The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry
Present address: UMR Génétique et Horticulture (GenHort), IFR149 QUASAV, Centre INRA Angers-Nantes, BP 60057, 49071 Beaucouzé, France.
Summary
Flowering is a key event in plant life, and is finely tuned by environmental and endogenous signals to adapt to different environments. In horticulture, continuous flowering (CF) is a popular trait introduced in a wide range of cultivated varieties. It played an essential role in the tremendous success of modern roses and woodland strawberries in gardens. CF genotypes flower during all favourable seasons, whereas once-flowering (OF) genotypes only flower in spring. Here we show that in rose and strawberry continuous flowering is controlled by orthologous genes of the TERMINAL FLOWER 1 (TFL1) family. In rose, six independent pairs of CF/OF mutants differ in the presence of a retrotransposon in the second intron of the TFL1 homologue. Because of an insertion of the retrotransposon, transcription of the gene is blocked in CF roses and the absence of the floral repressor provokes continuous blooming. In OF-climbing mutants, the retrotransposon has recombined to give an allele bearing only the long terminal repeat element, thus restoring a functional allele. In OF roses, seasonal regulation of the TFL1 homologue may explain the seasonal flowering, with low expression in spring to allow the first bloom. In woodland strawberry, Fragaria vesca, a 2-bp deletion in the coding region of the TFL1 homologue introduces a frame shift and is responsible for CF behaviour. A diversity analysis has revealed that this deletion is always associated with the CF phenotype. Our results demonstrate a new role of TFL1 in perennial plants in maintaining vegetative growth and modifying flowering seasonality.
Introduction
Obtaining plants that flower over a long period is the goal of many gardeners, so as to be able to achieve year-round fruit and flower production. The flowering period can be lengthened by exploiting the blossoming duration or number of flowering cycles. Most plants have a single annual flowering period (once-flowering habit, OF). Some perennial plants have the ability to flower again during the year: they can flower continuously during the favourable season (continuous flowering habit, CF) or they may only have a second bloom later in the season, which may be occasional (occasional re-blooming habit, OR). In some Rosoidae, such as rose and strawberry species, the CF trait is present, and is controlled by a recessive major gene named RECURRENT BLOOMING (RB) and SEASONAL FLOWERING LOCUS (SFL) in diploid rose (Semeniuk, 1971) and woodland strawberry (Fragaria vesca) (Brown and Wareing, 1965; Albani et al., 2004), respectively. In rose and woodland strawberry, the origin of continuous flowering is uncertain. The phenotype may have arisen from a mutation in natural populations. The rose mutant was thought to be selected from wild Rosa chinensis in China (Hurst, 1941), used in rose breeding as early as the Song Dynasty (ad 960–1279; Ogisu, 1996). Later, in the 18th century, cultivated CF Chinese roses were introduced to Europe and crossed with OF European roses to give rise to modern roses (Hurst, 1941). Although mentioned as early as 1553, the ‘perpetual’ woodland strawberry, native to the Alpine mountain region, was clearly described in 1766 and then introduced to gardens all over Europe (Duchesne, 1766). These F. vesca semperflorens plants were considered as mutants of OF woodland strawberry (Darrow, 1966). The potential of continuous flowering for flower and fruit production from spring to late fall explains the high gardening success of these CF roses and woodland strawberries.
In rose and woodland strawberry, the CF habit affects not only flowering but also other important developmental characters: CF plants have a short juvenile phase and flower rapidly after seed germination (Duchesne, 1766; Vries, 1976). In CF roses, all shoots are terminated in an inflorescence (determinate growth) (Figure 1b; Vries, 1976). In OF roses, the primary shoots remain vegetative with indeterminate growth, and flowering only occurs on axillary secondary shoots (Figure 1a). In woodland strawberry, the terminal meristem differentiates an inflorescence. Axillary meristems ensure the continuity of vegetative development in the OF habit, whereas in the CF habit, they also differentiate inflorescences as long as the growing conditions are favourable (Brown and Wareing, 1965; Savini et al., 2006). In OF roses and woodland strawberry, flowering is under environmental control (Battey et al., 1998; Foucher et al., 2008). CF roses are self-inductive and need no environmental control for flowering (Halevy, 1972). There have been conflicting reports on the environmental control of flowering in CF woodland strawberries, which could have lost photoperiodic and thermal control (Battey et al., 1998), or could have become long-day plants (Sonsteby and Heide, 2008).
