | M | 34 | 5 | 15 | Labrador retriever | 6 | M | 31 | 27 |
| 13 | Labrador retriever | 1.5 | MN | 30 | 10 | 17 | Weimaraner | 2 | F | 32 | 15 |
| 16 | Labrador retriever | 5 | M | 33 | 63 | 19 | Border terrier | 2 | M | 7 | 14 |
| 18 | Labrador retriever cross | 3.5 | M | 25 | 39 | 22 | Shetland sheepdog | 8 | M | 15 | 13 |
| 20 | Flat coated retriever | 1.5 | M | 36 | 7 | 23 | Labrador retriever | 1.5 | FS | 27 | 13 |
| 21 | Labrador retriever cross | 3 | M | 20 | 28 | 24 | West Highland white terrier | 3 | FS | 8 | 26 |
| 25 | Staffordshire bull terrier | 2 | MN | 23 | 5 | 27 | German shepherd dog | 4 | F | 35 | 8 |
| 26 | West Highland white terrier | 3.5 | M | 11 | 15 | 28 | Newfoundland | 2 | M | 65 | 24 |
| 29 | Labrador retriever | 2.5 | FS | 32 | 20 | ||||||
- F, female; FS, female spayed; M, male; MN, male neutered; LD, low dose group; SD, standard dose group.
- * Interval between the onset of clinical signs and the beginning of ASIT, expressed in months. Age is expressed in years. Bodyweight is expressed in kilograms.
Allergens for ASIT were selected based on the results of IDT in 25 dogs (13 dogs in the LD group, 12 dogs in the SD group) and of ASIgES in two dogs (one dog in each group). Five dogs reacted to more than eight allergens and required two vials of ASIT (four dogs in the LD group, one dog in the SD group). All dogs were hypersensitive to one or more dust mites and uncommonly to flower, grass, or weed pollens.
Six dogs (22.2%) did not complete the study (four dogs in the LD group, two dogs in the SD group). Two dogs (7.4%) were withdrawn due to lack of owner compliance (one dog in each group) and four dogs (14.8%) were withdrawn due to unacceptable discomfort (three dogs in the LD group, one dog in the SD group). There was no significant difference in the proportion of dogs withdrawn between the LD group and the SD group (P = 0.648).
Effectiveness of ASIT in the two groups
Changes in pruritus and mCADESI scores throughout the study in the LD and SD groups are summarized in Tables 4 and 5 and illustrated in Fig. 1. There was wide variation in the pruritus scores at the beginning and end of the study in both groups, while the mCADESI scores were more uniform, with the exception of two dogs in the SD group. The median pruritus scores at the beginning of the study were 3 in the LD group and 2 in the SD group. At the end of the study, the medians were 3 and 1 in the LD and SD group, respectively. The median mCADESI scores at the beginning of the study were 16 in the LD group and 16.5 in the SD group. At the end of the study, the medians were 12 and 8 in the LD and SD group respectively. There was no significant difference in the pruritus and mCADESI scores at the end of the study between the LD and SD group (MW < 1.419, P > 0.155). There was no significant difference in the change of pruritus score from the beginning to the end of the study in either group (Wp < 1.936, P > 0.052). In contrast, significant reductions in mCADESI scores in both groups were seen (Wp > 2.141, P < 0.032). The rate of improvement in either mCADESI or pruritus scores did not differ between the two groups (MW < 1.04, P > 0.296).
| LD group | SD group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion number | Day 0 | Day 90 | Day 180 | Day 270 | Inclusion number | Day 0 | Day 90 | Day 180 | Day 270 |
| Pruritus score | Pruritus score | Pruritus score | Pruritus score | Pruritus score | Pruritus score | Pruritus score | Pruritus score | ||
| 2 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | W 180 (5) | W 180 (5) |
| 3 | 4 | 1 | 3 | 3 | 4 | 1 | 1 | 2 | 1 |
| 6 | 3 | 2 | W (3) 180 | W (3) 180 | 5 | 1 | 0 | 2 | 1 |
| 7 | 2 | 1 | 0 | 0 | 8 | 1 | 0 | 0 | 0 |
| 10 | 4 | 4 | 3 | 2 | 14 | 3 | 1 | 0 | 0 |
| 12 | 3 | 3 | 3 | 3 | 15 | 1 | 2 | 1 | 0 |
| 13 | 3 | 4 | 2 | 2 | 17 | 3 | 3 | 0 | 2 |
| 16 | 1 | 2 | 1 | 3 | 19 | 3 | 2 | 4 | 3 |
| 18 | 3 | 3 | W 180 (3) | W 180 (3) | 22 | 2 | 2 | 1 | 0 |
| 20 | 2 | 1 | W 180 (3) | W 180 (3) | 23 | 3 | 1 | 2 | 1 |
| 21 | 3 | 2 | 3 | 3 | 24 | 1 | 1 | 1 | 1 |
| 25 | 2 | 0 | 0 | 0 | 27 | 1 | W 90 (2) | W 90 (2) | W 90 (2) |
| 26 | 2 | 1 | 1 | 1 | 28 | 3 | 2 | 3 | 4 |
| 29 | 4 | 3 | W 180 (5) | W 180 (5) | |||||
- LD, low dose group; SD, standard dose group.
