The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato
Summary
NHX-type antiporters in the tonoplast have been reported to increase the salt tolerance of various plants species, and are thought to mediate the compartmentation of Na+ in vacuoles. However, all isoforms characterized so far catalyze both Na+/H+ and K+/H+ exchange. Here, we show that AtNHX1 has a critical involvement in the subcellular partitioning of K+, which in turn affects plant K+ nutrition and Na+ tolerance. Transgenic tomato plants overexpressing AtNHX1 had larger K+ vacuolar pools in all growth conditions tested, but no consistent enhancement of Na+ accumulation was observed under salt stress. Plants overexpressing AtNHX1 have a greater capacity to retain intracellular K+ and to withstand salt-shock. Under K+-limiting conditions, greater K+ compartmentation in the vacuole occurred at the expense of the cytosolic K+ pool, which was lower in transgenic plants. This caused the early activation of the high-affinity K+ uptake system, enhanced K+ uptake by roots, and increased the K+ content in plant tissues and the xylem sap of transformed plants. Our results strongly suggest that NHX proteins are likely candidates for the H+-linked K+ transport that is thought to facilitate active K+ uptake at the tonoplast, and the partitioning of K+ between vacuole and cytosol.
Introduction
A critical process in the salt tolerance of plants is the compartmentation of Na+ in vacuoles, which provides an efficient mechanism to avert the deleterious effects of Na+ in the cytosol, while mediating vacuolar osmotic adjustment that facilitates water uptake into cells (Blumwald et al., 2000). The NHX proteins were originally described as Na+/H+ antiporters located in the tonoplast, driving Na+ accumulation into the vacuole, and conferring salt tolerance (Apse et al., 1999; Gaxiola et al., 1999; Quintero et al., 2000). However, in spite of numerous reports of improved halotolerance by the overexpression of NHX-type exchangers in various plant species, the mechanism underlying the enhancement of salinity tolerance by these transporters is not yet clear (Tester and Davenport, 2003; Pardo et al., 2006). Several reports failed to find the anticipated correlation between increased salt tolerance and enhanced accumulation of Na+ by NHX proteins (Ohta et al., 2002; Fukuda et al., 2004; Wu et al., 2009), whereas others found greater K+ contents, rather than greater Na+ contents, in tissues of the transgenic plants (Xue et al., 2004; Wu et al., 2005; Zhao et al., 2006; Rodriguez-Rosales et al., 2008). All plant NHX-type transporters characterized to date can be classified into two groups: class I and class II (Brett et al., 2005; Pardo et al., 2006). Proteins of class I localize to vacuoles and catalyze Na+/H+ and K+/H+ exchanges in vitro, whereas the endosomal (non-vacuolar) class-II proteins may show a preference for K+ over Na+ as the substrate (Venema et al., 2002, 2003; Apse et al., 2003; Rodriguez-Rosales et al., 2008). Biochemical studies have established that AtNHX1, a class-I protein, catalyzes both Na+/H+ and K+/H+ exchange with similar affinity (Venema et al., 2002; Apse et al., 2003; Yamaguchi et al., 2005). Moreover, binding of the calmodulin-like protein AtCaM15 of Arabidopsis thaliana to the C terminus of AtNHX1 lowered the maximal ion-exchange rate (Vmax) of the Na+/H+ exchange activity in isolated vacuoles, whereas the Vmax of the K+/H+ exchange activity was not significantly altered, thus leading to a decrease in the Na+ : K+ transport ratio (Yamaguchi et al., 2005). Indirect evidence from restored tolerance of yeast nhx1 mutants to NaCl and KCl suggested that the vacuolar proteins OsNHX1 from Oryza sativa (rice), and InNHX1 and InNHX2 from Ipomoea nil (Morning glory), also have dual Na+ and K+ specificity (Fukuda et al., 2004; Ohnishi et al., 2005). Thus, insufficient Na+–K+ discrimination brings into question the role of vacuolar NHX1-like proteins as effective Na+ scavengers in planta, as the cytosolic K+ concentration will most likely exceed that of Na+, even under salinity stress (Carden et al., 2003; Tester and Davenport, 2003). Cytosolic Na+ concentrations of 10–30 mm have been estimated in the root cells of salt-treated glycophytes by X-ray microanalysis and ion-sensitive electrodes, whereas cytosolic K+ will often remain above the 60 mm level (Hasegawa et al., 2000; Carden et al., 2003).
Besides their role in salinity tolerance, NHX proteins have been implicated in the control of vacuolar pH in petal cells of Ipomoea (Fukuda-Tanaka et al., 2000; Yamaguchi et al., 2001; Yoshida et al., 2005), and of leaf cell expansion in Arabidopsis (Apse et al., 2003). The participation of NHX proteins in these processes is most likely to be related to K+/H+ exchange, as Na+ concentrations in the cytosol of plant cells remain low in the absence of salinity stress. Vacuoles occupy most of the intracellular volume in many plant cells and are the main cellular reservoir for K+. Changes in tissue K+ concentration are largely a reflection of the dynamics of the vacuolar pool. By contrast, cytosolic K+ concentrations are tightly regulated through the integrated regulation of K+ uptake and efflux at the plasma membrane, and K+ import and export at the tonoplast (Leigh, 2001). Cytosolic K+ concentration is thought to decline only when vacuolar K+ has been depleted (Walker et al., 1996). Conversely, surplus K+ is placed into the vacuole to maintain cytosolic K+ within narrow limits, independently of K+ abundance in the growth medium. At the usual range of tonoplast membrane potential (20–40 mV, positive inside) and cytosolic K+ activity (80 mm), transport of K+ into the vacuole in K+-replete cells occurs against the electrochemical gradient, and must be energized (Walker et al., 1996; Martinoia et al., 2000). Two mechanisms have been proposed for active accumulation of K+ in the vacuole (Leigh, 2001). One is the vacuolar pyrophosphatase that may couple H+ and K+ pumping into the vacuole, but this has not been confirmed experimentally. A K+/H+ antiporter energized by the pH gradient across the tonoplast has also been suggested to control vacuolar K+ accumulation, albeit the molecular identity remains elusive (Walker et al., 1996; Carden et al., 2003). Vacuolar NHX-type exchangers could serve this critical function in plant cells.
To obtain further insights into the physiological role of NHX proteins, we undertook a detailed characterization of the ion relations of transgenic tomato overexpressing the vacuolar antiporter AtNHX1 of A. thaliana under different supplemental K+ and Na+ regimes. Our results show a critical involvement of AtNHX1 in the subcellular partitioning of K+ that, in turn, affects plant K+ nutrition and Na+ tolerance.