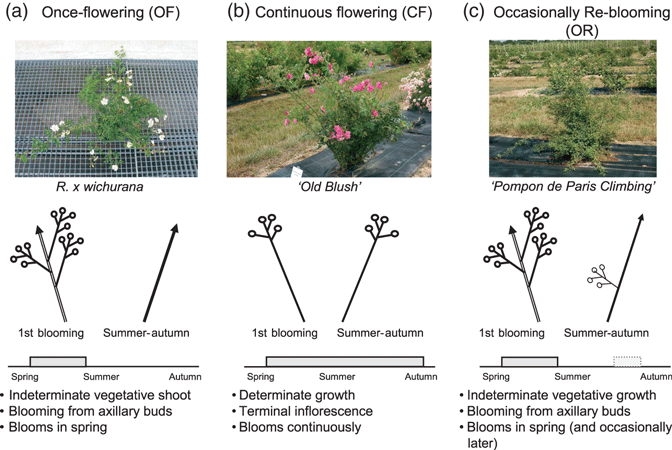
Different blooming modes in rose. (a) once-flowering genotypes (as Rosa wichurana) flower once a year in spring. Terminal inflorescences are borne by lateral shoots, arising from axillary buds of shoots from the previous year. Then, after the first blooming, new developing shoots (especially basal, reiteration shoots) have an indeterminate vegetative growth. In the next spring, from these shoots, axillary shoots will develop and terminate in an inflorescence. (b) Continuous flowering genotypes (such as the old Chinese cultivated variety ‘Old Blush’) flower during the growing seasons. All growing shoots terminate in an inflorescence (determinate growth). (c) Occasionally re-blooming (OR) genotypes, such as the vegetative mutants of continuous flowering genotypes (sports) ‘Pompon de Paris Climbing’, flower in spring from axillary shoots that develop terminal inflorescences. After spring, the new arising shoots have an indeterminate vegetative growth. Occasionally, the new arising shoots produce axillary shoots with terminal inflorescences during the late summer and early autumn (OR habit). Double and single lines represent shoots from the previous and current years, respectively. Arrows represent indeterminate vegetative growth. Flowers are represented by circles. For the three phenotypes, the duration of flowering is presented by a blue box. Occasional blooming is represented by a blue box with dotted lines.
In Chinese rose and woodland strawberry, as the mutation leading to CF is recessive and affects flowering and determinate/indeterminate growth, good candidate genes are TERMINAL FLOWER 1 (TFL1) homologues. Indeed TFL1 homologues control shoot meristem identity by repressing the floral transition. TFL1 homologues are required to maintain the inflorescence identity of the shoot apical meristem: examples are Arabidopsis thaliana (Bradley et al., 1997; Ratcliffe et al., 1998), Antirrhinum majus (Bradley et al., 1996), Pisum sativum (pea; Foucher et al., 2003) and Glycine max (soya bean; Liu et al., 2010; Tian et al., 2010). Furthermore, in Arabidopis and pea, TFL1 also influences the length of the vegetative phase (Bradley et al., 1997; Foucher et al., 2003). In Arabidospis, tfl1 mutants flower earlier. In perennial tomato, SELF PRUNING, the homologue of TFL1, controls the length of the sympodial unit. After flowering, sp mutants produce only a few sympodial units, with fewer and fewer nodes, leading to a terminal inflorescence with no repetition of the sympodial unit (Pnueli et al., 1998). In Populus sp. (poplar), TFL1 does not modify indeterminate growth, but rather regulates the first onset of flowering, axillary meristem identity and dormancy release (Mohamed et al., 2010). In Vitis vinifera (grapevine), a tfl1 mutant causes a modification in the inflorescence architecture (Fernandez et al., 2010). In Arabidposis thaliana, TFL1 belongs to a larger family, represented by six proteins (Kobayashi et al., 1999). Among them, FLOWERING LOCUS T (FT) and TWIN SISTER OF FT (TSF) are floral activators (Kardailsky et al., 1999; Kobayashi et al., 1999). Until recently, the biochemical nature of florigen remained elusive, until breakthrough experiments in A. thaliana (Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007; Notaguchi et al., 2008), Solanum lycopersicum (tomato; Lifschitz et al., 2006), Oryza sativa (rice; Tamaki et al., 2007) and Cucurbita moschata (pumpkin; Lin et al., 2007) revealed that the protein encoded by the FT gene is the florigen.
Here we investigated the function of TFL1 homologues in Rosoidae, and we demonstrated that continuous flowering in rose and woodland strawberry is controlled by a TFL1 homologue.
Results
SEASONAL FLOWERING (SFL) and RECURRENT BLOOMING (RB) are orthologous loci in strawberry and rose
As rose and strawberry are phylogenetically closely related species (both belonging to the Rosoideae tribe of the Rosaceae family; Potter et al., 2007), and as the genetic control is the same in both (recessive gene in both cases), we attempted to determine by comparison mapping if the two loci controlling continuous flowering in rose and woodland strawberry, i.e. RB and SFL, respectively, are orthologues. RB and SFL were mapped on the rose linkage group RG3 and the strawberry linkage group FG6, respectively (Sargent et al., 2006; Spiller et al., 2011). Eight Rosa markers located on RG3 had one significant match to the Fragaria genome, with a BLASTN score of <E-11. These eight Fragaria matches were located on the Fragaria chromosome FG6 (Figure S1). The colinearity of these loci was conserved, except for two loci that displayed small rearrangements. Therefore, a large segment of rose linkage group RG3 was orthologous to the upper part of the strawberry FG6, which gave rise to the hypothesis of an orthologous relationship between the genes controlling recurrent flowering in Rosa and Fragaria.
RoKSN and FvKSN are TFL1 homologues and co-localise with the RB and SFL loci, respectively
Using degenerate primers in rose and results from the strawberry genome sequence (Shulaev et al., 2011), we isolated four and seven TFL1/FT homologues in rose and strawberry, respectively. We identified two TFL1 homologues in rose and strawberry as good candidates for the continuous flowering gene, termed KSN (Koushin, an old Japanese name for the Chinese CF rose cultivar). RoKSN and FvKSN clearly belonged to the TFL1 clade, along with RoTFL1 and FvATC; whereas RoFT, FvFT, FvFT2 and FvFT3 were included in the FT clade and one (FvMFT) in the MFT clade (Figure 2). We also identified two TFL1/FT homologues (RoTFL1b and FvTFL1b) that are closely related to AtBFT.
