Evolution, structure and function of mitochondrial carriers: a review with new insights
Summary
The mitochondrial carriers (MC) constitute a large family (MCF) of inner membrane transporters displaying different substrate specificities, patterns of gene expression and even non-mitochondrial organelle localization. In Arabidopsis thaliana 58 genes encode these six trans-membrane domain proteins. The number in other sequenced plant genomes varies from 37 to 125, thus being larger than that of Saccharomyces cerevisiae and comparable with that of Homo sapiens. In addition to displaying highly similar secondary structures, the proteins of the MCF can be subdivided into subfamilies on the basis of substrate specificity and the presence of specific symmetry-related amino acid triplets. We assessed the predictive power of these triplets by comparing predictions with experimentally determined data for Arabidopsis MCs, and applied these predictions to the not yet functionally characterized mitochondrial carriers of the grass, Brachypodium distachyon, and the alga, Ostreococcus lucimarinus. We additionally studied evolutionary aspects of the plant MCF by comparing sequence data of the Arabidopsis MCF with those of Saccharomyces cerevisiae and Homo sapiens, then with those of Brachypodium distachyon and Ostreococcus lucimarinus, employing intra- and inter-genome comparisons. Finally, we discussed the importance of the approaches of global gene expression analysis and in vivo characterizations in order to address the relevance of these vital carrier proteins.
Introduction
Mitochondria are ubiquitous in eukaryotic cells and perform a wide range of essential cellular functions. In plants, in addition to respiration and cellular energy supply, they are involved in further metabolic tasks including nitrogen assimilation, photorespiration, C1 metabolism, photosynthesis in C4 plants, crassulacean acid metabolism and utilization of storage pools of carbon and nitrogen during seed germination (Douce, 1985). They also play a role in the biosynthesis of amino acids, tetrapyrroles, fatty acids and vitamin cofactors (Giege et al., 2003; Picault et al., 2004). While the outer mitochondrial membrane is permeable to solutes with a molecular mass of less than 4–5 kDa (Pfaff et al., 1968; Colombini, 1979; Ludwig et al., 1986; Benz et al., 1990), the inner membrane is impermeable. Indeed, only small and uncharged molecules such as O2 and CO2 can readily pass through this membrane. The passage of hydrophilic compounds across the inner mitochondrial membrane is catalyzed mainly by a family of nuclear-coded proteins known as the mitochondrial carrier family (MCF). MCs are small proteins normally possessing a molecular mass of about 30–34 kDa. All family members have common structural features which are different from those of any other known transporter family. Their primary structure consists of three tandemly repeated homologous domains about 100 amino acids in length, and each repeat contains two hydrophobic segments (spanning the membrane as α-helices) and a characteristic amino acid sequence motif PX[D/E]XX[K/R]X[K/R] (20–30 residues) [D/E]GXXXX[W/Y/F][K/R]G (PROSITE PS50920, PFAM PF00153 and IPR00193). Unlike the other members of the family, two subfamilies, the aspartate/glutamate and ATP-Mg/Pi carriers, have additional N-terminal regulatory domains (more than 150 amino acids), that usually contain Ca2+-binding motifs. Molecules transported by the MCF proteins are greatly variable in size and structure from H+ to NAD+ and coenzyme A. Most of them are negatively charged, but some are positively charged or zwitterions at physiological pH values. Many MC subfamilies catalyse a 1:1 exchange (antiport) reaction between substrates. However, other modes such as unidirectional substrate transport (uniport) and H+-compensated anion symport are also mediated by some MCs. Furthermore, MCs can be subdivided on the basis of the electrical nature of the reactions they catalyse with family members either being electrophoretic (electrogenic) or electroneutral. The ADP/ATP and aspartate/glutamate carriers, for example, drive electrogenic reactions as their operation results in net charge transfer. By contrast, the carrier subfamilies for Pi, glutamate and GTP/GDP as well as for oxoglutarate and ornithine are electroneutral.
Considerable research has been conducted on characterizing members of the MCF in both yeast and animals (see Klingenberg, 2008; Kunji and Robinson, 2010; Palmieri, 2004, 2006, 2008; Palmieri and Pierri, 2010a,b; Satrústegui et al., 2007; for reviews). In recent years the advent and exploitation of transcriptomic, proteomic and metabolomic technologies as well as the availability of knock-out collections in Arabidopsis have greatly aided in increasing our understanding of these proteins in plants (Picault et al., 2004; Haferkamp, 2007).
In this article we have reviewed our current understanding of the structure, biochemical characteristics, expression pattern, subcellular localization and in planta function of members of the MCF. In addition, intra- and inter-genome comparisons have allowed a first assessment of the evolution of this protein family.
Structure and Transport Mechanism
The atomic structure of the ADP/ATP carrier, a member of the MCF, in complex with its powerfull inhibitor carboxyatractyloside, has been solved to 2.2 Å (Pebay-Peyroula et al., 2003). This structure is composed of a six transmembrane α-helix bundle (H1–H6) and three short α-helices (h12, h34, h56) parallel to the membrane plane on the matrix side. H1–H6 line a water-accessible cavity (occupied by the inhibitor) which is open towards the cytosol and closed on the matrix side by a salt-bridge network formed by the charged residues of the first part of the three signature motifs, PX[D/E]XX[R/K]. The three-dimensional structure of the ADP/ATP carrier is critical in our understanding of MCF proteins in several ways. Firstly, it exhibits a three-fold pseudo-symmetry in line with the three-fold sequence repeats (Saraste and Walker, 1982), as was also observed by electron microscopy of the 2D crystals of the yeast ADP/ATP carrier (Kunji and Harding, 2003). Secondly, it roughly corresponds to the ‘c’ (cytosolic)-state of the ADP/ATP carrier as carboxyatractyloside is an inhibitor that blocks the carrier in this state (Klingenberg, 2008). Thirdly, this structure has been highly used as a template for building homology models of various carriers, thus greatly improving our understanding of the MC structure/function relationships (Walters and Kaplan, 2004; Wohlrab, 2004; Morozzo della Rocca et al., 2005; Tonazzi et al., 2005; Cappello et al., 2006, 2007; Robinson and Kunji, 2006; Robinson et al., 2008; Wibom et al., 2009; Giangregorio et al., 2010).
More recently further important structural information based on the available biochemical characterization (substrate specificity) of MCs, 3D comparative models and bioinformatics approaches has significantly contributed to deepen our understanding of MC structure and function. In addition to the salt-bridge network on the matrix side, suggested by Nelson et al. (1998) and experimentally demonstrated by the resolution of the 3D structure of the ADP/ATP carrier (Pebay-Peyroula et al., 2003), another salt-bridge network has been hypothesized to exist on the cytosolic side (Robinson et al., 2008). The latter is formed by the charged residues of the sequence motif [F/Y][D/E]XX[R/K] localized at the c-terminus of the even-numbered transmembrane α-helices. These networks constitute the cytosolic and matrix gates of MCs that close the protein central cavity in the ‘m’ (matrix)-state (in which the internal cavity is open towards the matrix and closed on the cytosolic side) and the c-state, respectively. Moreover, multiple sequence alignment of MCs of known function (substrate specificity) revealed residues of the three even-numbered transmembrane α-helices having the potentiality of discriminating the binding of three major classes of substrates: nucleotides, carboxylates or amino acids (Robinson and Kunji, 2006). Based on the 3D structure of the ADP/ATP carrier, these important residues protrude into the carrier cavity at approximately the midpoint of the membrane one-and-a-half helix turns above the matrix gate. They constitute the substrate binding site or part of it when, depending on the size, shape and chemistry of the substrate, residues of the odd-numbered transmembrane α-helices located in the cavity at the same level and/or other residues above and below are also involved in binding. Finally, in the odd transmembrane α-helices a well-conserved glycine is present nine residues before the prolines of the PX[D/E]XX[K/R]X[K/R] motif; and in the even transmembrane α-helices a conserved proline is present 10 residues after the glycine corresponding to the last residue of the second part ([D/E]GXXXX[W/Y/F][K/R]G) of the sequence motif (Palmieri and Pierri, 2010a). It is interesting that: (i) the Gly and Pro of the odd helices are aligned with the Gly and Pro of the even helices in an antiparallel fashion; (ii) the above-mentioned Pro and Gly are located strategically between the substrate binding site and the gates on both sides (Palmieri and Pierri, 2010a); and (iii) as assessed for the Pro of the odd helices (Pebay-Peyroula et al., 2003), the Gly of the odd helices and the Gly and Pro of the even helices may also act as hinges (Palmieri and Pierri, 2010a).
