Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development
Summary
Glutathione (GSH) homeostasis in plants is essential for cellular redox control and efficient responses to abiotic and biotic stress. Compartmentation of the GSH biosynthetic pathway is a unique feature of plants. The first enzyme, γ-glutamate cysteine ligase (GSH1), responsible for synthesis of γ-glutamylcysteine (γ-EC), is, in Arabidopsis, exclusively located in the plastids, whereas the second enzyme, glutathione synthetase (GSH2), is located in both plastids and cytosol. In Arabidopsis, gsh2 insertion mutants have a seedling lethal phenotype in contrast to the embryo lethal phenotype of gsh1 null mutants. This difference in phenotype may be due to partial replacement of GSH functions by γ-EC, which in gsh2 mutants hyperaccumulates to levels 5000-fold that in the wild type and 200-fold wild-type levels of GSH. In situ labelling of thiols with bimane and confocal imaging in combination with HPLC analysis showed high concentrations of γ-EC in the cytosol. Feedback inhibition of Brassica juncea plastidic GSH1 by γ-EC in vitro strongly suggests export of γ-EC as functional explanation for hyperaccumulation. Complementation of gsh2 mutants with the cytosol-specific GSH2 gave rise to phenotypically wild-type transgenic plants. These results support the conclusion that cytosolic synthesis of GSH is sufficient for plant growth. The transgenic lines further show that, consistent with the exclusive plastidic localization of GSH1, γ-EC is exported from the plastids to supply the cytosol with the immediate precursor for GSH biosynthesis, and that there can be efficient re-import of GSH into the plastids to allow effective control of GSH biosynthesis through feedback inhibition of GSH1.
Introduction
Glutathione (reduced form: GSH; oxidized form: GSSG) is a ubiquitous tripeptide formed from glutamate, cysteine and glycine. In plants a broad range of functions have been proposed for GSH, including redox control, detoxification of heavy metals and electrophilic xenobiotics, serving as electron donor for biochemical reactions, long-distance transport of reduced sulfur, and direct posttranslational modifications of proteins through reversible glutathionylation of thiol residues (Cairns et al., 2006; Grzam et al., 2007; Ito et al., 2003; Li et al., 2006; May et al., 1998; Michelet et al., 2005; Xiang et al., 2001). Because of this wide range of functions it is not surprising that GSH biosynthesis was found to be essential in plants (Cairns et al., 2006; Vernoux et al., 2000).
GSH is synthesized in two steps catalyzed by the enzymes glutamate cysteine ligase (GSH1, EC 6.3.2.2) and glutathione synthetase (GSH2, EC 6.3.2.3). In Arabidopsis, both enzymes are encoded by single-copy nuclear genes but, while GSH1 is exclusively targeted to plastids, GSH2 is located in both the cytosol and plastids (Wachter et al., 2005). A number of different GSH2 transcripts were identified and quantitative analysis revealed that only 8% encoded the entire chloroplast target peptide. Assuming similar translation efficiencies for the different mRNAs this would suggest that only a small fraction of GSH2 protein is localized to the plastids while the remainder is present in the cytosol. Similarly, in photoheterotrophic cell cultures of tobacco 24% of the GSH2 activity was allocated to the plastids (Hell and Bergmann, 1988) and in pea between 47% and 69% of GSH2 activity was plastidic (Klapheck et al., 1987). With GSH1 exclusively localized to the plastid, the cytosolic localization of GSH2 implies its substrate, γ-EC, must be transported from the plastids to the cytosol. This conclusion is surprising, since it is generally assumed that cysteine and GSH, the major cellular low molecular weight thiols, are exchanged between plastids and cytoplasm. The apparent sole function of γ-EC as a reaction intermediate of GSH biosynthesis and its low abundance make it an unlikely transport metabolite. Nevertheless, export of γ-EC from the plastids and subsequent GSH biosynthesis in the cytosol under conditions of increased demand for cytosolic GSH has been suggested (Meyer and Fricker, 2002) although not shown directly.
Glutathione can be visualized microscopically in living cells with monochlorobimane (MCB), a thiol-specific fluorescent tracer (Cairns et al., 2006; Meyer et al., 2001). Using MCB it has been shown that the cytosolic concentration of GSH is usually between 1 and 3 mm (Meyer and Fricker, 2002; Meyer et al., 2001). Conjugation of MCB with GSH leads to almost complete depletion of free GSH and increased de novo biosynthesis (Meyer and Fricker, 2002). GSH biosynthesis is regulated to a great extent by feedback inhibition of GSH1 by GSH (Hell and Bergmann, 1990; Jez et al., 2004). Consistent with such a feedback control mechanism, yeast and bacterial mutants deficient in GSH2 show an accumulation of γ-EC three- to fivefold greater than the normal GSH concentration (Grant et al., 1997; Harrison et al., 2005). For both the bacterium, Escherichia coli, and the yeast, Saccharomyces cerevisiae, glutathione synthetase is dispensable even under conditions of oxidative stress as the accumulation of γ-EC can compensate for the lack of GSH (Apontoweil and Berends, 1975; Grant et al., 1997). Similarly, in GSH2-deficient mutants of the symbiotic bacterium Sinorhizobium meliloti, accumulation of γ-EC to 2.5-fold the level of GSH in the wild-type is sufficient for survival of free-living bacteria. However, the mutant showed a delayed-nodulation phenotype indicating that the accumulation of γ-EC can only partially compensate for the lack of GSH (Harrison et al., 2005). The fact that γ-EC does not accumulate in an uncontrolled manner in bacteria and yeast gsh2 mutants indicates that γ-EC can, like GSH, exert a regulatory feedback inhibition of GSH1 in vivo. Efficient feedback control by γ-EC is also evident in halobacteria as these cells use γ-EC rather than GSH as their main internal thiol redox buffer (Sundquist and Fahey, 1989).
Arabidopsis embryos homozygous for T-DNA insertion mutations of GSH1 have a lethal phenotype (Cairns et al., 2006). Despite the fact that these embryos were almost completely devoid of GSH based on in situ labelling with MCB, they reached the same size as wild-type embryos. An impairment of metabolism was apparent from the bleaching of embryos soon after greening commenced and the lethal effect of GSH deficiency was manifested at some point during seed desiccation. In the present study we have investigated the effect of insertion mutations of GSH2 on viability and on the subcellular compartmentation of γ-EC. We found that, in contrast to yeast, the Arabidopsis thaliana gsh2 mutations are lethal, although the lethal effect occurs after germination rather than during the seed desiccation process. Loss of GSH2 leads to hyperaccumulation of γ-EC to levels 5000-fold in wild-type and orders-of-magnitude higher than normal levels of GSH. Complementation of this mutant with a cytosol-specific wild-type GSH2 fully rescued the mutant and thus provides evidence for export of γ-EC from the plastids and import of GSH into the plastids.
Results
gsh2 insertion mutations confer a seedling lethal phenotype
The enzyme glutathione synthetase (GSH2) in Arabidopsis is encoded by a single-copy gene. To study the importance of GSH2 in plants, we first identified insertion mutant lines for AtGSH2. A line containing a T-DNA insertion in the 7th exon of GSH2 was obtained from the SAIL Arabidopsis T-DNA collection (Sessions et al., 2002; SAIL 301_C06; here designated gsh2-T1; Figure 1a). A second allele obtained from the GABI-Kat T-DNA insertion collection (Rosso et al., 2003) has a T-DNA insertion within the 9th exon (line 393B05; here designated gsh2-T2; Figure 1a). The gsh2-T1 line segregated a ratio of 2 BASTA-resistant: 1 BASTA-sensitive plants (125:61, χ2 = 0.024; P = 0.88) expected if the gsh2 phenotype is lethal and the line contains a single T-DNA insertion. Progeny of reciprocal crosses of a gsh2-T1 heterozygote to the wild-type showed a 1:1 ratio of BASTA-sensitive to BASTA-resistant plants providing further evidence for a single insertion. The gsh2-T1 insertion mutant was generated in the qrt background that causes pollen tetrads to remain attached after the second meiotic division (Figure 1b; Sessions et al., 2002). In addition to the bar resistance marker the T-DNA also carries a GUS gene driven by a pollen-specific promoter (Sessions et al., 2002). Heterozygous gsh2-T1 plants produced 50% GUS-positive pollen, which is also consistent with a single locus T-DNA insertion (Figure 1c).
