The plastid-specific ribosomal proteins of Arabidopsis thaliana can be divided into non-essential proteins and genuine ribosomal proteins
Present address: Universität Erlangen-Nürnberg, Lehrstuhl für Molekulare Pflanzenphysiologie, Staudtstraße 5, D-91058 Erlangen, Germany.
Summary
Plastid translation occurs on bacterial-type 70S ribosomes consisting of a large (50S) subunit and a small (30S) subunit. The vast majority of plastid ribosomal proteins have orthologs in bacteria. In addition, plastids also possess a small set of unique ribosomal proteins, so-called plastid-specific ribosomal proteins (PSRPs). The functions of these PSRPs are unknown, but, based on structural studies, it has been proposed that they may represent accessory proteins involved in translational regulation. Here we have investigated the functions of five PSRPs using reverse genetics in the model plant Arabidopsis thaliana. By analyzing T-DNA insertion mutants and RNAi lines, we show that three PSRPs display characteristics of genuine ribosomal proteins, in that down-regulation of their expression led to decreased accumulation of the 30S or 50S subunit of the plastid ribosomes, resulting in plastid translational deficiency. In contrast, two other PSRPs can be knocked out without visible or measurable phenotypic consequences. Our data suggest that PSRPs fall into two types: (i) PSRPs that have a structural role in the ribosome and are bona fide ribosomal proteins, and (ii) non-essential PSRPs that are not required for stable ribosome accumulation and translation under standard greenhouse conditions.
Introduction
Plastids stem from formerly free-living cyanobacteria and arose through endosymbiosis. Present-day plastids possess only a vestigial genome, but expression of this genome is astonishingly complex and highly regulated at the transcriptional and post-transcriptional levels (Liere and Börner, 2007; Peled-Zehavi and Danon, 2007). As a result of endosymbiotic gene transfer (Bock and Timmis, 2008), the gene expression machinery of plastids is of dual genetic origin, with one part of its components being encoded by the plastid genome and the other part being encoded by nuclear genes. Translation in plastids occurs on prokaryotic-type 70S ribosomes. Most protein constituents of the plastid ribosome have orthologs in bacteria. The small (30S) subunit of the plastid ribosome contains 21 subunits that have orthologs in Escherichia coli. Twelve of these subunits are encoded by the plastid genome, whereas the remaining 9 subunits are encoded by the nuclear genome (Yamaguchi et al., 2000), exemplifying the dual genetic origin of the translational apparatus. The large (50S) subunit of the ribosome consists of 31 ribosomal proteins that have orthologs in E. coli. Nine of these are plastid-encoded and 22 are encoded by nuclear genes (Yamaguchi and Subramanian, 2000).
In addition to these ‘classical’ ribosomal proteins, plastid ribosomes also contain a small set of proteins that have no orthologs in E. coli (Yamaguchi and Subramanian, 2000; Yamaguchi et al., 2000). These proteins have been termed plastid-specific ribosomal proteins (PSRPs). Proteomics analysis of plastid ribosomes from spinach (Spinacia oleracea) has identified four PSRPs associated with the 30S ribosomal subunit (PSRP1, PSRP2, PSRP3 and PSRP4; Yamaguchi et al., 2000; Yamaguchi and Subramanian, 2003) and two PSRPs associated with the 50S ribosomal subunit (PSRP5 and PSRP6; Yamaguchi and Subramanian, 2000). The genome of the cyanobacterium Synechocystis sp. PCC6803 contains genes for only two of the six PSRPs present in higher plants (PSRP1 and PSRP3), and the genome of the unicellular green alga Chlamydomonas reinhardtii lacks genes for PSRP2 and PSRP5, possibly indicating that at least some PSRPs were acquired rather late in evolution (Yamaguchi and Subramanian, 2003).
The functions of PSRPs are largely unknown. PSRP1 is unusual in that it is present at a high concentration in the chloroplast stroma (Zhou and Mache, 1989; Yamaguchi and Subramanian, 2003). Recent data suggest that PSRP1 may not be a genuine ribosomal protein, but rather a ribosome-binding translation factor that acts as a functional homolog of the E. coli cold-shock protein pY (Sharma et al., 2007, 2010). It stabilizes the ribosome against dissociation and is recycled by the ribosome-recycling factor and translation elongation factor G.
Unlike PSRP1, all other PSRPs appear to be present in stoichiometric amounts with the classical ribosomal proteins (Yamaguchi and Subramanian, 2000, 2003; Yamaguchi et al., 2000), suggesting that they may represent bona fide ribosomal proteins. Cryo-electron microscopic studies have localized some PSRPs in the 3D structure of the chloroplast ribosome (Manuell et al., 2007; Sharma et al., 2007). Based on these studies, PSRP2 and PSRP3 have been suggested to structurally compensate for missing segments of the 16S rRNA in the 30S ribosomal subunit (Sharma et al., 2007). PSRP5 was tentatively localized near the tRNA exit site (E site) in the 50S ribosomal subunit and was therefore proposed to be involved in ejection of deacylated tRNAs from the ribosome (Sharma et al., 2007). Most PSRPs appear to be exposed at the surface of the chloroplast ribosome, and this finding has triggered speculation about their possible involvement in light-dependent regulation of chloroplast translation (Manuell et al., 2007; Sharma et al., 2007). However, to date, no functional data have been obtained for any of the stoichiometrically present PSRPs (PSRP2–6), and knowledge of their possible roles in translation and/or translational regulation remains elusive.
Here we have used a reverse genetics approach to study the functions of PSRPs in Arabidopsis thaliana. We wished to test whether these PSRPs are bona fide ribosomal proteins with essential functions in plastid protein biosynthesis, or function as accessory factors in the regulation of translation. To this end, we analyzed T-DNA mutants and generated RNAi lines for the five PSRPs, and compared their phenotypes with mutants for two classical ribosomal proteins. We show that, although knockdown of PSRP3, PSRP4 and PSRP5 leads to partial loss of chloroplast ribosomes, PSRP2 and PSRP6 can be knocked down without detectable effects on ribosome biogenesis and translation.
Results
Genes for PSRPs in the A. thaliana genome
All PSRPs are nuclear-encoded proteins that were initially identified by proteomic studies of spinach chloroplast ribosomes (Yamaguchi and Subramanian, 2000; Yamaguchi et al., 2000). Searches of the A. thaliana genome based on the spinach amino acid sequences revealed homologs of all six PSRPs. Five appear to be encoded by single-copy nuclear genes: PSRP1 (At5g24490), PSRP2 (At3g52150), PSRP4 (At2g38140), PSRP5 (At3g56910) and PSRP6 (At5g17870). For PSRP3, two nuclear genes were found, which are subsequently referred to as PSRP3/1 (At1g68590) and PSRP3/2 (At5g15760). Analysis of transcript accumulation using the Genevestigator (https://www.genevestigator.com/gv/index.jsp) and AtGenExpress (http://arabidopsis.org/portals/expression/microarray/ATGenExpress.jsp) datasets revealed that, in contrast to the PSRP3/1 gene, PSRP3/2 is not expressed at detectable levels under any of the many conditions tested, possibly suggesting that it does not represent a functional gene. This is consistent with our RT-PCR analyses, which failed to amplify a cDNA for PSRP3/2. We therefore used PSRP3/1 for our functional analysis of PSRPs. We also included all other PSRPs whose functions are not known, so that altogether five PSRP genes were investigated: PSRP2, PSRP3/1, PSRP4, PSRP5 and PSRP6.
