Dynamic changes of transcript profiles after fertilization are associated with de novo transcription and maternal elimination in tobacco zygote, and mark the onset of the maternal-to-zygotic transition
Summary
The maternal-to-zygotic transition (MZT) is characterized by the turnover of zygote development from maternal to zygotic control, and has been extensively studied in animals. A majority of studies have suggested that early embryogenesis is maternally controlled and that the zygotic genome remains transcriptionally inactive prior to the MZT. However, little is known about the MZT in higher plants, and its timing and impact remain uncharacterized. Here, we constructed cDNA libraries from tobacco (Nicotiana tabacum) egg cells, zygotes and two-celled embryos for gene expression profiling analysis, followed by RT-PCR confirmation. These analyses, together with experiments using zygote microculture coupled with transcription inhibition, revealed that a marked change in transcript profiles occurs approximately 50 h after fertilization, and that the MZT is initiated prior to zygotic division in tobacco. Although maternal transcripts deposited in egg cells support several early developmental processes, they appear to be insufficient for zygotic polar growth and subsequent cell divisions. Thus, we propose that de novo transcripts are probably required to trigger embryogenesis in later zygotes in tobacco.
Introduction
The maternal-to-zygotic transition (MZT) is characterized by the turnover of zygote development from maternal to zygotic control, and requires dramatic and complex cytological and molecular reconstruction. Investigating the timing and biological impact of such a transition is key to understanding the molecular mechanisms underlying fertilization and embryogenesis (Grimanelli et al., 2005; Minami et al., 2007; Ning et al., 2006; Schier, 2007; Sprunck et al., 2005).
The MZT has been intensively studied in animals, and occurs at different stages of embryogenesis in different species. These studies revealed that the MZT is defined by the elimination of maternal transcripts originally stored in the egg cells and by the de novo transcription of the zygote/embryo genome (Schier, 2007; Tadros and Lipshitz, 2009). Whereas zygotic genome activation (ZGA) is a hallmark of the MZT, it may precede the maternal elimination, depending on the species. ZGA can also follow a phase of transcriptional quiescence, which is also of variable duration, depending on the species (reviewed in Minami et al., 2007, Baroux et al., 2009). The timing of ZGA has primarily been investigated through the construction of cDNA libraries, or through the use of microarrays for global transcription analysis (Hamatani et al., 2004; Zeng and Schultz, 2005). The timing of the MZT has also been traditionally defined upon toxicological treatments. Use of transcriptional inhibitors, such as α-amanitin and actinomycin D, has supplied useful and unique information (Flach et al., 1982; Golbus et al., 1973; Warner and Versteegh, 1974) that has helped not only to distinguish the timing of the MZT, but also to reveal the developmental events associated with de novo transcription (Minami et al., 2007).
In comparison with animals, little is known about the MZT in plants because of the inaccessibility of egg cells and early embryos. Microarray analyses of precocious embryonic development in apomictic hybrids of Zea mays (maize), and its wild relative Tripsacum, have shown that early embryonic development occurs in the absence of significant changes in the transcript population until 3 days after pollination, compared with unfertilized ovules of sexual maize (Grimanelli et al., 2005). This observation suggests that maternal control persists until this stage, and the MZT may not occur until after this stage. Similarly, Arabidopsis embryos can develop until the pre-globular stage in the absence of de novo transcription, induced by RNA interference against RNA PolII (Pillot et al., 2010). This also suggests an MZT around this stage. Consistent with this, none of the paternally inherited alleles of 20 tested loci were expressed during early embryonic development in Arabidopsis (Vielle-Calzada et al., 2000).
However, this does not imply a lack of early zygotic expression. It has been proposed that ZGA in higher plants is a gradual process, similar to that of animals (Baroux et al., 2008), which consists of an early minor wave and a later major wave (Tadros and Lipshitz, 2009). Some evidence suggest that de novo transcription occurs at a much earlier stage in maize, Arabidopsis, Triticum spp. (wheat) and Nicotiana tabacum (tobacco) (see below and references therein). In maize, the de novo transcription of two genes probably involved in DNA replication (Dresselhaus et al., 1999) and three cyclins (Sauter et al., 1998) occurs immediately after fertilization. In addition, several transcripts known to be upregulated in the maize apical or basal cell of the two-celled proembryo have been shown to be expressed in the zygote, but are not present in the egg (Okamoto et al., 2005). Furthermore, in Arabidopsis, when wild-type eggs were fertilized with AtRPS5A::GUS transgenic sperm, the GUS signal was detected as early as the two-celled embryo stage (Weijers et al., 2001). De novo GUS activity in the Arabidopsis CYC::GUS transgenic line was also observed in zygotes (Baroux et al., 2001). In addition, several recessive mutants arresting at the zygote stage provide evidence for the requirement of early zygotic transcripts (Ronceret et al., 2005, 2008). In wheat, the transcript profile of two-celled proembryos was significantly different from that of egg cells (Sprunck et al., 2005). This indicates that the two-celled embryo stage may mark a major ZGA in wheat. Finally, in tobacco, we compared the transcript component of tobacco egg cells and zygotes by suppression subtractive hybridization in our previous work. Results from this study suggested that de novo transcripts were present even earlier, at the zygote stage (Ning et al., 2006). However, to confirm this hypothesis, one must increase the scale of the study to experimentally demonstrate that de novo transcripts are in fact synthesized and function in the zygote stage.
Here, we used expression profiling analysis of egg cells, zygotes and two-celled proembryos to show that the MZT is initiated prior to the first zygotic division. We also used zygote microculture paired with transcription inhibitor treatment (TIT) to show that de novo transcripts appearing in later zygotes are probably required to trigger embryogenesis. In addition, a possible role for maternal transcripts present in egg cells in zygotic development and embryogenesis was also experimentally evaluated.
Results
Cytological characteristics of developing tobacco zygotes
Morphological analysis revealed that zygotes undergo a series of morphological and structural changes prior to the first zygotic division, shrinking to a size smaller than the original egg cell. The large vacuole then gradually disappears, and the nucleus localizes near the center of the cell (Figures 1 and S1[link]). These findings were based on observations of both fixed material and isolated living cells, and were consistent with previous reports (Mogensen and Suthar, 1979; Sun et al., 2000). Between 72 and 96 h after pollination (HAP), the zygote exhibited more rapid growth and became elongated (Figure 1), and the nucleus moved to the chalazal end of the zygote. No obvious morphological or volume changes occurred in the zygote after 96 HAP. At 108 HAP, the two-celled proembryo was observed, and by 144 HAP, the embryo progressed to the four-celled proembryo stage (Table S1). Based on these observations, we used 96-HAP zygotes for transcription profile analysis and 72-HAP zygotes for RNA synthesis inhibitor treatment.
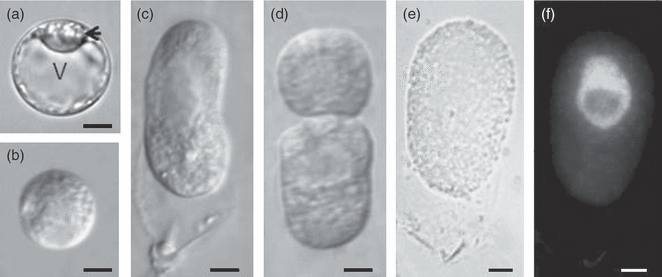
Cytological observation of egg cells, zygotes and two-celled embryos.(a) The egg cell was highly polarized before fertilization. Typically, the nucleus locates at one end of the cell (arrow).(b) After fertilization, the large vacuole gradually disappeared, and the nucleus localized at the center of the cell. The zygote appeared spherical and was much smaller in volume than the egg (zygote diameter = 15–20 μm, n = 30; egg cell diameter = 20–30 μm, n = 32).(c) From 72 h after pollination (HAP) to 96 HAP, as the zygote elongated, the nucleus moved toward the chalazal end.(d) A two-celled proembryo at 108 HAP.(e and f) A zygote at 96 HAP had already elongated, and the 4′,6-diamidino-2-phenylindole (DAPI)-stained nucleus was located at the chalazal end.Scale bar: (a) 15 μm; (b–f) 10 μm. V, vacuole.