It is believed that during the catalytic exchange transport cycle MCs undergo a conformational change from the c-state to the m-state and vice versa (see Figure 1a) (Kunji and Robinson, 2010; Palmieri and Pierri, 2010a,b). In brief, in the c-state the substrate enters the carrier from the cytosolic side and binds to the carrier (Figure 1a). As the substrate binds to the carrier, the protein rearranges until the transition state is reached in which a maximum of interactions between the protein and the substrate take place, according to the ‘induced transition fit’ of carrier catalysis (Klingenberg, 2005). In the transition state: (i) the substrate is bound in the cavity approximately at the center of the carrier, as predicted by the ‘single binding center-gating pore’ mechanism (Klingenberg, 1976); and (ii) the carrier is compactly structured around the substrate and almost entirely closed on either side of the membrane (Figure 1a). The total binding energy of the optimum fit interactions between the carrier and the substrate in the transition state triggers additional structural changes leading to the matrix conformation (in which the c-gate is closed and the m-gate is opened). At this stage the substrate, which entered the carrier from the cytosolic side, exits into the matrix and the catalytic cycle continues with the entry of another substrate from the matrix (Figure 1a). The above-reported mechanism describes the MC-mediated antiport mode of transport. However, some carriers may catalyze uniport, besides antiport, although at lower rates. This means that they are able to undergo a reversible transition between the c-state and the m-state in the absence of the substrate, because the activation energy barrier of the transition between the two states of these carriers is much lower than that of the obligatory 1:1 exchange carriers.
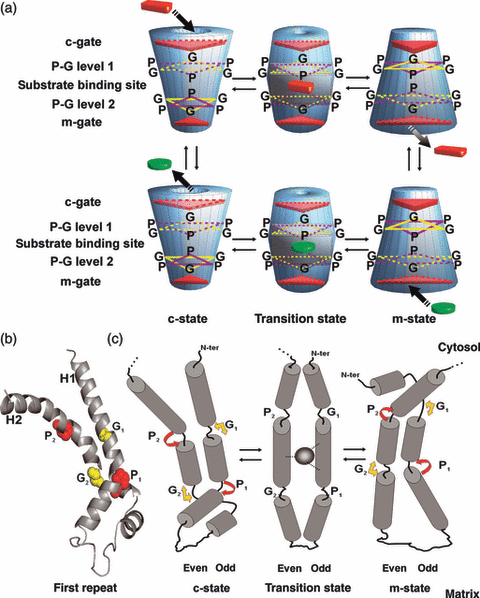
Mechanism of substrate translocation catalyzed by mitochondrial exchange carriers.(a) Scheme depicting the transition of MCs from the c-state to the m-state and vice versa. Truncated cones on the left show the c-state after the release of the substrate towards the cytosol (bottom) and immediately after the entry of the substrate from the cytosolic side (top); truncated cones on the right illustrate the m-state after the release of the substrate into the matrix (top) and immediately after the entry of the substrate from the matrix side (bottom); and the two central truncated bicone-shaped solids depict the transition states of the carrier with the bound substrate entered from the cytosol (top) and from the matrix (bottom). Red solid cuboids and green solid discs represent the substrate entering from the cytosol and from the matrix, respectively; red triangles indicate closed gates, and dotted red triangles open/partially closed gates. All transport steps are fully reversibile. The positions of the cytosolic gate, P-G level 1, substrate binding site, P-G level 2 and matrix gate are shown on the left.(b) Crystal structure of the bovine ADP/ATP carrier first repeat, taken from the published 3D structure of the carboxyatractyloside–ADP/ATP carrier complex. The conserved P and G positions in most MC odd and even transmembrane α-helices (Palmieri and Pierri, 2010a) are indicated by G1, P1, G2 and P2. In the ADP/ATP carrier A19 is present instead of G. A19 (G1) and G73 (G2) are shown in yellow and in surf representation; P28 (P1) and P83 (P2) in red and surf representation. Panel (c) The ‘flexible hinged helix movements’ of MCs occurring during their catalytic exchange transport cycle. For sake of clarity, only the movements of a single repeat are depicted. Subscripts 1 and 2 were added to the P and G to more easily identify the odd- and even-numbered transmembrane helix. The left side corresponds to the c-state, the right side to the m-state and the middle part to the transition state; the grey oval in the transition state denotes the bound substrate; the yellow arrows denote the ability of G to bend the helices; and the red arrows indicate kink/swivel at the P. The angle of observation is the same as that of panel (b). The carrier matrix axis is closer to the reader. Panels (b) and (c) are reproduced with permission (from Palmieri and Pierri, 2010a; Figure 2).
The large structural changes occurring during the transition from the c- to the m-state, and vice versa, are still unknown, in particular because the 3D structure of MCs in the m-state is not yet available. Based on current knowledge as well as on the role of Pro and Gly of the transmembrane α-helices, we have proposed that these conformational changes are largely due to the movements of the even and odd transmembrane α-helices (Palmieri and Pierri, 2010a). These ‘flexible hinged helix movements’ are schematically depicted in Figure 1c for one repeat of MCs. For comparative purposes Figure 1b illustrates the crystal structure of the first repeat of the ADP/ATP carrier in the c-state, taken from Pebay-Peyroula et al. (2003). The MC ‘hinged helix movements’ are the result of the substrate–protein interactions and in summary consist of a tilt of the entire helical segments and a kink/swivel of the helical termini at the level of their Pro and Gly. Viewing the carrier from the cytosol, even and odd helices would be seen rotating clockwise during the c- to m-state transition, counter-clockwise during the m- to c-state transition, and vice versa viewing the carrier from the matrix side. Furthermore, during the transition from the c-state to the m-state, the kink of the Pro in the even helices towards the cavity axis brings together the [F/Y][D/E]XX[R/K] portions closing the cytosolic salt-bridge network; the kink/swivel of the Gly in the odd helices towards the cavity axis rotates their N-termini behind the cytosolic salt-bridge network (Figure 1c, right side, upper segments), whereas the matrix termini of the even and odd helices move apart away from the cavity axis hinging on their Pro and Gly residues (Figure 1c, right side, lower segments). The opposite movements take place during the transition from the m- to the c-state (Figure 1c). In this way, during the transition from the c- to the m-state the matrix gate breaks and the cytosolic gate closes, and vice versa during the transition from the m- to the c-state (Figure 1).
Extension of the MCF
Only six MCs were sequenced after their purification from mitochondria by direct amino acid analysis or by DNA sequencing (Indiveri et al., 1997 and references therein). These early studies led to the conclusion that they all belonged to the same protein family which was named MCF. In the genomic era, many proteins of unknown function with the characteristic sequence features of the MCF have emerged from genome sequencing of various organisms. The genome of Saccharomyces cerevisiae encodes 35 MCs (Palmieri et al., 1996), that of Arabidopsis thaliana 58 (Picault et al., 2004) and the Homo sapiens genome about 50 (Palmieri, 2004).
The first step in elucidating MC function is to search for the substrate(s) transported by a particular carrier. Phylogenetic clustering, genetic information, knowledge of cell metabolism and complementation of phenotypes have often provided clues about the transported substrate. However, in this respect these methods are not conclusive. Until now, the best strategy employed to identify the substrate specificity of new MCs includes searching databases for carriers of unknown function, heterologous gene expression in Escherichia coli and reconstitution of purified recombinant carriers into liposomes, in which substrate transport is assayed by direct measurements (Fiermonte et al., 1993). In a few cases putative MCs were expressed in S. cerevisiae, purified from isolated mitochondria and reconstituted into liposomes (Palmieri et al., 1999a, 2001a,b). When using such gene-to-function strategies about half of the MCs in S. cerevisiae (Palmieri et al., 2006 and references in this work), a third in H. sapiens (Palmieri, 2004 and references in this work) and a quarter in A. thaliana (Picault et al., 2004 and references in this work) were identified.