PSRPs localize exclusively to plastids
As some ribosomal proteins are known to be dually targeted to plastids and mitochondria (Ueda et al., 2008), we tested whether or not the PSRPs are exclusively targeted to plastids. This is of critical importance to their functional analysis by reverse genetics and interpretation of any phenotypic data.
In order to determine the subcellular localization of all five PSRPs (PSRP2, PSRP3/1, PSRP4, PSRP5 and PSRP6), we constructed GFP fusions with the full-length PSRP coding regions obtained by cDNA amplification. The fusion genes (driven by the strong constitutively expressed CaMV 35S promoter) were introduced into tobacco protoplasts by transient transformation. As a control, two classic ribosomal proteins were selected (RPS17, a subunit of the small subunit of the plastid ribosome, and RPL24, a subunit of the large subunit of the plastid ribosome), and GFP fusions were also constructed using their genes. Investigation of the subcellular localization of GFP fusion proteins by confocal laser-scanning microscopy showed that all five PSRPs, as well as RPS17 and RPL24, localize exclusively to chloroplasts (Figure 1). This suggests that targeting the PSRP genes by reverse genetics represents a suitable means to reveal their specific functions in chloroplast translation.
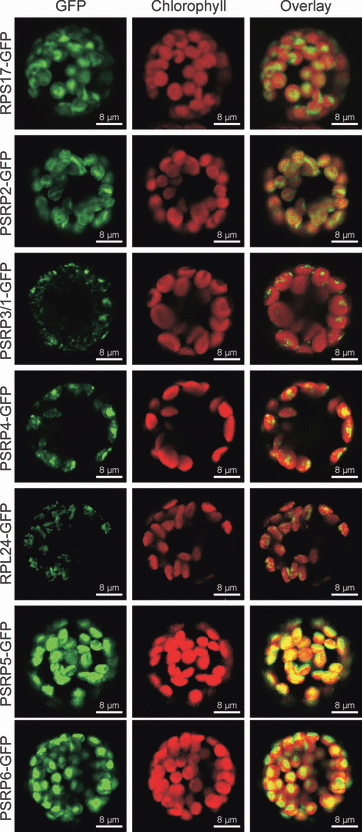
In vivo analysis of the subcellular localization of PSRPs. Fusion constructs of the full-length coding sequences of PSRPs from Arabidopsis thaliana to GFP were transiently expressed in tobacco protoplasts by polyethylene glycol-mediated DNA uptake. GFP fluorescence, chlorophyll fluorescence and the merged fluorescence signals are shown for all constructs. As a control, the subcellular localization of two classic ribosomal proteins (RPS17 and RPL24) was also determined. Co-localization of GFP fluorescence and chlorophyll fluorescence for all fusion constructs and the absence of fluorescence from mitochondria demonstrates that the PSRPs are exclusively targeted to chloroplasts. However, some fusion proteins are not homogeneously distributed within the chloroplast, as evidenced by punctate localization of the GFP fluorescence (e.g. RPL24–GFP, PSRP3/1–GFP and PSRP4–GFP). Images were obtained using a confocal laser scanning microscope 48 h after protoplast transformation. Scale bars = 8 μm.
Isolation of mutants for PSRPs in Arabidopsis
In order to address the functions of the five PSRPs, we isolated mutants for the corresponding genes in the A. thaliana nuclear genome (Figure 2). Searches of the publicly available collections of T-DNA insertion lines revealed that two independent T-DNA insertion lines in the coding region were only available for a single gene (PSRP3/1) (Figure 2 and Table 1). As two independently generated mutant lines per gene are required to draw reliable conclusions about phenotypes and gene functions, we also produced hairpin-type RNAi constructs targeted against RPS17, RPL24, PSRP2, PSRP4, PSRP5 and PSRP6 (Figure 2 and Table 1). Using these vectors, A. thaliana RNAi lines were generated by Agrobacterium-mediated transformation. The residual mRNA accumulation levels of the target genes were determined by quantitative RT-PCR, and lines showing a strong reduction in mRNA levels were chosen and included in our functional studies (Table 1). Quantitative RT-PCR was also used to measure residual expression of the target genes in all T-DNA insertion lines (Table 1). This was important because the insertion site was within the 5′ UTR in three of these lines (rps17-1, psrp2-1 and psrp5-1), leading to residual expression of the full-length mRNA. From these data, a final set of mutant lines was compiled that comprises two or three independently generated mutant lines per gene (Table 1). Residual RNA accumulation levels in the mutant collection varied between 0% in several T-DNA insertion lines and 20% in the weakest RNAi line (Table 1).
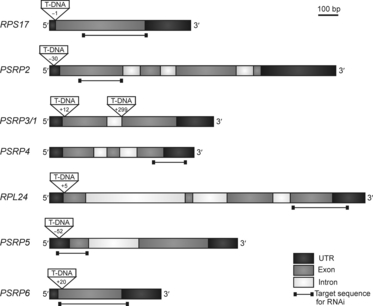
Plastid ribosomal protein genes targeted by reverse genetics. Gene structure, T-DNA insertion sites and target sequences for RNAi-mediated knockdown are shown for the five PSRP genes analyzed in this study and the two classic ribosomal proteins included as controls (RPS17 and RPL24). T-DNA insertion sites are indicated by their nucleotide positions relative to the translation initiation codon.
| Gene | AGI code | Mutant line | Mutation type | Residual expression (percentage of wild-type) |
|---|---|---|---|---|
| RPS17 | At1g79850 | rps17-1 | T-DNA | 15 |
| rps17-R2 | RNAi | 5 | ||
| PSRP2 | At3g52150 | psrp2-1 | T-DNA | 10 |
| psrp2-R2 | RNAi | 5 | ||
| PSRP3/1 | At1g68590 | psrp3/1-1 | T-DNA | 0 |
| psrp3/1-4 | T-DNA | 0 | ||
| PSRP4 | At2g38140 | psrp4-R1 | RNAi | 15 |
| psrp4-R2 | RNAi | 10 | ||
| RPL24 | At5g54600 | rpl24-1 | T-DNA | 0 |
| rpl24-R1 | RNAi | 15 | ||
| rpl24-R2 | RNAi | 19 | ||
| PSRP5 | At3g56910 | psrp5-1 | T-DNA | 5 |
| psrp5-R1 | RNAi | 20 | ||
| psrp5-R2 | RNAi | 18 | ||
| PSRP6 | At5g17870 | psrp6-1 | T-DNA | 0 |
| psrp6-R2 | RNAi | 18 |
In order to assess the phenotypic consequences of down-regulated PSRP gene expression, mutant lines were grown under standardized conditions (see Experimental procedures) and compared side-by-side with wild-type plants (Figure 3). Interestingly, widely different growth phenotypes were obtained. In general, when mutants for the same gene were compared, a good correlation between the level of down-regulation of the gene (Table 1) and the severity of the growth phenotype (Figure 3) was observed. Down-regulation of expression of the classic ribosomal protein gene RPS17 to 15% (T-DNA mutant rps17-1) or 5% (RNAi mutant rps17-R2) resulted in pale-green leaves and strongly impaired growth. Growth of the rpl24 mutants was also strongly retarded, and was extremely slow in the T-DNA insertion mutant rpl24-1 (Figure 3). It is important to note that this mutant line represents a null allele (Table 1), and yet the mutant plants are capable of autotrophic growth in soil. This suggests that L24 represents a non-essential ribosomal protein, at least as far as cellular viability and autotrophic growth are concerned. However, many of the homozygous mutant plants died before reaching the bolting stage, and the survivors did not produce seeds. In this respect, it is noteworthy that L24 is also a non-essential ribosomal protein in Escherichia coli. Bacterial strains carrying a null mutation in the rpl24 (rplX) gene have drastically reduced growth rates and display a temperature-sensitive phenotype (Dabbs, 1991).