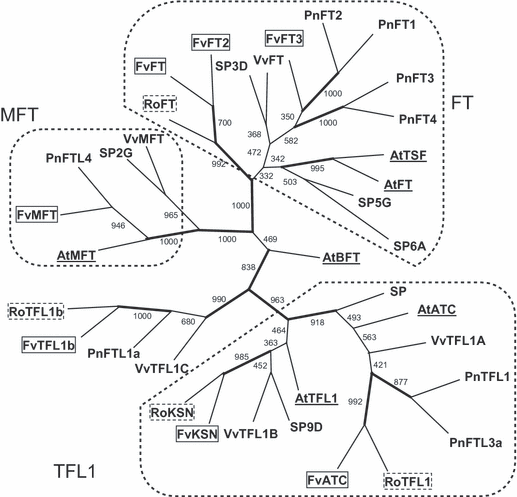
Phylogenetic analysis of members of the FT/TFL1 family in rose and woodland strawberry. The TFL1 homologues RoKSN and FvKSN belong to a small gene family. RoKSN (HQ174211), FvKSN (HQ378595), RoTFL1 (FM999796) and FvATC (21992) belong to the TFL1 clade. Four homologues [FvFT1 (04680), FvFT2 (28959), FvFT3 (21535) and RoFT (FM999826)] belong to the FT clade, one homologue [FvMFT (09405)] belongs to the MFT clade and two more homologues [FvTFL1b (13304) and RoTFL1b (HQ174212)] are related to AtBFT. The tree was constructed using the neighbour-joining method with the TFL1 family protein from rose (outlined with dotted lines), woodland strawberry (outlined with solid lines), Arabidopsis (underlined; Kardailsky et al., 1999; Kobayashi et al., 1999), tomato (Carmel-Goren et al., 2003), grapevine (Carmona et al., 2007) and poplar (Igasaki et al., 2008). Bootstrap values for 1000 re-samplings are shown on each branch. Branches with a bootstrap value >700 (of 1000) are shown with a thick line. The dashed lines delineate the different clades (TFL1, FT and MFT).
Using large populations that segregated for the OF/CF trait, we found that RoKSN and FvKSN co-localized with the RB and SFL locus in rose and woodland strawberry, respectively. No recombinant was found between the RoKSN/FvKSN and RB/SFL loci in 670 individuals from two F1 progenies obtained via the cross between CF and OF roses, and in 158 S1 selfed progeny of F. vesca Ilaria, respectively.
Insertion of a retrotransposon in RoKSN is responsible for the recurrent blooming character
By analysing seven CF roses of independent origins, ascertained by pedigree (Cairns, 2000), we detected the presence of a retrotransposon in the second intron of RoKSN (Figure 3). All CF roses were homozygous for the RoKSN allele with the retrotransposon. The 9-kbp long retrotransposon presented characteristics of the copia retrotransposon family (Kumar and Bennetzen, 1999). This large insertion may lead to an inactive RoKSN allele (see results below).
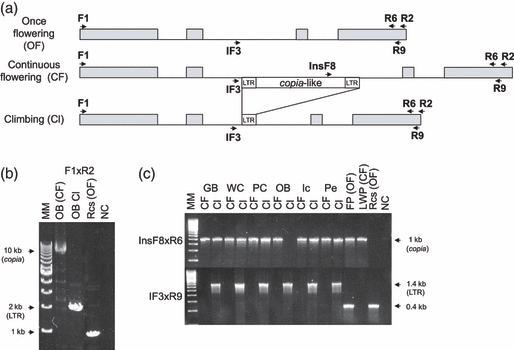
Insertional polymorphism at the RoKSN locus between once-flowering and continuous-flowering (OF and CF, respectively) mutant roses. (a) Schematic representation of the RoKSN genomic region for the three different alleles. In the second intron, the CF allele had a 9-kbp copia-like retrotransposon, the ‘climbing’ (Cl) allele just had a long terminal repeat (LTR) element, whereas the OF allele had no insertion. Exons are represented by grey boxes, introns are represented by black lines and primers are indicated with arrows. (b) PCR amplification of RoKSN in Chinese roses. The CF Chinese rose ‘Old Blush’ (OB), a progenitor used to introduce continuous flowering, had a copia-like retrotransposon inserted in the second intron, whereas OF wild Chinese rose (Rosa chinensis spontanea, Rcs) had no retrotransposon. The climbing vegetative mutant of ‘Old Blush’ (OB Cl) only had the LTR element. The F1 × R2 primer pair amplified the complete coding sequence (intron and exon) of RoKSN: 10 kb for the allele with the retrotransposon (copia), 2 kb for the allele with an LTR element and 1 kb for the ancestral allele. (c) Characterization of the retrotransposon insertion in different vegetative mutant pairs using different primer combinations. All tested climbing mutants showed recombination of the retrotransposon with the LTR motif remaining. InsF8 × R6 only amplified the sequence with the retrotransposon (1 kbp amplification, copia), whereas IF3 × R9 only amplified the sequence with the LTR motif (1.4-kbp amplification, LTR) or the allele without insertion (400-bp amplification). In the CF/Cl mutant pairs, the CF rose varieties and their corresponding climbing (Cl) mutants are ‘Gold Bunny’ (GB), ‘Wendy Cusson’ (WC), ‘Pink Chiffon’ (PC), ‘Old Blush’ (OB), ‘Iceberg’ (Ic) and ‘Peace’ (Pe). The OF/CF mutant pair is ‘Félicité et Perpétue’ (FP) and its mutant ‘Little White Pet’ (LWP). MM, molecular marker; NC, negative control (water).