Construction of cell type-specific cDNA libraries and bioinformatics analysis of expressed sequence tags and contigs
Mature egg cells, zygotes and two-celled proembryos were isolated for library construction. During isolation, two inhibitors of transcription, actinomycin D and cordycepin, were applied to avoid changes in gene expression arising from potential stresses (Leonhardt et al., 2004; Ning et al., 2006). The isolated cells remained viable and exhibited intact morphology (Figure 1). The cDNA libraries were generated from 159 egg cells, 143 zygotes and 149 two-celled proembryos. Expressed sequence tag (EST) clones from the unamplified libraries were selected and sequenced from their 5′ ends in a single run. After quality assessment, 6469 ESTs, corresponding to 1000 contigs and 2247 singletons, were used for analysis (Table 1). The zygote contig population shows 22.1% similarity with the egg. By contrast, the transcript profile of two-celled embryos changed less dramatically, as the two-celled embryo contig population shows 33.6% similarity with the zygote.
| Library | No. of ESTs | No. of contigs (formed by 2 or more EST) | No. of singletons (% contigs) | No. of contigs | Contigs/ESTs (%) |
|---|---|---|---|---|---|
| Egg cell | 1964 | 295 | 1012 (77) | 1307 | 67 |
| Zygote | 2356 | 391 | 1192 (75) | 1583 | 67 |
| Two-celled proembryo | 2149 | 350 | 898 (72) | 1248 | 58 |
| Total | 6469 | 1000 | 2247 (69) | 3247 | 50 |
- cDNA was directionally cloned into the vector. EST clones from three unamplified libraries were randomly picked and were sequenced from their 5′ ends in a single run. After quality assessment, low-quality sequences and sequences of less than 100 bp (excluding poly-A tails) were removed. In total, 6469 ESTs were used for analysis. The cleaned EST sequences were assembled into contigs using Cap3 according to their sequence connection.
The identified contigs were further used for BLASTN and BLASTX searches. In total, 5849 ESTs (2729 contigs) matched an entry, whereas another 620 ESTs (10%), forming 518 contigs (15%), did not, and were thus considered ‘novel’ transcripts (Table S2). Contigs matching characterized proteins or proteins with putative functions were grouped according to functional categories (Figure 2b,c). Contigs encoding proteins involved in translation (including ribosomal proteins) comprised the largest functional group in each library (Figure 2b: category 1). Compared with the egg cell library, the proportion of ESTs related to translation (Figure 2c: category 1) increased in the zygote and two-celled proembryo libraries. Consistent with these observations, a previous ultrastructural analysis of egg cells revealed that a high density of cytoplasmic ribosomes was present in all flowering plant eggs analyzed, but polyribosomes were rarely observed (Russell, 1993). Together with the present transcript analysis, these data suggest that although few proteins are actively synthesized in egg cells, the cells are well prepared for protein synthesis immediately after activation. Contigs or ESTs related to primary and secondary metabolism comprised the second largest category in the two-celled proembryo library (Figure 2b,c: category 2).
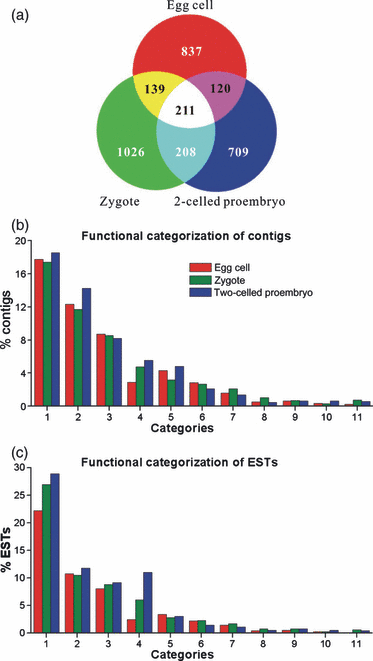
Comparison of contigs and expressed sequence tags (ESTs) among three cDNA libraries.(a) Distribution of contigs derived from egg cells (red), zygotes (green) and two-celled proembryos (blue).(b) Functional classification of 1569 contigs.(c) Functional classification of 3960 ESTs. Contigs and ESTs were grouped into different categories based on their biological functions: (1) translation (including ribosomal proteins); (2) primary and secondary metabolism; (3) protein folding, modification and degradation; (4) DNA and chromatin structure (including histones); (5) transcription and RNA processing; (6) signal transduction; (7) intracellular trafficking, secretion and vesicular transport; (8) cell cycle regulation; (9) cell rescue and defense; (10) cell wall, membrane and envelope biogenesis; and (11) cytoskeleton. Notice that the EST percentage of zygotes in categories 1, 4, 7 and 8 is obviously different from that of egg cells.
Composition analysis reveals changes in transcriptional profiles across developmental stages
The most predominant difference among the three libraries was present in category 4, which was composed of transcripts involved in DNA and chromatin structure, including histones. Every subunit constituting nucleosomes was found in the egg cell ESTs, but the numbers of these ESTs increased in the zygote and two-celled proembryo. Other transcripts related to DNA and chromatin structure were also present in the zygote and two-celled proembryo ESTs. Increases in transcript types and quantities linked to translation, metabolism and DNA structure suggested that the transcript profile changed after fertilization, and that this change may correlate with the process of zygote development.
The 20 largest contigs from each library were also distinct (Tables 2–4), indicating that the transcript profile varies substantially between the stages analyzed. For example, some transcripts present in the egg disappeared soon after fertilization. In egg cells, 185 ESTs (9.4% of the egg ESTs), made up of five different contigs, showed similarity to Early Culture Abundant 1 (ECA1; Table 2), but these ESTs decreased to 10 in the zygote library, and to only one in the two-celled proembryo library. ECA1 encodes a small protein with a putative signal peptide for extracellular localization (Sprunck et al., 2005). Other large contigs present at high numbers in eggs, such as microtubule-binding protein, small nuclear ribonucleoprotein G, glucan endo-1,3-β-glucosidase precursor and NLI interacting factor (NIF) family protein, were not present in the most prevalent contigs in zygotes and two-celled embryos.