Table 1 lists the main subfamilies into which MCs can be divided according to their specificity. Four considerations should be made. First, some substrates, and even certain defining substrates, are transported by more than one subfamily. Second, the best transported substrate in reconstituted liposomes might not always be the most important substrate under physiological conditions. This situation is particularly true in different tissues, or specialized cells, in which the transported substrates may well be present in the cytosol and/or in the mitochondrial matrix at different concentration ratios. Third, some subfamilies may transport additional, yet untested substrates (see Marobbio et al., 2008). Fourth, most of the subfamilies reported in Table 1 are present in all eukaryotes. Furthermore, the carrier subfamilies defined on the basis of their function (substrate specificity) are also characterized by specific amino acid triplets (Figure 2 and Table 1). As mentioned above, MCs are three-fold pseudo-symmetric. Therefore, each carrier displays symmetry-related triplets of amino acids when its three repeat sequences are aligned (Robinson et al., 2008). Figure 2 shows the triplets protruding into the carrier cavity of all MCs present in A. thaliana, H. sapiens and S. cerevisiae. These residues belong to the odd-numbered (triplets 11–34) and even-numbered (triplets 73–96) transmembrane α-helices of the above carriers. The Pro and Gly triplets (19, 28, 73 and 83) are also shown in Figure 2, although they do not protrude into the cavity. Each subfamily is characterized by the complete set of triplets listed in Table 1 that are not present, altogether, in any other subfamily. For example, all the members of the AAC subfamily exhibit triplets 11 (DNS), 19 (AGT), 23 (KL[G/S]), 84 (TYG), 85 (QRX) and 88 (NYV); and all the members of the AGC subfamily triplets 22 (GQA), 77 (QCR), 84 (EFQ), 85 (KSF) and 88 (KYT). The number of characterizing triplets ranges from two to eight in the various subfamilies (Table 1). As can be seen in Figure 2, related subfamilies transporting structurally related substrates may share some triplets. For example the NAD+, PyC and FAD subfamilies share triplet 19 (GGK) amongst their three to six characterizing triplets. It is worth mentioning that the NAD+ and PyC subfamilies, which have been characterized in greater depth than the FAD subfamily, share some substrates as well (Marobbio et al., 2006; Palmieri et al., 2009; Todisco et al., 2006; S. Todisco and M.A. Di Noia, personal communication). The OGC and DTC subfamilies also share two triplets (KLK and GTY) of the eight characterizing triplets, as well as some transported substrates (Fiermonte et al., 1993; Picault et al., 2002). Given the importance of these structural features some additional remarks should be made. Although all five biochemically characterized members of the ornithine carrier subfamily (ORC1 and ORC2 in H. sapiens, BAC1 and BAC2 in A. thaliana and Ort1p in S. cerevisiae) transport ornithine, some differences in substrate specificity have been noticed. It is likely that this subfamily will be divided into additional subfamilies as there are significant differences in triplets 23, 26 and 84 (see Figure 2 and Table 1), in particular with respect to Ort1p and BAC2. The GGC subfamily is defined by transporting GTP and GDP specifically and by a specific set of triplets (Figure 2 and Table 1). Surprisingly, however, certain GGC triplets are very similar to some of three subfamilies transporting carboxylates (citrate, SFC and ODC). Moreover, the UCP subfamily and an ‘unnamed’ family have been included in Table 1 for their relevance, although the substrate specificity of several of their members is yet to be determined.
| Subfamilies | Aliases | Main substrates | References | Triplets |
|---|---|---|---|---|
| 1. For nucleotides and dinucleotides | ||||
| ADP/ATP | AAC | ADP, ATP | Klingenberg (2008) and Fiore et al. (1998) | 11(DNS), 19(AGT), 23(KL[G/S]), 84(TYG), 85(QRX), 88(NYV) |
| Coenzyme A/PAP | Coa/PAP | Coa, PAP, dephospho-coa, AXP | Prohl et al. (2001) and Fiermonte et al. (2009) | 23 (K[V/A]Q), 34 (IVR), 88 ([K/Q]SS) |
| ATP-Mg/Pi | APC | ATP-Mg, Pi, AXP | Fiermonte et al. (2004) and Traba et al. (2008, 2009) | 23 (RQ[Q/A]), 30(DE[A/T/N]), 84(EYA), 88(KDS) |
| Thiamine pyrophosphate | TPC | Thpp, thmp; (d)NDP, (d)NTP | Dolce et al. (2001), Marobbio et al. (2002) and Lindhurst et al. (2006) | 23(R[T/S]K), 34(IT[K/R]), 80 (L[A/T]K), 85(GAT) |
| Pyrimidine nucleotides | PNC | Pyrimidine (deoxy)nucleotides | Marobbio et al. (2006) and Floyd et al. (2007) | 19 (G[G/A]K), 27 (CNY), 30 ([D/E]WE), 37 (QQR), 83 ([PEP), 85 (R[I/V][S/T]) |
| FAD/folate | FAD | Folates, FAD | Tzagoloff et al. (1996), Titus and Moran (2000) and Bedhomme et al. (2005) | 19 (GGK), 27 (HNY), 30 (DWQ) |
| ANT | ANT | ATP, ADP, AMP | Palmieri et al. (2001b) | 19 (SAK), 30 (DAI), 33 (KAK), 37 (QKR) |
| NAD+ | NDT | NAD+, (d)AMP, (d)GMP | Todisco et al. (2006) and Palmieri et al. (2009) | 19 (GGK), 27 (CNY), 30 (DWE), 89 (FP[L/F]) |
| GTP/GDP | GGC | GTP, GDP, dgtp, dgdp, ITP, IDP | Vozza et al. (2004) | 22 (EGS), 23 (IEL), 84 (QGK), 85 (RSL), 88 (KLS) |
| 2. For di-/tri-carboxylates and keto acids | ||||
| Dicarboxylates | DIC | Malate, succinate, phosphate, sulfate, thiosulfate | Palmieri et al. (1996), Fiermonte et al. (1998b) and Palmieri et al. (2008a) | 26 (TG[C/S]), 27 (H[N/T][S/Q/N]), 33 (K[N/M]K), 88 (RQ[I/L/T]) |
| Oxoglutarate | OGC | Oxoglutarate, malate | Indiveri et al. (1987) and Fiermonte et al. (1993) | 26 (VGS), 27 (QTM), 33 (KLK), 35 (RRR), 77 (GTY), 84 (YVH), 88 (RQT), 93 (TSE) |
| Di-/tri-carboxylates | DTC | Oxoglutarate, citrate | Picault et al. (2002) | 26 (IGS), 27 (QSL), 33 (KLK), 35 (RRQ), 77 (GTY), 84 (YLH), 88 (RMT), 93 ([K/R]DN) |
| Succinate/fumarate | SFC | Succinate, fumarate | Palmieri et al. (1997b) and Catoni et al. (2003) | 22 (EAG), 84 (KNG), 88 (RNT) |
| Citrate | CTP | Citrate, malate, isocitrate, cis-aconitate, PEP | Kaplan et al. (1993, 1995) | 22 (E[A/S][S/T]), 84 (KN[S/D]), 88 (RRV) |
| Oxodicarboxylates | ODC | Oxoadipate, oxoglutarate | Palmieri et al. (2001a) and Fiermonte et al. (2001) | 22 (EE[A/G]), 77 (PTK), 81 (E[H/N]L) 84 (K[F/W]G), 85 (RNG), 88 (KY[M/L]) |
| Oxaloacetate/sulfate | OAC | Oxaloacetate, sulfate, thiosulfate, α-isopropylmalate | Palmieri et al. (1999b) and Marobbio et al. (2008) | 23 (VAA), 26 (TGM), 30 (E[F/Y]D), 80 (YRR), 84 ([L/M]GH), 88 (RQ[C/S]) |
| 3. For amino acids | ||||
| Glutamate | GC | Glutamate | Fiermonte et al. (2002) | 22 (GQA), 77 (NTR), 80 (LRV), 84 (EFL), 85 (KSF), 88 (KYA) |
| Aspartate/glutamate | AGC | Aspartate, glutamate, cysteinesulfinate | Palmieri et al. (2001c) and Cavero et al. (2003) | 22 (GQA), 77 (QCR), 84 (EFQ), 85 (KSF), 88 (KYT) |
| Ornithine | ORC | Ornithine, (lysine, citrulline, arginine, histidine) | Palmieri et al. (1997a), Hoyos et al. (2003) and Fiermonte et al. (2003) | 23 ([V/I][A/S]W) but (KSN) in S. cerevisiae, 26 (GL[V/C]) but (ELI) in S. cerevisiae, 84 (EGA), but (QAV) in atbac2 |
| Carnitine | CAC | Carnitine, acylcarnitines | Indiveri et al. (1990) and Palmieri et al. (1999a) | 23 (VTW), 85 (FSN) |
| S-adenosylmethionine | SAMC | S-adenosylmethionine, S-adenosylhomocysteine | Marobbio et al. (2003), Agrimi et al. (2004), Palmieri et al. (2006a) and Bouvier et al. (2006) | 19 (G[E/G]G), 23 ([D/E][C/S][A/G]), 26 ([L/F]RT), 80 ([G/A]RW), 85 ([A/S][S/T/D]X), 88 (FQF) |
| 4. For other substrates | ||||
| Phosphate | Pic | Phosphate | Wohlrab and Briggs (1994) and Fiermonte et al. (1998a) | 19 (CEG), 23 (HDA), 80 (G[R/K]M), 88 (KKQ) |
| UCP | UCP, KMCP | 23 ([D/E][V/I/S/Q][A/V/T/S]), 88 ([R/K] [D/E][F/M]) | ||
| Unnamed | MRS3-4, MFRN1-2 | 19 (GTG), 22 (E[S/A/H][A/C]), 23 (HDA), 27 ([F/Y][T/N]T) | ||
- With a few exceptions, the substrates transported by each carrier subfamily were identified in liposomes reconstituted with the recombinant protein. The characterizing triplets of each carrier subfamily are the triplet sets present in the functionally identified MCs of each family. The acronyms of the indicated subfamilies are: AAC, ADP/ATP carrier; AGC, aspartate/glutamate carrier; ANT, peroxisomal adenine nucleotide translocator; APC, ATP-Mg/Pi carrier; CAC, carnitine carrier; CoA/PAP, coenzyme A /adenosine 3′,5′-diphosphate carrier; CTP, citrate carrier; DIC, dicarboxylate carrier; DTC, di-/tri-carboxylate carrier; FAD, FAD carrier; GC, glutamate carrier, GGC, GTP/GDP carrier; NDT, NAD+ carrier; OAC, oxaloacetate/sulfate carrier; ODC, oxodicarboxylate carrier; OGC, oxoglutarate carrier; ORC, ornithine carrier; PiC, phosphate carrier; PNC, pyrimidine nucleotide carrier; SAMC, S-adenosylmethionine carrier; SFC, succinate/fumarate carrier; TPC, thiamine pyrophosphate carrier; UCP, uncoupling protein. AXP, adenine nucleotides; dNDP, deoxynucleoside diphosphates; dNTP, deoxynucleoside triphosphates; PEP, phosphoenolpyruvate; Pi, phosphate; ThMP, thiamine monophosphate; ThPP, thiamine pyrophosphate.