- W 90, withdrawn on day 90; W 180, withdrawn on day 180.
| LD group | SD group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion number | Day 0 | Day 90 | Day 180 | Day 270 | Inclusion number | Day 0 | Day 90 | Day 180 | Day 270 |
| mCADESI score | mCADESI score | mCADESI score | mCADESI score | mCADESI score | mCADESI score | mCADESI score | mCADESI score | ||
| 2 | 37 | 15 | 14 | 4 | 1 | 156 | 33 | W 180 (136) | W 180 (136) |
| 3 | 29 | 18 | 12 | 14 | 4 | 84 | 16 | 51 | 29 |
| 6 | 35 | 17 | W 180 (32) | W 180 (32) | 5 | 17 | 7 | 9 | 4 |
| 7 | 33 | 32 | 9 | 7 | 8 | 14 | 7 | 4 | 3 |
| 10 | 35 | 5 | 7 | 7 | 14 | 9 | 10 | 6 | 5 |
| 12 | 16 | 12 | 7 | 9 | 15 | 23 | 14 | 12 | 13 |
| 13 | 16 | 16 | 8 | 15 | 17 | 15 | 13 | 6 | 9 |
| 16 | 19 | 8 | 9 | 33 | 19 | 36 | 20 | 40 | 25 |
| 18 | 39 | 11 | W 180 (42) | W 180 (42) | 22 | 47 | 21 | 15 | 5 |
| 20 | 25 | 22 | W 180 (20) | W 180 (20) | 23 | 33 | 20 | 14 | 8 |
| 21 | 19 | 14 | 16 | 8 | 24 | 21 | 12 | 8 | 5 |
| 25 | 15 | 10 | 9 | 7 | 27 | 10 | W 90 (12) | W 90 (12) | W 90 (12) |
| 26 | 13 | 5 | 4 | 5 | 28 | 12 | 9 | 19 | 19 |
| 29 | 16 | 8 | W 180 (16) | W 180 (16) | |||||
- mCADESI, modified canine atopic dermatitis extent and severity index; LD, low dose group; SD, standard dose group.
- W 90, withdrawn on day 90; W 180, withdrawn on day 180.
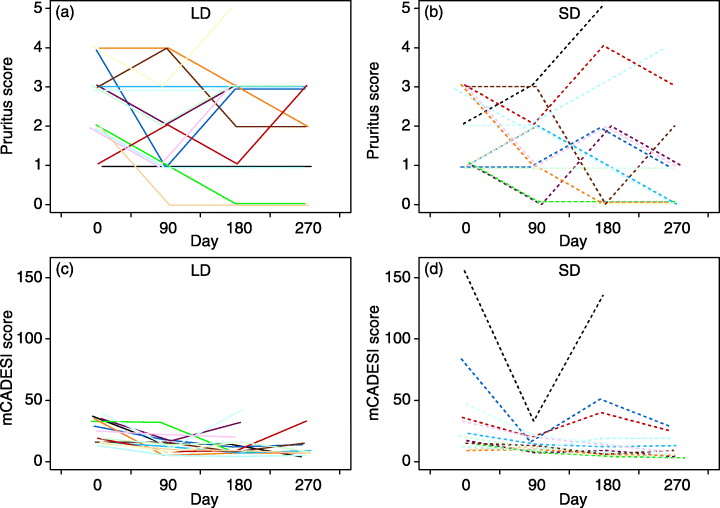
Changes in pruritus and mCADESI scores throughout the study in the LD (a, c) and SD (b, d) groups. Each coloured line refers to one dog. The y-axis on (c) and (d) (0–150) represents the highest score achieved. The maximum possible score was 540. LD, low dose group; SD, standard dose group; mCADESI, modified canine atopic dermatitis extent and severity index.