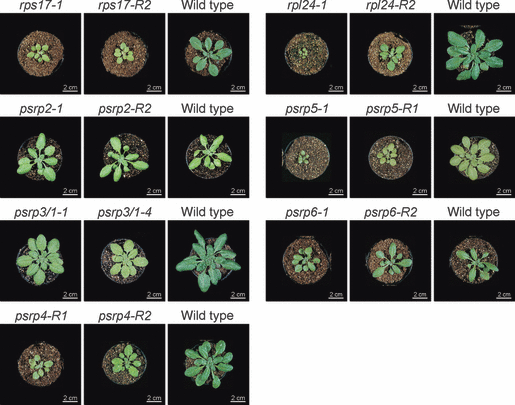
Phenotypes of psrp mutants in comparison with wild-type plants and mutants for the two classic ribosomal proteins RPS17 and RPL24. Plants were grown under long-day conditions at a light intensity of 120 μE m−2 sec−1. For each set of mutants, a corresponding wild-type control plant (grown side-by-side) is shown. Mutant numbers are indicated by numerals following the gene names. ‘R’ indicates RNAi lines; all other mutants are T-DNA insertion lines.
Knockdown of the three PSRPs of the small ribosomal subunit had very different phenotypic consequences. Reduction in PSRP2 gene expression to 10% (T-DNA mutant psrp2-1) or 5% (RNAi mutant psrp2-R2) of wild-type mRNA levels (Table 1) did not cause a detectable alteration in growth phenotype, possibly indicating that this PSRP has limited importance in plastid translation. In contrast, down-regulation of PSRP4 gene expression to 15% (RNAi mutant psrp4-R1) or 10% (RNAi line psrp4-R2) of wild-type mRNA levels (Table 1) led to pale-green leaves and severe growth retardation, resulting in a similar phenotype to the rps17 mutant lines (Figure 3). Complete loss of PSRP3/1 gene expression (in T-DNA mutants psrp3/1-1 and psrp3/1-4) resulted in a much less severe mutant phenotype, and demonstrates that PSRP3/1 represents a non-essential PSRP gene. Successful isolation of null mutants for PSRP3/1 prompted us to investigate the expression status of the suspected pseudogene PSRP3/2 in the psrp3/1-1 and psrp3/1-4 backgrounds. RT-PCR analyses revealed that PSRP3/2 expression was undetectable in the two homozygous knockout mutants, indicating that PSRP3/2 cannot compensate for loss of PSRP3/1 function.
Knockdown of the two PSRPs of the large ribosomal subunit also produced very different phenotypes. Reduction of PSRP5 gene expression to 5% (T-DNA mutant psrp5-1) produced plants that resembled rpl24-1 mutants and resulted in severely delayed plant growth and development. Plants often did not reach maturity and produced no seeds. Somewhat less severe phenotypes were obtained in RNAi mutants that retained 20% (mutant psrp5-R1) of wild-type expression levels (Figure 3 and Table 1). In contrast, knockout mutants of PSRP6 were phenotypically indistinguishable from wild-type plants (Figure 3), possibly indicating that PSRP6 serves no important function in translation, at least not under standard growth conditions in the greenhouse.
Leaf anatomy in psrp mutants
Chloroplast function and leaf development are intimately linked. A number of mutants have been described that are affected in chloroplast development and/or function and show defects in leaf cell differentiation, especially palisade cell development. These include mutants in metabolic functions of the chloroplast (Streatfield et al., 1999), mutants in plastid gene expression (Wycliffe et al., 2005; Hricováet al., 2006) and mutants in chloroplast biogenesis (Chatterjee et al., 1996). This phenomenon is usually attributed to retrograde signaling from the plastid to the nucleus (Rodermel, 2001; Yu et al., 2007). To analyze whether psrp mutants show defects in leaf development, we investigated leaf anatomy by epifluorescence microscopy of toluidine blue O-stained cross-sections of mutant and wild-type leaves. Interestingly, the psrp3/1 and psrp5 mutants showed severe alterations in leaf anatomy (Figure 4). Cylindrical palisade cells were not recognizable, and all mesophyll cells were much smaller than in the wild type. Somewhat less severe defects were seen in the rpl24 mutant and the psrp4 mutant, and the rps17 mutant was only mildly affected (Figure 4). The severely disrupted mesophyll differentiation and altered cell size in leaves of the psrp3/1-1 mutant are particularly striking, because this mutant has a relatively mild growth phenotype compared to the rps17-1 mutant (Figure 3).
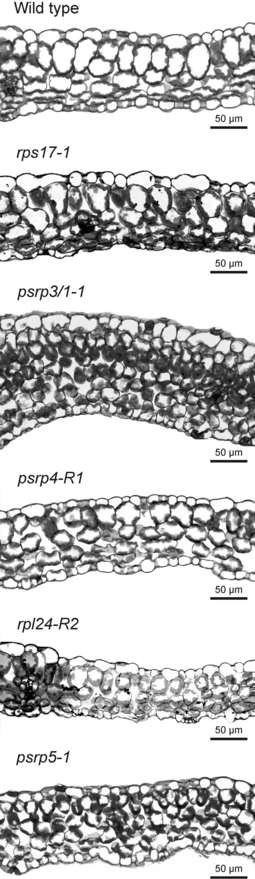
Leaf anatomy in ribosomal protein mutants. Transverse leaf sections are shown for the wild type and for all psrp mutants and conventional ribosomal protein mutants that showed phenotypic alterations (see Figure 3). Note the drastically altered cellular organization of the leaves in all ribosomal protein mutants and the severely disturbed palisade cell development.
Photosynthesis in psrp mutants
The growth phenotypes and pigment deficiency of some of the Arabidopsis psrp mutants (Figure 3) suggest that impaired chloroplast translation has a direct impact on photosynthesis. This is certainly conceivable, as most plastid genome-encoded proteins are involved in either photosynthesis or gene expression. We therefore wished to obtain quantitative data on photosynthetic performance in the psrp mutants. To this end, the chlorophyll content, efficiency of photosynthetic electron transport and contents of the protein complexes of the photosynthetic electron transport chain in the thylakoid membrane were determined using spectroscopic methods. One mutant line per ribosomal protein was characterized in detail. As these analyses required substantial amounts of leaf material (including sufficient material for thylakoid isolations), the most severely affected mutants (rpl24-1 and psrp5-1) could not be included.
As suggested by their light-green phenotype, the chlorophyll content per leaf area was found to be significantly reduced in the rps17-1, psrp3/1-1, psrp4-R1, rpl24-R2 and psrp5R1 mutants (Figure 5). Likewise, the maximum quantum efficiency of photosystem II (FV/FM), a standard measure of photosystem II integrity, was significantly reduced in these mutants (Figure 5).