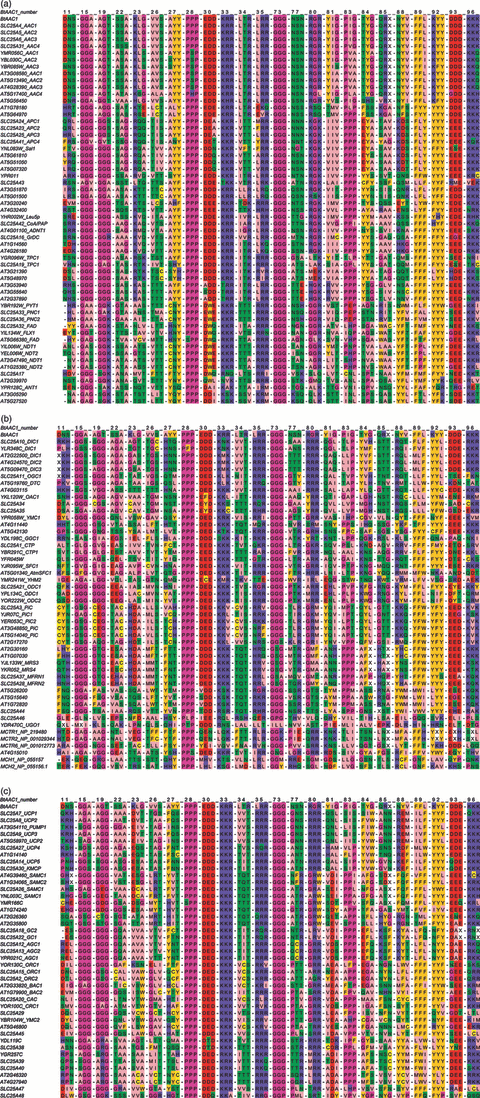
Alignment of symmetry-related amino acid triplets of all the MCs of H. sapiens (53), S. cerevisiae (35) and A. thaliana (58).Each triplet is formed by the three aligned residues of each carrier, which are derived from the inter-repeat multiple sequence alignment of the MCs indicated above. The triplets are ordered horizontally according to the number of the first-repeat amino acids of the bovine ADP/ATP carrier sequence (NP_777083). Amino acids are coloured according to the default Jalview–Zappo style. The carriers are listed according to the following major groups of substrates: nucleotides (panel a); carboxylic acids including keto acids (panel b); amino acids and other substrates (panel c).
Size of the MCF in Plant Genomes
The MCF is probably the largest family of membrane metabolite transport proteins. MCs are also highly abundant in the genome of several species of dicots, monocots and algae ranging in number from 37 to 125 (Table 2). This number is comparable with or higher than that found in S. cerevisiae (35) and H. sapiens (53). In general, in the plant species whose genomes have been assembled, MCs are distributed almost uniformly in all the chromosomes (Table 2). A high number of MCs is also detectable in the genomes of dicots, monocots and algae species which are currently being assembled: 66 MCs in Arabidopsis lyrata, 57 in Carica papaya, 81 in Morchella esculenta, 61 in Vitis vinifera, 65 in Mimulus guttatus, 65 in Ricinus communis, 61 in Cucumis sativus for dicots; 64 in Oryza sativa, 91 in Populus trichocarpa for monocots; 60 in Selaginella moellendorfi and 93 in Physcomitrella patens for algae.
| Dicots | Monocots | Algae | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. thaliana | M. truncatula | G. max | B. distachyon | S. bicolor | Z. mays | O. lucimarinus | C. reinhardtii | |||||||||
| Chr | mbp | MC No. | mbp | MC No. | mbp | MC No. | mbp | MC No. | mbp | MC No. | mbp | MC No. | mbp | MC No. | mbp | MC No. |
| 0 | – | – | 17 | 0 | 29 | 0 | – | – | 755 | 0 | 14 | 0 | – | – | – | – |
| 1 | 30 | 9 | 33 | 5 | 55 | 6 | 74 | 17 | 73 | 14 | 300 | 15 | 1 | 4 | 9 | 2 |
| 2 | 19 | 10 | 32 | 3 | 51 | 10 | 59 | 17 | 77 | 5 | 234 | 4 | 0.895 | 2 | 9 | 3 |
| 3 | 23 | 9 | 44 | 9 | 47 | 6 | 59 | 10 | 74 | 10 | 230 | 7 | 0.982 | 4 | 7 | 3 |
| 4 | 18 | 10 | 41 | 7 | 49 | 9 | 48 | 8 | 68 | 9 | 247 | 6 | 0.930 | 0 | 3 | 3 |
| 5 | 26 | 20 | 43 | 7 | 41 | 8 | 28 | 4 | 62 | 2 | 216 | 9 | 0.847 | 0 | 3 | 0 |
| 6 | – | – | 23 | 1 | 50 | 8 | – | – | 62 | 5 | 169 | 8 | 0.818 | 1 | 7 | 9 |
| 7 | – | – | 35 | 2 | 44 | 9 | – | – | 64 | 3 | 170 | 4 | 0.783 | 3 | 6 | 3 |
| 8 | – | – | 37 | 3 | 46 | 15 | – | – | 55 | 1 | 174 | 7 | 0.701 | 1 | 4 | 0 |
| 9 | – | – | – | – | 46 | 4 | – | – | 59 | 7 | 152 | 10 | 0.670 | 1 | 4 | 4 |
| 10 | – | – | – | – | 50 | 3 | – | – | 60 | 4 | 149 | 3 | 0.613 | 3 | 6 | 3 |
| 11 | – | – | – | – | 39 | 3 | – | – | – | – | – | – | 0.593 | 2 | 2 | 0 |
| 12 | – | – | – | – | 40 | 2 | – | – | – | – | – | – | 0.538 | 4 | 9 | 0 |
| 13 | – | – | – | – | 44 | 5 | – | – | – | – | – | – | 0.528 | 3 | 6 | 0 |
| 14 | – | – | – | – | 49 | 6 | – | – | – | – | – | – | 0.708 | 1 | 4 | 0 |
| 15 | – | – | – | – | 50 | 3 | – | – | – | – | – | – | 0.468 | 1 | 3 | 1 |
| 16 | – | – | – | – | 37 | 7 | – | – | – | – | – | – | 0.428 | 2 | 6 | 3 |
| 17 | – | – | – | – | 41 | 5 | – | – | – | – | – | – | 0.366 | 2 | 6 | 0 |
| 18 | – | – | – | – | 62 | 4 | – | – | – | – | – | – | 0.149 | 0 | – | – |
| 19 | – | – | – | – | 50 | 6 | – | – | – | – | – | – | 0.154 | 0 | – | – |
| 20 | – | – | – | – | 46 | 6 | – | – | – | – | – | – | 0.549 | 1 | – | – |
| 21 | – | – | – | – | – | – | – | – | – | – | – | – | 0.321 | 3 | – | – |
| Unknown | – | – | – | – | – | – | 1 | 0 | – | – | – | – | – | – | 10 | 3 |
| Total | 119 | 58 | 308 | 37 | 979 | 125 | 272 | 55 | 1414 | 60 | 2061 | 73 | 14 | 38 | 112 | 37 |
- The data were retrieved from Ensembl-plants (http://plants.ensembl.org/index.html) for A. thaliana and B. distachyon, NCBI and PGDD (http://chibba.agtec.uga.edu/duplication/) for O. lucimarinus, and Phytozome (http://www.phytozome.net/) for the other species. The ID limit used with Biomart/Ensembl was the IPR-ID ‘IPR001993’ and with Biomart/Phytozome the PFAM-ID ‘PF00153’. All the sequences were validated by protein blast analysis on the non-redundant database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The number of MCs refers to sequences which are longer than 265 amino acids and non-redundant. chr, chromosome; mbp, mega base pairs.