Results
Phenotype of transgenic lines
The cDNA corresponding to the Arabidopsis AtNHX1 gene (At5g27150) (Yokoi et al., 2002) was expressed under the cauliflower mosaic virus (CaMV)35S gene promoter in transgenic tomato plants (Solanum lycopersicum cv. MicroTom). Transgene expression in kanamycin-resistant plants was analyzed by northern blotting and RT-PCR with AtNHX1-specific probes and primers, respectively. Four independent homozygous lines (N-367, -374, -376 and -380) were selected for further study because of they showed accumulation of the AtNHX1 transcript (Figure S1). The abundance of the AtNHX1 mRNA in these transgenic plants was not affected by salt stress (5 h in 150 mm NaCl; Figure S1). Among the transgenic lines tested, line N-367 had the highest expression level of AtNHX1. Western blotting of microsomal fractions from line N-367 probed with polyclonal antibodies raised against AtNHX1 demonstrated the accumulation of the Arabidopsis protein (Figure 1a). Tonoplast vesicles from the roots (Figure 1b) and leaves (data not shown) of line N-367 showed greater Na+/H+ and K+/H+ exchange activity than vesicles from control plants. Both the exchange of Na+/H+ and K+/H+ increased twofold in transgenic plants over the background of the endogenous antiporter activity in tomato (Figure 1c). No significant difference was found in vacuolar ATPase activity, and in the magnitude of inside-acid pH gradients performed in tonoplast vesicles from control and transgenic plants (Figure 1c). The commensurate gain in Na+/H+ and K+/H+ exchanging capacities in tonoplast membranes of the N-367 plants is in agreement with the low Na+–K+ selectivity of AtNHX1, previously determined in artificial proteoliposomes and Arabidopsis vacuoles (Venema et al., 2002; Apse et al., 2003). Rates of Na+/H+ and K+/H+ exchange in the vacuoles of control plants were subtracted from the rates in transgenic plants and fitted to Michaelis–Menten kinetics. The Km of AtNHX1 protein was estimated at 22 ± 12.8 mm for Na+ and 20.9 ± 14.7 mm for K+. Vmax was also similar for both cations [116 ± 20 for Na+ and 96.5 ± 19.9 for K+; measured as the relative change in fluorescence (ΔF/Fmax) mg−1 min−1]. Together, these results demonstrate that transgenic lines had a greater number of active NHX1-type transporters than control plants, and that they had low Na+–K+ selectivity.
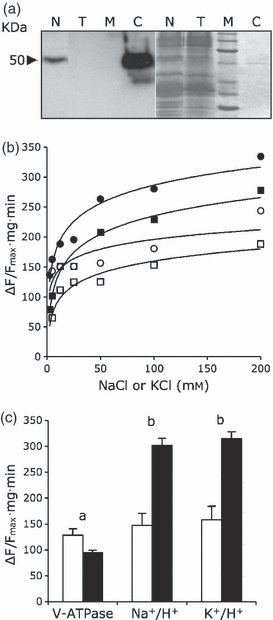
Na+/H+ and K+/H+ exchange in tonoplast vesicles.(a) Microsomal proteins (30 μg) from wild-type (T) and N-367 transgenic plants (N) were probed with antibodies against the AtNHX1 protein. C, 100 ng of purified AtNHX1; M, molecular size markers. Left, chemiluminiscence after western blotting; right, Coomassie staining.(b) ATP-dependent inside-acid pH gradients were preformed in tonoplast vesicles isolated from roots of wild-type (○, □) and N-367 transgenics (•, ), and the rate of dissipation was measured after the addition of NaCl (circles) or KCl (squares) salts at the indicated final concentrations. Exchange activity is expressed as arbitrary units (fluorescence recovery per minute and milligram of protein). Curves were fitted by the least-squares method.(c) Vacuolar H+-ATPase and Na+-K+/H+ exchange activities. Inside-acid pH gradients were established in tonoplast vesicles purified from the roots of wild-type (white bars) and N-367 transgenic plants (black bars) after the addition of ATP. Proton pumping by the V-ATPase was calculated from initial rates of fluorescence decline. Once fluorescence was stabilized, 100 mm of NaCl or KCl salts were added, and fluorescence recovery indicating cation/proton exchange was monitored for 2 min. Activities are expressed in arbitrary units as the relative change in fluorescence (ΔF/Fmax) per minute and milligram of membrane protein. Values are means of three independent biological replicates, different to those shown in (b). Letters a and b on top of pair-wise comparisons indicate: a, no significant difference, by the least significant difference test; or b, statistically different values at P < 0.05.
Overexpression of AtNHX1 has been shown to convey salt tolerance to tomato plants (cv. Moneymaker) (Zhang and Blumwald, 2001). Microtomato plants overexpressing AtNHX1 were more salt tolerant than control plants when the stress treatment was applied as salt-shock (Figure 2). By contrast, when salinity was increased stepwise at 25-mm increments twice a day, to reach final concentrations of 75 or 150 mm NaCl, plant growth was similarly inhibited in control and all transgenic lines tested (Tables 1 and S1). In these conditions, both control and transgenic plants completed their life cycle and set fruits after 8 weeks (Table S1). Notably, no consistent enhancement of Na+ accumulation was observed in the AtNHX1-overexpressing transgenic lines under all saline regimes tested, including the salt-shock treatments to which the transgenic lines showed greater tolerance than control plants (Figure 2; Tables 1 and S1). Instead, K+ contents were consistently greater in transgenic lines under nearly all growth conditions analyzed, including the absence of saline stress and varying K+ availability (Tables S2 and S3). To test how this differential accumulation of K+ affected the mineral nutrition of transgenic lines, plants were transferred to low K+ concentration (0.1 mm K+) hydroponic medium and grown until K+ was nearly depleted in the medium. Transgenic plants exhibited earlier development of K+-deficiency symptoms (crinkled upper leaves, yellowish bronze mottling and chlorosis along the leaf margin; Figure 2c) relative to control plants, despite the fact that leaves of transgenic plants contained greater total K+ concentrations than those of control plants at the end of the experiment (70.2 mm in N-367 and 42.8 mm in control plants). Together, these results indicate that AtNHX1 overexpression has a greater impact on K+ homeostasis than on Na+ accumulation under the growth regimes tested. Moreover, these results suggest that the greater K+ levels may not be physiologically accessible to transgenic plants growing in a K+-limiting environment, presumably because of enhanced compartmentation into the vacuole at the expense of the cytosolic K+ pool.