In order to validate the function of RoKSN in continuous flowering control in roses, we studied vegetative mutant pairs showing different flowering behaviours. Frequently, CF roses mutate into climbing roses (Lewis, 1994). These climbing roses have primary shoots with indeterminate vegetative growth, whereas inflorescences are produced by axillary secondary shoots. They bloom in spring, and occasionally they can flower later in autumn (OR; Figure 1c). Only one mutation from OF to CF was described. An OF rose, i.e. ‘Félicité et Perpétue’ (FP), gave a CF dwarf mutant ‘Little White Pet’, LWP (Roberts et al., 1999). Each of the seven mutants studied showed a difference at the RoKSN locus when compared with the wild type. All six climbing mutants had a new allele at the RoKSN locus with a 1-kbp insertion at the same place as the 9-kpb insertion in the second intron (Figure 3). This 1-kbp sequence corresponded to the long terminal repeat (LTR) element of the retrotransposon. Five climbing mutants (‘Gold Bunny Cl.’, ‘Wendy Cusson Cl.’, ‘Pink Chiffon Cl.’, ‘Iceberg Cl.’ and ‘Peace Cl.’) had both the 9-kbp retrotransposon and 1-kbp LTR insertions. One climbing mutant (‘Old Blush Cl.’) only had the 1-kbp insertion (Figure 3). Mutation in climbing roses can be explained by the recombination of the retrotransposon in the climbing mutants. This recombination restores an active RoKSN allele (see discussion below). In the rare OF/CF mutation, FP was found to be heterozygous at the RoKSN locus (allele with and without the retrotransposon), whereas LWP only had the allele with the retrotransposon (Figure 3c). In LWP, by sequencing and Southern blot analyses, only one allele has been found, i.e. that with the retrotransposon (Figures 3c and S2). In this latter genotype, the absence of the active allele (without the retrotransposon) can be explained by the deletion of the allele or a somatic chromatid exchange. Analysis of these independent mutants demonstrated that recombination of the retrotransposon restored an OF phenotype, whereas deletion of the functional allele led to CF roses.
Full-length RoKSN mRNA is not accumulated in CF roses as a result of retrotransposon insertion
We were unable to detect RoKSN mRNA accumulation in CF roses, whereas the transcript accumulated in OF roses (Figure 4b). mRNA synthesis or accumulation might be blocked by the copia-like retrotransposon insertion. To test this hypothesis, RoKSN transcript accumulation was studied by one-step reverse transcription (RT)-PCR in an OF/climbing mutant pair, i.e. ‘Peace’/’Peace Climbing’. In one-step RT-PCR, cDNA synthesis and PCR were performed at the same time using RoKSN-specific primers. As described before, ‘Peace Climbing’ has an allele with the LTR element at the RoKSN locus (Figure 3c). Using primer combination F9XR11 (surrounding intron 1), one-step RT-PCR products were observed in both ‘Peace’ and ‘Peace Climbing’, suggesting that nascent RNA is transcribed and the first intron is spliced out in both (Figure 4a and c). Using primer combination F9XR13 (surrounding intron 2, where the retrotransposon is inserted), one-step RT-PCR products were observed in ‘Peace Climbing’ and in an OF rose (‘Park Yellow Tea Scent’); no product was detected in the CF rose (Figure 4c). Then, using primer combination F9 and primers in the retrotransposon (InsR3, InsR4, InsR5 or InsR8), we observed one-step RT-PCR products in ‘Peace’ corresponding to PCR products that would be expected if the second intron was not spliced out (Figure 4a and d). We conclude that, as a result of the retrotransposon insertion, the second intron is not spliced out in CF roses and no full-length mRNAs are accumulated. In climbing mutants, the recombination of the retrotransposon restored splicing of the second intron and an active RoKSN allele, leading to indeterminate growth and non-continuous flowering.
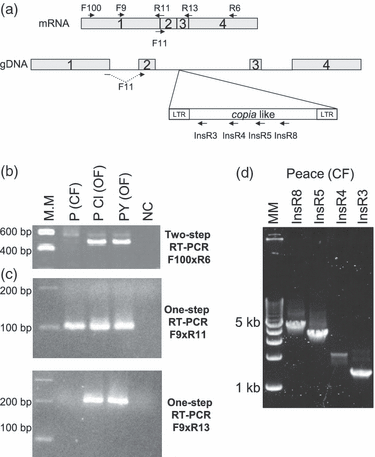
Effect of the insertion of the copia-like retrotransposon on splicing of the second intron of RoKSN. (a) Schematic representation of RoKSN mRNA and genomic (gDNA) region with exons (boxes numbered from 1 to 4), introns (lines) and retrotransposon (insert). Primers used are indicated by arrows. (b) Two-step RT-PCR using an F100 × R6 primer combination (c). One-step RT-PCR using different RoKSN primer pairs. The F9 × R11 primer pair amplified the 100-bp product only if the first intron was spliced out, as the R11 primer spanned the first intron; the F9 × R13 primer pair amplified the 200-bp product only if the second intron was spliced out, as the R13 primer spanned the second intron. (d) One-step RT-PCR using primer pairs of F11 in combination with InsR8, InsR5, InsR4 and InsR3, respectively, located within the retrotransposon. F11 spanned the first intron. RT-PCR was performed on total RNA from terminal shoots of ‘Peace’ (P), a continuous-flowering (CF) rose, its climbing mutant (‘Peace Climbing’, PCl) and a once-flowering (OF) Chinese rose ‘Park Yellow Tea Scented China’ (PY). MM, molecular marker; NC, negative control (water).