| Contig ID | Number of ESTs | Accession | BLASTX resulta | E value |
|---|---|---|---|---|
| EC-1b | 185 | ABK28267 | Arabidopsis thaliana unknown protein | 3.00E−25 |
| EC-15c | 10 | AY310769 | Nicotiana benthamiana putative 40S ribosomal protein S29 | 1.00E−94 |
| EC-5 | 8 | AAF64449 | Euphorbia esula glutathione S-transferase | 1.00E−90 |
| EC-7 | 7 | ABB72814 | Solanum tuberosum putative 40S ribosomal protein S8-like protein | 1.00E−67 |
| EC-8 | 6 | AK224716 | Solanum lycopersicum cDNA | 1.00E−107 |
| EC-9 | 6 | AY962601 | Nicotiana tabacum nucleoside diphosphate kinase mRNA | 0 |
| EC-10 | 6 | BAD05590 | Oryza sativaputative microtubial binding protein | 4.00E−50 |
| EC-11 | 5 | AAR22555 | Lactuca sativa 60S acidic ribosomal protein P3 | 6.00E−22 |
| EC-12 | 5 | L18915 | Nicotiana tabacum ribosomal protein L17 mRNA | 0 |
| EC-13 | 5 | ABB72816 | Solanum tuberosum ribosomal protein L24-like protein | 7.00E−58 |
| EC-14 | 5 | DQ138111 | Nicotiana tabacum ubiquitin extension protein (UBQ2) mRNA | 0 |
| EC-16 | 5 | AAU93594 | Solanum demissum putative 40S ribosomal protein S9 | 1.00E−95 |
| EC-18 | 5 | ABD32677 | Medicago truncatula probable small nuclear ribonucleoprotein G | 2.00E−31 |
| EC-19 | 5 | AAL92578 | Arabidopsis thaliana Glucan endo-1,3-beta-glucosidase precursor | 4.00E−20 |
| EC-20 | 5 | AAW50980 | Triticum aestivum ribosomal protein L36 | 2.00E−47 |
| EC-21 | 5 | DQ235186 | Solanum tuberosum ribosomal protein L11-like protein mRNA | 0 |
| EC-22 | 5 | AY489135 | Capsicum annuum ubiquitin-conjugating enzyme 8 mRNA | 0 |
| EC-23 | 5 | AAM47581 | Arabidopsis thaliana NLI interacting factor (NIF) family protein | 5.00E−43 |
| EC-24 | 4 | D85912 | Nicotiana tabacum mRNA for cytosolic ascorbate peroxidase | 0 |
| EC-25 | 4 | AY310762 | Nicotiana benthamiana putative 60S ribosomal protein L23 | 0 |
- aNCBI database nr.
- bShows significant similarity with ECA1 from barley. Five different contigs have significant similarity with ECA1.
- cContain two different contigs of significant similarity with glutathione-S-transferase.
| Contig ID | Numbers of EST | Accession | BLASTX resulta | E value |
|---|---|---|---|---|
| ZY-1 | 11 | AY912494 | Nicotiana benthamiana ubiquitin/s27a 40S ribosomal protein | 0 |
| ZY-2b | 10 | ABK28267 | Arabidopsis thaliana unknown protein | 3.00E−25 |
| ZY-3 | 10 | AY962601 | Nicotiana tabacum nucleoside diphosphate kinase mRNA | 0 |
| ZY-4 | 10 | ABB72816 | Solanum tuberosum ribosomal protein L24-like protein | 7.00E−58 |
| ZY-5 | 9 | L18915 | Nicotiana tabacum ribosomal protein L17 mRNA | 0 |
| ZY-6 | 9 | NP_563961 | Arabidopsis thaliana 10 kDa chaperonin | 2.00E−38 |
| ZY-7 | 9 | Not hit | ||
| ZY-8 | 8 | CAA49175 | Mesembryanthemum crystallinum ribosomal protein L6 | 1.00E−80 |
| ZY-9 | 8 | DQ056435 | Lycopersicon esculentum 60S ribosomal protein L10 | 0 |
| ZY-10 | 8 | NM_111364 | Arabidopsis thaliana 40S ribosomal protein S24 | 2.00E−101 |
| ZY-11 | 8 | AY495970 | Capsicum annuum small subunit ribosomal protein S16 | 1.00E−114 |
| ZY-12 | 8 | AB032543 | Nicotiana tabacum A34 mRNA for H2A histone | 0 |
| ZY-13 | 8 | AY730335 | Solanum tuberosum genome sequence | 1.00E−39 |
| ZY-14 | 7 | AY310769 | Nicotiana benthamiana putative 40S ribosomal protein S29 | 1.00E−94 |
| ZY-15 | 7 | DQ222516 | Solanum tuberosum putative 40S ribosomal protein S15a | 1.00E−125 |
| ZY-16 | 7 | AY491666 | Capsicum annuum mRNA 60S ribosomal protein L37a | 2.00E−62 |
| ZY-17 | 7 | X60007 | Nicotiana sylvestris Glycine-rich protein 2 | 0 |
| ZY-18 | 7 | DQ228344 | Solanum tuberosum 40S ribosomal protein S23 | 1.00E−122 |
| ZY-19 | 7 | Z31720 | Nicotiana tabacum ribosomal protein L19 | 0 |
| ZY-20 | 7 | AC144343 | Medicago truncatula putative histone H4 | 1.00E−63 |
- aNCBI database nr.
- bShows significant similarity with ECA1 from barley and belongs to the biggest contig of E1.
| Contig ID | Numbers of EST | Accession | BLASTX resulta | E value |
|---|---|---|---|---|
| 2C-1 | 19 | AK224698 | Solanum lycopersicum 60S acidic ribosomal protein P3 | 1.00E−91 |
| 2C-2 | 17 | AY368274 | Nicotiana tabacum cyclophilin-like (CYP1) mRNA | 0 |
| 2C-3 | 16 | AK224716 | Solanum lycopersicum cDNA | 1.00E−107 |
| 2C-4 | 15 | D85912 | Nicotiana tabacum mRNA for cytosolic ascorbate peroxidase | 0 |
| 2C-5 | 15 | AB331236 | Nicotiana tabacum mRNA for histone H3.1 | 2.00E−119 |
| 2C-6 | 13 | NP563961 | Arabidopsis thaliana 10 kDa chaperonin | 2.00E−38 |
| 2C-7 | 12 | EF051133 | Nicotiana tabacum histone H3.3-like mRNA | 0 |
| 2C-8 | 11 | No hit | ||
| 2C-9 | 11 | AY310762 | Nicotiana benthamiana 60S ribosomal protein L23 | 0 |
| 2C-10 | 11 | DQ138111 | Nicotiana tabacum ubiquitin extension protein (UBQ2) | 0 |
| 2C-11 | 11 | AJ718017 | Nicotiana tabacum Probable histone H2B.1 | 1.00E−100 |
| 2C-12 | 10 | AK224728 | Solanum lycopersicum 60S acidic ribosomal protein P1 (L12) | 1.00E−105 |
| 2C-13 | 10 | DQ016993 | Ipomoea batatas putative L24 ribosomal protein mRNA | 1.00E−100 |
| 2C-14 | 10 | DQ191633 | Solanum tuberosum 60s acidic ribosomal protein-like protein | 3.00E−71 |
| 2C-15 | 10 | AJ718017 | Nicotiana tabacum Probable histone H2B.1 | 1.00E−121 |
| 2C-16 | 9 | AY495970 | Capsicum annuum small subunit ribosomal protein S16 (SRP2) | 1.00E−114 |
| 2C-17 | 9 | AAX92952 | Zea mays histone H3 | 2.00E−54 |
| 2C-18 | 9 | DQ252521 | Solanum tuberosum putative 40S ribosomal protein S10-like | 0 |
| 2C-19 | 9 | AF154663 | Nicotiana tabacum 60S ribosomal protein L15 | 0 |
| 2C-20 | 9 | DQ191664 | Solanum tuberosum ribosomal protein S27-like protein | 1.00E−91 |
- aNCBI database nr.
In addition to the disappearance of egg-specific transcripts, those that could represent de novo transcription appeared in zygotes. Transcripts with similarity to ribosomal proteins (Table 3) were the most prevalent contigs in the zygote, but were not found at high prevalence in egg cells. ESTs encoding proteins with similarity to a 10-kDa chaperonin were abundant in zygotes, and were also present in two-celled proembryos, but were not found in the most prevalent contigs of egg cells. Transcripts encoding the histones H2A and H4 were the two largest contigs in the zygote, but were not found in the most predominant contigs of the egg cells.