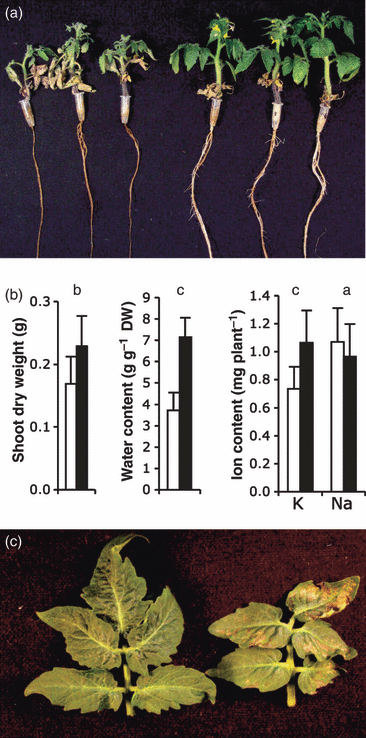
Salt tolerance and K+ deficiency.(a) Wild-type and transgenic N-367 plants were treated with 150 mm Na2SO4 for 6 days, and were then allowed to recover for 4 days without salt. Shown are three representative plants from the controls (left) and from line N-367 (right).(b) Shoot dry weight, water content, and K+ and Na+ contents of wild-type (white bars) and N-367 transgenic plants (black bars), treated as described in (a). Values are means and SDs of eight plants. Letters a, b and c on top of pair-wise comparisons indicate: a, no significant difference by the least significant difference test; b, statistically different values between genotypes at P < 0.05; and c, statistically different values between genotypes at P < 0.01.(c) Symptoms of K+ deficiency. Plants were grown for 2 weeks in hydroponic culture containing 0.1 mm K+, without renewal of the hydroponic solution. Representative leaves of control (left) and transgenic (right) plants are depicted. The concentrations of K+ were 42.8 mm and 70.2 mm in leaves of control and N-367 plants, respectively, at the end of the experiment.
| NaCl (mm) | 0 | 75 | 150 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FW | K+ | Na+ | FW | K+ | Na+ | FW | K+ | Na+ | |
| WT | 1.76a | 103.7a | 3.2a | 1.48a | 40.8a | 93.4a | 0.83a | 19.3a | 252.7a |
| N-367 | 2.88b | 142.6b | 3.4a | 1.53a | 56.9b | 103.9a | 0.61b | 34.7b | 221.7a |
| N-374 | 1.48a | 171.0c | 4.1a | 1.10a | 61.4b | 101.8a | 0.36c | 33.5b | 177.5a |
| N-376 | 1.43a | 169.0c | 4.1a | 1.19a | 48.8a | 145.8b | 0.42c | 24.4a | 225.7a |
| N-380 | 2.31c | 145.6b | 3.7a | 1.31a | 54.2b | 117.1a | 0.67ab | 36.4b | 171.2a |
K+ uptake
Consistent with the hypothesis of the reduced cytosolic availability of K+ in the AtNHX1 transgenic lines under K+ limitation, roots of line N-367 that had been starved for K+ for 48 (data not shown) or 96 h (Figure 3a) showed greater rates of Rb+ uptake (a tracer for K+) than control roots. The rate of Rb+ uptake was higher in the transgenic plants at all concentrations tested (10 μm–10 mm range). Fitting these data to Michaelis–Menten kinetics indicated similar apparent affinities for Rb+ in control and N-367 plants (Km 58.4 and 62.5 μm, respectively), but twice the Vmax in the transgenics relative to control plants (4.48 versus 2.26 μmol Rb+ g−1 min−1), suggesting that N-367 plants had more active root Rb+ (K+) transporters than control plants, i.e. transgenic plants were quantitatively but not qualitatively more responsive to K+ starvation. Similar results were obtained with plants of transgenic line N-380 (data not shown), supporting that this phenotype arose from the overexpression of AtNHX1. Equivalent results were obtained when K+ uptake was measured by monitoring K+ withdrawal from the hydroponic solution with a K+-specific electrode. As shown in Figure 3(b), rates of K+ uptake were greater in plants of the transgenic line relative to the control line. Consistent with enhanced root K+ uptake, xylem sap collected from excised roots of transgenic line N-367 plants grown in 4 mm K+ contained much greater K+ concentrations than sap from control roots (32.9 and 8.4 mm, respectively). Non-starved plants of various independent transgenic lines in standard growth conditions (4 mm K+) showed significantly higher K+ concentrations in leaves and roots than control plants (Table S2). Together, these results demonstrate that plants overexpressing AtNHX1 take up K+ more rapidly, and are more responsive to K+ deprivation, than control plants.
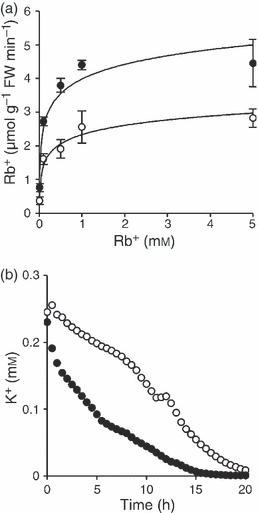
Rb+ and K+ uptake.(a) Plants were starved for 96 h in K+-free hydroponic solution, and were then transferred to fresh media with 0.01, 0.1, 0.5, 1 and 5 mm RbCl. After 10 min, roots of wild-type (○) and N-367 transgenics (•) were washed, and the Rb+ content was determined by AAS. Values are averages of three biological replicas. Uptake rates were fitted to Michaelis–Menten kinetics by using nonlinear regression.(b) Time-course of K+ withdrawal from hydroponic solution with 0.25 mm KCl, recorded with a K+-specific electrode, in mature wild-type plants (○) and N-367 transgenics (•).
High-affinity K+-uptake systems mediate K+ nutrition at low external concentrations, and are induced by K+ deprivation (Rodriguez-Navarro, 2000). The HAK5 gene is induced in K+-starved tomato plants, and its expression pattern parallels the presence of high-affinity K+ uptake in tomato roots, suggesting that this gene encodes a component of K+ uptake in the micromolar range of K+ concentrations (Nieves-Cordones et al., 2007, 2008). Control and N-367 plants growing in 4 mm K+ were transferred to K+-free hydroponic solution, and the expression of HAK5 was analyzed by RT-PCR in roots after 48 and 96 h of starvation. As depicted in Figure 4(a), HAK5 expression was induced earlier in the transgenic plants than in controls, indicating that plants overexpressing AtNHX1 sensed K+ deficiency before the controls. Induction of HAK5 in wild-type plants occurred only after several days of K+ starvation (Nieves-Cordones et al., 2007; data not shown). In a control experiment, Rb+ uptake by roots of plants starved as described above were transferred to a fresh hydroponic culture containing either 10 or 100 μm RbCl, and the rate of Rb+ accumulation in roots was determined by atomic absorption spectrometry. As shown in Figure 4(b), transgenic plants had a greater capacity for Rb+ uptake in the micromolar range that was statistically significant at 96 h of starvation, in accordance with the upregulation of gene HAK5.