FvKSN, the RoKSN orthologue in woodland strawberry controls continuous flowering
Sequencing the entire FvKSN revealed that CF genotypes showed a 2-bp deletion in the first exon that caused a frame shift, which led to the rapid introduction of a stop codon in the putative translated protein and to a non-functional allele named ksn (Figure 5d). In the 158 S1 seedlings, all homo-zygous individuals with a 2-bp homozygous deletion in the recessive locus (ksn/ksn) had a CF habit, whereas all homozygous individuals without a 2-bp deletion (KSN/KSN) or heterozygous (ksn/KSN) had an OF habit.
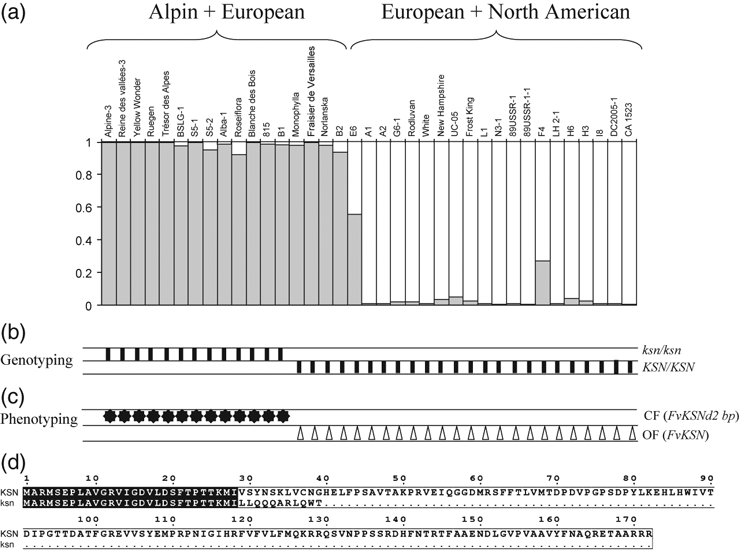
(a) Relationship between the genetic structure of 37 woodland strawberry genotypes, FvKSN genotyping and continuous-flowering (CF)/once-flowering (OF) phenotyping. Estimated genetic structure of woodland strawberry genotypes, based on 11 neutral microsatellites (Table S3). The structure is represented by vertical lines, in which the ancestry proportions of each genotype are indicated by the length of the white and grey segments. (b) Genotypes are distributed in K = 2 ancestry groups (black and white). KSN and ksn are, respectively, the allele without and with the 2-bp deletion. The ksn/ksn or KSN/KSN genotyping of each genotype is indicated by vertical bars. (c) CF/OF phenotyping, indicated by stars and triangles, respectively, for each genotype. (d) Alignment of the protein encoded by FvKSN and the version encoded by the allele with the 2-bp deletion (FvKSNd2bp).
In order to confirm the role of the 2-bp deletion of FvKSN in continuous flowering, we conducted a diversity analysis between the 2-bp deletion and flowering phenotypes using 37 accessions of F. vesca, including 13 CF genotypes. The 37 accessions were available from different genetic resources (Table S1). The sequencing of the entire FvKSN gene in all accessions revealed a total of six SNPs located in introns and the 2-bp deletion in the first exon, which led to six haplotypes (Table S1). Within this genetic polymorphism, only the 2-bp deletion was associated with the CF habit (Figure 5b and c). Using 11 neutral microsatellites, we investigated the population structure in these 37 accessions using structure 2.2. The most likely number of potential clusters (K) was estimated as two. All genotypes except one were clearly ranked within one of the two groups. The first group included 17 genotypes from Europe, mainly from the Alps, whereas the second group included 19 genotypes that originated from Europe/North America (Figure 5a). All CF strawberries, with the 2-bp deletion, were included in the former group, suggesting that in F. vesca continuous flowering may have originated from Alpine genotypes.