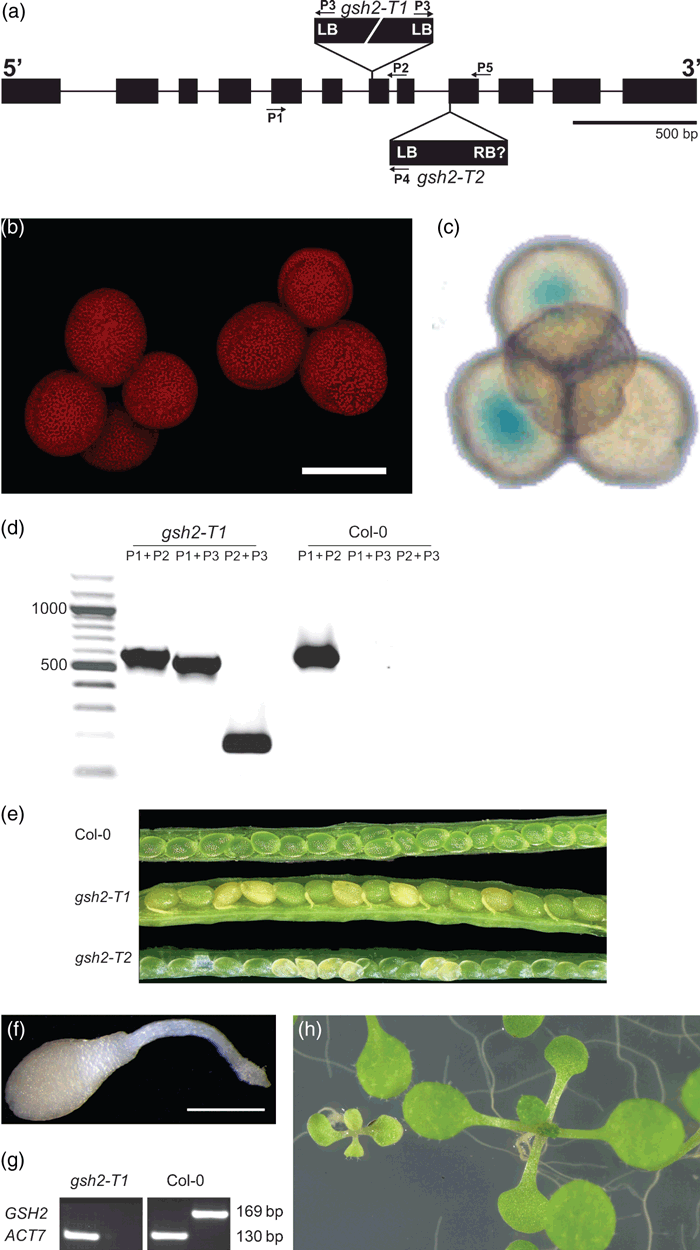
Isolation and characterisation of Atgsh2 T-DNA insertion alleles.(a) Physical map of the GSH2 gene (At5g27380) and insertion sites for the T-DNA insertion alleles, gsh2-T1 and gsh2-T2. Exons are represented as boxes and introns as lines. Positions of primers used for genotyping of gsh2 lines are indicated as arrows.(b) gsh2-T1 pollen tetrads visualised by CLSM based on pollen autofluorescence (λex = 405 nm). Scale bar = 20 μm.(c) GUS labelling of the gsh2-T1 pollen resulted in two GUS positive pollen grains (blue staining) within a single tetrad.(d) Genotype determination of a segregating population of heterozygous gsh2-T1 plants. This line contains two T-DNA insertions head to head. The primer for the left border of the T-DNA (P3) amplifies a DNA fragment in combination with gene-specific primers, P1 and P2, from either side of the insertion.(e) Opened siliques from a Col-0 wildtype and from two different gsh2-T1 and gsh2-T2 heterozygous plants. Homozygous mutants bleached during embryogenesis from torpedo stage onwards and can be distinguished from green, phenotypically wild-type ovules in the same silique.(f) Homozygous gsh2-T1 seedling 10 days after germination. Scale bar = 1 mm.(g) Semi-quantitative RT-PCR on homozygous gsh2-T1 seedlings showed absence of GSH2 mRNA. Amplification of AtACT7 was used as a constitutively-expressed control.(h) Homozygous gsh2-T1 seedlings can be partially rescued by GSH. A segregating population of seedlings was grown on full strength MS-medium supplemented with 0.8 mm GSH for 11 days.
Progeny from gsh2-T1 and gsh2-T2 heterozygous individuals were genotyped by PCR with primer pairs specific for mutant or wild-type GSH2 alleles (Figure 1d; data not shown). No mature homozygous mutant progeny were recovered in either population. Immature siliques of both insertion lines had ∼25% bleached embryos from 7 days post-fertilization onwards (Figure 1e). This phenotype was recently reported for gsh1 T-DNA insertion mutants (Cairns et al., 2006), suggesting a common effect due to GSH deficiency. Among 66 progeny of crosses between heterozygous gsh2-T1 and heterozygous gsh2-T2 insertion lines no viable plants carrying both insertion alleles were identified, confirming that the two mutants were unable to complement and that the mutations are allelic. The segregation pattern of 75% green and 25% bleached embryos in the gsh2-T2 line would be consistent with the presence of a single T-DNA insertion. The initial screen of seedlings derived from these seeds, however, resulted in a segregation of 3 sulfadiazin-resistant: 1 sulfadiazin-sensitive seedlings. This segregation is inconsistent with a single insertion causing a lethal phenotype for which a segregation of 1 sensitive: 2 resistant : 1 resistant, but lethal would be expected. Although this segregation is also inconsistent with multiple insertions, it was recently confirmed that this line contains a second T-DNA insertion in the gene At1g80320 coding for an oxidoreductase (http://www.gabi-kat.de) and an overlapping potential natural antisense gene (At1g80325; http://www.arabidopsis.org). For both genes no phenotype has been described. Because of the second insertion in the gsh2-T2 line all further work was done with the gsh2-T1 line. Seeds in siliques of self-fertilized heterozygous plants reached their full size and turned brown during normal seed desiccation after day 16 (not shown). Greater than 95% of these seeds germinated on agar medium and segregated wild-type and small, white seedlings that ceased growth immediately after germination (Figure 1f) in a 3:1 ratio (150:48; χ2 = 0.06; P = 0.81). All white seedlings tested were homozygous for the T-DNA insertion mutation. RT-PCR analysis of gsh2-T1 seedlings confirmed the absence of a functional GSH2 mRNA (Figure 1g).
To further test whether the lethal phenotype results from the inability to synthesise GSH, developing ovules were isolated 8–12 days after self pollination of a gsh2-T1 heterozygous individual and cultured in liquid medium containing 1 mm GSH. White embryos continued their development and germinated (not shown). However, in the absence of GSH no further growth was observed after germination. Germinating a segregating population on agar medium containing 0.8 mm GSH resulted in partial rescue of the homozygous mutants. Under these conditions seedlings started growing and showed partial greening (Figure 1h). Nevertheless, growth was retarded in comparison with the wild-type and the mutants failed to reach maturity even when seedlings were transferred to freshly made medium at 4–5 days intervals (not shown).