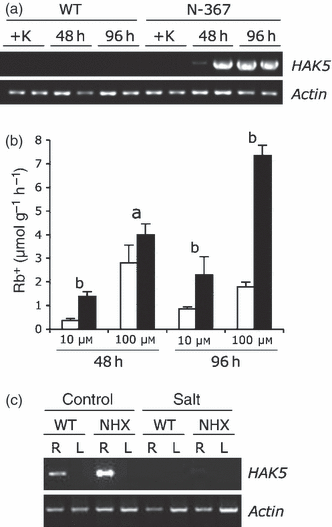
HAK5 expression induced by K+ starvation.(a) Plants growing in 4 mm K+ were transferred to K+-free medium for 48 or 96 h, or were kept in the presence of K+ (+K) until all samples were collected. Shown is the amplification (25 cycles) by RT-PCR of HAK5 in roots of duplicate biological samples. The actin TOM41 gene was used as an internal standard.(b) Root Rb+ uptake in wild-type (white bars) and N-367 plants (black bars) after being transferred to K+-free hydroponic solution for 48 and 96 h, and then to the same solution supplemented with 10 or 100 μm RbCl for 10 min. Roots were excised and the Rb+ content was determined by atomic absorption spectrophotometry. Values are means and SEs of Rb+ uptake per gram of fresh weight of roots of triplicate biological samples. Within treatments, the letters a and b on top of pair-wise comparisons indicate: a, no significant difference by the least significant difference test; or b, statistically different values at P < 0.05.(c) Wild-type (WT) and N-367 (NHX) plants were transferred to fresh hydroponic media with (salt) or without (control) 150 mm SO4Na2, and were then grown for 2, 4 and 6 days. Shown is the amplification (35 cycles) by RT-PCR of HAK5 in root (R) and leaf (L) samples after 2 days in salt. HAK5 could not be amplified from samples corresponding to 4 and 6 days of salt treatment. The actin TOM41 gene was used as the internal standard.
Vacuolar compartmentation of K+ and Na+
A combination of scanning electron microscopy and energy dispersive X-ray microanalysis (SEM-EDX) was used on frozen sections to measure the vacuolar ion contents in various leaf and root cell types of salt-treated tomato plants (Table 2). Although this technique does not enable the quantification of absolute ion concentrations, changes in the relative abundance of various mineral elements can be assessed. As net K+ concentration in plant tissues is largely a reflection of the vacuolar pool, the actual K+ and Na+ concentrations in the remaining parts of plants processed for SEM-EDX were determined by atomic absorption spectrometry to ensure that SEM-EDX results correlated with tissue ion contents. Linear correlations between actual element concentrations (mM) and EDX recordings (counts) were statistically significant (K+, r = 0.68, P < 0.05; Na+, r = 0.85, P < 0.01). Vacuoles of leaf mesophyll cells of N-367 plants showed consistently greater signals for K+ ions in SEM-EDX than control plants in two different saline regimes. By contrast, Na+ content in leaf vacuoles did not show significant differences among lines. Vacuolar contents in the root cortex and vascular cylinder of plants grown in 75 or 150 mm NaCl showed a similar pattern, with greater K+ accumulation in cells of AtNHX1-overexpressing plants than in controls, albeit mean values were not statistically different in cortical cells. At 150 mm NaCl, both the cortical and vascular cells of N-367 plants showed reduced vacuolar Na+ contents relative to controls. Of the eight pair-wise comparisons of Na+ vacuolar contents in Table 2, in only one instance (cortical cells at 75 mm NaCl) was the vacuolar content of Na+ greater in the transgenics. These results, together with the total Na+ concentrations determined in whole plants as described above, indicate that overexpression of AtNHX1 did not appreciably enhance the capacity of Na+ sequestration into vacuoles.
| K+ | Na+ | K+ | Na+ | |
|---|---|---|---|---|
| 75 mm NaCl | Leaf | Root | ||
| Palisade | Cortex | |||
| WT | 0.02a | 0.63a | 0.32a | 0.36a |
| N-367 | 0.41b | 0.83a | 0.48a | 0.62a |
| Lagunar | Vascular cylinder | |||
| WT | 0.09a | 0.23a | 0.17a | 0.86a |
| N-367 | 0.36b | 0.83a | 0.24b | 1.11a |
| 150 mm NaCl | Palisade | Cortex | ||
| WT | 0.15a | 0.75a | 0.86a | 1.24a |
| N-367 | 0.24b | 0.36b | 1.95a | 0.63b |
| Lagunar | Vascular cylinder | |||
| WT | 0.13a | 0.67a | 0.36a | 1.60a |
| N-367 | 0.44b | 0.73a | 2.99b | 1.08b |
The sequestration of ions in the vacuolar lumen is accompanied by the concurrent synthesis and accumulation of compatible solutes in the cytosol (Hasegawa et al., 2000). Therefore, the concentrations of free sugars and of the amino acid proline were determined in control and transgenic plants of line N-367 with and without 150 mm NaCl. Sugars were significantly greater (P < 0.05) in leaf and roots of transgenic plants in the absence of salt stress (Table 3). The greatest increase (62%) was found in leaves of transgenic plants relative to control leaves. After 30 days in salt, transgenic plants still had slightly greater concentrations of free sugars in leaves and in roots, albeit the differences in roots were not statistically different. Proline concentrations, which were not different among plant lines without salt stress, were significantly greater in leaves and roots of the transgenic plants under salt stress (Table 3). As expected, the osmotic potentials in both control and transgenic plants were more negative under salt stress than in regular growth medium. However, the organic (proline and sugars) and inorganic (K+ and Na+) solute concentration differences among plant lines did not result in different osmotic potentials of the transgenic plants relative to controls (Table 3).
| Line and treatment | Proline leaf | Proline root | Sugars leaf | Sugars root | Ψo leaf | Ψo root |
|---|---|---|---|---|---|---|
| No salt | ||||||
| WT | 6.55 | 0.51 | 60.5 | 12.1 | −1.01 | −0.60 |
| N-367 | 6.26 | 0.73 | 98.2a | 16.5a | −1.09 | −0.72 |
| 150 mm NaCl | ||||||
| WT | 7.7 | 2.28 | 28.0 | 10.1 | −1.52 | −1.12 |
| N-367 | 10.2a | 3.48b | 33.8a | 14.2 | −1.52 | −1.01 |
Next, we tested whether the differential upregulation of HAK5 in the transgenic lines also occurred under salinity stress. Enhanced expression of HAK5 could, theoretically, contribute to better Na+–K+ discrimination in saline media (Rodriguez-Navarro, 2000). Control and N-367 plants were subjected to salt-shock with 150 mm Na2SO4 (i.e. similar conditions to the experiment shown in Figure 2) for 2, 4 and 6 days. As depicted in Figure 4c, roots of transgenic plants had a slightly higher HAK5 mRNA level before stress treatment. However, the low mRNA levels in both plant lines (RT-PCR needed 35 amplification cycles instead of the 25 cycles used in panel A) were further repressed by salinity stress. Salt-induced repression of HAK5 has been previously reported (Nieves-Cordones et al., 2008).