Overall effectiveness of ASIT
In this study, complete response to ASIT (pruritus score of 0 at the end of the study) was obtained in six dogs (22.2%), two in the LD group and four in the SD group (Table 4). Six dogs (22.2%), four in the LD group and two in the SD group, had a partial response (lower pruritus score on day 270 but not reaching a score of 0). No response to ASIT (the same or a higher pruritus score at the end of the study compared to day 0) was observed in 15 dogs (55.6%), eight in the LD group and seven in the SD group, including the six withdrawn patients.
Correlations between bodyweight, age and interval between the onset of clinical signs and the beginning of ASIT with changes in pruritus and mCADESI scores in the two groups
There was a significant correlation between bodyweight and the change in pruritus score when intention-to-treat analysis was applied (Sr = 0.462, P = 0.019), with lower weight dogs in the LD group having a greater reduction in their pruritus scores. This correlation disappeared when per protocol analysis was performed (Sr = 0.315, P = 0.160). No correlations with age or interval between the onset of clinical signs and the beginning of ASIT and the change in pruritus score were found (Sr < |0.422|, P > 0.060). In addition, there were no other significant correlations within the two groups between change in pruritus score and weight, age or interval between the onset of clinical signs and the beginning of ASIT (Sr < |0.646|, P > 0.055).
There was, however, a significant correlation between bodyweight and change in mCADESI score, when the intention-to-treat analysis was performed (Sr = 0.496, P = 0.011), with lower weight dogs having a greater reduction in their mCADESI score. This correlation disappeared with per protocol analysis (Sr = 0.387, P = 0.084). No correlations between the change in mCADESI and age or interval between the onset of clinical signs and the beginning of ASIT in either group was observed (Sr < 0.456, P > 0.116).
DISCUSSION
In this study, no difference in effectiveness could be demonstrated between the LD and SD protocols when treating canine AD with ASIT. However, four dogs in the SD group achieved a final pruritus score of 0, while the same result was observed in only two dogs in the LD group (Table 4). It is possible, therefore, that a larger study might have been able to demonstrate significant differences between the two protocols. The mCADESI scores improved significantly in both groups and no difference between the LD and SD groups was found. This may be related to the fact that dogs in both groups received concurrent treatment for secondary infections (superficial bacterial pyoderma, Malassezia dermatitis, bacterial/Malassezia otitis externa) throughout the study, as well as regular ectoparasite control.
The study was designed as a prospective, randomized, double-blinded, controlled study in which LD ASIT was administered from the beginning and SD ASIT was used as control. In the present study, a significant correlation was found between the dogs’ bodyweight and the change in pruritus scores, albeit only when intention-to-treat analysis was applied, suggesting that tailoring the dose of ASIT depending on the dog's weight could result in a better success rate. This observation has not been previously reported and warrants further larger studies.
A retrospective study reported increased effectiveness of 10-fold lower doses of ASIT than in the LD group in the present study, but that study design was different in that the lower doses were introduced in the maintenance stage of ASIT and various concentrations of allergens were used to obtain good to excellent results (20–10 000 PNU).25 An unpublished study compared high dose ASIT (maintenance concentration 40 000 PNU) and LD ASIT (maintenance concentration 10 000 PNU) to ASIT using nonspecific allergens, and found that the high dose protocol was more effective than the LD and the nonspecific protocol,27 although 68% of the dogs included in the LD group also achieved a good to excellent response.27 These studies used aqueous allergens.25,27 Another retrospective uncontrolled study on LD ASIT, with lower concentrations (100–1000-fold less than the concentration used for IDT) used from the beginning of treatment, reported a good response in 71.6% of the dogs.26 The latter study was carried out with alum-precipitated ASIT obtained from a different manufacturer (ALK®, Hoersholm, Denmark).26 The results obtained in the present study are therefore likely to be related to the different study design, ASIT protocol and type, concentration and source of allergens utilized.