Function Prediction of MCs in A. thaliana, B. distachyon and O. lucimarinus
To further characterize plant MCs we investigated their symmetry-related triplets and compared these to MC subfamilies for which substrate specificity was determined in H. sapiens, S. cerevisiae and A. thaliana (Table 3). In other words, after having identified numerous MC subfamilies on the basis of substrate specificity and symmetry-related triplets, we predicted a function (e.g. the transported substrates) for many MCs of A. thaliana, B. distachyon and O. lucimarinus. To the best of our knowledge, none of the B. distachyon and O. lucimarinus MCs have been functionally characterized until now. In A. thaliana the function of several members of the family has been experimentally investigated. In Table 3 the conclusions of these studies have been compared to the function predicted in this review. In many cases there is a perfect or partial agreement, in others a disagreement, between the experimentally tested and predicted functions. The transporters which displayed unexpected functions can be split into two groups; those displaying novel substrate specificity and those demonstrating similar substrate specificity to that expected but which reside at different organellar locations. In the case of the former it is, however, important to note that substrate specificity is difficult to determine in the absence of highly comprehensive experiments offering a wide range of potential substrates under appropriate conditions.
| Genes | Function | References | ||||
|---|---|---|---|---|---|---|
| A. thaliana | B. distachyon | O. lucimarinus | Predicted | Experimentally tested | ||
| In (partial) agreement | In disagreement | |||||
| At3g08580 | BD1G29260 | OL10G01660 | AAC1 | AAC1 | – | Millar and Heazlewood (2003) and Haferkamp et al. (2002) |
| At5g13490 | BD3G53520 | – | AAC2 | AAC2 | – | Millar and Heazlewood (2003) and Haferkamp et al. (2002) |
| At4g28390 | – | – | AAC3 | AAC3 | – | Haferkamp et al. (2002) |
| At5g17400 | – | – | AAC4 | – | ER-ANT1 | Leroch et al. (2008) |
| At5g56450 | BD2G18347 | OL03G05390 | Unknown1 (AAC?) | – | – | – |
| At1g78180 | BD1G71800 | – | Unknown2 (AAC?) | – | – | – |
| At5g64970 | – | – | Unknown3 (AAC?) | – | – | – |
| At5g61810 | BD1G36570 | – | APC1 | – | – | – |
| At5g51050 | – | – | APC2 | – | – | – |
| At5g07320 | – | – | APC3 | – | – | – |
| At3g51870 | BD2G09790 | OL10G01620 | Unknown4 (CoA/PAP?) | – | – | – |
| At5g01500 | – | – | Unknown5 (CoA/PAP?) | – | TAAC | Thuswaldner et al. (2007) |
| At3g20240 | BD3G40497 | OL02G00320 | Unknown6 (Nt) | – | – | – |
| – | BD4G34100 | OL12G03180 | Unknown7 (Nt) | – | – | – |
| – | BD5G26776 | – | Unknown8 (Nt) | – | – | – |
| At3g05290 | BD2G26480 | – | Unknown9 (Ant1?) | – | PNC1 | Arai et al. (2008) and Linka et al. (2008) |
| At5g27520 | – | – | Unknown10 (Ant1?) | – | PNC2 | Arai et al. (2008) and Linka et al. (2008) |
| At4g32400 | BD2G34270 | OL03G01320 | Unknown11 (Nt) | – | ATBT1 | Kirchberger et al. (2008) |
| – | BD1G36670 | OL12G00350 | Unknown12 (Nt) | – | – | – |
| – | BD3G07500 | OL15G01450 | Unknown13 (Nt) | – | – | – |
| At4g01100 | BD2G14840 | OL03G04280 | Unknown14 (CoA/PAP?) | – | ADNT1 | Palmieri et al. (2008b) |
| At1g14560 | BD2G47880 | OL08G02620 | Unknown15 (CoA/PAP?) | – | – | – |
| At4g26180 | BD3G08557 | – | Unknown16 (CoA/PAP?) | – | – | – |
| – | BD2G41500 | – | Unknown17 (CoA/PAP?) | – | – | – |
| At3g53940 | BD2G02880 | OL07G00540 | Unknown18 (Nt) | – | – | – |
| At3g55640 | BD1G66990 | – | Unknown19 (Nt) | – | – | – |
| At2g37890 | – | – | Unknown20 (Nt) | – | – | – |
| At3g21390 | BD2G59930 | OL13G02340 | Unknown21 (TPC?) | – | – | – |
| At5g48970 | – | OL21G00660 | Unknown22 (TPC?) | – | – | – |
| At5g66380 | BD1G09560 | OL13G02140 | FAD1 (folate?) | Folate (folt1) | – | Bedhomme et al. (2005) |
| – | – | OL21G00840 | FAD2 | – | – | – |
| At2g47490 | BD2G28120 | OL11G01860 | NDT1 | NDT1 | – | Palmieri et al. (2009) |
| At1g25380 | – | – | NDT2 | NDT2 | – | Palmieri et al. (2009) |
| At2g39970 | BD1G69370 | OL20G02530 | Unknown23 (NT) | – | PMP38 | Fukao et al. (2001) and Linka et al. (2008) |
| – | BD1G67180 | – | Unknown24 (NT) | – | – | – |
| At2g22500 | BD3G38410 | – | DIC1 | DIC1 | – | Palmieri et al. (2008a) |
| At4g24570 | BD4G32510 | – | DIC2 | DIC2 | – | Palmieri et al. (2008a) |
| At5g09470 | – | – | DIC3 | DIC3 | – | Palmieri et al. (2008a) |
| – | – | OL14G02490 | Unknown25 (DIC?) | – | – | – |
| At5g19760 | BD2G06520 | OL10G00520 | DTC | DTC | – | Picault et al. (2002) |
| At4g03115 | BD2G32600 | – | Unknown26 (DTC?) | – | – | – |
| At5g01340 | BD1G65510 | OL07G03670 | SFC1 | ATMSFC1 | – | Catoni et al. (2003) |
| At4g11440 | – | – | Unknown27 (acids) | – | – | – |
| At5g42130 | BD2G61677 | OL16G01220 | Unknown28 (acids) | – | – | – |
| – | – | OL03G02490 | Unknown29 (acids) | – | – | – |
| – | – | OL11G02620 | Unknown30 (acids) | – | – | – |
| At2g33820 | BD1G15960 | OL01G00440 | ORNITHINE1 | BAC1 | – | Hoyos et al. (2003) |
| At1g79900 | BD2G07420 | – | ORNITHINE2 | BAC2 | – | Hoyos et al. (2003) and Palmieri et al. (2006b) |
| – | – | OL02G00080 | Unknown31 (ornithine) | – | – | – |
| At5g46800 | BD3G34077 | – | Unknown32 (amino acid) | – | Carnitine (CAC) | Lawand et al. (2002) and Millar and Heazlewood (2003) |
| At4g27940 | BD3G34090 | OL12G01640 | Unknown33 (amino acid) | – | – | – |
| At2g46320 | BD2G07390 | OL01G03790 | Unknown34 (amino acid) | – | – | – |
| – | BD3G34820 | – | Unknown35 (amino acid) | – | – | – |
| At4g39460 | BD1G71410 | OL01G03770 | SAMC1 | SAMC1 | – | Palmieri et al. (2006a) and Bouvier et al. (2006) |
| At1g34065 | BD1G01770 | – | SAMC2 | SAMC2 | – | Palmieri et al. (2006a) and Bouvier et al. (2006) |
| At1g74240 | BD1G70017 | OL13G02410 | Unknown36 (samc?) | – | – | – |
| At2g26360 | BD4G45190 | OL21G00590 | Unknown37 (samc?) | – | – | – |
| At2g35800 | – | – | Unknown38 (samc?) | – | – | – |
| – | BD5G16330 | OL17G01690 | Unknown39 (amino acid) | – | – | – |
| At3g54110 | BD4G09060 | OL07G04120 | UCP1 | PUMP1 | – | Boreckýet al. (2001) and Hanak and Jezek (2001) |
| At5g58970 | BD2G40390 | – | UCP2 | UCP2 | – | Hanak and Jezek (2001) |
| At1g14140 | BD5G11757 | – | UCP3 | – | – | – |
| At2g30160 | BD1G65170 | OL06G04180 | MRS3 | – | – | – |
| At1g07030 | – | – | Mrs4 | – | – | – |
| At2g17270 | BD4G37420 | OL17G01410 | Unknown40 (phosphate?) | – | PIC | Hamel et al. (2004) and Millar and Heazlewood (2003) |
| At5g14040 | BD3G57890 | – | PIC1 | PIC1 | – | Hamel et al. (2004) and Millar and Heazlewood (2003) |
| At3g48850 | BD1G67330 | – | PIC2 | PIC2 | – | Hamel et al. (2004) and Millar and Heazlewood (2003) |
| – | BD4G32020 | OL12G02770 | Unknown41 (phosphate?) | – | – | – |
| – | BD5G11740 | OL01G01320 | Unknown42 (phosphate?) | – | – | – |
| At5g26200 | BD1G72940 | OL09G02540 | Unknown43 | – | – | – |
| At1g72820 | BD1G08301 | OL16G02350 | Unknown44 | – | – | – |
| At5g15640 | BD4G31560 | – | Unknown45 | – | – | – |
| At4g15010 | BD2G12390 | – | Unknown46 | – | – | – |
| – | BD3G51060 | – | Unknown47 | – | – | – |
- The function of A. thaliana, B. distachyon and O. lucimarinus MC genes was predicted on the basis of the symmetry-related triplet sets reported in Table 1, and the predicted function was compared with the experimentally tested function if available. When the function could not be predicted, the most likely function based on similarity to the triplet sets of Table 1 was given in parenthesis with a question mark; in some cases only the most probable class of substrates (nucleotides (Nt), acids or amino acids) was indicated. For the acronyms under the heading ‘predicted’ see Table 1; for the acronyms under the heading ‘experimentally tested’ see references.