Cytosolic K+ activity in K+-limited plants
Presumably, the enhanced activity of AtNHX1 in the tonoplast of transgenic tomato plants promoted the accumulation of K+ into the vacuole at the expense of a diminishing cytosolic K+ pool. To test this hypothesis, electrophysiological measurements were performed to assess K+ activity in the cytosol of transgenic plants. First, epidermal root cells of K+-limited (0.1 mm KCl) seedlings of wild-type and N-367 lines were probed using single electrodes. No significant differences were observed in the plasma membrane potentials (Em) between seedlings of the wild type and line N-367 (−168 ± 10 and −163 ± 11 mV, respectively) (Figure 5). After the addition of 1 mm NaCN and 1 mm salicylhydroxamic acid (SHAM) to the assay media, to inhibit ATP synthesis and H+ pumping, new resting membrane potentials (apparent diffusion potentials, ED) were reached (Figure 5). Notably, ED values were lower in the wild type (−100 ± 7 mV; n = 5) than in N-367 plants (−77 ± 14 mV; n = 5). Under resting conditions in ATP-depleted cells, cytosolic K+ serves as a charge balance that counteracts membrane depolarization in response to the increase in the external K+ concentration, until equilibrium is reached (ED = 0) (Mithöfer et al., 2005). The less negative ED (i.e. more depolarized) value in transgenic N-367 plants suggests a lower cytosolic K+ concentration relative to control plants. As expected, ED values showed a strong dependency on external K+, the most permeable ion in plant cell membranes, in both transgenic and wild-type cells (Figure 5a,b). The slopes of changes in ED in response to external K+ were similar (25–30 mV for a 10-fold increase in extracellular K+), indicating that the permeability of the plasma membrane to K+ did not differ among plant lines (Figure 5c). However, root cells of N-367 plants showed less negative ED values than wild-type cells over all external K+ concentrations tested (Figure 5c), indicating that K+ activity in the cytosol of transgenic plants was diminished (Mithöfer et al., 2005). On the other hand, similar increases in external Na+ concentrations elicited equivalent depolarization of membranes of only 10 mV in both wild-type and N-367 epidermal root cells (data not shown).
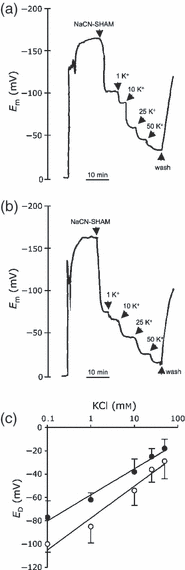
Diffusion potentials (ED) at different external K+ concentrations.(a) Representative recording of the membrane potentials in single epidermal root cells of wild-type plants. After the addition of 1 mm NaCN and 1 mm SHAM, plasma membranes depolarized until a new resting membrane potential was reached. Then, the external K+ concentration was increased step-wise, as indicated by the arrow heads; wash indicates the onset of an NaCN-SHAM wash.(b) Representative recording of transgenic N-367 seedlings.(c) Diffusion potentials (ED) at different external K+ concentrations measured in epidermal root cells of wild-type (○) and N-367 transgenic seedlings (•). Values are means of five independent experiments, similar to those shown in (a) and (b).
To measure the actual cytosolic K+ activity (K+cyt), double-barreled K+-selective microelectrodes were used to impale epidermal root cells of control and transgenic plants grown in 0.1 mm KCl (Figure 6). K+cyt activities were calculated from microelectrode calibration curves (slopes were close to 51 mV/pK+). There were no significant differences between Em measurements in transgenic and control plants (−168 ± 3 mV in the wild type and −165 ± 5 mV in N-367; n = 3) when the double-barreled K+-selective microelectrodes were used, and the Em values were similar to those registered with single-barrel microelectrodes, as described above. By contrast, K+cyt was significantly different between genotypes (Figure 6). The K+cyt determined in impaled wild-type seedlings was 98 ± 1.3 mm, but was only half that, i.e. 55 ± 2.2 mm (n = 3), in the N-367 seedlings. Together, these results demonstrate that overexpression of AtNHX1 reduced K+ activity in the cytosol of transgenics plants under limiting (0.1 mm) levels of external K+.
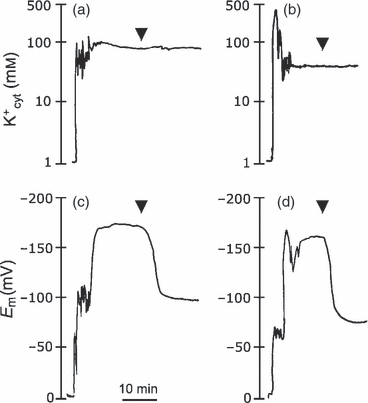
Simultaneous measurement of membrane potential and cytosolic K+ concentration. Arrowheads indicate the addition of 1 mm NaCN and 1 mm SHAM to the assay medium to inhibit respiration and suppress the activity of the plasma membrane proton pump. Membrane potentials depolarized to diffusion level, whereas cytosolic K+ activity remained unchanged. Shown are representative traces of three replicates.(a) Cytosolic K+ activity was determined with a double-barreled electrode in single epidermal root cells of the wild type.(b) Cytosolic K+ activity in transgenic N-361 seedlings.(c) The membrane potential was recorded in a wild-type seedling with the double-barreled electrode.(d) Membrane potential recorded in transgenic N-367 seedling.