The continuous flowering gene shows seasonal regulation in rose
We monitored RoKSN transcript accumulation during the floral process by quantitative (q)RT-PCR. In OF Rosa wichurana, new shoots arose from axillary buds of shoots from the previous year (Figure 1a). After a short vegetative phase (first three stages, W1, W2 and W3), these new axillary vegetative shoots underwent floral differentiation, with transformation of the apical meristem into a dome-like structure (stages W4 and W5). Then the new shoot was terminated by an inflorescence (stages W6–W9). RoKSN transcripts were barely detectable in axillary vegetative shoots before floral differentiation (stages W1–W3). RoKSN transcripts were transiently accumulated during floral differentiation (stage W4) and later during inflorescence development (stages W7 and W8; Figure 6a). During the floral process, RoFT, a floral activator, and later RoLFY, a homologue of the floral identity gene LEAFY (Remay et al., 2009), were progressively accumulated (Figure 6b and 6c). After the first flowering, new shoots arose from the base of the bush and had indeterminate vegetative growth during the rest of the growing season (Figure 1a). An 80-fold increase in RoKSN transcript accumulation was detected when spring (stage W1) and autumn (stage W10) vegetative shoots were compared (Figure 6a). After flowering, RoFT and RoLFY had the same level compared with spring vegetative shoots (stage W1; Figure 6). Therefore, low RoKSN expression in spring was associated with floral transition and determinate growth, whereas high expression of RoKSN in autumn was associated with vegetative and indeterminate growth. In CF roses, functional RoKSN transcripts were not detected (because of retrotransposon insertion), and all new shoots terminated in an inflorescence.
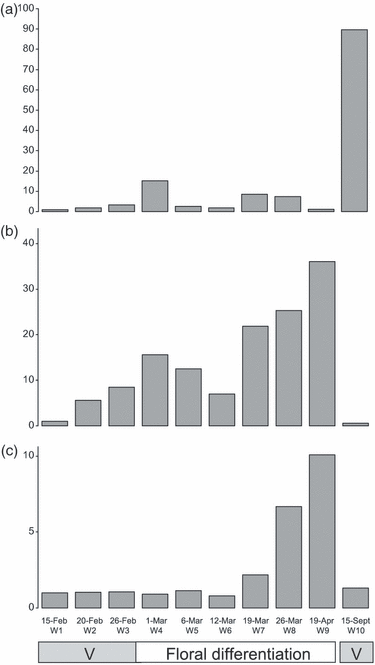
Seasonal accumulation of (a) RoKSN, (b) RoFT and (c) RoLFY transcripts in the once-flowering (OF) genotype Rosa wichurana. Transcript accumulation was monitored by two-step RT-PCR in shoots emerging in spring and later in autumn. In spring, the axillary buds borne on shoots from the previous year burst open and gave new shoots. After a vegetative phase (stages W1, W2 and W3), the meristem became competent for flowering. Then floral differentiation took place (transformation of the apical meristem into a dome-like structure, stages W4 and W5), followed by the development of a terminal inflorescence (stages W6–W9). After the first flowering, new shoots arose and remained vegetative (OF genotypes; Figure 1a, stage W10). The x-axis indicates the dates at which shoot terminal parts were sampled in 2007 and the different stages (W1–W10). The transcript accumulation levels are expressed relative to the first sample for each gene (stage W1, base value = 1). Floral differentiation and inflorescence development stages were determined by histological and microscopic analysis, as previously described (Foucher et al., 2008). For the different stages, the terminal part of the shoot was harvested after rapid dissection (removal of young leaves). Tissues studied represent the shoot apical meristem, the shoot and the new developing leaves (primordia and very young leaves).
Discussion
A floral repressor regulates continuous flowering in rose and woodland strawberry
In rose, we demonstrated that the continuous flowering phenotype was explained by the insertion of a copia-like retrotransposon in the second intron of RoKSN, i.e. a TERMINAL FLOWER 1 homologue. Insertion of the retrotransposon modified RNA maturation. The second intron was not spliced out, and full-length RoKSN RNA was not accumulated (Figure 4). In six independent vegetative mutants, climbing mutant roses differed from CF roses in the size of the insertion, probably as a result of recombination between LTR elements (Figure 3). After retrotransposon recombination, only the solo LTR element remained and the second intron was spliced out, thus restoring a functional RoKSN allele (Figure 4). Such a process has been described in grapevine, where the recombination of a Ty3-gypsy-type retrotransposon restores MYB transcription factors responsible for fruit colour (Kobayashi et al., 2004). In the CF/climbing mutant pair, the restoration of RoKSN in climbing mutants may not be complete as flowering can occur occasionally in the autumn (Figure 1c). The presence of the LTR element in the second intron may modify the level or the spatiotemporal pattern of RoKSN expression. Indeed, LTR elements contain signals for transcription regulation (Kumar and Bennetzen, 1999), which may modify RoKSN expression in climbing mutants. In another vegetative mutant pair (changing from OF to CF behaviour), the functional allele was deleted and only the non-functional allele was present, leading to the continuous flowering phenotype (Figure 3c and S2). Furthermore, the role of KSN in continuous flowering control was strengthened by the absence of recombination between RoKSN and the continuous flowering locus in large F1 progenies segregating for the CF/OF phenotype (in 670 individuals). These results strongly suggest that in rose the continuous flowering gene is encoded by RoKSN, i.e. a TFL1 homologue. Functional validation still needs to be performed in rose by knocking out the gene in OF roses. Presently, no protocols for the genetic transformation of OF roses are available (Debener and Hibrand-Saint Oyant, 2009). However, the conclusion drawn in rose was reinforced by the results obtained in woodland strawberry.