Homozygous gsh2-T embryos and seedlings hyperaccumulate γ-EC
Since both gsh2 mutants exhibit the same bleached embryo and seedling lethal phenotype, both insertion mutations lie within exon sequences and wild-type transcripts were not detected by using RT-PCR, we believe it likely that these are null mutations. Nonetheless, in contrast to the embryo-lethal phenotype of gsh1 insertion mutants (Cairns et al., 2006), both gsh2-T mutants have a seedling lethal phenotype. This might be explained either by residual amounts of GSH synthesised by other pathways (Spector et al., 2001) or by partial compensation of GSH functions by accumulating γ-EC, as shown for bacteria and yeast. We measured the content of low molecular weight thiols in Col-0 ovules and bleached ovules of a gsh2-T1 heterozygous individual harvested 8–12 days post-self-fertilization. While the pool of low molecular weight thiols in Col-0 ovules was dominated by GSH, mutant ovules contained γ-EC as the most prominent thiol (Figure 2a), up to 600-fold the level of γ-EC in the wild-type. Cysteine was slightly increased in the mutant while the cysteinyl-glycine content was unaffected. Although the GSH content in mutant ovules was decreased to only 50% that in the wild-type, GSH was also found in bleached ovules from gsh1-T1 heterozygotes (Figure 2a) and it has been shown previously that the residual GSH in gsh1-T1 ovules was almost exclusively located in the maternal tissues of the ovule, the integuments (Cairns et al., 2006). HPLC analysis of low molecular weight thiols extracted from single isolated embryos confirmed both the absence of detectable GSH and the presence of high levels of γ-EC in the mutant (Figure 2b). At the U-turn stage mutant embryos contained 192 ± 60 pmole γ-EC (embryo)−1 compared with 16.6 ± 4.1 pmole GSH (embryo)−1 in green embryos collected from the same silique. A similar HPLC signature of low molecular weight thiols was found in mutant embryos of a gsh2-T2 individual (not shown).
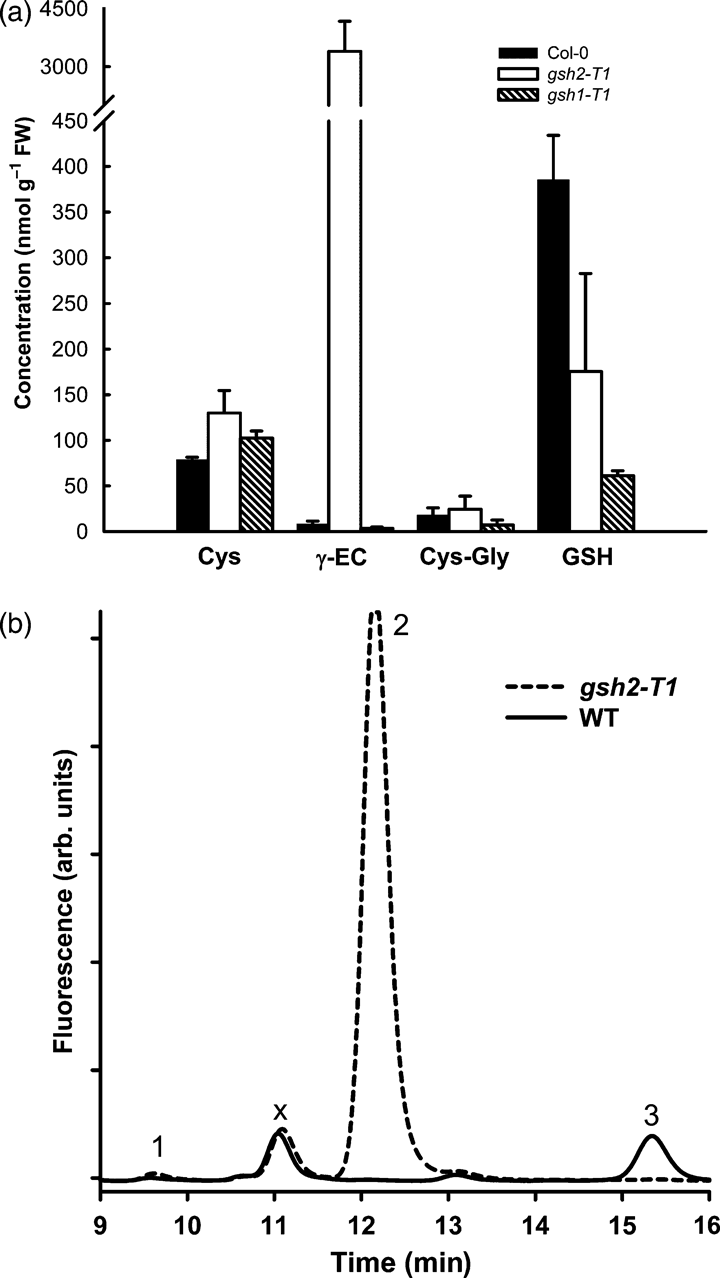
Low molecular weight thiols in ovules and isolated embryos.(a) Green ovules from a Col-0 plant and segregating bleached ovules from heterozygous gsh2-T1 and gsh1-T1 plants were dissected from siliques 8 to 12 days after self-fertilization, extracted and analysed for their thiol content by HPLC; n = 6 from two independent experiments.(b) Representative HPLC traces for low molecular weight thiols extracted from isolated U-turn stage embryos. Homozygous gsh2-T1 embryos were isolated based on their bleached phenotype. Peak 1 = cysteine; peak 2 = γ-EC; peak 3 = GSH; peak ‘X’ is an unknown compound from the buffers used for derivatization. This peak is independent of the sample.
We also measured low molecular weight thiols in gsh2-T1 and Col-0 seedlings 10 days post-germination. Mutant seedlings at this stage contained ∼20 000 nmol (g FW)−1γ-EC and no detectable GSH compared with 6 nmol (g FW)−1γ-EC and 120 nmol (g FW)−1 GSH in wild-type seedlings (Figure 3). 63% of the γ-EC in the mutant was in the reduced state with the remainder as oxidized bis-γ-glutamylcystine [(γ-EC)2]. Cysteine content was increased 30-fold in the mutant. Seeds were also germinated on medium containing 1 mm l-buthionine (S,R)-sulfoximine (BSO), a specific inhibitor of GSH1, and thiols were measured after 9 days. The level of GSH in the wild-type and of γ-EC in the mutant decreased to ∼16% and 2%, respectively, compared with seedlings germinated in the absence of BSO (Figure 3). The levels of cysteine in the wild-type and mutant increased three- and sixfold, respectively.
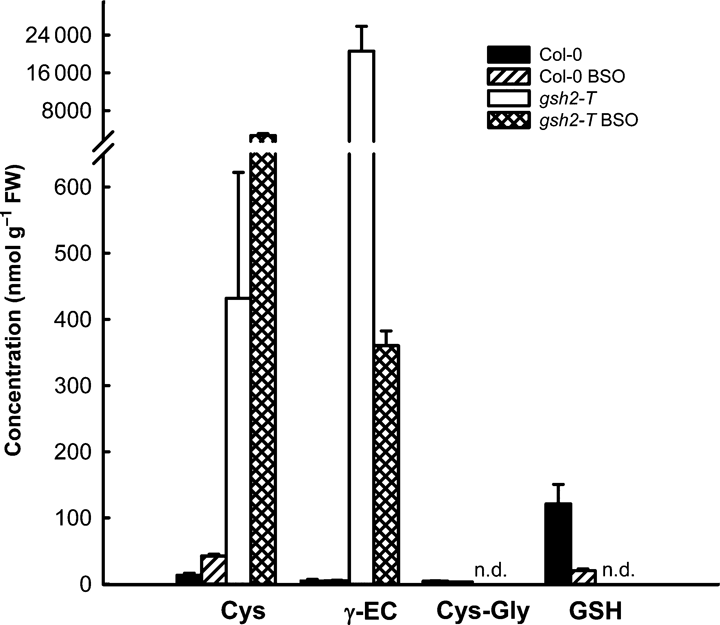
Analysis of low molecular weight thiols and the effect of BSO in homozygous gsh2-T1 seedlings.Seedlings germinated on growth medium with and without 1 mm L-BSO for 9–10 days were extracted and analysed for their content of low molecular weight thiols by HPLC. Values show means ± SD (n = 8 from 2 independent experiments for controls and n = 3 for BSO treatment).
Homozygous gsh2-T embryos show intense cytosolic labelling with MCB
Intact U-turn stage embryos were dissected from ovules and labelled with 100 μm MCB, which in the wild-type results in fluorescence almost entirely due to GSH labelling. Notwithstanding that the homozygous gsh2-T1 embryos contained no GSH as measured by HPLC, when labelled with 100 μm MCB they showed much stronger fluorescence than wild-type embryos imaged under the same conditions (Figure 4a). No obvious difference in fluorescence intensity was observed between green embryos from a wild-type plant and green embryos dissected from a segregating population of ovules from a heterozygous gsh2-T1 plant suggesting that heterozygous embryos behave like wild-type (not shown). A lack of chlorophyll autofluorescence was also observed for the mutant embryos (Figure 4c,e,g). Counterstaining embryos and seedlings with propidium iodide (PI) resulted in exclusive labelling of cell walls (Figure 4) showing that cells of the mutants were viable at this stage. In wild-type embryos the fluorescent GSB conjugate was almost entirely sequestered to the vacuole within 30 min (Figure 4d,f). In contrast, high resolution imaging of gsh2-T1 embryos showed a significant part of the fluorescence was retained in the cytosol (Figure 4e,g). Retention of the bimane-labelled compounds in the cytosol can be seen by weakly labelled vacuoles and visualization of plasmodesmatal connections between cells (Figure 4g). Strong labelling with 100 μm MCB was also observed for seedlings 5 days after germination (Figure 4h) and was almost completely abolished when seedlings were germinated in the presence of 1 mm BSO and imaged under the same conditions as the controls (Figure 4i).