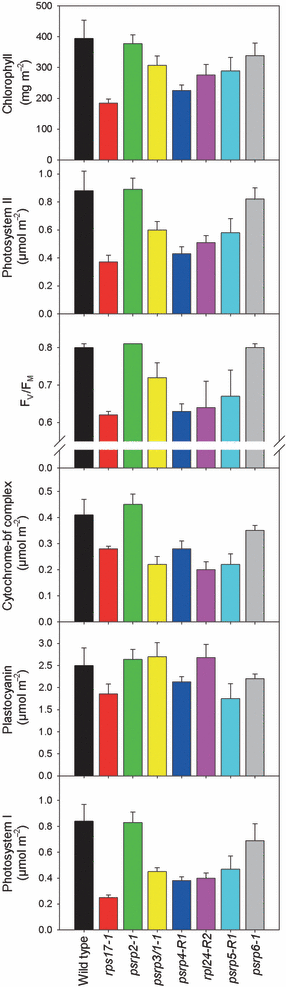
Analysis of photosynthetic parameters in wild type and selected ribosomal protein mutants. Values represent the means of at least three biological replicates. The error bars indicate the standard deviation.
To assess the consequences of impaired PSRP function at the protein level, the contents of the protein complexes participating in photosynthetic electron transport (photosystem II, cytochrome b6f complex, photosystem I) were determined by difference absorption spectroscopy (Schöttler et al., 2007a,b). The rps17-1, psrp3/1-1, psrp4-R1, rpl24-R2 and psrp5-R1 mutants showed strongly reduced accumulation of these three complexes (Figure 5), consistent with the plastid encoding essential core subunits of all thylakoid protein complexes. Some of the mutants (rps17-1, psrp4-R1 and psrp5-R1) also showed a moderate reduction in their plastocyanin content. Plastocyanin is a nuclear-encoded protein in the thylakoid lumen that functions as an electron carrier between the cytochrome b6f complex and photosystem I. It is not expected to be directly affected by reduced plastid translational activity, but is known to be tightly regulated and respond sensitively to alterations in chloroplast function (Sullivan and Gray, 2002; Schöttler et al., 2004).
The deficiency of the ribosomal protein mutants in core complexes of the photosynthetic electron transport chain was further confirmed by chlorophyll a fluorescence emission measurements at 77 K. At this low temperature, the fluorescence emission from the two photosystem cores can be readily analyzed (Krause and Weis, 1991). Compared to the wild-type spectrum, the 77 K chlorophyll a fluorescence emission in the rps17-1, psrp3/1-1, psrp4-R1, rpl24-R2 and psrp5R1 mutants was reduced, and the severely affected mutants rps17-1, psrp3/1-1 and rpl24-R2 also showed a shifted emission maximum of photosystem I fluorescence towards shorter wavelengths (Figure S1). This indicates an altered functional organization of the photosystems (Jensen et al., 2004; Schöttler et al., 2007a,b), and suggests that a substantial proportion of the photosystem I antenna is not connected to reaction centers, probably due to deficiency in photosystem I core accumulation in the mutants (Figure 5).
Consistent with their wild-type-like phenotypes, the psrp2 and psrp6 mutants did not show significant alterations in any of the photosynthetic parameters measured. The psrp2 mutant was virtually identical to wild-type plants for all parameters, whereas the psrp6 mutant showed a subtle trend toward slightly lower contents of thylakoid protein complexes. However, all these changes are within the standard deviation of the wild-type, and therefore not statistically significant (Figure 5).
Finally, we wished to confirm these data by direct analysis of thylakoid protein accumulation. Using antibodies against diagnostic subunits of photosystem II, the cytochrome b6f complex, photosystem I and chloroplast ATP synthase, we performed a series of western blot experiments in which we compared the results for the various ribosomal protein mutants with those for a dilution series of the wild type (Figure 6a). Although the limited dynamic range of western blots does not allow accurate quantification of protein accumulation levels, the observed changes relative to wild-type correlated well with the severity of the phenotypes of the various mutants and the spectroscopic data (3, 5, 6). Moreover, when electrophoretically separated samples of total protein were analyzed by Coomassie staining (Figure 6b), all the ribosomal protein mutants that displayed an abnormal phenotype showed a significant reduction in the most abundant chloroplast protein, Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase).
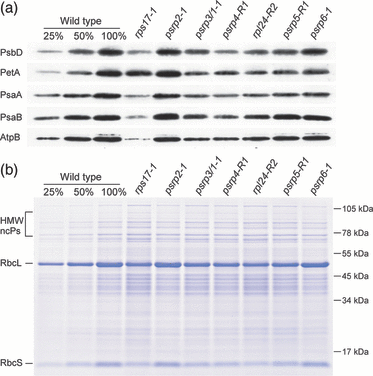
Protein accumulation in ribosomal protein mutants. (a) Accumulation of thylakoid protein complexes. Western blot analyses were performed using antibodies against diagnostic subunits of photosystem II (PsbD subunit), the cytochrome b6f complex (PetA, i.e. cytochrome f), photosystem I (PsaA and PsaB) and the chloroplast ATP synthase (AtpB). For the wild-type (WT), a dilution series is included to enable quantitative assessments. (b) Analysis of separated total protein extracts by Coomassie staining. Reduced accumulation of chloroplast protein complexes in all ribosomal protein mutants that display an abnormal phenotype (see Figure 3) is clearly visible for the most abundant protein in the plant cell, Rubisco. Its large subunit (RbcL) and small subunit (RbcS) are indicated. HMW ncPs, high-molecular-weight non-chloroplast proteins. Note their over-representation in the mutants showing the most severe reduction in Rubisco.
Chloroplast translation in psrp mutants
Impaired growth and reduced photosynthetic performance in the psrp3, psrp4 and psrp5 mutants (3, 5) suggest that the mutant phenotypes are due to defects in chloroplast translation. To assess translational activity in the mutants, the ribosomal association of the rbcL mRNA was comparatively analyzed in wild-type plants, mutant lines for the classic ribosomal proteins S17 and L24, and the psrp2, psrp3, psrp4, psrp5 and psrp6 mutants.
Polysomes were isolated from wild-type and mutant plants and separated in sucrose density gradients by preparative ultracentrifugation. Polysomes are complexes of mRNAs covered with translating ribosomes, and their migration into continuous sucrose gradients upon centrifugation correlates with the number of ribosomes associated with the mRNA molecule. Heavily translated mRNAs are loaded with many ribosomes and migrate deeply into the gradient, whereas poorly translated mRNAs and untranslated mRNAs accumulate in the upper fractions of the gradient. When RNA samples extracted from individual gradient fractions were hybridized to an rbcL-specific probe, identical polysome profiles were obtained for the wild-type and the psrp2 and psrp6 mutants (Figure 7a). This suggests that chloroplast translation proceeds normally in the psrp2 and psrp6 mutants, consistent with their wild-type-like phenotype (Figure 3) and photosynthetic performance (Figure 5). In contrast, the distribution of the rbcL transcript was clearly shifted to lighter fractions in the rps17, rpl24, psrp3/1, psrp4 and psrp5 mutants (Figure 7a). Together with the clearly observable global changes in chloroplast polysome distribution (Figure 7b), this suggests that the growth phenotypes of these mutants are indeed due to impaired plastid translation.