In F. vesca, which also showed continuous flowering in certain genotypes, we identified FvKSN, a gene showing high similarity with RoKSN (Figure 2). Using comparative mapping, we showed that the two genes were located in a syntenic and co-linear region, suggesting that they are orthologues. Furthermore, all CF individuals from an S1 segregating population had a 2-bp deletion in the coding sequence of the gene, whereas the deletion was absent in all OF homozygous genotypes. This deletion induced a frame shift and a stop codon leading to a truncated protein (Figure 5d). We propose that this 2-bp deletion in the coding sequence is responsible for continuous flowering in F. vesca. As no mutant was available in strawberry, we performed a diversity analysis. Among the 37 accessions studied, the 2-bp deletion and the continuous flowering phenotype were always associated (Figure 5). We concluded that FvKSN was the orthologue of RoKSN, and that both genes control the continuous flowering trait.
KSN and the seasonality of flowering
The main consequence of the continuous flowering mutation is the modification of flowering seasonality. Perennial plants repeatedly cycle between vegetative and floral development. Most perennial plants have a single reproductive phase that is synchronized to the changing seasons. OF plants bloom in spring or early summer, and further developments are vegetative. This seasonality might be explained by seasonal regulation of KSN. We showed a seasonal regulation of RoKSN transcript accumulation in OF roses: before the first bloom, RoKSN was barely expressed; in parallel, RoFT and RoLFY, homologues of the floral integrator FT and the floral identity gene LFY (Remay et al., 2009), were progressively accumulated (Figure 6). The low RoKSN level in spring may increase the flowering competence of the apical meristem. Opposite results have been found in other Rosaceae (Japanese pear and quince) where TFL1 homologues are expressed before floral differentiation and are repressed during floral differentiation (Esumi et al., 2007). In all cases, flowering is associated with LFY induction (Figure 6c; Esumi et al., 2007). TFL1 might be differently regulated between rose and pear. The RoKSN expression during floral differentiation (stages W4, W7 and W8; Figure 6a) suggests a role of RoKSN in inflorescence development. Genetic analysis reveals that a quantitative trait locus (QTL) involved in the inflorescence architecture is centred on the RB locus in rose (Kawamura et al., 2011).
After the first blooming, newly arising shoots remained vegetative and RoKSN transcripts accumulated. No RoFT and RoLFY transcripts accumulated. In continuous flowering roses, this regulation was disrupted by the absence of RoKSN transcript accumulation. The floral repressor was absent and flowering occurred during all seasons. This report and a previous study in Arabis alpina (Wang et al., 2009) highlighted the importance of floral repressors in the control of flowering seasonality. In A. alpina, PERPETUAL FLOWERING 1 (PEP1), a homologue of the floral repressor FLOWERING LOCUS C (FLC), limited the duration of flowering and facilitated the return to vegetative development. PEP1 is transiently repressed by low temperature, thereby allowing seasonal flowering of A. alpina (Wang et al., 2009).
KSN and other aspects of plant development
The continuous flowering mutation affects different developmental processes In rose and woodland strawberry, mutation of TFL1 homologues reduces the vegetative phase. CF genotypes can flower rapidly after seed germination (in a few weeks), whereas OF genotypes have a juvenile period of 1 or 2 years (Duchesne, 1766; Vries, 1976). Expression of TFL1 may avoid precocious flowering, as recently demonstrated in A. alpina (Wang et al., 2011). Furthermore, the extinction of the TFL1 homologue in trees (apple and poplar) reduced the juvenile phase (Kotoda et al., 2006; Mohamed et al., 2010). In Citrus and Lolium perenne, accumulation of TFL1 transcripts in juvenile stems was proposed to prevent precocious flowering (Jensen et al., 2001; Pillitteri et al., 2004). All of these results suggest that TFL1 homologues have an important role in controlling juvenility in plants.
Continuous flowering also involves GA signalling, as CF roses are insensitive to GA for flowering, whereas GA inhibits flowering in OF roses (Roberts et al., 1999). KSN might be an integrator of endogenous signals, such as plant hormones, although the interaction with hormones is so far unknown in TFL1 homologues. In tomato, it was proposed that SFT (the FT homologue) may interact with auxin to regulate the sympodial cycle and leaf architecture (Shalit et al., 2009). In Arabidopsis, MOTHER OF FT AND TFL1 (MFT) was shown to regulate seed germination via the GA and ABA signalling pathway (Xi et al., 2010).
Origin of continuous flowering in horticulture
Our results highlighted the origin of CF in two important horticultural plants. In woodland strawberry, CF was well described from the 18th century, and was proposed to have originated in the Alps (Duchesne, 1766). The hypothesis of only one mutation is strengthened by the presence of only one haplotype in CF genotypes with the 2-bp deletion in the FvKSN coding sequence, even though the OF displayed five haplotypes (Figure 5; Table S1). Only one mutation might be responsible for the CF habit in the wood strawberry genotypes studied, despite the fact it has been found growing wild at various sites in the European Alps (De Vilmorin, 1898). This hypothesis has to be validated on a larger number of genotypes. In rose, CF flowering was thought to arise from a mutation in wild Chinese rose and then introduced in old Chinese cultivated roses (Hurst, 1941; Ogisu, 1996). We hypothesize that the mutation may result from the insertion of a retrotransposon into the RoKSN locus in wild R. chinensis spontanea, as the retrotransposon was absent in the RoKSN locus in this wild species (Figure 3b), i.e. an OF rose that is supposed to be an ancestor of the original CF roses (Martyn, 2005).
In conclusion, KSN may be an integrator of endogenous and environmental factors. Understanding the regulation of KSN by these different factors may provide new prospects for controlling flower and fruit production in perennial plants.