Discussion
Based on biochemical and physiological data, a critical role for tonoplast Na+/H+ antiporters in minimizing cytoplasmic Na+ concentration through the sequestration of this ion in vacuoles has been proposed (Blumwald et al., 2000). Thus far, the only Na+–K+/H+ antiporters known to localize to the tonoplast of plants belong to the NHX family. Overexpression of NHX proteins from various sources have been shown to increase salt tolerance in a range of plant species (Pardo et al., 2006; Munns and Tester, 2008). However, only a few of these studies have addressed the ion relations of the transgenics overexpressing NHX proteins. Results have been disparate, ranging from enhanced Na+ or K+ accumulation in the transgenics to no significant differences with controls (reviewed by Pardo et al., 2006). Moreover, it should be noted that K+ and Na+ ions are unevenly distributed between the vacuole and cytoplasm of plant cells, and none of these studies addressed the number of ions compartmentalized in the vacuoles relative to their net tissue content. We show here that the expression of the vacuolar Na+–K+/H+ antiporter AtNHX1 in tomato increased the K+ concentration in leaves and roots in all growth conditions tested, with negligible impact on the capacity for Na+ sequestration inside vacuoles when plants were subjected to salinity stress. The larger vacuolar K+ pools detected in transgenic plants, together with the reduced K+ cytosolic activity under K+ limitation, indicate that K+ was preferentially accumulated in vacuoles even at the expense of depleting the cytosolic compartment when external K+ was low. Diminished cytosolic K+ activity in K+-starved transgenic plants resulted in the earlier development of K+ nutrient deficiency symptoms, faster transcriptional activation of HAK5 and greater K+ uptake by roots. Thus, transgenic plants intensified the response to K+ starvation despite the greater overall tissue K+ content of the transgenics. Vacuoles are K+ reservoirs that ensure an adequate supply to the cytosol in a low-K+ environment (Leigh, 2001). In barley roots, cytosolic K+ concentration declined only when vacuolar K+ concentration decreased to values around 20 mm, or when the whole-root K+ concentration fell below 25 mm (Walker et al., 1996; Leigh, 2001). Although we could not measure the precise concentration of K+ in the vacuoles of plants because of the semi-quantitative nature of EDX analyses, the relative K+ contents were significantly greater in the transgenics than in control plants in the cell types tested (Table 2). Electrophysiological studies suggest the suitability of tonoplast K+/H+ antiporters for the homeostatic regulation of cytosolic K+ when cells are provided with surplus concentrations of K+ (Walker et al., 1996). Assuming a tonoplast membrane potential of 30 mV (positive inside), the vacuolar K+ concentration would be 20 mm at equilibrium with the cytosol if the cytosolic concentration was ∼100 mm. Any further increase of the vacuolar concentration would necessitate energized K+ transport across the tonoplast, presumably mediated by K+/H+ antiporter activity (Walker et al., 1996). Our data strongly support the notion that NHX-type proteins serve this function. Recently, the K+/H+ exchange activity of the ItNHX1 protein has been implicated in the increase of the vacuolar pH, K+ accumulation, cell expansion and color transition from red to blue in petals of Ipomoea tricolor (Yoshida et al., 2009). Also, Arabidopsis nhx1 mutant plants had reduced leaf area and smaller epidermal cells (Apse et al., 2003), suggesting that these mutant plants do not supply the vacuoles with sufficient osmotic potential for the normal expansion of leaf cells, which largely depends on K+ accumulation (Maggio et al., 2006).
Under salt stress, K+ concentrations in different parts of tomato plants decreased, whereas the Na+ concentration increased in both transgenic and control lines, following well-known patterns of salt-related mineral changes in tomato (Rush and Epstein, 1981; Olias et al., 2009). However, no consistent differences in Na+ contents among transgenic and control lines were observed after saline treatments (Tables 1 and S1), despite the significant enhancement of Na+/H+ antiporter activity in tonoplast vesicles purified from these transgenic plants (Figure 1). By contrast, the expression of AtNHX1 led to the enhanced retention of K+ in transgenic lines. The dual affinity of AtNHX1 for Na+ and K+ (Figure 1) (Venema et al., 2002; Apse et al., 2003; Yamaguchi et al., 2005) and its Km for K+ (21 mm, this work; 12.2 mm, Yamaguchi et al., 2005) imply that under regular growth conditions AtNHX1 mediates the uptake of K+ into vacuoles. Under salinity stress, Na+ transport will also take place because of the rising concentration of this ion in the cytosol. However, the cytosolic concentration of K+ would still be higher than that of Na+ under most circumstances, including when plants are growing under salinity stress (Carden et al., 2003; Tester and Davenport, 2003; Munns and Tester, 2008). For instance, single-cell measurements of cytosolic activities of Na+ and K+ in the root cortex of two varieties of barley subjected to severe salt stress combined with low external K+ (200 mm NaCl and 0.1 mm K+), showed that the Na+ activity in the cytosol ranged from 2 to 28 mm, depending on the plant variety and the duration of the treatment, whereas K+ the activity was 40–63 mm. By contrast, Na+ activity in vacuoles was 26–88 mm, which would require active transport, i.e. against the electrochemical gradient of Na+, to compartmentalize cytosolic Na+ into the vacuole. In this scenario, this likely occurs in the cytosol of many plants thriving under salt stress (Munns and Tester, 2008): K+/H+ exchange by NHX1-like proteins could predominate over, but not suppress, Na+/H+ exchange. The relative prevalence of each exchange would be dependent on the selectivity of the particular NHX protein being considered, and the ionic environment in the cytosol, which in turn will be dependent on the plant species and the intensity and duration of the salinity stress. In addition, Na+ transport across the tonoplast is bidirectional (Demidchik et al., 2002; Tester and Davenport, 2003). Backflow from the vacuole to the cytosol, probably through ion channels, demands sustained influx by Na+/H+ exchange to keep Na+ compartmentalized. Thus, even if the K+ : Na+ ratio in the cytosol is high, and the NHX1-like proteins do not discriminate between K+ and Na+, they can still contribute to significant Na+ compartmentation provided that the ‘leak’ from the vacuole to the cytosol is low. These variables, and the conceivable diverse biochemical behavior of NHX-type proteins isolated from various sources, and ectopically expressed in a range of plant species, could explain the disparate results reported regarding the enhanced accumulation of Na+, K+ or both, in the transgenic plants overexpressing NHX proteins. To complicate matters further, Yamaguchi et al. (2005) have shown that the ion selectivity of AtNHX1 can be modulated through a regulatory mechanism involving binding of a calmodulin-like protein to the C-terminal part of the transporter. This interaction resulted in a further gain of K+ selectivity over Na+, albeit these results were obtained in yeast and have not been confirmed in planta. Interestingly, a genetic screen for mutants of AtNHX1 that enhanced the salt tolerance of yeast cells resulted in the isolation of protein variants with improved discrimination of K+ over Na+ (Hernandez et al., 2009). Together, these data and the results shown here support the notion that significant transport of K+ into vacuoles by AtNHX1 is an essential component of the salt tolerance conveyed by this exchanger. Another layer of regulation of the NHX proteins is imparted by the SOS pathway regulating the efflux and net content of Na+ in plants. The tonoplast Na+/H+ exchange attributed to NHX proteins in Arabidopsis was, when compared with the wild type, significantly higher, greatly reduced and unchanged in the sos1, sos2 and sos3 mutants, respectively (Qiu et al., 2004). An activated form of the SOS2 protein kinase added in vitro increased tonoplast Na+/H+ exchange activity in vesicles isolated from sos2, but did not have any effect on the activity in vesicles isolated from the wild type, sos1 or sos3. Moreover, SOS2 interacts with and upregulates the transport activity of the vacuolar H+-ATPase (Batelli et al., 2007). These results demonstrate that tonoplast Na+–K+/H+ exchange is a target of the SOS regulatory pathway, and that there may be coordinate regulation of ion transporters in the tonoplast and plasma membrane.