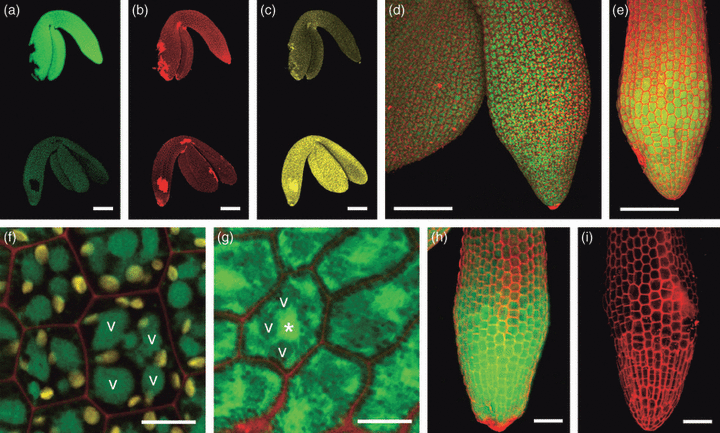
In situ labelling of isolated intact gsh2-T1 embryos and seedlings with MCB.Embryos and seedlings were labelled with 100 μm MCB (green) and 50 μm PI (red) for 25 min.(a–c) U-turn stage homozygous gsh2-T1 embryo (top) and a phenotypically wild-type embryo (bottom) dissected from a silique of a heterozygous gsh2-T1 plant after self-pollination; (a) MCB labelling; (b) PI labelling; (c) chlorophyll autofluorescence recorded on a separate channel.(d) Wild-type embryo; chlorophyll autofluorescence appears red together with the PI signal.(e) Hypocotyl of a gsh2-T1 embryo.(f, g) High-resolution images of hypocotyl cells of wild-type (f) and gsh2-T1 (g) embryos; vacuoles in one cell of Col-0 and the gsh2 mutant are labelled with a ‘v’ and the nucleus in the mutant with an asterisk (*). Chlorophyll autofluorescence in the wild-type is shown in yellow, but was not detectable in the mutant imaged with similar sensitivity settings.(h) gsh2-T1 seedling 5 days after germination on MS medium.(i) gsh2-T1 seedling 5 days after germination on MS medium supplemented with 1 mm BSO.Scale bars = 100 μm (a–e), 10 μm (f, g), and 50 μm (h, i).
To evaluate the in situ staining with MCB in more detail, seedlings were first treated with MCB and then in situ labelled thiols were extracted and assayed by HPLC. Treatment of Col-0 seedlings with 100 μm MCB resulted in almost exclusive labelling of GSH with only trace amounts of labelled cysteine and γ-EC (Figure 5a). In contrast, MCB treatment of mutant seedlings labelled ∼850 nmol (g FW)−1γ-EC, a trace of cysteine and no detectable GSH. Incubation with the more reactive dye monobromobimane (MBB) resulted in much stronger thiol labelling. This labelling further increased with increasing MBB concentration, but even with 1 mm MBB only half the reduced γ-EC pool (12 600 nmol g FW−1) was labelled during the 25 min incubation period (Figure 5a). High resolution imaging of bimane-dependent fluorescence showed that even after incubation of U-turn stage embryos with 1 mm MBB the labelling was predominantly in the cytosol. Bleached plastids identified by their residual autofluorescence showed significantly less bimane-dependent fluorescence than the surrounding cytosol (Figure 5b,c) suggesting that labelling of γ-EC in the plastid was not occurring to any great extent.
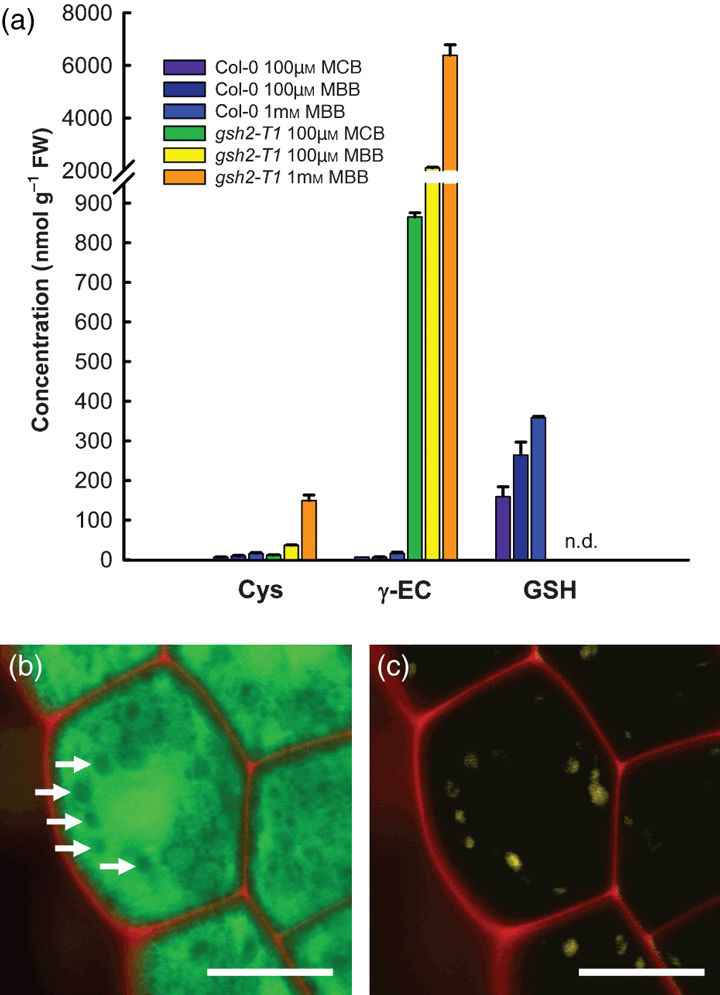
Analysis of low molecular weight thiols after fluorescent in situ labelling with bimane.(a) 10 days after germination Col-0 and homozygous gsh2-T1 seedlings were labelled in situ with 100 μm MCB, 100 μm MBB or 1 mm MBB, respectively, for 25 min. Subsequently, the seedlings were extracted and the labelled thiols analysed by HPLC (mean ± SD, n = 2).(b, c) Intact homozygous gsh2-T1 embryos dissected from ovules were labelled with 1 mm MBB (green) and 50 μm PI (red) for 25 min. (b) Merged image for MBB and PI labelling. Arrows indicate less intensely labelled areas, which can be clearly identified as bleached chloroplasts due to their residual autofluorescence (yellow) (c). Scale bars = 10 μm.