Evolution of MCs Deduced by Phylogenetic Trees
A phylogenetic tree of all the MCs of A. thaliana, H. sapiens and S. cerevisiae deduced from genomic analysis is presented in Figure 3a (the protein sequences can be found in Supporting Information). This figure shows that the MCs of these three evolutionarily distant species (146 carriers altogether), based on their sequence similarity, cluster into many different clades suggesting a large variety of specialized functions. Indeed, the MCF is highly divergent, full alignment showing only five identical amino acids (with a frequency between 92 and 99% of the analyzed samples) and only 11 highly conserved amino acids. However, nearly all clades include members of A. thaliana, H. sapiens and S. cerevisiae, with the following exceptions: the GTP/GDP carrier belongs only to fungi, the AT3G20240 and AT4G32400 gene products only to plants, the succinate-fumarate clade to plants and fungi, and the clade of UCP to animals and plants. The fact that the great majority of closely related sequences are present in all three kingdoms shows that the common ancestor of all eukaryotes already possessed many MC functions that have been retained in animals, plants and yeast. In other words, many MC functions existed before the speciation events that have produced the three kingdoms.
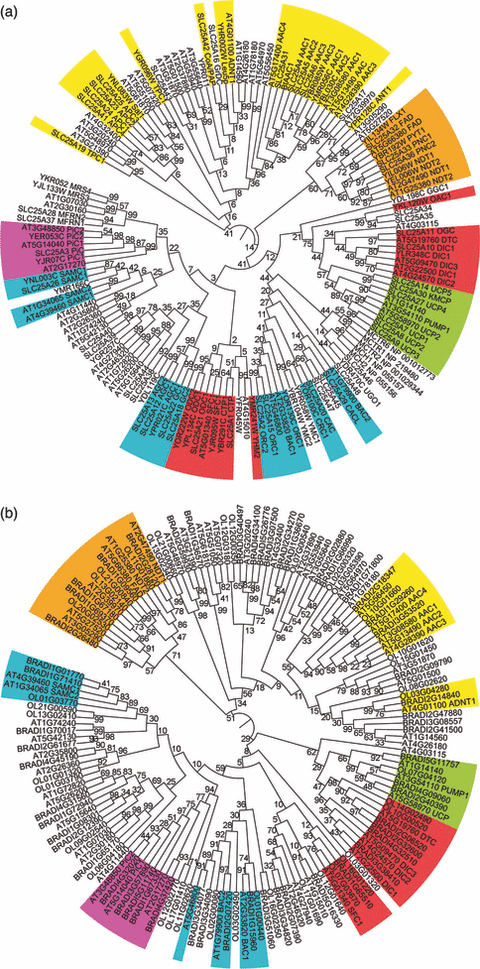
Phylogenetic trees of MCs.The trees of H. sapiens (53), S. cerevisiae (35) and A. thaliana (58) MCs (a) and of B. distachyon (55), O. lucimarinus (40) and A. thaliana (58) MCs (b) originated from ClustalW multiple-sequence alignments by using the neighbor-joining method implemented in MEGA4 (Tamura et al., 2007). Bootstrap values for 1000 replicates are reported on each node; gene names and aliases describing the function on each terminal node. MC subgroups are coloured in yellow (transporting nucleotides), orange (dinucleotides), red (acids), green (UCP), blue (amino acids) and purple (phosphate).
Several other interesting features became apparent from this analysis. The most recent branches of the tree cluster species-specific carriers. These intraspecies parologs are more similar to each other than to their interspecies potential/verified orthologs. For example, AtSAMC1 is closer to AtSAMC2 than to human SAMC1 or yeast Sam5p. This suggests that intraspecies paralogs originated from duplications which occurred independently in each of the three lineages. Interestingly, in several clades A. thaliana exhibits a larger number of paralogs than do H. sapiens and S. cerevisiae. The duplication, retention and differentiation of the A. thaliana paralogs could reflect specific functional requirements of plants as compared to fungi and animalia (which could, for example, be due to the presence of different plastid types in plants).
A phylogenetic tree of all the MCs of A. thaliana, B. distachyon and O. lucimarinus (Figure 3b; the protein sequences can be found in Tables S2–S6) shows that the possibility to correctly detect orthologs between plants is greater than between plants and animalia or yeast, which is in agreement with the fact that the three analyzed plant species are more similar to each other than to species of different kingdoms. Therefore, at this level of resolution, once a MC is characterized in a model species (e.g. A. thaliana), the one-to-one ortholog may most likely be traced in another member of the green lineage such as B. distachyon or O. lucimarinus. Further points of interests concern the repertoire of MCs which is not identical in different plants as independent duplications occurred in all the species (less in A. thaliana as confirmed by synteny analysis; see below). Furthermore, like A. thaliana (Figure 3a), B. distachyon and O. lucimarinus also display more paralogs than H. sapiens and S. cerevisiae, particularly in the clades grouping carriers for nucleotides and amino acids. Therefore, this trait is common in plants as compared with animals and fungi. By contrast to Figure 3a, Figure 3b shows that the most recent branches frequently group carriers from different plant species although some lineage-specific duplications are detectable. Finally, to summarize, the repertoire of MCs in the existing eukaryotes derives from a set of carriers which were already functionally specialized in the ancestral eukaryote and later, after several speciation events, underwent independent rounds of duplication in all the kingdoms including plants.
A few additional remarks concerning the early MC evolution are worth mentioning. MCs are unambiguously recognized by their sequence features: a tripartite structure, a three-fold repeated signature motif, and six transmembrane α-helices (two in each of the three repeats) separated by hydrophilic loops. These structural features clearly indicate that MCs result from the tandem triplication of a primordial 100 amino acid two-helix domain (Kuan and Saier, 1993; Palmieri, 1994; Fiermonte et al., 1999). Moreover, given that a low-grade sequence similarity is also detectable in the two helices of each repeat, it might be hypothesized that the primordial repeat may itself have evolved by duplication of a DNA sequence encoding a single transmembrane segment. It should be noted, however, that in the MCs identified in plants, fungi and animalia there is insufficient evidence to assess whether this similarity results from homology or convergent evolution.
In the past, besides sequence similarity, the location of the introns in MC genes has also been exploited to investigate the evolution of MCF members. It was observed that introns tend to interrupt the coding sequence of the human citrate, carnitine and dicarboxylate carrier genes at positions corresponding to protein folding in or near the hydrophilic loops in the MC amino acid sequences (Iacobazzi et al., 1997, 1998; Fiermonte et al., 1999). To verify this tendency at a genome-wide level, we extended the analysis to the entire set of 58 A. thaliana MC genes. After having generated the multiple protein sequence alignment of the 58 MCs, assigned the predicted membrane folding and the position of the intron sites relative to the aligned residues, the intron density (i.e. the number of introns per residue) in protein regions with distinct structure assignment was measured. The results revealed that hydrophilic loops host a notable 1.8-fold excess in intron density (0.021 intron sites/residue) compared with transmembrane helices (0.012 intron sites/residue, chi-squared test P-value <10–4). The most straightforward interpretation of this finding is that intron gain events, which may also modify the coding sequence, are more frequently retained in loop-coding sequences than in helix-coding sequences. In other words, given that transmembrane helices are the most conserved regions in MCs, intron sites can be under-represented in sequences encoding transmembrane helices due to negative selection.
Evolution of MCs Deduced by Comparative Genomics
Whole genome comparisons reveal the existence of collinear regions, which consist of genetic loci that co-localize in the same or similar order between distinct genomic portions. We exploited the presence of MC genes in collinear regions of the alga O. lucimarinus, the dicot A. thaliana and the monocot B. distachyon as model systems of green plants (Figure 4). In O. lucimarinus six MC genes (FAD1–FAD2, unk21–unk22, unk36–unk37) are traced in collinear regions found between chromosomes 13 and 21 (Figure 4a). It is known that O. lucimarinus chromosome 21 derives from a duplication event and subsequent fusion of the ancestral chromosomes 9 and 13 and that this event post-dates the divergence between O. lucimarinus and O. tauri (Palenik et al., 2007). Therefore, the chromosome 21-inserted FAD2, unk22 and unk37 are a recent acquisition of O. lucimarinus, while their counterparts on chromosome 13 are the one-to-one orthologs of O. tauri. These three gene pairs in O. lucimarinus are identical both at nucleotide and amino acid levels. Therefore the increase in gene dosage should produce higher levels of gene products unless mechanisms of dosage compensation silence one of the two paralogs.