If NHX-type proteins can increase salt tolerance without necessarily enhancing Na+ accumulation into vacuoles, the question then remains of what is the biochemical or cellular mechanism that conveys halotolerance to the tomato plants overexpressing the AtNHX1 protein. We suggest that this tolerance derives from the significant role that the AtNHX1 antiporter plays in K+ homeostasis by capturing K+ in the vacuoles, as well as the concurrent increment of compatible solutes (free sugars and proline) in the cytosol. It is generally accepted that Na+ inhibits K+ uptake and elicits K+ loss (Maathuis and Amtmann, 1999; Shabala et al., 2006). Consequently, the enhanced capacity of K+ uptake and subsequent retention is a critical adaptation to salinity stress (Maathuis and Amtmann, 1999; Borsani et al., 2001; Maggio et al., 2006). A negative correlation between Na+-triggered K+ loss and salt tolerance has been described previously (Chen et al., 2005; Shabala et al., 2006). Owing to the enlarged K+ pool created before the onset of salinity stress, and the subsequent retention afterwards, the transgenic plants would be better poised to withstand the imposition of salt stress, particularly if applied as a salt-shock (Figure 2). The larger vacuolar pool of K+ could sustain and stabilize the cytosolic K+ concentrations for longer periods of time in the transgenic plants. Only later, as Na+ concentrations increase within plant cells and the apoplast, and the cytosolic and vacuolar pools of K+ are eventually depleted, would the contribution of AtNHX1 to salt tolerance gradually decline, and salt-specific maladies appear (Munns, 2002). Greater K+ contents in salt-tolerant transgenic plants expressing NHX proteins have also been described by others (Xue et al., 2004; Wu et al., 2005; Zhao et al., 2006; Rodriguez-Rosales et al., 2008). In addition, the enlarged pool of K+ in the vacuoles of tomato plants expressing AtNHX1 required the coordinated accumulation of compatible solutes (Table 3). Transgenic AtNHX1 plants showed a significantly greater accumulation of free sugars even if they were not transferred to salt. Under salt stress, the transgenic plants accumulated more proline than controls, another feature often linked to salt tolerance (Hasegawa et al., 2000). However, the combined accumulation of K+ and compatible osmolytes in the transgenic plants did not result in a significantly different osmotic potential compared with control plants (Table 3). Therefore, we posit that the survival of AtNHX1-expressing plants under salt-shock was unlikely to be caused by improved osmotic adjustment, but instead by the greater K+ accumulation before salt treatment and K+ retention afterwards, together with the well-known protective effects of compatible solutes against the dehydration and oxidative stress associated with salinity. The additive effect of each of these individual features may contribute to improve the salt tolerance of the transgenics.
Concluding remarks
Our results reveal that NHX proteins are likely candidates for the H+-linked K+ transport responsible for active K+ uptake at the tonoplast, to facilitate the partitioning of K+ between the vacuole and the cytosol. This critical function should be taken into consideration for understanding the salt-tolerant phenotype widely reported for plants overexpressing NHX-type proteins, some of which are not significantly affected in Na+ compartmentation.
Experimental procedures
Tomato transformation and molecular methods
For plant transformation, the AtNHX1 open reading frame (ORF) was inserted into vector pBI321 (Martínez-Atienza et al., 2007) between the CaMV 35S gene promoter and the nopaline synthase gene (NOS) terminator. The resulting plasmid was electroporated into Agrobacterium tumefaciens strain LBA4404 for transformation of cotyledon pieces. The protocol used was as described by Meissner et al. (1997), except that 150 mg ml−1 or 60 mg ml−1 ticarcillin were used. In this study, only homozygous T2 and T3 plants (established by segregation analysis) of transgenic lines were used in physiological or phenotypic experiments. Reverse transcription (RT)-PCR for the detection of gene transcripts was performed as described in Ruiz et al. (1998). The PCR reactions were performed with primers annealing to genes AtNHX1 (AF106324), tomato HAK5 (DQ489721) and tomato TOM41 (U60480). For western blotting, microsomes were purified as described below and probed with antibodies raised against AtNHX1 (Quintero et al., 2000). The AtNHX1 protein was purified as described by Venema et al. (2002).
Plant culture and chemical analyses
Seeds were germinated at 28°C in Petri dishes, and 3-day-old seedlings were transplanted into plastic holders containing rockwool, and were then transferred to 8-L plastic containers with modified Long Ashton solution, containing: 4 mm NO3−, 1 mm H2PO4−, 3 mm SO42−, 4 mm K+, 2 mm Ca2 + , 1 mm Mg2 + , 50 μm Fe (as FeEDDHA), 10 μm Mn, 1 μm Cu, 5 μm Zn and 30 μm B. In the standard medium, the K+ concentration was 4 mm. In experiments with different K+ levels, the required concentration was obtained by adding 0.5 m K2SO4. Salinity treatments were administered to 2-week-old plants in hydroponic culture. Salt was added at 25 mm increments every 12 h until reaching 75 or 150 mm Na+ (as NaCl), or as a salt-shock with 300 mm Na+ (as Na2SO4), in the nutrient solution. Plants were kept in a growth chamber with the following conditions (day/night): 25/20 ± 2°C; 40/60% relative humidity; 14-h light; photosynthetically active radiation (PAR), 250 μmol m−2 s−1. For ion analyses, plants were separated into leaves, stems (including petioles), fruits and roots, and were weighed. Roots were washed thoroughly in tap water and blotted dry before weighing. All plant parts were dried at 70°C for 48 h in a forced-air oven. From fresh- and dry-weight measurements, water contents (in g H2O (g dry weight)−1) were obtained. Potassium and sodium were extracted from finely ground material with hot water under pressure by autoclaving samples, and then measured by atomic absorption spectrophotometry (AAS) (Perkin-Elmer 1100B, http://www.perkinelmer.com). Sugar content was estimated by the phenol-sulfuric assay using glucose as standard (Ashwell, 1969). Proline was determined by reverse-phase HPLC following pre-column derivatization with phenylisothiocyanate (Heinrikson and Meredith, 1984). Osmotic potentials were determined from saps extracted from frozen tissues using a Wescor psychrometer HR-33T and sample chamber C-52. All statistical analyses were performed with the software package Statistix (http://www.statistix.com).