γ-EC inhibits glutamate cysteine ligase in vitro
Glutathione biosynthesis is normally regulated through feedback inhibition of GSH1 by GSH (Griffith and Mulcahey, 1999). Unlike in the Arabidopsis gsh2 mutants, in gsh2 mutants of yeast (Grant et al., 1997) and Sinorhizobium (Harrison et al., 2005) γ-EC was elevated above wild-type levels but did not hyperaccumulate. This suggests that in these organisms γ-EC can also exert feedback control on GSH1, albeit with reduced efficiency compared with GSH. In halobacteria a steady state concentration of γ-EC in the cytosol of 4 mm without any further hyperaccumulation has been calculated (Sundquist and Fahey, 1989) also suggesting feedback control of GSH1 by γ-EC. Thus, if γ-EC were accumulating within the plastids where it is synthesized it might inhibit GSH1 activity in the same way that γ-EC appears to inhibit GSH1 in vivo in gsh2 mutants of bacteria and yeast. To investigate this possibility we measured the inhibition of purified recombinant GSH1 from Brassica juncea, which has 91% identity to Arabidopsis GSH1, by GSH and γ-EC. It was observed that γ-EC was an effective inhibitor of BjGSH1 (Table 1) with 10 mmγ-EC inhibiting the activity to the same extent as 5 mm GSH. Assuming that the observed inhibition of BjGSH1 by γ-EC in vitro parallels the effect on AtGSH1, the extreme levels of γ-EC accumulated in the gsh2 mutant suggest that γ-EC is not accumulating in the plastids to levels sufficiently high to inhibit GSH1.
| Thiol concentration | GSH | γ-EC |
|---|---|---|
| 0 mm | 100 | 100 |
| 5 mm | 33 ± 7 | 61 ± 6 |
| 10 mm | 21 ± 9 | 34 ± 4 |
- All values are given as percent activity compared with the control activity in the absence of reduced GSH or γ-EC. 100% correspond to a specific activity of 561 ± 38 nmol γ-EC min−1 (mg protein)−1 in the absence of inhibiting thiols. Means and standard deviation are based on five independent replications of activity measurements.
Complementation of gsh2-T1 with a cytosol-specific enzyme
Expression of GSH2 exclusively in the cytosol would require the export of γ-EC from the plastids for GSH biosynthesis. It has previously been shown that GSH2 transcripts are of different lengths most of which are truncated for the sequence coding for the N-terminal plastid transit peptide (Wachter et al., 2005). Such a partial transit peptide sequence is insufficient to direct a fused protein into the plastids (Figure 6). To ensure cytosol-specific complementation, gsh2-T1 heterozygous plants were transformed with a construct expressing the GSH2 cDNA lacking the entire plastid transit peptide from the CaMV 35S promoter. Homozygous gsh2-T1 progeny carrying the transgene were identified by PCR genotyping. These lines were indistinguishable from Col-0 under standard growth conditions (Figure 7a). Blotting of proteins extracted from chloroplasts isolated from cytosolic complementation lines showed almost no GSH2 protein when compared with total leaf extract (Figure 7b). A very faint band visible may be attributed to a non-specific binding of anti-GSH2 antibody or to contamination of the plastidic fraction with adhering cytosol. To further confirm that restriction of GSH biosynthesis to the cytosol fully complements the mutant, the gsh2-T1 mutant was also complemented with a fusion protein of E. coli GSHB and GFP expressed from the CaMV 35S promoter. Stable expression of this GSHB:GFP fusion also resulted in a wild-type phenotype (not shown). The content of low molecular weight thiols in these plants was similar to the wild type with the exception of increased amounts of CysGly (Table S1). Plastids were clearly characterized by their autofluorescence and showed no GFP signal indicating the fusion protein was exclusively located in the cytosol (Figure 7c).
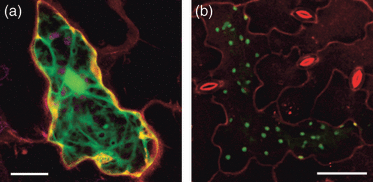
The full length transit peptide of AtGSH2 targets a fused GFP exclusively to the plastids. Arabidopsis leaves were bombarded with different constructs described by Wachter et al. (2005).(a) GFP fused behind a truncated transit peptide sequence is exclusively located in the cytosol. Nucleotides 121–204 counted from the start of the translation initiation site of the AtGSH2 cDNA were fused to GFP. Plastids, detected by their autofluorescence and depicted in magenta, show no GFP fluorescence. The image is a maximum projection of single optical sections collected along the z-axis. Scale bar = 20 μm.(b) GFP fused behind the full-length transit peptide sequence of AtGSH2 (nucleotides −22 to 204 counted from the translation initiation side) is targeted exclusively to the plastids. The image is a maximum projection of single optical sections collected along the z-axis. Scale bar = 50 μm.
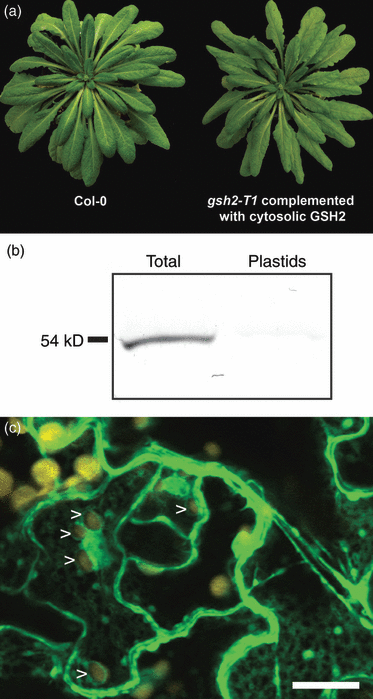
Complementation of gsh2-T1 plants with a wild-type GSH2 expressed in the cytosol.(a) Eight week old wild-type (left) and a homozygous gsh2-T1 plant expressing an Arabidopsis GSH2 cDNA lacking the entire target peptide (right).(b) Immunodetection of GSH2 in total protein fractions extracted from mature leaves or protein fractions extracted from purified chloroplasts of a cytosolic complementation line. In all cases 5 μg of protein was used per lane and resolved by SDS-PAGE. The GSH2 protein was detected on gel blots with a polyclonal antibody raised against Arabidopsis GSH2.(c) Expression of a GSHB:GFP fusion generated from E. coli GSHB resulted in exclusive cytosolic localization of the fusion protein. Images were taken by CLSM with λexc = 488 nm. Chloroplasts (shown in yellow) were always devoid of GFP fluorescence (arrowheads).
All cytosolic complementation lines tested contained at least 70% of the wild-type GSH level (Figure 8a). While cysteine levels were indistinguishable from the wild type in all lines, higher γ-EC levels correlated with lower GSH concentrations were observed in some lines (lines 4–4 and 4–32; Figure 8a). This may be due to low GSH2 activity and thus insufficient capacity to synthesise GSH. To test this, GSH2 activity was measured in the wild type and the different complementation lines. While one line had GSH2 activity similar to the wild type, the lines 4–4 and 4–32 showed a mean activity of <50% of the wild-type GSH2 activity (Figure 8b). Although these differences were not statistically significant it is interesting to note that the lower mean activity was observed in the lines with lower GSH and increased γ-EC levels. None of the cytosolic complementation lines tested showed an extractable GSH2 activity greater than the wild-type, as might have been expected with expression from the strong constitutive CaMV 35S promoter. This was further corroborated by protein gel blot analysis, which showed that the amount of GSH2 protein in the complementation lines was similar to the wild type (Figure 8c).
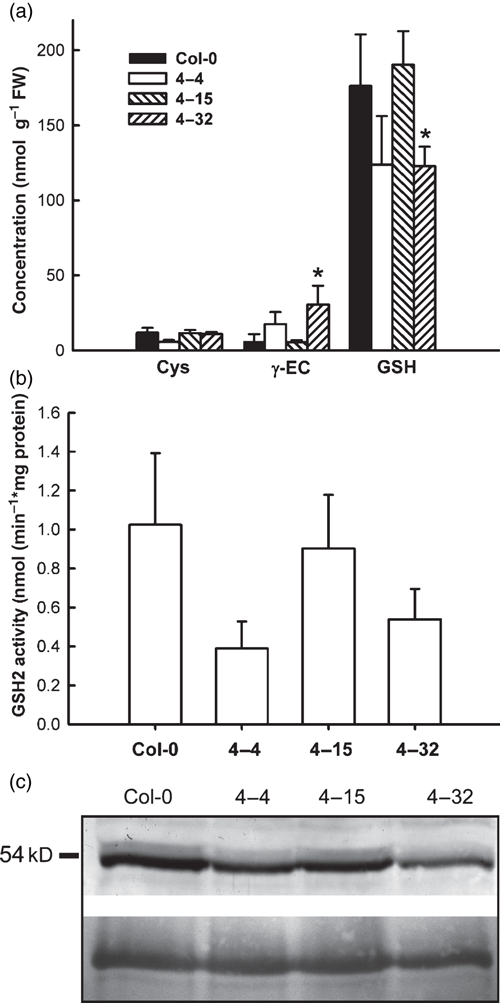
Low molecular weight thiols, GSH2 activity and GSH2 protein abundance in transgenic Arabidopsis lines expressing GSH2 exclusively in the cytosol.(a) Low molecular weight thiols extracted from leaves of wild type and of three cytosolic complementation lines (lines 4–4, 4–15 and 4–32; mean ± SD n = 4). *Significant differences from the wild type at P < 0.05.(b) GSH2 activity in total protein extracts of leaves from the same batch of plants as used in (a; mean ± SD, n = 3–5).(c) Immunodetection of GSH2 from the wild type and transgenic lines. Proteins from leaf extracts were resolved by SDS-PAGE and GSH2 protein was detected on gel blots with a polyclonal anti-GSH2 antibody. In transgenic lines GSH2 protein migrated at 54 kDa, the size of native mature GSH2. The bottom panel shows the loading control as visualised by Ponceau staining of the membrane.