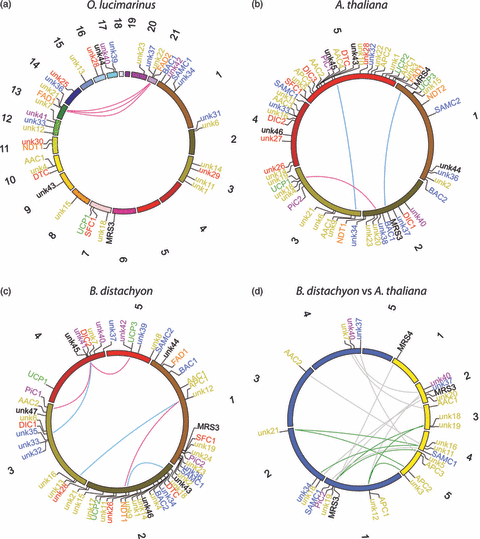
MCs in collinear regions.(a–c) Intra-genome comparison. Genes encoding MCs are superimposed on the chromosomes of O. lucimarinus (a), A. thaliana (b) and B. distachyon (c) according to the genomic coordinates (chromosome size is in scale). Lines connect paralogs in collinear regions duplicated by segmental duplication (red lines) and whole genome duplication (WGD) (blue lines). Regions duplicated by WGD were gathered from literature data on A. thaliana (Van de Peer et al., 2009) and B. distachyon (International Brachypodium Initiative., 2010). (d) Inter-genome comparison. The circle represents the chromosomes of A. thaliana (yellow) and B. distachyon (blue). Genes in regions with conserved gene order between the two genomes (synteny blocks) with lines pairing orthologs are reported. One-to-one relationships between orthologs are indicated by gray lines and one-to-two relationships by green lines. For all panels, circles were generated with Circos (Krzywinski et al., 2009); gene pairs in collinear regions were recovered from PLAZA (http://bioinformatics.psb.ugent.be/plaza/; Proost et al., 2009) for O. lucimarinus and from PGDD (http://chibba.agtec.uga.edu/duplication/) for A. thaliana and B. distachyon; for gene names and colors see Table 3 and Figure 3, respectively (unk, unknown).
In angiosperms recurrent events of whole genome duplications (WGDs) occurred at different evolutionary times (see Soltis et al. (2008) and Freeling (2009) for reviews). In spite of several WGD events, only three pairs of MC paralogs (MRS3–MRS4, unk9–unk10, unk18–unk20) are detectable in collinear regions of A. thaliana (Figure 4b). This apparent discrepancy can be explained by the high rate of gene loss and gene rearrangements in A. thaliana that reduced the total number of genes and degenerated homologous segments (Vandepoele et al., 2002; Thomas et al., 2006). According to a model of the ancestral A. thaliana genome (Van de Peer et al., 2009), the location of MRS3–MRS4 and unk9–unk10 on chromosome pairs 1–2 and 3–5, respectively, should indicate that these genes represent a lineage-specific acquisition not shared with other dicots. By contrast, the location of the unk18–unk20 pair on chromosomes 2–3 should be the result of a segmental duplication.
The genome of B. distachyon hosts more pairs (six) of MC paralogs in collinear segments compared to the A. thaliana genome (Figure 4c), although the latter underwent a higher number of WGDs. The higher conservation of collinearity in B. distachyon should reflect, at least in part, a lower rate of gene loss and chromosome rearrangement in B. distachyon than in A. thaliana. Among the B. distachyon MC paralog pairs, three (DIC1–DIC2, DTC–unk26 and unk12–unk13) are located in regions duplicated by the WGD at the base of cereal diversification (International Brachypodium Initiative 2010). These paralogs are therefore a common characteristic of cereals that is not expected in more evolutionary distant angiosperms. The expansion of the PiC subfamily and the acquisition of unk11–unk12 in B. distachyon post-dates the divergence of cereals, showing that they are recent gains of the B. distachyon lineage. In summary, the intra-genome comparisons discussed above show that WGDs and segmental duplications have contributed to expand the gene repertoire of MCs in green plants. The observation that some MC paralogs escaped the large-scale gene loss following WGD events (Freeling, 2009) reinforces the idea that these paralogs are functionally important and contribute to plant complexity and diversification.
A major goal of inter-genome comparisons is to unveil orthology relationships. Conserved synteny (i.e. detection of collinear segments between different genomes) is a reliable tool to identify one-to-one orthologs, especially in the case of large gene families such as that of MCs. Extensive conserved synteny is reported within dicots and monocots, but much less between the two clades because breaks in collinearity are the natural effect of a greater evolutionary distance (Tang et al., 2008). Even though approximately 500 million years separate monocots and dicots from the common ancestor of the angiosperm, 15 MCs in A. thaliana and 13 in B. distachyon are present in conserved synteny blocks (Figure 4d). Most of these genes are related by a one-to-one relationship that connects the (two most likely) orthologs in the two species. In addition, one-to-two relationships were also found, which associate 10 MCs in the two genomes, nine of which are specific for nucleotides (Figure 4d). The preferential retention of nucleotide carriers suggests that their expansion is tolerated in angiosperms or, more likely, that they functionally contribute to angiosperm evolution.
Physiological Characterization of Plant MCs
Expression and localization
Despite the fact that only a relatively limited number of plant transporters have been characterized at the biochemical level, a wealth of information is available in databases such as ATGenExpress, Aramemnon and SUBA. Figure 5 displays a heatmap documenting the relative expression levels of 56 members of this family in A. thaliana within a wide range of tissues. When these data are evaluated from a functional perspective the following conclusions can be drawn. Several of the transporters (for example AT5G66380_FOLT1, AT5G01340_SFC1, AT4G39460_SAMC2, AT1G25380_NDT2 and AT4G27940) are expressed constitutively across tissue types indicating that they perform essential housekeeping functions. However, the vast majority of the biochemically characterized members of the MCF are differentially expressed among different cell types. Notably, AT4G39460_SAMC1 (Bouvier et al., 2006; Palmieri et al., 2006a) is highly expressed in mature pollen and developing seeds commensurate with the acknowledged import of the precursor methionine to these tissues (Gallardo et al., 2002; Palmieri et al., 2006a). Of the other transporters three separate classes were distinguishable. Those which were predominantly expressed in pollen, seeds and vegetative rosettes were AT5G46800_CAC, AT2G22500_DIC1, AT4G24570_DIC2, AT2G47490_NDT1, AT3G08580_AAC1, AT5G13490_AAC2, AT1G79900_BAC2 and a few, as yet, uncharacterized proteins, whilst those which were predominantly expressed in embryo and seedling stages as well as those expressed in heterotrophic root and stem tissue generally corresponded to genes of unknown function. The only clear exception to this observation is the fact that the functionally characterized dicarboxylate transporters (Palmieri et al., 2008a) display differential behavior, with AT2G22500_DIC1 and AT4G24570_DIC2 being highly expressed in pollen and seeds but not in roots or stems whereas AT5G09470_DIC3 is massively expressed in roots and stems but not in pollen, demonstrating the importance of both tissue specific expression and plant-tissue communication.
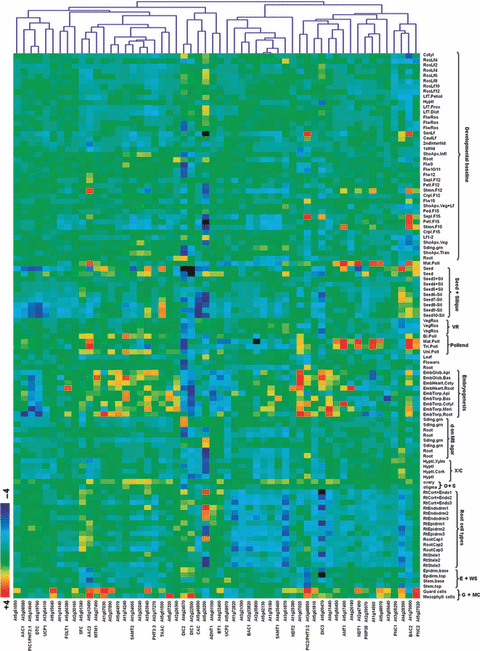
Co-expression analysis of genes encoding MCs found in genomic databases of A. thaliana.Heatmap of gene expression data and clustering analysis of the corresponding gene expression dataset obtained from the expression browser tool at the Bioarray Resource (BAR; http://www.bar.utoronto.ca; Toufighi et al., 2005) were performed using MultExperiment Viewer software (Saeed et al., 2003). The analysis was performed with 56 carriers of A. thaliana. The conditions tested are according to the AtGenExpress Plus-Extended Tissue Series dataset defined by BAR including both a developmental series and a wide variety of tissue types. Abbreviations: VR, vegetative rosette; d, development; X/C, xylem/cork; O + S, ovary + stigma; E + WS, epidermis + whole stem; G + MC, guard + mesophyll cells.