Finally, some transcripts not present in eggs and zygotes appeared in proembryos. In two-celled proembryos, one of the largest contigs encoded cyclophilin-like protein 1 (CYP1) (Table 4). However, CYP1 was not present in the most prevalent contigs in the zygote, and only two ESTs in egg cells shared similarity with CYP1. In addition, histones were highly represented (five contigs) in two-celled proembryos (Table 4). These characteristic transcript profile changes from egg cell to zygote to two-celled proembryo could reflect both active maternal transcript degradation and/or de novo transcription in early embryogenesis.
To verify the expression patterns of transcripts identified in different cell types, RT-PCR analysis was performed (Figure 3). We tested the expression of 55 candidate genes from the egg, zygote and two-celled libraries in sperm, egg, zygote and two-celled proembryos, as well as in other floral or vegetative tissue (17, 20 and 18 transcripts from the egg, zygote and proembryo libraries, respectively), and are shown Figures 3 and S2[link].

Expression of a subset of transcripts in eggs, zygotes and two-celled proembryos. Transcript expression in cells at the indicated developmental stages or from the indicated tissues was analyzed by RT-PCR. Most of the transcripts shown in this figure have no similarity to annotated database entries. EC-1, ECA1; EC-276 and EC-327, integral membrane proteins; EC-100, dehydrin; EC-19, β-1, 3-glucanase; EC-861, non-cell-autonomous pathway protein 1 (NCAPP1); EC-844, LEA-domain-containing protein; EC-910, A-type cyclin; Zc1520, AP2/EREBP transcription factor. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control.
From 17 egg library transcripts, five showed a clear extinction after fertilization (EC218, EC247, EC276, EC841, EC100; Figure 3), whereas seven transcripts showed downregulation after fertilization (two of which were not detectable in two-celled proembryos; Figure 3), and five showed an increase or semi-constant level (Figure S2). The largest contig in the egg cell cDNA library, ECA1-like, was strongly expressed in egg cells, and was downregulated in zygotes and two-celled proembryos. These results suggest that degradation of egg mRNAs is initiated in the zygote as an early step in the MZT, and may contribute to differences in transcript profiles after fertilization.
Of the 20 transcripts analyzed from the zygote cDNA library, nine transcripts were not detected in egg cells, and were thus considered to be candidate de novo transcripts produced after fertilization (Figure 3). The majority of these transcripts corresponded to novel genes. Similarly, of the 18 transcripts from the two-celled proembryo cDNA library, five new transcripts were present only after fertilization. The expression patterns of the other genes analyzed are selectively shown in Figure S2. The presence of de novo transcripts identified in early zygotes implies that zygotic gene activation may begin shortly after fertilization in tobacco. These results further confirmed that genes identified from the three cDNA libraries were indeed expressed in the corresponding cell stages. Furthermore, these results also support the idea that degradation of maternal transcripts and de novo transcription are initiated at the zygote stage.
Actinomycin and cordycepin completely block cell division in 72-HAP zygotes, and greatly suppress division in 96-HAP zygotes
To further confirm that the MZT is initiated in the zygote, and to estimate the role of the de novo transcripts in zygote development and embryogenesis, we carried out a series of experiments using the microculture technique combined with the application of transcription inhibitors. To test inhibitor efficiency, we tried different combinations of actinomycin D and cordycepin concentrations on ovule cultures for primary optimization (Figure S3), on protoplast cultures for further optimization (Figure S4A) and on zygote cultures for final confirmation. Combination C2 (20 μg ml−1 actinomycin D + 40 μg ml−1 cordycepin) was chosen for further experiments. Under these conditions, cells remained viable and divided after the removal of the inhibitors, indicating that the potential toxic effects of the inhibitors on cell division were reversible (Figure S4B,C).
We then isolated 103 inhibitor-treated zygotes from cultured ovary cells to test the effectiveness of the applied inhibitors by monitoring the expression patterns of selected genes. Based on this work and our previous data (Ning et al., 2006), 10 de novo transcripts were analyzed in zygotes. Among these transcripts, some are known to be homologous with transcripts that play important roles in embryogenesis and post-embryonic development (Bartee et al., 2001; Collings et al., 2008; Konopka and Bednarek, 2008; Richmond and Somerville, 2000; Xiao et al., 2006). Therefore, expression of these transcripts may mark the onset of embryogenesis in 96-HAP zygotes. RT-PCR analysis revealed that de novo transcription was indeed suppressed by these transcription inhibitors (Figures 4 and S5[link]).

Confirmation of transcript inhibitor efficiency by RT-PCR. In vivo zygotes at 96 h after pollination (HAP; left panel) were used as a control. Translation inhibitors were applied to ovules at 42 HAP, and treated zygotes at 144 HAP were isolated for comparison (right panel). ZC1, ZC7, ZC8, ZC18 and ZC35 (Ning et al., 2006) encode proteins that are homologous with Arabidopsis dynamin family protein, the tobacco chromomethylase-like (CMT-like) protein, the Lycopersicon esculentum putative glucosyltransferase (GTF), tobacco α-tubulin and the Arabidopsis transcription factor WUSCHEL-related homeobox 9 (WOX9), respectively. ZY-267, ZY-833, ZY-840, ZY-931 and ZY-1255 encode unknown proteins. Untreated proembryos at 144 HAP were also used as controls (Figure S5).
Based on our well-established zygote culture system (He et al., 2007), zygotes at 72 HAP were chosen to test the effects of inhibition of de novo transcription. After 48 h of culture under control conditions, almost 90% of zygotes exhibited normal development (Figure 5a: A1–A3), and approximately 45% of the zygotes had divided (Figure 5a: A3). However, in the presence of TIT, only 10% of the zygotes exhibited limited elongation, and no cell division was observed, although 75% of the zygotes were enlarged or extruded small bubbles (Figure 5a: A4). This observation implies that de novo transcription is required for zygote directional elongation at this stage. Furthermore, maternal transcripts present in the egg cell appear to be insufficient for the first cell division of zygotes.
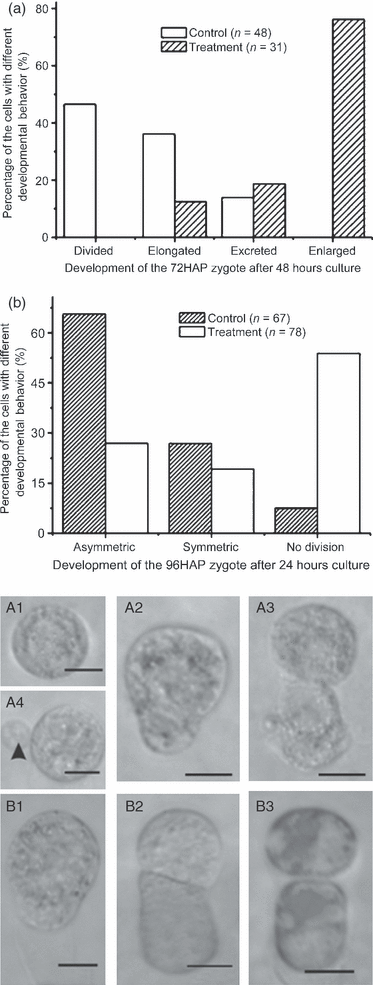
Transcription inhibitor treatment (TIT) blocks zygote development in vitro.(a) Development of a 72-h-after-pollination (HAP) zygote after inhibitor treatment. The first division was prevented by TIT.(b) Comparison of the frequency of the first division in control and inhibitor-treated 96-HAP cultivated zygotes. P < 0.001.(A1–A4) Zygotes (72 HAP) after 48 h of culture. (A1) Inhibitor-treated zygote. (A2) Elongated control zygote. (A3) Control zygote after cell division. (A4) An inhibitor-treated zygote. The zygote extruded small bubbles (arrowhead), but did not divide.(B1–B3) Zygotes (96 HAP) after 24 h of culture. (B1) An undivided zygote after TIT. (B2) A control zygote after cell division. (B3) A TIT zygote after cell division, indicating that 96-HAP zygotes were not completely inhibited. Scale bar: 10 μm.