Discussion
Endogenous glutathione biosynthesis is essential for normal development
In plants, GSH biosynthesis occurs in both plastids and the cytosol (Hell and Bergmann, 1988; Wachter et al., 2005). The compartmentation of GSH biosynthesis between different organelles appears to be a unique feature of plant cells. Previous observations have shown that gsh1 mutants of Arabidopsis are not viable (Cairns et al., 2006). This was confirmed for the gsh2 mutants analysed in this study but, in contrast to the embryonic lethal effect of gsh1 mutations, the lethal effect of gsh2 mutations is established only several days after germination. This suggests that accumulation of γ-EC can partially compensate for the lack of GSH.
The partial compensation of GSH-deficiency by γ-EC has been described for bacteria and yeast. The absence of hyper-sensitivity of S. cerevisiae gsh2 mutants grown in rich glucose-based medium to oxidative stress has been attributed to the antioxidant properties of the elevated levels of γ-EC (Grant et al., 1997). γ-EC also functions as the main antioxidant thiol in halobacteria, which lack GSH and where auto-oxidation of thiols is low under conditions of high salt, γ-EC can functionally replace GSH (Sundquist and Fahey, 1989). The identification of a high copy-number suppressor for the gsh2 knockout in S. cerevisiae which further elevated the level of γ-EC (Wheeler et al., 2002) and the observation that externally supplied γ-EC can rescue the limited growth of S. cerevisiae gsh2 mutants on minimal medium (Grant et al., 1997) suggest that in yeast γ-EC can substitute for GSH provided it is present in sufficient quantities. Similarly, γ-EC in Schizosaccharomyces pombe gsh2 mutants can partially replace the role of GSH in GST-dependent conjugation of red pigments and their vacuolar sequestration (Chaudhuri et al., 1997). In contrast, in Arabidopsis, hyperaccumulation of γ-EC did not compensate for the lack of GSH and prevent the lethal phenotype, although the lethality was delayed to post-germination. The lethal effect may be because of deleterious effects of the hyperaccumulation itself or, alternatively, because the accumulated γ-EC is not present in all compartments where GSH is normally required. The observation that accumulation of γ-EC after the probable silencing of the GSH2 transgene caused lethality despite significant residual amounts of GSH (Figure S1) strongly supports the first hypothesis. Germination of gsh2 mutants on medium containing BSO significantly decreased γ-EC to only threefold wild-type GSH-levels after 10 days. In this case, however, cysteine hyperaccummulated to levels of >8000 nmol (g FW)−1, which is also likely to be toxic due to the high sensitivity of cysteine to auto-oxidation in the presence of transition metals (Park and Imlay, 2003).
Lack of full complementation of gsh2 mutants on externally supplied GSH provides clear evidence that plants do not possess sufficient transport capacity to sustain growth without endogenous GSH biosynthesis. Similar observations have been described for gsh1 null mutants (Cairns et al., 2006) and rml1 mutants that can mature in the presence of externally-supplied GSH, but exhibit decreased fertility (Vernoux et al., 2000). That gsh2 lines complemented with cytosol-specific GSH2 are fully viable (see below) suggests GSH transport capacity is not limited internally between different organelles but rather at the plasma membrane. Glutathione has been discussed as a long distance transport metabolite for reduced sulfur between shoots and roots and into developing seeds (Cairns et al., 2006; Herschbach and Rennenberg, 2001), but very little is known about transporters involved in the uptake of GSH into the cells. Putative GSH transporters have been cloned from different species and shown to complement the S. cerevisiae hgt1 mutant, that is unable to take up external GSH (Bogs et al., 2003; Cagnac et al., 2004; Zhang et al., 2004). The Km values of these transporters for GSH of 0.4–23 mm make them unlikely to be efficient uptake systems.
Hyperaccumulation of γ-glutamylcysteine
The rate-limiting step for GSH-biosynthesis is catalyzed by GSH1 (Noctor et al., 1996). Heterologous overexpression of bacterial GSH1 in poplar and tobacco as well as homologous overexpression of AtGSH1 in wild-type Arabidopsis led to enhanced GSH levels (Creissen et al., 1999; Noctor et al., 1996; Xiang et al., 2001). Increased enzyme activity and increased GSH levels were observed independently of whether the protein was targeted to the cytosol or the plastids. In poplar expressing bacterial GSH1, the content of γ-EC increased more than 10-fold (Noctor et al., 1996). Because this accumulation of γ-EC was even more pronounced at the end of the dark period it was attributed partially to the limitation of glycine for GSH-biosynthesis (Noctor et al., 1997). The data reported here for the cytosolic complementation lines for GSH2 in a gsh2 background also indicate that GSH2 can become rate-limiting when its activity is slightly below the wild-type level. At the same time the complementation experiments indicate that cytosolic GSH2 activity might be subjected to an additional post-translational control mechanism that limits the total amount of native GSH2 protein and thus also limits the enzyme activity to values typical for wild-type plants. No similar control of protein abundance and hence activity was observed after expression of bacterial glutathione synthetase in the cytosol of poplar leaves where the extractable GSH2 activity was up to 300-fold higher than in untransformed controls (Foyer et al., 1995). At this stage it remains unclear, however, how such tight regulation of GSH2 in Arabidopsis might be achieved.
Sulfate assimilation is considered to be controlled at several points and through several potential signals (Kopriva, 2006). One of these signals might be GSH, which when applied to plants externally leads to a decrease in sulfate uptake. Consistent with this effect, depletion of GSH relieved the repression of adenosine 5′-phosphosulfate reductase (APR; Lappartient et al., 1999; Vauclare et al., 2002). Hyperaccumulation of γ-EC in gsh2 knockouts clearly indicates that the regulatory effects of GSH on sulfate assimilation are not met by γ-EC. The electrons required for reduction of sulfate to sulfite are derived from GSH and are transferred to sulfate by a glutaredoxin-like domain of APR (Bick et al., 1998). In contrast, this function of GSH as an electron donor for sulfate assimilation can clearly be covered by γ-EC, which is consistent with the report that the Vmax for APR with γ-EC as electron donor was similar to the Vmax achieved with GSH (Bick et al., 1998).
It has long been assumed that GSH-biosynthesis is regulated by direct feedback of GSH on GSH1 (Hell and Bergmann, 1990; Jez et al., 2004). The observed inhibition of GSH1 by γ-EC in vitro would thus strongly suggest that γ-EC cannot hyperaccumulate within the plastids without feedback inhibiting GSH1 in vivo. It should be noted, however, that the observed inhibitory effect of both GSH and γ-EC on GSH1 in the experiments reported here is much weaker than the inhibition reported for tobacco (Hell and Bergmann, 1990), which might indicate that different plant GSH1 proteins are regulated differently. For the Brassicaceae it was shown recently that GSH1 may be controlled by two independent disulfide bonds that affect enzymatic activity (Hothorn et al., 2006). Structural analysis of GSH1 crystals and site-specific mutagenesis indicate that both disulfide bridges need to be in the oxidized state for full enzyme activity (Hicks et al., 2007; Hothorn et al., 2006). While the first of these disulfide bridges is surface-exposed the second is buried at the interface of a homodimer formed in the oxidized state. Redox titrations of wild-type and mutant GSH1 identified the latter disulfide (Cys186-Cys406) as the redox-responsive switch for GSH1 (Hicks et al., 2007). The midpoint potential of GSH1 of −318 mV at pH 7.0 and −365 mV at pH 7.9 (Hicks et al., 2007) is very similar to the glutathione redox potential in non-stressed cells (Meyer et al., 2007; Schwarzländer et al., 2008). Although no information on the interaction of GSH1 with glutaredoxins is available, it is apparent that a link of GSH1 activity to the actual glutathione redox potential through glutaredoxins as the mediator would allow for a rapid response of GSH1 activity to adverse environmental conditions. The highly oxidized γ-EC pool indicates that the gsh2 mutant seedlings are suffering from oxidative stress. Under these conditions it is likely that GSH1 is present in its oxidized and thus highly active form.