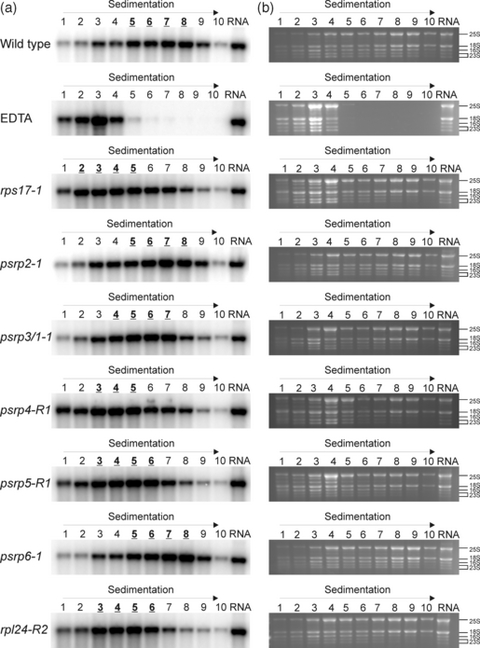
Analysis of polysome loading in psrp mutants. Polysomes were separated in sucrose gradients, and gradient fractions are numbered from top to bottom. Equal aliquots of extracted RNAs from all fractions were separated by denaturing agarose gel electrophoresis. A control gradient containing EDTA (causing polysome disassembly) identifies fractions 1–4 as the fractions that mainly contain free ribosomes. ‘RNA’ denotes a sample of extracted total cellular RNA that was included as a control in all blots. (a) Analysis of polysome loading of the rbcL mRNA by hybridizing RNA gel blots of gradient fractions to an rbcL gene-specific radiolabeled probe. For each plant line analyzed, the three or four fractions containing the bulk of rbcL transcripts are indicated in bold and underlined. Note the virtually identical polysome profiles in the wild type and the psrp2 and psrp6 mutants, but a shift of the peak of rbcL accumulation to lighter fractions in the rps17, rpl24, psrp3/1, psrp4 and psrp5 mutants. (b) Assessment of global changes in polysome distribution by analysis of ribosomal RNA profiles in polysome gradient fractions from wild-type and mutant plants. RNA samples representing one-third of the volume of each gradient fraction were subjected to denaturing gel electrophoresis in 1.5% agarose gels and stained with ethidium bromide. rRNA species are labeled on the right according to their sedimentation coefficients. For the plastid 23S rRNA, the two largest hidden break products (Delp and Kössel, 1991) are indicated. Depletion of plastid rRNA species in the lower gradient fractions (especially in fractions 8-10) is clearly visible for all lines showing mutant phenotypes.
Accumulation of chloroplast ribosomes in psrp mutants
The observed chloroplast translational deficiency in some of our psrp mutants (Figure 7) has two possible molecular causes. First, the PSRPs could be required for plastid ribosome assembly and/or stability, in which case their knockdown would result in chloroplast ribosome deficiency. Alternatively, they could be involved in the regulation of chloroplast translation, as has been proposed previously (Manuell et al., 2007; Sharma et al., 2007). In this case, ribosome accumulation would be normal and the reduced translational activity would solely be due to reduced recruitment of ribosomes to mRNAs (i.e. a reduced efficiency of translation initiation or a defect in translation elongation).
To distinguish between these two possibilities, we used a microfluidics-based platform suitable for sizing and quantification of ribosomal RNA (rRNA) species (Walter et al., 2010). Accumulation of a given rRNA species serves as a proxy for accumulation of the respective ribosomal subunit, because rRNAs do not stably accumulate unless they are incorporated into ribosomal subunits. As the two ribosomal subunits only associate with each other during translation, and otherwise are present in the dissociated state, accumulation of the large ribosomal subunit is independent of accumulation of the small ribosomal subunit and vice versa. Therefore, rRNA quantification is also suitable to distinguish between defects in biogenesis of the large subunit versus the small subunit of the ribosome (Walter et al., 2010).
The ratios of cytosolic to chloroplast ribosomal subunits and the ratios of large (50S) to small (30S) subunits of the chloroplast ribosome were determined for all mutants and compared to those of wild-type plants (Figure 8 and Figure S2). In agreement with the wild-type-like phenotype and chloroplast translational activity of the psrp2 and psrp6 mutants, their plastid ribosome and ribosomal subunit accumulation was nearly indistinguishable from that of wild-type plants, providing further evidence against a fundamental role for these two PSRPs in chloroplast protein biosynthesis. Interestingly, all other ribosomal protein mutants showed pronounced changes in chloroplast ribosome accumulation. As expected, the rps17 mutant showed a specific reduction in the small subunit of the chloroplast ribosome (determined as the ratio of 18S rRNA:16S rRNA), and no significant change in the large subunit of the chloroplast ribosome (determined as the ratio of 18S rRNA:23S rRNA). Consequently, the ratio of 30S:50S subunits was greatly reduced in this mutant (determined as the ratio of 16S rRNA:23S rRNA). Conversely, the rpl24 mutant showed a drastic reduction in the large subunit of the chloroplast ribosome (determined as the ratio of 18S rRNA:23S rRNA) and a greatly increased ratio of 30S:50S subunits (Figure S2). Ribosomal subunit accumulation in the psrp4 mutant was very similar to that in the rps17 mutant. This is in line with the association of PSRP4 with the small subunit of the plastid ribosome. Moreover, this result shows that PSRP4 behaves like a bona fide ribosomal protein, and suggests that this protein is required for stable accumulation of the 30S subunit. Similarly, ribosomal subunit accumulation in the psrp5 mutant was very similar to that in the rpl24 mutant (Figure S2). This is consistent with the association of PSRP5 with the 50S subunit of the plastid ribosome, and suggests that PSRP5 represents a genuine ribosomal protein that is required for assembly and/or stability of the large ribosomal subunit. These changes in ribosomal subunit accumulation became even more apparent when the data were normalized to those for the wild-type and expressed as relative changes (Figure 8). When displayed in this way, a 16S:18S ratio below 1.0 indicates depletion of 30S subunits, a 23S:18S ratio below 1.0 indicates depletion of 50S subunits, and the 16S:23S ratio is a direct measure of the stoichiometry of the two plastid ribosomal subunits (Figure 8).
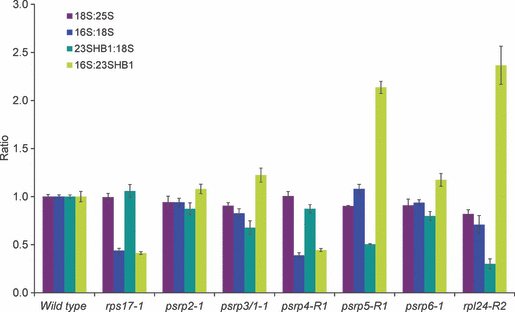
Accumulation of ribosomal RNAs as a proxy for the corresponding ribosomal subunits. Changes in rRNA ratios relative to the wild-type are shown (see text for details and Figure S2 for unnormalized data). 25S, 25S rRNA in the 60S subunit of the cytosolic ribosome; 18S, 18S rRNA in the 40S subunit of the cytosolic ribosome; 23HB1, 1.1 kb fragment of the (post-transcriptionally cleaved) 23S rRNA in the 50S subunit of the chloroplast ribosome (‘hidden break’ product 1); 16S, 16S rRNA in the 30S subunit of the chloroplast ribosome. Data comprise three technical replicates for each plant line, and error bars indicate standard deviation.