In addition, 96-HAP zygotes (Figure 5b: B1) were also subjected to TIT. Zygotes at this stage were all elongated. In control cultures, approximately 90% of the zygotes divided (Figure 5b: B1, B2). After TIT, the division frequency was reduced to approximately 45% (Figure 5b: B3). That 45% of zygotes still divide may suggest that the genes regulating cell division are already activated before/around 96 HAP. Partially inhibited division may result from asynchronous development of individual zygotes. This hypothesis was further supported by observations on asynchronous nuclear division at this stage (Figure S6).
Further confirmation of inhibition of zygotic division by actinomycin and cordycepin using an in vitro–in vivo embryogenesis system
To reduce the potential influence of in vitro manipulation and culture conditions on zygote development, and to further confirm the effect of TIT on blocking zygote division and embryogenesis, we employed an in vitro–in vivo embryogenesis system, ovary culture, for further confirmation (Figure S7). The development of embryos in cultured ovaries was normal, with the exception of a 12- to 24-h delay, exhibiting no differences from development in vivo (Figure 6a), with regard to both the division plane pattern and morphology (Figure 6b).
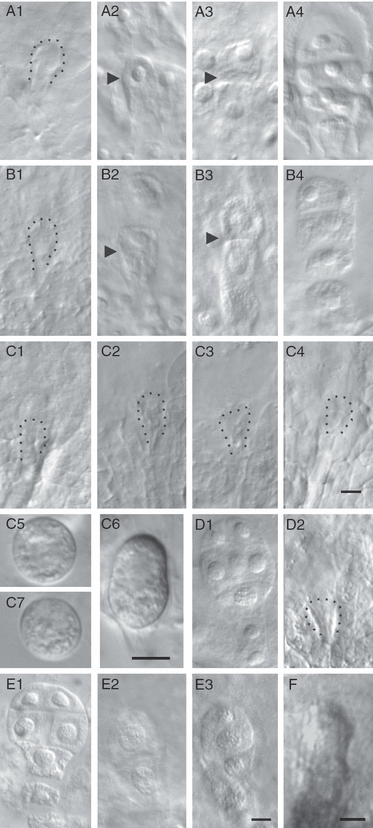
Characterization of control and transcription inhibitor treatment (TIT) zygote development in vivo and in cultured ovaries by whole-mount clearing and zygote isolation.(A1–A4) The ovules in vivo. (A1) Zygote at 72 h after pollination (HAP; circled). (A2) Zygotes at 96 HAP are elongated (arrowhead). (A3) Two-celled proembryo (arrowhead shows the division plane) at 108 HAP. (A4) A four-celled proembryo formed by 144 HAP. (B1–B4) Control cultured 42-HAP ovules.(B1) Small zygote cultured for 54 h. (B2) Elongated zygote cultured for 78 h. (B3) The zygote divided after 78 h in culture. (B4) The four-celled proembryos after 102 h of culture.(C1–C4) The 42-HAP ovules treated with inhibitors for 30–102 h. Development arrested at an early stage. (C5, 7) Zygotes isolated from the 144-HAP ovaries after TIT beginning at 36 (C5) or 42 HAP (C7). Note that both zygotes in C5 and C7 arrested at a similar stage appear morphologically similar to the 72-HAP zygote. (C6) Zygote (96 HAP) in vivo.(D1) Control ovaries (67 HAP) cultured for 77 h showing the formation of an eight-celled embryo. (D2) Ovaries (67 HAP) treated with inhibitors for 77 h. Zygotic development is blocked.(E1) Control ovaries (91 HAP) cultured for 53 h showing the formation of an eight-celled embryo. (E2) Ovaries (91 h) treated with inhibitors for 53 h. Zygote developed into a two-celled proembryo. (E3) Ovaries (91 HAP) treated with inhibitors for 53 h. Zygote developed into a four-celled proembryo.(F) Elongated zygotes are observed after the removal of inhibitors (n = 120).Scale bars: 10 μm. (A–C) n = 100–120 for each stage. (D, E) n = 120–150 for each material.
Before fertilization, we treated ovaries with inhibitors at 42 HAP to guarantee the complete inhibition of de novo transcription. To avoid the potential influence of delayed action of the inhibitors caused by differences in absorption by different ovaries, ovaries at 36 HAP were also used for TIT. Initial experiments confirmed that treatment of 42 HAP zygotes was sufficient to block the fertilization-induced de novo transcription (Figure 6c). At the beginning of the culture (42–72 HAP), the ovaries developed in a manner similar to control ovaries. After 96 HAP, control ovules exhibited obvious growth, whereas inhibitor-treated ovaries were arrested, and their ovule volumes remained similar to those of 72-HAP ovules in vivo (Figure S8). The zygotes remained at a similar stage from 72 to 144 HAP (Figures 6c and S9[link]).
We then treated ovaries at different stages after fertilization and compared them with those treated before fertilization. Ovules in 67-HAP ovaries treated for 77 h developed to a stage similar to those in 42-HAP ovaries treated for 102 h (Figure 7a). Zygote development inside the ovaries was also arrested at a similar stage, and no cell division was observed (Figure 6d). However, when 91-HAP ovaries were treated, the embryos developed into two- or four-celled proembryos (6, 7). These results were also confirmed by inhibitor treatment at both 36 and 42 HAP. Subsequent to treatment, the ovules did not develop properly, evident by comparison with control 96-HAP ovaries (Figure 7c). Additional TIT experiments, in which 108-HAP ovaries were subjected to treatment, revealed that eight-celled embryos were well developed (Figure S10). These results suggest that after initiation of the MZT, de novo transcripts are successively required during later embryogenesis.
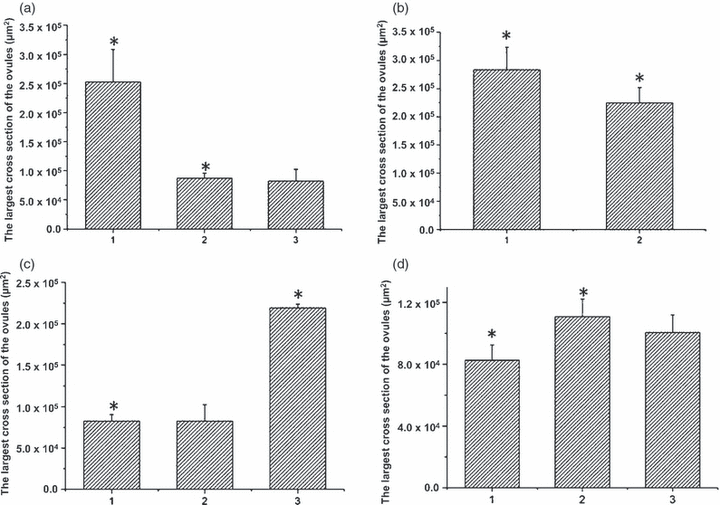
Effects of application and removal of transcription inhibitors on ovarian growth.(a) Volume of inhibitor-treated ovules (calculated by measuring the area of the largest cross section of the ovules). (1) Control ovules (67 h after pollination, HAP) cultured for 77 h. (2) Ovules (67 HAP) treated with inhibitors for 77 h. (3) Ovules (42 HAP) treated with inhibitors for 102 h. P < 0.05.(b) Increase in ovule volume. (1) Control ovules (91 HAP) cultured for 53 h. (2) Ovules (91 HAP) treated with inhibitors for 53 h. P < 0.05.(c) Comparison of ovule volumes at the end of culture (144 HAP). (1) Inhibitor-treated ovules from 42 HAP. (2) Inhibitor-treated ovules from 36 HAP. (3) Control ovules (96 HAP) in vivo. P < 0.05. The time treatment initiated had no obvious influence on ovule growth.(d) Ovule volumes increased after inhibitors were removed. (1) Ovules treated for 102 h. (2, 3) Recovery groups. The inhibitors were removed after 54 h (2) or 78 h (3) of treatment. P < 0.05, n = 60–100.