Experimental Procedures
Plant material
Rosa wichurana originated from the ‘Jardin de Bagatelle’ (Paris, France). Rosa hybrida‘Félicité et Perpétue’ and its mutant ‘Little White Pet’ were obtained from Loubert Nursery (Les Rosiers sur Loire, France). Rosa chinensis spontanea was a gift from Mr Ogisu. ‘Gold Bunny’, ‘Wendy Cussons’, ‘Pink Chiffon’, ‘Old Blush’, ‘Iceberg’, ‘Peace’ and the corresponding climbing mutants were obtained from Hiroshima Botanical Garden (Japan), and are of independent origins (Cairns, 2000). The mutants are vegetative mutants, also known as sports. A sport of a clonally propagated plant arises as a mutant cell in the shoot meristem (Lewis, 1994). Rose F1 progenies were obtained from a cross between R. whichurana and H190 (575 individuals; Hibrand-Saint Oyant et al., 2008) and R. wichurana and R. hybrida‘The Fairy’ (95 individuals; Kawamura et al. 2011). In strawberry, the 158 S1 progeny was obtained from F. vesca Ilaria, issued from a cross between Fragolina di Ribera and Alpine (Consiglio per la Ricerca e la Sperimentazione in Agricoltura, Forli, Italy). The collection of the 37 genotypes listed in Table S1 was obtained from Dresden, INRA and USDA-Corvallis.
Cloning and phylogenetic analysis
TFL1 homologues in rose were isolated by PCR on R. chinensis spontanea using the primers UtflF1 and UtflR1. All primers are listed in Table S2. In strawberry, FvKSN was first isolated using primers from rose, KSNqPCRF2 and KSNqPCRR2. Other TFL1 homologues were isolated using sequences obtained via Fragaria genome sequencing (Shulaev et al., 2011). Amino acid sequences of FT and TFL1 homologues in Arabidopsis, tomato, poplar and grapevine were aligned with predicted FT/TFL1 proteins in strawberry and rose using clustalw (Thompson et al., 1997). A phylogenetic tree was produced from 1000 bootstrap replicates by applying the neighbour joining (NJ) method with phillips software. The phylogenetic tree was displayed using treeview (Page, 1996).
Phenotyping, mapping and genetic structure
Segregating populations were phenotyped for CF. In rose, genotypes were considered as CF when plants flowered in the first year after germination, and were confirmed by the presence of flowers in October and November over a 4-year period (2006–2009). In strawberry, genotypes were considered as CF when plants had inflorescences in late summer and fall. In the seven pairs of mutants, the climbing rose genotypes, classified as OF, may occasionally flower late in the summer. In rose, based on polymorphism caused by the retrotransposon insertion, the RoKSN genetic marker was developed using a multiplex kit (Qiagen, http://www.qiagen.com) with F3, InsR3 and R1 primers. In strawberry, FvKSN was genotyped based on the 2-bp deletion identified in the coding region. The relatedness of 37 wood strawberry genotypes was studied with 11 neutral microsatellite loci (Table S3), distributed throughout the genome, using structure software (Pritchard et al., 2000). Comparative mapping between RG III and FG VI was based on the anchorage of eight rose sequence markers, genes or microsatellites, on the strawberry genome (http://www.strawberrygenome.org) using blastn. Gene markers were localized precisely, whereas SSR markers were attributed to scaffolds.
Gene expression analysis
Total RNA extraction was performed as previously described by Remay et al. (2009). To test the effect of the retrotransposon on mRNA accumulation, a one-step RT-PCR was performed using the one-step RT-PCR kit (Qiagen), according to the manufacturer’s recommendations. Briefly, both cDNA synthesis and PCR were performed in a single tube using gene-specific primers and target RNA. To study RoKSN accumulation during the floral process, a two-step RT-PCR was carried out as previously described (Remay et al., 2009). Total RNA was reverse transcribed using oligo-dT primers and subjected to quantitative PCR to analyse transcript accumulation. The level of RNA was normalized using the TCTP gene as a reference, after checking the homogeneity of the cycle threshold (Ct) variation with a second housekeeping gene: i.e. EF1α (Remay et al., 2009). Terminal parts of the shoots were harvested in spring and autumn from R. wichurana at different time points (see details in the legend of Figure 6). The terminal part of the shoot was dissected (removal of young leaves), and therefore corresponds to the shoot apical meristem, the leaf primordia and the stem.
Accession Numbers
Acknowledgements
A. Gaston and A. Remay were supported by a joint grant from Région Aquitaine and Pays de la Loire, respectively, as well as the French ‘Institut National de la Recherche Agronomique’. We thank the experimental unit ‘Horticole’, N. Dousset and J. Chameau from the experimental team of GenHort, and A. Bonnet, N. Pedeprat and S. Schafleitner from UR419 for growing and phenotyping the plants. We thank the Strawberry Sequencing Consortium for access to the sequences before publication of the strawberry genome, and V. Shulaev and K. Folta in particular. H. Iwata was encouraged and supported by the late Dr Susumu Ohno and the late Gisuke Wakunaga, Chairman of Wakunaga Pharmaceutical Co. Ltd. We thank M. Ogisu for providing R. chinensis var. spontanea, P. Heitzler for critical comments on the article and D. Manley for correcting the English.