Unexpectedly, the psrp3/1 mutant did not behave like a small subunit mutant, but was more similar to the large subunit mutants. Its 16S rRNA:23S rRNA ratio was elevated, indicating that accumulation of the 50S subunit is more strongly affected than accumulation of the 30S subunit (Figure 8 and Figure S2). Thus, although PSRP3 was clearly shown to be physically associated with the small subunit rather than the large subunit of the chloroplast ribosome (Yamaguchi and Subramanian, 2000, 2003; Yamaguchi et al., 2000), its knockout somehow appears to impair accumulation of the large subunit. This could indicate a dual role for the PSRP3 protein in ribosome assembly and/or stability.
rRNA processing defects in psrp mutants
Previous work has established that defects in plastid translation can result in reduced efficiency of chloroplast ribosomal RNA processing, particularly with regard to precursors of the 23S rRNA, 16S rRNA and 4.5S rRNA, which over-accumulate upon impairment of plastid translation, while the 5S rRNA is not affected (Yu et al., 2008). To test whether or not reduced ribosomal subunit accumulation and reduced plastid translation levels in our ribosomal protein mutants are accompanied by defects in rRNA processing, Northern blot experiments with specific probes for all four plastid rRNA species were performed. These analyses revealed partially defective rRNA processing, the intensity of which largely correlated with the severity of the mutant phenotype (Figure 9). The observed defects were very similar to those reported previously for the chloroplast translation mutant svr1, which harbors a disrupted gene for a chloroplast-localized homolog of pseudouridine synthase (Yu et al., 2008). Specifically, over-accumulation of the 4.5S +23S rRNA precursor was seen, as well as over-accumulation of several other 23S rRNA precursor species and/or processing intermediates and a specific 16S rRNA precursor (Figure 9). Overall, the similar patterns of these rRNA processing defects in several otherwise unrelated translation mutants suggest that the defects represent a secondary consequence of impaired chloroplast translation.
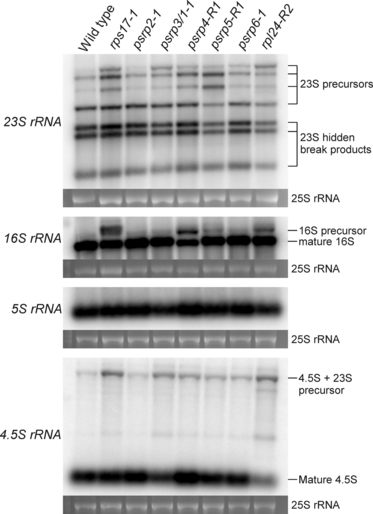
Plastid ribosomal RNA accumulation and rRNA processing defects in psrp mutants. Samples of 2 μg total cellular RNA were electrophoretically separated in denaturing 1.5% agarose gels. Northern blots were hybridized to specific probes for all four rRNA species of the chloroplast ribosome as indicated on the left. Precursors and mature rRNA species are labeled on the right. To confirm equal loading, the ethidium bromide-stained agarose gel was photographed prior to blotting, and the 25S rRNA of the cytosolic ribosome is shown below each blot.
Discussion
Similar to bacteria, most protein subunits of the plastid 70S ribosome are encoded by essential genes, and only few knockouts of plastid ribosomal protein genes give rise to viable plants in the model species A. thaliana and Nicotiana tabacum (Pesaresi et al., 2001; Morita-Yamamuro et al., 2004; Rogalski et al., 2006, 2008b). This is consistent with the absolute requirement for plastid translation for cell survival in most dicotyledonous plants (Ahlert et al., 2003; Rogalski et al., 2008a). In contrast, at least some monocotyledonous plants (Han et al., 1992; Hess et al., 1993) and a few exceptional dicot species (Zubko and Day, 1998) appear to be capable of heterotrophic growth in the absence of plastid translation.
An enigmatic aspect of plastid ribosomes is the existence of a set of unique ribosomal proteins, so-called plastid-specific ribosomal proteins (PSRPs). In this study, we have considered the functions of all PSRPs that are present in stoichiometric amounts with the classic ribosomal proteins (Yamaguchi and Subramanian, 2000, 2003; Yamaguchi et al., 2000). This is the case for five of the six PSRPs identified in higher plants, with PSRP1 (recently revealed as a functional homolog of the E. coli cold-shock protein pY; Sharma et al., 2007, 2010) being the only exception. Based on structural studies, PSRPs were proposed to participate in light-dependent regulation of chloroplast translation (Manuell et al., 2007). However, our reverse genetics analyses of five PSRPs in Arabidopsis suggest that at least three of them (PSRP3, PSRP4 and PSRP5) behave like bona fide ribosomal proteins and are unlikely to solely play a regulatory role in translation. Their knockout by T-DNA insertion or knockdown by RNAi results in partial loss of 30S or 50S ribosomal subunits (Figure 8 and Figure S2), and consequently reduced plastid translation (Figure 7). This general role in plastid protein biosynthesis most likely explains the impaired growth as well as the pigment deficiency and reduced photosynthetic performance of the psrp3, psrp4 and psrp5 mutants (3, 5).
The loss of ribosomal subunits in mutants for psrp3, psrp4 and psrp5 (Figure 8 and Figure S2) raises the interesting question why plastid ribosomes need these additional ribosomal proteins, whereas ribosomes in E. coli can stably assemble without these proteins. One possibility is that differences in the amino acid sequence of ribosomal proteins and/or the nucleotide sequence of rRNAs result in structural or conformational differences (Sharma et al., 2007) that create the need for additional ribosomal proteins in plastids. Alternatively, the roles played by PSRPs in chloroplasts may be played by auxiliary ribosome assembly factors in E. coli that are not stably associated with ribosomes.
In contrast to PSRP3, PSRP4 and PSRP5, proteins PSRP2 and PSRP6 appear to be dispensable for normal growth of Arabidopsis plants. None of the growth conditions tested so far revealed any phenotypic difference between wild-type plants and psrp2 or psrp6 mutants. Also, the photosynthetic parameters and chloroplast translational efficiency are comparable with those of wild-type plants, arguing against an important role for these two PSRPs in chloroplast translation and/or translational regulation. Most importantly, the maximum quantum efficiency of photosystem II (FV/FM), a sensitive measure of photosystem II integrity, was unaltered in both mutants compared to the wild-type. The D1 reaction center protein of photosystem II has the highest turnover of all plastid proteins, and requires constant re-synthesis during repair of photooxidatively damaged photosystem II. Therefore, the parameter FV/FM is highly sensitive to reduced chloroplast translational activity (Rogalski et al., 2008b), and the absence of change in FV/FM in the psrp2 and psrp6 mutants, in addition to the unaltered polysome loading, provides strong evidence of wild-type-like translation capacity in these mutants (5, 7).
Based on structural studies, PSRP6 has been suggested to be only relatively loosely bound to the ribosome, while PSRP2 has been proposed to structurally compensate for a missing segment of the 16S rRNA in the 30S ribosomal subunit (Sharma et al., 2007). Our data do not exclude the possibility of such a structural function, but do argue against a strong importance of PSRP2 in ribosome function or assembly. However, it remains possible that PSRP2 or PSRP6 play a more significant role under special environmental conditions, and thus confer some selective advantage.