Characterization of the role of egg cell transcripts in zygote development
To evaluate the role of maternal transcripts stored in egg cells in early zygotic development, cytological observations of fertilization and zygote development in ovules treated with inhibitors before fertilization were made. Several critical developmental events were characterized, based on our previous observations. We found that the fertilization process was always normal and that volume reduction and vacuole disappearance in fertilized egg cells were also normal in the presence of inhibitors (6, 5, 7). The nucleus still occupied the central part of the cell, but directional elongation did not occur. These observations indicate that without zygotic transcription, fertilization can be completed, and that early developmental events marking the transition from egg cell to zygote can occur (Table S3).
The cell wall of the zygote at 72 HAP is very thin and easily removed. Therefore, through an isolation procedure, the zygotes were converted into protoplasts. During cell culture, zygotes rapidly resynthesized cell walls in the control experiments. However, after TIT, cell wall synthesis was mostly blocked. Compared with 72-HAP zygotes, 96-HAP zygotes showed obvious thick and polar-deposited cell wall material, and were able to maintain relative integrity during isolation. In both control and inhibitor-treated zygotes, the basal part of the cell wall and the newly synthesized portions had brighter cell wall fluorescence (Figure S11). This observation suggests that the typical polar deposition of cell wall material was almost complete in 96-HAP zygotes, and that de novo transcripts after 72 HAP are likely to be required for such deposition.
During cultivation, the 72-HAP zygote could carry out limited elongation, as described above (Figures 5a and S12[link]), but this elongation was greatly blocked by the inhibitors. However, in 96-HAP zygotes, such directional elongation was almost complete, implying that new transcripts appearing after 72 HAP may be necessary for full elongation of the zygotes.
In treated 72-HAP zygotes, cell division was never observed, but 45% of the treated 96-HAP zygotes could divide. In addition, control two-celled proembryos could further develop into four-celled proembryos, but in treated two-celled proembryos, no further division was observed. These results were further confirmed using the in vivo–in vitro culture system (data not shown). These data again suggest that the de novo transcripts at 96 HAP are likely to be essential for triggering embryogenesis.
When 42-HAP ovaries were cultured for 54 and 78 h, followed by the removal of inhibitors, both ovules and zygotes showed further growth (6, 7), but could not develop into embryos. FM4-64 incubation of the inhibitor-treated zygotes showed that the cell membrane was both intact and active, as the rate of endocytosis was similar to that of control cells (Figure S13). This result indicates that membrane activity was essentially normal in these cells, suggesting that the inhibitor had no obvious influence on zygote viability during treatment. Fluorescein diacetate (FDA) staining coupled with live microscopy further confirmed cell viability (Figure S14; Video Clip S1). Furthermore, leaf protoplasts continued to divide and form multicellular structures after the same treatment. These observations may indicate that the process for triggering zygotic embryogenesis is highly pre-programmed and sensitive, and that once this process is interrupted, it cannot easily recover.
Discussion
Because of its key role in the molecular mechanisms of fertilization and early embryogenesis, the MZT has been extensively investigated in mammals, particularly in mice. The MZT has primarily been investigated using two strategies. First, global transcription analysis has revealed that the transcriptional profile greatly changes during the MZT (Minami et al., 2007; Schier, 2007) because of both maternal transcript degradation and zygotic gene activation (Baroux et al., 2008). Second, in vitro culture systems combined with the application of transcription inhibitors have provided unique evidence indicating that de novo transcription occurs in early-stage embryos, and is a prerequisite for subsequent embryogenesis (Minami et al., 2007). These available data suggest that transcript profiling analysis alone is not sufficient for the characterization of the MZT, particularly for the full exploration of the biological significance of the MZT in embryogenesis. Thus, a combination of these two strategies is necessary for the complete characterization of the MZT. Using these strategies, the timing of the MZT has been clearly revealed in several animals.
In contrast to mammals, little is known about the MZT and its relationship with embryogenesis in angiosperms, partly because the employment of similar techniques is significantly more difficult in plants (Pillot et al., 2010). The inaccessibility of the egg cell and early embryo greatly limits these investigations. In addition, setting up a reliable culture system for zygote development is rather difficult in plants. As a result of technical improvements in sexual cell isolation in plants over the last 10 years, we have been able to successfully develop techniques for the construction of a cell-specific cDNA library from gamete cells (Ning et al., 2006), and for the microculture of zygotes (He et al., 2007) in tobacco. Therefore, as a result of the development of these techniques, characterization of the MZT in tobacco became feasible.
The MZT is initiated prior to zygotic division in tobacco
Comparing the three cDNA libraries from developmentally consecutive cells (egg cell, zygote and two-celled proembryo), the transcript profiles in egg cells before and after fertilization differ substantially. First, some maternal transcripts stored in egg cells begin to disappear. As a representative example, in egg cells the most abundant ESTs were from the ECA1-like gene, representing approximately 9.4% (185) of the total ESTs. Importantly, the numbers of these ECA1-like ESTs decreased to 10 in zygotes and only one in two-celled proembryos. Gradual degradation of maternal transcripts is a key signal for MZT initiation. If some of the maternal transcripts were degraded after fertilization and replaced by new transcripts from the zygotic genome, the proportion of degraded transcripts could be much higher than what we observed.
Second, zygotic gene activation, evident as de novo transcription, provides further support that the MZT is initiated in zygotes in tobacco. Analysis of transcript profiles from each cDNA library revealed the presence of transcripts in zygotes and two-celled proembryos that were not found in egg cells. These transcripts encoded proteins involved in DNA and chromatin structure (including histones). Transcripts encoding each subunit of the nucleosome were found in egg cells, but the numbers of these transcripts increased dramatically in zygotes and two-celled proembryos. Transcripts encoding a protein with similarity to a 10-kDa chaperonin were not found in the most prevalent contigs of egg cells, but were abundant in zygotes. Additionally, transcripts encoding histones H2A and H4 comprised the two largest contigs in zygotes, but were not present in the most common contigs in egg cells. RT-PCR analysis provided further evidence that some transcripts not detected in egg cells were present in zygotes. These results indicate that the zygotic genome is activated prior to the first zygotic division in tobacco.