Since no significant difference was found between the two dose groups, overall effectiveness of ASIT in canine AD could be evaluated. Results are presented based on data rather than on predefined criteria, in comparison to most published studies.9,14,15,17,18,24 Effectiveness of ASIT in controlling pruritus and clinical signs are presented separately, since clinical improvement is extensively influenced by concurrent treatment for secondary infections. Moreover, dogs were treated for secondary infections throughout the study, and did not have bacterial pyoderma, Malassezia dermatitis or bacterial/Malassezia otitis externa at the time of the last clinical assessment. For these reasons, overall effectiveness of ASIT was based on the final pruritus score.
In this study, the pruritus score reached a final value of 0 in only six dogs (22.2%). The success rate is comparable with some other studies, in which 19.5% to 21.5% of the cases fully responded to ASIT alone or combined with topical nonsteroidal treatments.23,24 These two studies were carried out using aqueous23 or alum-precipitated ASIT.24 ASIT was ineffective in controlling pruritus in 15 cases (55.6%) in the present study, a much higher percentage than in most studies.8–11,13–18,23,24 However, the present study had a strict experimental design and inclusion and assessment criteria. With the exception of the report by Willemse et al.,9 all previous studies have been retrospective and based on owner questionnaires.
This study monitored the progress of the dogs over 9 months, as recommended previously.9 Dogs were enrolled consecutively and assessed in parallel, and the influence of seasonal variations in the allergen load on the final results cannot be ruled out.
The itching scale developed to assess the severity of pruritus was generally considered easy to use by both the investigator and the owners. However, an occasional owner found it too rigid and had problems making a decision between two consecutive pruritus grades.
Preliminary statistical analyses confirmed that the dogs were equally distributed for age, sex, bodyweight, duration of clinical signs before the initiation of ASIT, and pruritus and mCADESI scores at the beginning of the study. However, despite the use of block randomization, the dogs were not equally distributed according to breed, and in many cases, only one dog represented a particular breed. Therefore, an assessment of the response to ASIT in relation to the breeds could not be carried out.
In conclusion, there is no evidence to recommend the use of LD ASIT from the outset over SD ASIT. However, although the results were not significantly different, there was some evidence to suggest that the SD protocol was superior: first, the number of complete responses (four dogs in the SD group vs. two dogs in the LD group); second, the number of withdrawals due to unacceptable discomfort (three dogs in the LD group vs. one dog in the SD group); and third, the greater reduction in pruritus and mCADESI scores in the SD group. However, this remains to be proven in a larger study. In this study, ASIT was effective in controlling pruritus due to AD in 22.2% of cases, with 22.2% of dogs exhibiting a partial response and 55.6% showing no response.
ACKNOWLEDGEMENTS
The authors are very grateful to Drs Adri van den Broek and Ariane Neuber and Mr Andrew Carter for their help in selecting and enrolling the cases. The authors also thank Joan Freeman, Diane McDonald and the other nursing staff at the Royal (Dick) School of Veterinary Studies’ Hospital for Small Animals, for ensuring the blinding of the study and administering the immunotherapy injections, and Mr Michael Thrusfield of the Division of Veterinary Clinical Studies for prior randomization of the the cases. SC was supported by the British Small Animal Association's Petsavers.
REFERENCES
Résumé Il existe des rapports anecdotiques de l’augmentation d’efficacité de l’immunothérapie spécifique d’allergènes (ASIT) chez le chien en utilisant des doses plus faibles que celles recommandées par les fabricants. Cependant, aucune étude controlée na été réalisée. Le but de cette étude, en double aveugle, était d’évaluer si l’induction et la maintenance avec une ASIT à dose faible (LD) permettait un taux de succès différent que celui du protocole standard (SD). Vingt-sept chiens souffrant de dermatite atopique ont été divisés en deux groupes par randomisation. Un groupe (n = 13) a reçu SD ASIT; l’autre groupe (n = 14) a reçu LD ASIT (1/10 de la SD), en utilisant le même protocole de fréquence d’injections. Les cas ont été gradés à 0, 3, 6, et 9 mois pour les signes cliniques en utilisant un canine atopic dermatitis extent and severity index modifié (mCADESI) et pour le prurit en utilisant une échelle de 0 à 5. Aucune différence n’a été observée entre les deux groupes pour le prurit et le mCADESI (P > 0.155) à la fin de l’étude. Les modifications du prurit (P > 0.920) et du mCADESI (P > 0.296) entre le début de l’essai et la fin étaient identiques dans les deux groupes; les scores de prurit n’ont pas changé pendant l’essai dans les deux groupes (P > 0.052). Cependant des réductions significatives des scores du mCADESI ont été notées dans les deux groupes (P < 0.032). Six chiens ont atteint un score de prurit final de 0.6, et 15 n’ont pas été amélioré ou se sont détériorés. Cette étude montre qu’il n’existe pas de preuve que LD ASIT est plus efficace que le protocole standard.