PSRP3 is unusual in that its knockout does not specifically affect the small (30S) subunit of the chloroplast ribosome. Instead, the psrp3 mutant lines are more similar to mutants for constituents of the large (50S) ribosomal subunit (Figure 8 and Figure S2). The reason for this unusual behavior of PSRP3 is currently unknown. From biochemical studies (Yamaguchi and Subramanian, 2000, 2003; Yamaguchi et al., 2000), it appears clear that PSRP3 is physically associated exclusively with the small subunit of the chloroplast ribosome. Likewise, structural studies have shown that PSRP3 is located in the 30S subunit, where it appears to form a protrusion together with PSRP2 (Sharma et al., 2007). As the 30S and 50S subunits assemble and accumulate largely independently of each other (Kaczanowska and Rydén-Aulin, 2007), the effect of the psrp3 knockout on the large subunit of the plastid ribosome is somewhat puzzling. Recent work in both prokaryotes and eukaryotes has revealed a growing number of ribosomal proteins that have dual functions, and, in addition to their role in the ribosome, are involved in other processes, including various steps in gene expression (Wool, 1996; Revenkova et al., 1999; Warner and McIntosh, 2009). An example for plastids is ribosomal protein L4, which, in addition to its structural function in the 50S subunit of the plastid ribosome, may be involved in transcriptional regulation (Trifa et al., 1998; Trifa and Lerbs-Mache, 2000). An additional function of PSRP3 in the expression of plastid rpl genes or assembly of the 50S subunit of the chloroplast ribosome could provide a possible explanation for the observed effect on the large ribosomal subunit in the psrp3 knockout mutant. However, whether or not PSRP3 really represents a dual-function ribosomal protein remains to be investigated.
Another interesting aspect of the psrp3 knockout mutants lies in the disproportionately strongly affected leaf anatomy (Figure 4). Although showing only a moderate reduction in chloroplast ribosome content (Figure 8 and Figure S2) and a weaker growth phenotype than the rps17-1, psrp4-R1 and rpl24-1 mutants, for example (Figure 3), palisade parenchyma development was strongly affected. In fact, the psrp3 knockout mutants had a very similar leaf anatomy to the mutant with the most severe phenotype, psrp5-1 (Figure 3), in that palisade cell differentiation was virtually completely blocked and there was no recognizable visual difference between palisade cells and spongy parenchyma cells with respect to either size or cell shape (Figure 4). This may indicate that the retrograde signaling pathway required for palisade cell development (Rodermel, 2001) is largely disrupted in these two mutants, and, moreover, may be further evidence that, in addition to being a ribosomal protein of the 30S subunit, PSRP3 has another cellular function(s) that has not yet been recognized.
In summary, our data suggest that the five stoichiometrically present PSRPs of higher plants fall into two distinct types. PSRP3, PSRP4 and PSRP5 qualify as bona fide ribosomal proteins. They are important constituents of the chloroplast ribosome, and their deficiency results in loss of the 30S or 50S ribosomal subunits, and consequently reduced plastid translation. In contrast, PSRP2 and PSRP6 are non-essential ribosomal proteins that are not required for stable ribosome assembly and translation under greenhouse conditions. Finally, PSRP3 represents a special case and may be a dual-function ribosomal protein with ‘moonlighting’ functions in gene expression or signaling that remain to be discovered.
Experimental procedures
Plant material and growth conditions
Arabidopsis thaliana Heynh. wild-type and mutant plants (ecotype Col-0) were grown in controlled environment chambers. Phenotypic assays were performed under long-day conditions (16 h light/8 h dark regime) at a light intensity of 120 μE m−2 sec−1. Homozygous T-DNA insertion lines from the Salk (Alonso et al., 2003) and GABI-Kat (Rosso et al., 2003) collections were identified by PCR using genomic DNA- and T-DNA-specific primers (LB-SALK-b1.3, LB-GABI and LB-SAIL_1; see Table S1 for sequences) in combination with gene-specific primers (Table S1). Wild-type alleles were amplified using the gene-specific RP primer in combination with the gene-specific LP primer (Table S1). T-DNA insertion sites were determined by sequencing of PCR products generated with LB and RP primers using LB as the sequencing primer. Physiological measurements were performed on plants that had fully developed rosettes, and material for molecular analyses was collected from plants that had developed one pair of true leaves.
Plant transformation and isolation of RNAi lines
The transformation vectors used for down-regulation of plastid ribosomal proteins were obtained from the AGRIKOLA collection of RNAi vectors (Hilson et al., 2004). The gene-specific tag of PSRP5 (CATMA 3c57833) was recombined from the pENTR207 vector into pK7GWIWG2(I) (Karimi et al., 2002). The length of the gene-specific tags and their positions within the coding regions of the corresponding plastid ribosomal protein genes are listed in Table S2 and shown schematically in Figure 2. The plasmids were transformed into Agrobacterium tumefaciens strain GV3101::pMP90::pSOUP by electroporation. A. thaliana Col-0 plants were transformed by the floral-dip method (Clough and Bent, 1998). Transgenic pAGRIKOLA plants were selected for BASTA resistance by spraying seedlings with phosphinotricine at a concentration of 40 μg ml−1 five times at 2-day intervals. Transgenic pK7GWIWG2(I) plants were selected for kanamycin resistance by germination of surface-sterilized seeds on agar-solidified half-strength MS medium (Duchefa, http://www.duchefa.com/) containing 1% sucrose, 50 mg L−1 kanamycin and 125 mg L−1 Betabactyl (Duchefa). Integration of the hairpin-containing T-DNA was confirmed by PCR using genomic DNA and the primer combinations P51/P56, P64/P69, P51/P64 and P56/P69 for transgenic pAGRIKOLA plants and PK1/PK2, PK3/PK4, PK1/PK3 and PK2/PK4 for transgenic pK7GWIWG2(I) plants (see Table S3 for sequences).
Isolation of nucleic acids and hybridization procedures
Total plant DNA was isolated from fresh leaf material by a rapid mini-prep procedure (Doyle and Doyle, 1990). Total cellular RNA for quantitative RT-PCR applications was isolated using a NucleoSpin RNA plant kit (Macherey-Nagel, http://www.mn-net.com/) according to the manufacturer’s instructions. RNA for Northern blotting was extracted using the peqGold TriFast reagent (Peqlab, http://www.peqlab.de/).
For Northern blot analysis, RNA samples were electrophoretically separated in formaldehyde-containing 1.5% agarose gels and transferred onto Hybond XL membranes (GE Healthcare, http://www.gehealthcare.com) by capillary blotting using standard protocols. A 565 bp SacII/PstI restriction fragment covering part of the rbcL coding region was used as a hybridization probe for polysome analysis. Hybridization probes for plastid ribosomal RNAs were generated by PCR amplification using specific oligonucleotides (Table S4). Prior to labeling, DNA fragments were purified by agarose gel electrophoresis followed by extraction from an excised gel slice using a NucleoSpin Extract II kit (Macherey-Nagel). Hybridization probes were labeled with α[32P]dCTP by random priming (Multiprime DNA labeling system; GE Healthcare). Hybridizations were performed at 65°C in Rapid-Hyb buffer (GE Healthcare) according to the manufacturer’s instructions.