Having studied the tissue specificity of the MCF protein expression we next turned our attention to their levels of expression under a range of stresses by evaluating data from AtGenExpress concerning exposure to cold, osmotic, salt, drought, genotoxic, oxidative, UV-B, wounding and heat stress (Figure S1). Interestingly, of the 28 A. thaliana MCF members whose function has been experimentally investigated, relatively few (six or seven) are strongly transcriptionally regulated (either in root or shoot tissue). These MCs were AT5G09470_DIC3, AT5G13490_AAC2, AT3G48850_PiC2, AT1G79900_BAC2, AT2G22500_DIC1, AT4G24570_DIC2 and AT2G47490_NDT1 with all other members largely exhibiting constitutive patterns of expression. Of the seven transporters highlighted above, four (AT3G48850_PiC2, AT1G79900_BAC2, AT2G22500_DIC1 and AT4G24570_DIC2) display broadly similar expression profiles being highly upregulated under conditions of cold, osmotic and salt stress whereas the other three were generally characterized as displaying fewer and more specific changes. Indeed, they were generally downregulated. The transcript levels of AT3G54110_UCP1 and AT5G58970_UCP2 were not induced under the different stress conditions tested (Figure S1). This finding is in agreement with a recent study by Van Aken et al. (2009) that suggests that UCP proteins are not amongst the most widely stress-responsive mitochondrial proteins.
Notably, the expression profile in roots under different stress conditions seems to be independent of the changes observed in shoots (Figure S1). For example, when the expression profile of AT3G48850_PiC2 is compared distinct patterns are apparent in roots and shoots. While in roots only a moderate increase in expression was observed after 6-h exposure to salt stress, in shoots gene expression was highly upregulated by cold, osmotic, salt, oxidative, UV-B and wounding stresses. Differential expression patterns between roots and shoots are also apparent for AT2G22500_DIC1, AT4G24570_DIC2 and AT5G27520_PNC2 under cold and salt stresses in roots and cold, osmotic, salt, oxidative, UV-B and wounding stresses in shoots. Thus, under the stress conditions tested the expression responses in shoots seem to be more affected by environmental changes than in roots.
Whilst these database resources are highly useful in obtaining an overview of transcriptional regulation of the MCs, further information can be obtained from focused studies. For example GUS staining of Arabidopsis plants transformed with a AT4G39460_SAMC1 promoter-GUS fusion revealed a clear upregulation of its expression following wounding (Palmieri et al., 2006a), whereas GUS staining and RT-PCR analysis indicated that transcription of AT1G79900_BAC2 is upregulated by stress (Toka et al., 2010). It thus seems likely that the sensitivity of targeted studies may well be necessary to elucidate subtle differences in the level and site of expression of at least some of the plant MCs. Additionally, as mentioned above, Van Aken et al. (2009) demonstrated that the MCs are one of the most represented families of mitochondrial proteins under conditions of stress. The MCF members found to be upregulated in response to stress were the phosphate transporter (AT3G48850_PiC2), ADP/ATP exchanger (AT4G28390_AAC3), dicarboxylic acid transporter (AT2G22500_DIC1, AT4G24570_DIC2, AT4G27940), the peroxisomal adenine nucleotide carrier (AT5G27520_PNC2) and a mitochondrial substrate carrier of unknown function (AT5G61810). However, it is clear that these studies only cover a minority of members of plant MCs and that much research is required to improve our understanding of the precise function of each and every member of the family.
Despite the difficulties encountered in studying isolate, intact and highly purified organelles, several proteomics studies have been performed with isolated organelles; the results obtained have been used to generate databases, such as SUBA (http://suba.plantenergy.uwa.edu.au/) and ARAMEMNON (http://aramemnon.botanik.uni-koeln.de/request.ep). These studies have revealed the presence of MCF members in several different plant organelles (Millar and Heazlewood, 2003; Eubel et al., 2008; Linka et al., 2008). We have summarized all available information concerning the subcellular location of MCF proteins in plants (Table S1). There exists a minority of MCs that are not localized to mitochondria as their name would suggest (Palmieri, 1994; Haferkamp, 2007). Recently it has been demonstrated that MCF proteins are localized in plant plastids (Bedhomme et al., 2005; Bouvier et al., 2006; Kirchberger et al., 2008; Palmieri et al., 2009), peroxisomes (Fukao et al., 2001; Palmieri et al., 2001b; Arai et al., 2008; Eubel et al., 2008; Linka et al., 2008) and the endoplasmic reticulum of Arabidopsis (Leroch et al., 2008). In many cases these findings were in contrast to their bioinformatically predicted locations and thus strongly support the contention that the determination of subcellular localization of MCF proteins should be confirmed experimentally by methods beyond those afforded by potentially ambiguous computer-based predictions (Millar et al., 2009).
In planta function
The above sections have detailed impressive advances in identifying the evolution of the MCF in plants as well as its biochemical properties, characteristics of expression and subcellular location of its members. Whilst our level of knowledge lags behind that apparent in the microbial and mammalian fields, the recent adoption of genomic and comparative-genomic approaches has ensured immense progress in furthering understanding of the plant MCF in recent years. The availability of knock-out mutants in Arabidopsis and rice has additionally facilited studies to ascertain the in planta role of the various MCF proteins. To date, the reverse genetic analysis of five MCF proteins has been reported as summarized below.
In the knockout mutant of AT3G54110_UCP1, which dissipates the proton gradient across the inner mitochondrial membrane, a restriction in photorespiration and a subsequent reduction in the rate of photosynthetic carbon assimilation were detected (Sweetlove et al., 2006). The knockout mutant of AT4G01100_ADNT1, which catalyzes the exchange of AMP for ATP (Palmieri et al., 2008b), displayed no clear photosynthetic phenotype, but it was characterized by a remarkable reduction in root growth suggesting an important role for this protein in supporting growth in plant heterotrophic tissues (Palmieri et al., 2008b). The knockout mutant of AT5G66380_FOLT1, which catalyzes the transport of folate into chloroplasts, displayed no difference in terms of germination efficiency, growth rate, morphology, seed production and fertility or rates of photosynthesis and respiration (Bedhomme et al., 2005), which seemingly indicates that its function can be compensated for by another folate chloroplast transport protein, for example by the AT2G32040 protein which does not belong to the MCF and is structurally similar to cyanobacterial plasma membrane folate transporters (Klaus et al., 2005). The characterization of a knockout mutant for AT1G79900_BAC2 (Toka et al., 2010) showed that AT1G79900_BAC2 can work as a hyperosmotic stress-inducible transporter of basic amino acids and that it contributes to proline accumulation in response to hyperosmotic stress in Arabidopsis. The phenotype of the ‘à bout de souffle’ Arabidopsis mutant is similar to that of mutants defective in peroxisomal fatty acid β-oxidation suggesting that it is an acyl-carnitine carrier (Lawand et al., 2002). However, the transported substrate for this protein has not yet been identified. Given that AT5G46800_CAC is structurally highly similar to BAC carriers of plants it remains possible that this carrier is also involved in amino acid transport (Hoyos et al., 2003; Palmieri et al., 2006b; Linka and Weber, 2010).
Concluding Remarks and Outlook
Understanding the evolution, structure and function of the MCF members in plants, in general, is strongly tied not only to understanding their role in the model plant A. thaliana but also in microbial and mammalian systems. Impressive progress has recently been made on substrate specificity, modes of transport, expression and localization of these carriers in Arabidopsis (Picault et al., 2004; Linka and Weber, 2010). However, increased utilization of advanced genomic tools applied to a wider range of green lineages will likely allow us to address numerous functional genomic issues. What is clear from the information presented in this study is that there is a high level of conservation in gene function across species and even kingdoms with many of the MCF members clearly having been in existence in the ancestral eukaryote from which all kingdoms derive. This important finding suggests that it is often relatively easy to predict one-to-one orthologs of MCs from different species in the green lineage. However, due to different rates of genome-wide duplication and/or gene loss this is not always the case. Despite the great progress made in recent years, a number of important questions need to be answered in order to understand the function of the plant MC subfamilies of various divergent plant species. The most pressing challenge is probably to gain more information concerning the substrate specificities of each and every transporter. In order to achieve this, heterologous expression of transporters and detailed biochemical characterization will be required as well as experiments involving isolated plant mitochondria. An additional and equally important question is to elucidate the in vivo function of all MCs which will require the isolation and characterization of knockout mutants of all the family proteins. Due to the possibility of functional redundancy, the generation and characterization of multiple mutants will also be necessary. The type of characterization will be largely dependent on their location of expression; a survey of the data presented here will be an useful resource to obtain information about which tissues and/or cellular conditions are the focus of future studies. Once this essential information is achieved we will be able to clarify the functional importance of the events underlying the evolution of this gene family.
Acknowledgements
This work was supported by grants from MIUR, the Center of Excellence in Genomics (CEGBA), the Fondazione Cassa di Risparmio di Puglia, the Apulia Region, and the Italian Human ProteomeNet No. RBRN07BMCT_009.
This paper is dedicated to the memory of Professor Gian Tommaso Scarascia Mugnozza.