Resumen Existen descripciones anecdóticas de mayor efectividad de la inmunoterapia alérgeno-específica (ASIT) en perros con dosis de vacuna inferiores a las recomendadas por los productores de la vacuna. Sin embargo, no se han realizado estudios controlados. El objetivo de este estudio prospectivo, doble-ciego fue evaluar si la inducción y mantenimiento con una dosis baja (LD) ASIT llevaba a un resultado diferente comparando con la dosis estándar (SD). Se distribuyeron al azar veintisiete perros con dermatitis atópica confirmada, en dos grupos. Un grupo (n = 13) recibió SD ASIT; el otro (n = 14), LD ASIT (1/10 de la SD), siguiendo el mismo protocolo de frecuencia. Se evaluaron los casos a los 0, 3, 6, y 9 meses para los síntomas clínicos utilizando un índice de la extensión y severidad de la dermatitis atópica canina (mCADESI) y para el prurito utilizando una escala de 0–5. No se hallaron diferencias significativas entre los grupos con los valores de prurito y del mCADESI (P > 0.155) al final del estudio, y los cambios en el prurito (P > 0.920) y la valoración de mCADESI (P > 0.296) desde el principio al final del estudio eran similares en ambos grupos, con las valoraciones del prurito en ambos grupos sin cambiar durante el estudio (P > 0.052). Sin embargo, se observaren reducciones significativas en las valoraciones de mCADESI en ambos grupos (P < 0.032). Seis perros alcanzaron una puntuación del prurito de 0, 6 una reducción de la valoración del prurito y 15 no mejoraron ni empeoraron. Así, no se produjo ninguna evidencia de que el LD ASIT fuera más efectivo que el protocolo estándar.
Zusammenfassung Es gibt anekdotenhafte Berichte über erhöhte Wirksamkeit von allergenspezifischer Immuntherapie (ASIT) bei Hunden mit niedrigeren als den vom Hersteller empfohlenen Mengen an Vaccinen. Jedoch wurden noch keine kontrollierten Studien durchgeführt. Das Ziel dieser prospektiven Doppelblindstudie war es nachzuprüfen, ob Induktion und Erhaltung mit niedrig dosierter (ND) ASIT im Vergleich zur Standarddosis (SD) zu einer anderen Erfolgsrate führen. Siebenundzwanzig Hunde mit bestätigter atopischer Dermatitis wurden durch Blockrandomisierung zwei Gruppen zugeordnet. Eine Gruppe (n = 13) erhielt SD ASIT; die andere Gruppe (n = 14) erhielt ND ASIT (1/10 der ND) mit derselben Häufigkeit. Die Fälle wurden nach 0, 3, 6, und 9 Monaten hinsichtlich klinischer Anzeichen anhand des modified canine atopic dermatitis extent and severity index (mCADESI) und hinsichtlich des Juckreizes anhand einer von 0–5 reichenden Skala eingeteilt . Es gab am Ende der Studie zwischen den Gruppen keine signifikanten Unterschiede bezüglich der Juckreiz- und mCADESI-Punktzahlen (P > 0.155) und die Veränderungen der Juckreiz- (P > 0.920) und mCADESI- Punktzahlen (P > 0.920) von Beginn bis zum Ende der Studie waren in beiden Gruppen ähnlich, wobei sich die Juckreizpunktzahlen in beiden Gruppen während der Studie nicht änderte P > 0.052). Jedoch wurde eine signifikante Reduktion des mCADESI-Punktzahl in beiden Gruppen (P < 0.032) beobachtet. Sechs Hunde erreichten eine Endpunktzahlvon 0, 6 eine Reduktion der Juckreizpunktzahl und 15 zeigten weder Verbesserung noch Verschlechterung. Folglich ergab sich kein Hinweis, dass ND ASIT effektiver als das Standardprotokoll war.