Vacuolar membrane isolation and ion transport
Isolation of tonoplast membranes and measurement of cation/proton exchange were as described by Barkla et al. (1999), except that fluorescence quenching of 9-amino-6-chloro-2-methoxy-acridine (ACMA) was used to monitor the formation and dissipation of pH gradients. Purified tonoplast vesicles (50 μg of protein) were added to a buffer containing 250 mM mannitol, 10 mM bis-tris-propane-2-(N-morpholine)-ethanesulphonic acid (BTP-MES) (pH 8.0), 100 mm tetramethyl ammonium chloride, 3 mm MgSO4 and 1 μm ACMA (1 ml final volume). Fluorescence was recorded with a Hitachi fluorescence spectrophotometer (FL-2500; http://www.hitachi.com) in a thermostated cell (26°C) at excitation and emission wavelengths of 415 and 485 nm, respectively (slit width, 10 nm). The reaction mixture was stirred and maintained at 26°C throughout the transport assays. Formation of acid-inside pH gradients were started with the addition of 1.5 mm ATP-BTP (pH 8.0). When fluorescence stabilized, the initial rate of dissipation was measured after the addition of NaCl or KCl salts. Exchange rates are expressed as fluorescence recovery relative to that prior to ATP addition (ΔF/Fmax) per minute and milligram of protein. To estimate the kinetic parameters of AtNHX1, the rates of transport in wild-type vesicles were subtracted from the rates obtained for vacuoles containing AtNHX1. Exchange rates at each substrate concentration were fitted to Michaelis–Menten kinetics by using nonlinear regression with kaleidagraph (Synergy Software, http://www.synergy.com).
Root K+ uptake
Root K+ uptake was estimated by measuring Rb+ uptake (Benlloch et al., 1989) or measuring K+ depletion from the incubation solution with a K+-specific electrode. Tomato plants grown in full nutrient solution were transferred to K+-free solution for 48 and 96 h before measuring Rb+ uptake in nutrient media containing various concentrations of RbCl for 10 min. Then, roots were immersed in ice-cold 5 mm CaSO4 for 5 min, blotted on filter paper, cut off and their weight determined before being frozen at −70°C. The Rb+ concentration was determined in centrifuged homogenates by AAS. Accumulation rates at each Rb+ concentration were fitted to Michaelis–Menten kinetics by using nonlinear regression with kaleidagraph (Synergy Software). For K+-uptake measurements using the K+-selective electrode, plants were transferred to a medium containing 0.25 mm KCl and 2 mm CaCl2. Variation in K+ concentration was continuously recorded using an ion meter equipped with K+ and reference electrodes (Crison). An additional estimate of K+ uptake was performed by AAS analysis of K+ concentration in the xylem sap collected from de-topped plants at harvest.
Vacuolar compartmentation
Vacuolar contents in leaves and roots were analyzed by EDX. For EDX analysis, pieces of freshly harvested fully expanded leaves or the main root were used from plants grown in different salinity levels. The plant pieces were mounted in slots of copper holders and fixed with optimal cutting temperature compound (BDH, http://uk.vwr.com). The copper holder containing the samples was dipped into a bath of slush N2 before transferring it under vacuum into the cryopreparation chamber (CT1500; Oxford Instruments, http://www.oxford-instruments.com) attached to the SEM (DSM 960; Zeiss, http://www.zeiss.com). The temperature in the chamber was left to rise from −163 to −90°C and was then set for 10 min for ice sublimation before sputter-coating with gold (2 min). The SEM was fitted with an ATW detector interfaced with a Link ISIS analyzer (http://www.oxford-instruments.com). Samples were analyzed keeping the following parameters constant: accelerating voltage, 15 kV; take-off angle 42°; collecting time of X-ray counts, 100 s; working distance between sample and detector, 24 mm. Measurements were performed by focusing on exposed vacuoles of specific cells (mesophyll parenchyma in leaves, or cortical and vascular cylinder cells in roots), or covering cell groups from different tissues in roots (cortex and vascular cylinder). Solutions containing known ion concentrations and plant extracts obtained from the remaining parts of leaves and roots, in which ion concentration was determined by AAS, were used to determine the response of the analyzer to K+ and Na+. The linear correlation between actual element concentration (mM) and EDX recordings (counts) was statistically significant (K+, r = 0.68, P < 0.05; Na+, r = 0.85, P < 0.01).
Electrophysiological experiments
For electrophysiological experiments, tomato seeds were germinated in distilled water and transferred for 3–4 days to media containing 2 mm CaCl2 and 0.1 mm KCl. Membrane potentials (Em) were measured in epidermal root cells of tomato seedlings, as described previously (Rubio et al., 2004). Diffusion potentials (ED) were reached after the addition of 1 mm NaCN and 1 mm SHAM (Fernández et al., 1999) to the above assay medium, buffered with 9 mm BTP-MES, pH 6.0. Cytosolic K+ activities were determined by two different approaches. First, diffusion potentials were measured at increasing external K+ concentrations (1, 10, 25 and 50 mm KCl) with single-barrel microelectrodes. Different diffusion potentials in control and transgenic roots indicated unequal cytosolic K+ activities (Mithöfer et al., 2005). Second, cytosolic K+ activities were directly measured by double-barreled K+-selective microelectrodes. The microelectrode pre-treatment and backfilling was similar to the H+- or Na+-selective microelectrodes described previously (Fernández et al., 1999; Rubio et al., 2005). The microelectrodes were filled with a K+-sensor cocktail containing potassium ionophore I (cocktail B, cat. no. 60398; Fluka, now part of Sigma-Aldrich, http://www.sigmaaldrich.com) dissolved in a mixture of polyvinylchloride/tetrahydrofuran (40 mg ml−1) at a ratio 30/70 (v/v) (Mithöfer et al., 2005). The signals from the K+-selective and voltage barrels were recorded and simultaneously subtracted by a high-impedance differential amplifier (FD223; World Precision Instruments, http://www.wpiinc.com). The difference was calibrated before and after the experiments with different KCl solutions (from 1 to 500 mm KCl) maintained at a constant ionic strength by the addition of MgCl2 (Walker et al., 1995). Calibration curves showed slopes around 51 mV/pK+. The impalements were stable for at least 30 min.
Acknowledgements
We are indebted to Bronwyn Barkla and Rosario Herrera-Estrella (Instituto de Biotecnologia, UNAM, Cuernavaca, México) and Andrés Belver (EEZ, CSIC, Granada, Spain) for their helpful advice in the measurement of antiporter activity. This work was supported by grants BFU2006-06968 from ‘Ministerio de Educación y Ciencia’ and AGR-1482 from ‘Junta de Andalucía’ to JMP. Additional support was obtained from CSIC-CONACYT Bilateral Cooperation Grant 2004MX0021.