Polysome loading assays and quantification of ribosomes
Isolation of polysomes and RNA extraction from sucrose gradient fractions were performed as described previously (Barkan, 1998; Kahlau and Bock, 2008). Gradient fractionation was performed using an Auto Densi-Flow (Labconco, http://www.labconco.com/) and a Pharmacia LKB RediFrac fraction collector (GE Healthcare). RNA pellets were dissolved in 30 μl sterile Tris/HCl pH 7.6, and 10 μl aliquots were used for Northern blot analyses. Control gradients for identification of fractions containing free mRNAs contained 20 mm EDTA.
Ribosomal RNAs were analyzed and quantified in total seedling RNA preparations using an Agilent Technologies 2100 Bioanalyzer (http://www.agilent.com), the Agilent RNA 6000 Nano kit and the software provided by the supplier.
PCR, cDNA synthesis and DNA sequencing
RNA samples treated with rDNase (Macherey-Nagel) were reverse-transcribed using SuperScript III reverse transcriptase (Invitrogen, http://www.invitrogen.com/) and oligo(dT)18 primer according to the manufacturer’s instructions. cDNAs were used as templates for quantitative real-time PCR with gene-specific primers (Table S5). Real-time PCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems, http://www.appliedbiosystems.com/) using Absolute SYBR Green ROX mix (Thermo Scientific, http://www.thermofisher.com/). EF-1α (At5g60390) and UBQ10 (At4g05320) were detected in parallel and used as internal standards (Table S5). Three biological and three technical replicates were analyzed. The 2−ΔΔCT method was used to determine the relative transcript levels (Livak and Schmittgen, 2001).
For DNA sequencing, amplification products were separated by electrophoresis in 1.0% agarose gels and purified from excised gel slices using a NucleoSpin Extract II kit (Macherey-Nagel).
Subcellular localization analyses and microscopic techniques
For construction of GFP fusions, the complete coding sequences (lacking the stop codon) of the plastid ribosomal proteins were amplified using Phusion DNA polymerase (http://www.finnzymes.com) and the gene-specific primers listed in Table S6. The primer sequences introduced restriction sites for XhoI and SpeI into the PCR product. After digestion with the restriction enzymes XhoI and SpeI, the amplification products were cloned into the respective restriction sites of the pA7-GFP vector (Voelker et al., 2006), generating in-frame translational fusions between the ribosomal protein gene and the coding sequence of GFP. Preparation and transient transformation of tobacco (Nicotiana tabacum L.) protoplasts were performed as described previously (Huang et al., 2002). Subcellular localization of GFP fluorescence was determined by confocal laser-scanning microscopy (TCS SP5; Leica Microsystems CMS GmbH, http://www.leica.com/) using a HCx PL APO 63x W objective lens. Excitation wavelengths and emission filters were 488 nm/band-pass 505–530 nm for GFP, and 488 nm per long-pass 650–710 nm for chlorophyll fluorescence. Image processing was performed using Leica confocal LAS AF version 2.3.1 build 5194 software (http://www.leica-microsystems.de).
For light microscopic analysis of leaf anatomy, tissue samples of approximately 1 mm3 were fixed with 4% paraformaldehyde and 0.2% glutaraldehyde in 0.1 m phosphate buffer (pH 7.4), vacuum-infiltrated and incubated at 4°C overnight. The samples were then briefly rinsed in the same buffer and dehydrated in an ethanol series (30, 50, 70, 80, 90 and 100%) with incubation times of 1 h in each solution. Subsequently, the samples were infiltrated with Technovit 7100 resin (Heraeus Kulzer, http://www.kulzer-technik.de) for up to 24 h, followed by polymerization at room temperature (Beeckman and Viane, 2000). Light microscopic analysis was performed on 5 μm cross-sections cut using a rotary microtome (RM 2265; Leica) and placed on poly-l-lysine-coated glass slides (Sigma, http://www.sigmaaldrich.com/). The slides were dried at 37°C for 2 h on a heating plate, and then stained with toluidine blue O (0.05%). Finally, the sections were examined with a motorized epi-fluorescence microscope (Olympus BX 61, http://www.olympus-global.com/) using cell∧P software (Olympus, http://www.olympus.de).
Protein isolation and immunoblotting
Thylakoid proteins from wild-type and transplastomic plants were isolated from total leaf material as described previously (Machold et al., 1979). Samples were loaded on Tricine/SDS polyacrylamide gels (Schägger and von Jagow, 1987) on the basis of equal leaf area, electrophoretically separated, and transferred to Hybond-P PVDF membranes (GE Healthcare) using a Trans-Blot cell (Bio-Rad, http://www.bio-rad.com/) and a standard transfer buffer (192 mm glycine, 25 mm Tris, pH 8.3). Immunoblot detection was performed with specific antibodies using an enhanced chemiluminescence system (ECL® PLUS system; GE Healthcare). Total plant protein was isolated, electrophoretically separated and analyzed by Coomassie staining as described previously (Zhou et al., 2008).
Determination of chlorophyll contents, fluorescence measurements and difference absorption spectroscopy
Chlorophyll contents were determined in 80% v/v acetone (Porra et al., 1989). Chlorophyll fluorescence was recorded using a pulse-amplitude modulated fluorimeter (DUAL-PAM-100; Heinz Walz GmbH, http://www.walz.com) at room temperature using intact plants. Plants were dark-adapted for 1 h prior to determination of the maximum quantum efficiency of photosystem II (FV/FM). The contents of photosystem II, the cytochrome b6f complex and photosystem I were determined in thylakoids isolated according to published procedures (Schöttler et al., 2004). Photosystem I content was quantified on the basis of P700 difference absorption signals at 830-870 nm in solubilized thylakoids using the DUAL-PAM instrument (Schöttler et al., 2007a,b). Plastocyanin was quantified by measuring difference absorption changes at 870–950 nm. Contents of photosystem II and the cytochrome b6f complex were determined by difference absorption measurements of cytochrome b559 (photosystem II) and cytochromes f and b6 (cytochrome b6f complex). Measurement and de-convolution methods have been described in detail previously (Kirchhoff et al., 2002; Schöttler et al., 2007a,b).
77 K chlorophyll a fluorescence emission spectra were recorded using a Jasco F-6500 fluorimeter (Jasco GmbH, http://www.jasco.de) using isolated thylakoids (equivalent to 10 μg chlorophyll ml−1). Chlorophyll a fluorescence was excited at 430 nm wavelength (10 nm spectral bandwidth). Fluorescence emission was determined using a spectral bandwidth of 1 nm at wavelengths of 660–800 nm, and normalized to the photosystem II signal.
Acknowledgements
We thank Linda Bartezko for help with plant transformation, the Max Planck Institut für Molekulare Pflanzenphysiologie Green Team for plant care, Josef Bergstein for photography, Yvonne Weber for help with protoplast transformation and Marc Lohse (all Max-Planck-Institut für Molekulare Pflanzenphysiologie) for help with microscopy. We are grateful to Rodrigo Caroca and Alois Schweighöfer (both Max-Planck-Institut für Molekulare Pflanzenphysiologie) for helpful discussion. We thank the Salk Institute Genomic Analysis Laboratory and the GABI consortium for providing sequence-indexed Arabidopsis T-DNA insertion mutants. This research was supported by the Deutsche Forschungsgemeinschaft (BO1482/15 within FOR 804 and SFB 429 A12).