Accumulation of (γ-EC)2 has also been observed in transgenic tobacco plants overexpressing GSH1 in the chloroplasts (Creissen et al., 1999). This accumulation indicates that no enzyme for efficient reduction of (γ-EC)2 is present and, thus, the only way for removal of the oxidized form is degradation or sequestration to the vacuole. Oxidized glutathione can be a substrate for ABC-transporters on the tonoplast and transport to the vacuole may be a mechanism for removal of high GSSG concentrations in the cytosol under conditions of oxidative stress (Foyer et al., 2001). Although not tested experimentally it is possible that such transport mechanisms might also accept (γ-EC)2 as a substrate, especially when present at very high concentrations.
Compartmentation of GSH biosynthesis
Immunodetection of GSH2 in proteins extracted from mature leaves and isolated plastids and confocal images of GFP-tagged protein all support the cytosol-specific location of GSH2 in the transgenic lines generated in this work. A requirement for efficient export of γ-EC from plastids was previously proposed based on long-term labelling experiments of Arabidopsis suspension culture cells with MCB (Meyer and Fricker, 2002) and can be inferred from the localisation of the biosynthetic enzymes for GSH (Wachter et al., 2005). In intact cells and tissues MCB primarily labels GSH, which normally is by far the most prominent low molecular weight thiol (Meyer et al., 2001). This was convincingly shown by the absence of detectable MCB-dependent fluorescence in homozygous gsh1 knockout embryos (Cairns et al., 2006). In the gsh2 mutant analysed here, however, γ-EC is intensely labelled although it is apparent that only a small fraction of the entire γ-EC pool can be labelled with the 100 μm MCB routinely used for effective GSH-labelling, which is presumed to occur through a GST-catalysed reaction (Meyer et al., 2001). The function of γ-EC as a substrate for GSTs in the absence of GSH has been shown in S. pombe (Chaudhuri et al., 1997). Thus, it is likely that with the extreme concentrations of γ-EC Arabidopsis GSTs might also use γ-EC as a substrate for conjugation reactions. The extremely high concentration of γ-EC in seedlings could not be labelled to more than 50% of the reduced γ-EC pool even with the faster-reacting MBB derivative at a concentration of 1 mm within 25 min. Despite this partial labelling of the γ-EC pool the imaging data clearly show a very strong fluorescent signal in the cytosol and little or no labelling in the plastids, which would be consistent with export of γ-EC from the plastids to the cytosol. A second possible explanation for this observation may be that even at high MBB concentrations most of the MBB is conjugated to the very high γ-EC concentrations in the cytosol before reaching the plastid. In any case, the strong cytosolic labelling suggests export of γ-EC from the plastids and is consistent with earlier observations by Wachter et al. (2005) and Meyer and Fricker (2002). While GSH-bimane adduct in wild-type cells is quickly sequestered into the vacuole (Meyer et al., 2001) sequestration of γ-EC-bimane conjugates was restricted. Slow vacuolar sequestration of γ-EC-bimane formed within the cytosol as a degradation product of GSH-bimane through action of phytochelatin synthase was also recently shown under conditions of heavy metal stress (Grzam et al., 2006).
Uptake of GSH into isolated plastids has been indicated before (Noctor et al., 2002). Transgenic expression of native GSH2 in the cytosol of a gsh2 knockout mutant complemented the mutant and led to near-wild-type levels of thiols. This demonstrates export of γ-EC from the plastids to act as a substrate for the cytosolic GSH2 and suggests efficient import of GSH into the plastids to re-establish feedback control over GSH1 activity. Nonetheless, the γ-EC level in some complemented lines was three- to fivefold higher than in wild-type controls. The concomitant 25% decrease in GSH content in these lines indicates that the increase of γ-EC was due to limited GSH2 activity, which was further corroborated by direct glutathione synthetase activity measurements. The fact that the 25% decrease in GSH did not lead to a stronger accumulation of γ-EC also indicates efficient feedback control of GSH1 and thus further corroborates the uptake of GSH into the plastids.
In summary, our studies on a seedling-lethal gsh2 mutant strongly suggest a mechanism for either passive or active export of γ-EC from the plastids, where it is synthesised, to the cytosol. Export of γ-EC from the plastids prevents feedback control on GSH1 in the gsh2 mutant and thus leads to hyperaccumulation of γ-EC during embryogenesis and after germination. Export of γ-EC was further corroborated through cytosol-specific GSH biosynthesis engineered in the GSH2-deficient background. Cytosol-specific complementation of the knockout mutant also provides clear evidence for efficient uptake of GSH into the plastids leading to re-establishment of feedback control on GSH1.
Experimental procedures
Plant material and growth conditions
Arabidopsis (Arabidopsis thaliana [L.] Heynh.) plants were grown on soil in controlled growth chambers with a diurnal cycle of 16 h light at 22°C and 8 h dark at 19°C. The light intensity was 120 μmol photons m−2 sec−1. For isolation of homozygous mutant seedlings segregating populations of seeds were moistened in sterile water with 0.4% (v/v) Tween-20 for 15 min, then surface sterilized with 70% EtOH for 1 min and then 6% sodium hypochlorite for 5 min. Finally the seeds were washed four times with sterilized water. Sterilized seeds were dried on filter paper for 15 min and then transferred to Arabidopsis growth medium solidified with 1% phytagel as described earlier (Meyer and Fricker, 2000). Plates were kept at 4°C for 1 day before placing them in vertical orientation in a growth cabinet with short day conditions 8 h light and 16 h dark at reduced light intensity of 25–50 μmol photons m−2 sec−1.
Screening for T-DNA insertions and genotyping
Genotyping of T-DNA insertional lines was done with the genomic primers P1 (5′-TTC CAC TTG TTT GCA GGT CAT TGC-3′) and P2 (5′-AAT AAA CCA CTG CGA CTG CTT GGC-3′) and the primer P3 (SAIL-LB3; 5′-TAG CAT CTG AAT TTC ATA ACC AAT CTC GAT ACA C-3′) for the line gsh2-T1 and the genomic primer P1 together with the left border primer P4 (5′-CCC ATT TGG ACG TGA ATG TAG ACA C-3′) for the line gsh2-T2. In the latter case the wild-type allele was amplified with primers P1 and P5 (5′-TCT TCA GTA TCA GCC TCC ATT TAC-3′).
RNA isolation and semi-quantitative RT-PCR analysis
Segregating seeds were sterilized and plated on non-selective Agar plates. 5 days after germination RNA was isolated from 5 mg of homozygous seedlings using RNeasy Plant Mini Kit (Qiagen, http://www1.qiagen.com) according to the manufacturer’s protocol, but with RNeasy MinElute columns (Qiagen) to facilitate elution with a very small volume. First strand cDNA was synthesised from 1 μg RNA using M-MLV Reverse Transcriptase kit (Promega, http://www.promega.com) according to the manufacturer’s protocol. For specific amplification of wild-type GSH2 the following primers were used: forward (5′-TTG GAG TAC AGT AAC CCA AGA GCG GTA GT-3′) and reverse (5′-CAT CCT CTT GTA CAC TCC CTT CTT TTT CGA CTT-3′). The transcript of the constitutively expressed actin 7 (AtACT7) was used as an internal control with the following primers: forward (5′-CAA CCG GTA TTG TGC TCG ATT C-3′) and reverse (5′-GAG TGA GTC TGT GAG ATC CCG-3′). The PCR was carried out using Taq polymerase (New England Biolabs, http://www.neb.com) with following conditions: initial denaturation 5 min at 94°C was followed by 30 cycles of 30 sec denaturation step at 94°C, 30 sec annealing step at 56°C and 15 sec extension step at 72°C. The final extension step was for 5 min at 72°C.