Together, results indicating that the degradation of maternal transcripts and de novo transcription occur in zygotes suggest that the MZT may be initiated prior to the first zygotic division in tobacco. However, one concern is that in zygotes, de novo transcripts not present in egg cells could be confused with transcripts delivered by the sperm via fertilization. Therefore, further confirmation is necessary to ensure that these are truly de novo-synthesized transcripts. Thus, next we carefully compared the expression pattern of a subset of transcripts by RT-PCR (Figures 3 and S2[link]) in sperm, egg, zygote, two-celled proembryos and vegetative tissues. These results showed that the majority of these transcripts were not found in sperm, indicating their emergence at the zygote stage. Some of these transcripts were either only or dominantly expressed in zygotes and two-celled proembryos. Therefore, these transcripts probably play a role in later zygote development and/or embryogenesis. In fact, the Polycomb group proteins EMF2 and FIE2, which negatively regulate embryogenesis (Danilevskaya et al., 2003; Yoshida et al., 2001), were identified in zygote and two-celled proembryo cDNA libraries, respectively. Arabidopsis thaliana MERISTEM LAYER 1 (ML1), which is essential for the formation of the epidermis in developing embryos (Sessions et al., 1999), was found in zygote cDNA libraries. As ML1 was shown to be required for epidermal development, the presence of homologous transcripts in tobacco zygotes may indicate that embryonic patterning begins to be established as early as shortly after fertilization in tobacco.
Recently, a study reported that the SHORT SUSPENSOR (SSP) gene in Arabidopsis is essential for regulating the asymmetric first division of the zygote (Bayer et al., 2009). Interestingly, transcripts from this gene were found in both sperm and zygotes, but were only translated in zygotes. The subsequent translation of SSP activates downstream proteins in the zygote stage to ensure proper zygotic division. This result provides strong evidence for a functional requirement for paternal transcripts inherited by the sperm cytoplasm translated after fertilization. In the present study, we also identified transcripts that accumulated specifically in the sperm prior to fertilization, such as ZY-931 (Figure 4), which was subsequently depleted in embryos. This observation suggests that this paternally derived transcript may also play a role in zygote development and/or early embryogenesis.
In both in vitro and in vivo–in vitro culture systems, cell elongation, cell division and embryogenesis could be completely blocked by treatment with transcription inhibitors in zygotes at developmental stages prior to 72 HAP (around 24–27 h after fertilization). However, after 96 HAP (48–51 h after fertilization), zygotic cell division could only be partially blocked, and after 108 HAP, zygotes could develop into up to four-celled embryos. These results suggest that a change in the transcript profile must occur at approximately 96 HAP in zygotes, as de novo transcripts should be gradually generated for subsequent zygotic development. Evidence from the cell culture models coincides with that of transcript profile analysis (Figure 8). However, considering that inhibitors may play multiple roles, e.g. not only mRNA but also rRNA inhibition (Perry and Kelley, 1968), and that possible side effects may also contribute to the arrest of zygote development after TIT, we cannot definitively conclude that zygotic control has totally overtaken maternal control at the zygote stage. Whether reliance on maternal information ends completely during early embryogenesis still requires further investigation. In fact, a recent study involving the downregulation of RNA POLII in Arabidopsis demonstrated prolonged reliance on maternal transcripts for zygote/proembryo development (Pillot et al., 2010), indicating that in at least some plant species, the elimination of maternal transcripts could occur over an extended time period. Thus, future investigations using specific inhibition of RNA POLII will offer more solid evidence for the arrest of zygote development after TIT, and will further elucidate whether ribosomal or messenger RNA have a distinct role in MZT.
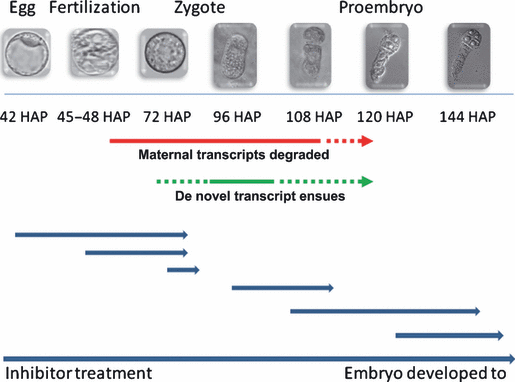
Schematic illustration of results from transcript profiling and zygote culture after transcription inhibitor treatment. Red and green lines indicate the periods during which maternal transcript elimination and de novo transcription were initiated, respectively, according to our findings. The red and green dotted lines with arrows indicate possible extensions of the periods for maternal transcript elimination and de novo transcription. Blue lines indicate the primary results from inhibitor treatments. The starting point of each line shows the stage at which inhibitors were applied. The lengths of the lines denote the duration of the cell culture. Arrowheads indicate the developmental stage that zygotes and embryos reached after treatment.
The first zygotic division in tobacco requires global transcriptional processes
Maternal transcripts in egg cells are expected to support early development after fertilization. However, the developmental processes that these maternal transcripts regulate and support remain unknown. To define these processes, we carefully observed critical developmental events occurring during fertilization and zygote growth using the microculture system and inhibitor treatments (Table 5).
| Start point of TITa | Duration of culture (h) | Final developmental status of the treated cells | Development status of control at the same timeb |
|---|---|---|---|
| Before fertilization | |||
| 36-HAP ovary | 108 | Successful fertilization; non-elongated zygote morphologically similar to that of 72 HAP in control | Four-celled proembryo |
| 42-HAP ovary | 102 | Successful fertilization; non-elongated zygote morphologically similar to that of 72 HAP in control | Four-celled proembryos |
| After fertilization | |||
| 67-HAP ovary | 77 | Non-elongated zygote without division and morphologically similar to that of 72 HAP in control | Four- to eight-celled proembryos |
| 72-HAP zygote | 48 | Zygote limitedly elongated but never divided | Almost 90% zygotes normally developed |
| 91-HAP ovary | 53 | Two- to four-celled proembryo | Eight-celled embryos |
| 96-HAP zygote | 24 | The zygote first division greatly decreased | 90% zygote developed to the two-celled proembryos |
- aTIT, transcription inhibitor treatment.
- bControl is the same material without TIT.
- HAP, hours after pollination; n = 100–150.
Detailed cytological observation of zygote development in ovaries treated with inhibitors before fertilization showed that some early developmental events, such as gamete fusion, zygote volume reduction, complete cell wall formation, large vacuole disappearance and limited cell enlargement, could still proceed after the inhibition of transcription (5, 6[link]). Although de novo transcription was inhibited, the zygote could grow to a stage morphologically similar to a 72-HAP zygote cultured under control conditions, but no further development was observed. Therefore, these early developmental steps (prior to 72 HAP) are primarily maternally controlled. However, maternal transcripts deposited in the egg cell are not likely to be sufficient for triggering embryogenesis.
At 72 and 96 HAP, zygotes exhibited rapid growth in control cultures. The cell elongated, and the nucleus localized to the pole in preparation for the first zygotic division. At this stage, cell division was no longer completely blocked by the same inhibitors, signifying that embryogenesis had already been initiated around 96 HAP. These data from both zygote and ovule cultures suggest that the de novo transcripts produced during this period are probably required for triggering embryogenesis.
The underlying reason for maintaining the silence of the zygotic genome prior to the one-celled proembryo stage remains unknown. In animals, a study reported that the nucleocytoplasmic ratio and titration of a transcriptional repressor are potential factors that determine the timing of the MZT (Schier, 2007). MZT initiation has also been shown to coincide with lengthening of the first cell cycle. Specifically, mice embryos undergo a lengthened cell cycle at the beginning of embryogenesis, and this extended time period is considered to be the reason why the MZT occurs at a much earlier stage in mice compared with most other animals (O’Farrell et al., 2004). Interestingly, in tobacco zygotes, a 4N complement of nuclear DNA is present in the zygote because tobacco gametes undergo cell fusion after the completion of the S phase, apparently during G2 (Tian et al., 2005). Thus, the tobacco zygote may have a higher nucleocytoplasmic ratio compared with many other plants, including maize, wheat and Arabidopsis. In addition, tobacco zygotes undergo the first cell cycle over an extended period of several days. These characteristics may explain why the MZT is initiated before the first zygotic division in tobacco.