Reverse-phase HPLC analysis of low molecular weight thiols
Three to four milligram of isolated ovules or seedlings, respectively, were extracted with 0.1 N HCl. Subsequently, all low molecular weight thiols were reduced by addition of DTT and then derivatized with 10 mm monobromobimane as described previously (Cairns et al., 2006). Samples were analysed by reverse-phase HPLC and fluorescence excitation at 380 nm. For HPLC analysis of samples labelled with MCB in situ the tissue was extracted in 0.1 N HCl. After centrifugation the supernatant was directly analysed by HPLC as described above. Analysis of the ratio of oxidized to reduced γ-EC was carried out as described (Fey et al., 2005).
Plasmid-construction and Arabidopsis transformation
The AtGSH2 ORF excluding the plastid target peptide was PCR amplified from a cDNA clone (U14457, TAIR) using Phusion polymerase (Finnzymes, http://www.finnzymes.fi) with forward primer (5′-GGA TCC ATG GAA TCA CAG AAA CC-3′) and reverse primer (5′-GTC GAC TCA AAT CAG ATA TAT GC-3′), thereby introducing BamH1 and Sal1 restriction sites. PCR product was gel-purified, cloned into pCAPS vector (Roche, http://www.roche.de) and sequenced to exclude the possibility of amplification errors (Seqlab, http://www.seqlab.de). Using BamH1 and Sal1 restriction sites AtGSH2 was cloned into pBinAR (Höfgen and Willmitzer, 1992). Transformation of gsh2-T1 Arabidopsis plants was carried out as described (Clough and Bent, 1998). Transformants were selected using kanamycin and PCR genotyped for the insertion. For the wild-type GSH2 allele the following primers were used: forward primer P6 (5′-AGG TTA GCC TGG AAC ACA AAT CAA GA-3′) and reverse primer P7 (5′-TGG AAA CTT GAC GGC AAC TTA CTG A-3′). For the gsh2-T1 allele primer P6 was used together with the left border primer P3.
The GSHB open reading frame was amplified from E. coli K12 strain MG1655 using gene-specific primers: forward (5′-CCG CTC GAG AAA AAA TGA TCA AGC TCG GCA TCG T-3′) and reverse (5′-CGG ACT AGT GGG TAC TGC TGC TGT AAA CGT GC-3′), thereby introducing Xho1 and Spe1 restriction sites and ligated in pGEM-T. Positive clones were screened by PCR and sequenced to exclude the possibility of amplification errors (AGRF, Australia). Using Xho1 and Spe1 restriction sites EcGSHB was cloned into the plant expression vector JH322 fused to the 5′ of GFP and transformed into plants. Transformation of Columbia wild-type Arabidopsis plants was carried out as described (Clough and Bent, 1998). Transformants were selected using glufosinate ammonium crossed to heterozygous Arabidopsis gsh2-T1 individuals and the F1 progeny were self-fertilized to obtain homozygous gsh2 individuals containing the EcGSHB transgene.
CLSM imaging
Imaging of isolated embryos was carried out after dissecting intact embryos from ovules. Isolated embryos were imbibed in 100 μm MCB and 50 μm PI for 25 min and then transferred to aqueous medium on a slide. Imaging was done on a confocal microscope (Zeiss LSM510META scan head attached to an inverted microscope Axiovert200M; Zeiss, http://www.zeiss.com) equipped with lasers for 405 and 543 nm excitation. Images were collected with either a 25× water immersion lens (LCI Plan-Neofluar 0.8NA; Zeiss) or a 63× water immersion lens (C-Apochromat 1.2NA; Zeiss) and line scan mode with an averaging of four. After simultaneous excitation of PI with 543 nm and MCB and chloroplast autofluorescence both with 405 nm the three signals were collected on separate channels. MCB-dependent fluorescence was collected at 475–525 nm, PI-signal from the band 560–615 nm, and chloroplast autofluorescence at 678–753 nm. Some bleed-through occurred between the MCB-channel and the PI channel and between PI and the chloroplast autofluorescence. This bleed-through was corrected for by subtracting the respective signals from each other. For presentation of entire embryos stacks of images were collected with a z-increment of 1 μm. Stacks were projected as a maximum projection.
Inhibition assay for GSH1
Recombinant GSH1 protein from Brassica juncea was isolated according to Hothorn et al. (2006). Enzyme activity was measured spectrophotometrically in a coupled assay with pyruvate kinase and lactate dehydrogenase as described earlier (Hothorn et al., 2006). After determination of the kinetic parameters for the isolated enzyme inhibition by GSH and γ-EC was determined after subsequent addition of the respective thiols at increasing concentrations.
Immunodetection of GSH2
Total proteins were isolated from leaves and separated as described earlier (Wirtz and Hell, 2007). For isolation of chloroplasts leaves were homogenized in slushy frozen buffer A (340 mm sorbitol, 2 mm HEPES, 0.4 mm KCl, 0.04 mm EDTA, pH 7.8) for 5 to 10 sec using a blender. Ice-cold homogenate was filtered through a cheese-cloth/viscose/cheese-cloth sandwich rinsed with cold buffer A. The filtrate was then centrifuged at 2.000 g and 4°C for 60 sec. The chloroplast pellet was re-suspended in 50 ml buffer B (330 mm sorbitol, 50 mm HEPES, 2 mm EDTA, 1 mm MgCl2, 1 mm MnCl2, pH 7.6). After a second centrifugation at 4.000 g the chloroplast pellet was re-suspended in 5 ml buffer B and gently loaded onto a Percoll gradient. Samples were centrifuged for 10 min at 7000 g and 4°C without braking. Intact chloroplasts migrated in the Percoll gradient and concentrated near the bottom, while damaged chloroplasts did not enter the Percoll. Chloroplasts were collected and washed with volumes of buffer B and centrifugation at 2500 g for 60 sec at 4°C without braking. The chloroplast pellet was resuspended in protein extraction buffer and homogenised on ice for 1 min by sonication. The homogenate was centrifuged for 30 min at 16 000 g and 4°C and desalted on NAP5 columns. The eluate was concentrated using Amicon-10 concentrators (Millipore, http://www.millipore.com) and analysed by SDS-PAGE.
Separated proteins were blotted to nitrocellulose using the Mini Trans-Blot system (BioRad, http://www.bio-rad.com). To visualize the marker and check transfer quality, the nitrocellulose membrane was stained with Ponceau (0.1% Ponceau S (w/v) in 5% acetic acid) for 5–10 min and was documented photographically. The membrane was incubated in blocking solution (5% BSA in TBS) overnight in the fridge. The blocking solution was washed twice for 10 min with TBS (20 mm Tris, 137 mm NaCl, pH 7.6) and incubation with a primary antibody against AtGSH2 for 3 to 4 h at RT. After washing twice for 10 min with TBS the membrane was incubated with secondary antibody (AntiRabbit IgG– Alkaline Phosphatase Conjugate, 1:10 000; Sigma, http://www.sigmaaldrich.com) for 30 min and washed twice with TBS. For the alkaline phosphatase reaction the nitroblue tetrazolium/5-bromo-5-chloro-3-indolyl phosphate substrate system (Roche) was used. Blots were documented by scanning.
GSH2 enzyme assay
Crude protein extracts from Arabidopsis were analysed for GSH2 activity as described (Arisi et al., 1997). The reaction was started by adding 135 μl of crude protein extract. After 5, 15 and 30 min 25 μl aliquots were taken out of the reaction and immediately added into a MBB mixture containing 215 μl H2O, 20 μl 1 m Tris pH 8,3, 10 μl 10 mm DTT and 25 μl 10 mm MBB. Conjugation of thiols to bimane was carried out at RT for 15 min until the reaction was stopped by adding 705 μl of 5% acetic acid. The amount of GSH formed in the assay was analysed by HPLC as described above. Enzyme activity was calculated using the initial velocity estimated by linear regression of the three time points measured.
Acknowledgements
We are thankful to Andreas Wachter and Roland Gromes for providing recombinant GSH1 protein and plasmids encoding GSH2 reporter gene fusions. We also thank Thorsten Brach for help with the crosses of the allelic gsh2 knockout lines. This study was financially supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to AJM (grant No. ME1367/3-2).