Experimental Procedures
Isolation of eggs, zygotes and two-celled proembryos from tobacco
Tobacco (N. tabacum cv SR1) plants were grown in a glasshouse at 25°C with a light period of 16 h. Egg and sperm cells were isolated according to previously described procedures (Sun et al., 2000; Tian and Russell, 1997). The zygotes and two-celled proembryos were isolated by enzymatic maceration combined with manual dissection (Fu et al., 1996; He et al., 2007). Two transcription inhibitors, actinomycin D (50 mg L−1) and cordycepin (100 mg L−1), were added to all solutions during the isolation process (Leonhardt et al., 2004; Ning et al., 2006) to inhibit changes in gene expression caused by potential stresses incurred during physical isolation and enzymatic digestion. The isolated cells were immediately transferred to 2× lysis/binding buffer (from Dynabeads mRNA DIRECT Micro kit; Dynal Biotech, now part of Invitrogen, http://www.invitrogen.com/dynal) in 0.2-ml tubes, and were frozen in liquid nitrogen for subsequent mRNA isolation and cDNA library construction.
cDNA library construction and sequencing
mRNA was isolated from 159 eggs, 143 zygotes or 149 two-celled proembryos using the Dynabeads mRNA DIRECT Micro kit (Dynal Biotech) following the manufacturer’s instructions. The only deviation from the recommended protocol was a reduction in the annealing volume to 50 μl. Subsequently, a SMART cDNA Library Construction Kit (Clontech Laboratories, http://www.clontech.com) was used for cDNA library construction. Ligations were packaged with Gigapack III Gold packaging reagents (Stratagene, now part of Agilent Technologies, http://www.genomics.agilent.com). The unamplified library titers were 4.43 × 106 for the egg library, 4.4 × 106 for the zygote library and 5.4 × 106 for the two-celled embryo library. Individual cloned cDNAs were obtained by in vivo mass excision, followed by random selection and sequencing using a DNA capillary sequencer (ABI 3730XL; Applied Biosystems, http://www.appliedbiosystems.com). All EST sequences from representative cDNA libraries were deposited in NCBI dbEST. The accession numbers for the apical cell ESTs are HS080288–HS083059, those for the basal cell ESTs are HS083060–HS085835, and those for the two-celled proembryo ESTs (tc0001-tc0007) are HO844849, HO844115, HO845026, HO843422, HO844377, HO844521, and HO844358.
Bioinformatics
We combined ESTs from three individual libraries for the following processes. PHRED was used for base calling and trimming of low-quality sequences. Cloning vectors and linkers were masked with the cross-match program. The cleaned EST sequences were clustered into non-redundant sets (cluster) using Uicluster2, or were assembled into contigs using Cap3, according to their sequence connection. All the contigs were given numbers for the following analysis. Groups that contained only one sequence were classified as singletons. For annotation, the assembled consensus sequences and singletons were used as queries for BLASTN and BLASTX searches (http://www.ncbi.nlm.nih.gov). The E values were 10−5 for both BLASTN and BLASTX. Contigs encoding proteins of known function were manually categorized into broad functional groups using the Munich Information Centre for Protein Sequences classification as a guide.
Expression analysis by RT-PCR
For each type of isolated cell, cDNA was synthesized twice on independent occasions for comparison and confirmation of results. mRNA was isolated from 110 or 102 egg cells, 109 or 108 zygote cells, and 100 or 88 two-celled embryos, respectively, as described above. The Super SMART™ cDNA PCR Synthesis kit (Clontech Laboratories) was used for cDNA amplification.
RNA from pistils, anthers, shoot tips, young leaves and roots was isolated using TRIzol reagent (Invitrogen, http://www.invitrogen.com). Then, 1 μg of total RNA was digested with DNAse (Ambion, http://www.ambion.com) and then used for first-strand cDNA synthesis using Oligo (dT)15 (Promega, http://www.promega.com) and Superscript III reverse transcriptase (Invitrogen). The quality and quantity of cDNAs were tested using PCR with primers for the tobacco G3PDH gene as a control (Ning et al., 2006) (accession no. AJ133422; G3PDH primers, 5′-TCCACTCCATCACAGCCACA-3′ and 5′-AGACTCCTCACAGCAGCACC-3′). Specific primer pairs were designed for each selected contig using Primer Premier 5 (Premier Biosoft International, http://www.premierbiosoft.com). GAPDH PCR reactions were carried out for 25 cycles, using 0.5 μl of cDNA as a template. PCR reactions for contig-specific primers were carried out for 30–35 cycles.
Ovary culture and zygote culture
The general method for ovary culture was performed according to Wang et al. (1997), with minor modifications. Briefly, flowers at 42–43 HAP containing unfertilized ovules were obtained and inserted into Eppendorf tubes containing MS medium for 24 h of primary culture to ensure completion of fertilization. Next, the ovaries were removed, and the surfaces were sterilized with 75% alcohol. After three washes with sterilized water, the pedicels were cut off, and the ovaries were placed onto Petri dishes for further culture in the dark at 25°C. Culture medium consisted of MS, basic elements and 2% sucrose, pH 5.8.
For the zygote recovery test, inhibitors were removed after treatment for 54 or 78 h. The ovaries were then cultured for an additional 120 h. Freshly isolated zygotes were cultured according to the method described by He et al. (2007). During the cultures, 20 μg ml−1 actinomycin D and 40 μg ml−1 cordycepin were added to inhibit transcription. Actinomycin D and cordycepin were dissolved in dimethyl sulfoxide and sterilized water, respectively. Controls were treated with the appropriate vehicles.
Cytological observations
Ovules were fixed in Carnoy’s fluid (3:1 absolute ethyl alcohol:glacial acetic acid) for 1 h, and then rehydrated with 75, 50 and 25% ethyl alcohol, for 20 min at every step. Ovules were cleared with Hoyer’s solution overnight for observation. The embryo sacs were released according to Fu et al. (1996).
Observations of cell walls, nuclear location and vesicles followed protocols described by He et al. (2007). For cell wall observations, calcofluor white ST (CW; America Cyanamid Co., now part of BASF Co., http://www.basf.com) was used to fluorescently label cell walls. CW (0.1 mg ml−1) was dissolved in 0.5 m mannitol (pH 5.8). Stained zygotes were washed twice in 0.5 m mannitol prior to visualization. To observe nuclei, zygotes were incubated in 10 mg ml−1 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, supplied by Invitrogen) in 0.5 m mannitol for 15–20 min, followed by two washes with 0.5 m mannitol. To visualize endomembrane vesicles, the procedure reported by Hadley et al. (2006) was employed. Briefly, isolated zygotes were incubated with 1 μg ml−1 FM4-64 [(N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl) pyridinium dibromide; Molecular Probes] for 5 min. The zygotes were then washed twice with 0.5 m mannitol prior to observation. Images were acquired using a Leica DMIRE2 inverted microscope equipped with a cooled charge coupled device (CCD) (model RTE/CCD-1300-Y/HS; Roper Scientific Co., http://www.roperscientific.com).
Acknowledgements
The project was supported by the ‘973’ Project (2007CB108700), National Natural Science Fund of China (30521004, 30771131) and the key grant project of Chinese Ministry of Education (307018). We thank Sheila McCormick from U.C. Berkeley for her useful suggestions and critical reading of the manuscript.




