Silencing of the major family of NBS–LRR-encoding genes in lettuce results in the loss of multiple resistance specificities
Summary
The RGC2 gene cluster in lettuce (Lactuca sativa) is one of the largest known families of genes encoding nucleotide binding site–leucine-rich repeat (NBS–LRR) proteins. One of its members, RGC2B, encodes Dm3 which determines resistance to downy mildew caused by the oomycete Bremia lactucae carrying the cognate avirulence gene, Avr3. We developed an efficient strategy for analysis of this large family of low expressed genes using post-transcriptional gene silencing (PTGS). We transformed lettuce cv. Diana (carrying Dm3) using chimeric gene constructs designed to simultaneously silence RGC2B and the GUS reporter gene via the production of interfering hairpin RNA (ihpRNA). Transient assays of GUS expression in leaves accurately predicted silencing of both genes and were subsequently used to assay silencing in transgenic T1 plants and their offspring. Levels of mRNA were reduced not only for RGC2B but also for all seven diverse RGC2 family members tested. We then used the same strategy to show that the resistance specificity encoded by the genetically defined Dm18 locus in lettuce cv. Mariska is the result of two resistance specificities, only one of which was silenced by ihpRNA derived from RGC2B. Analysis of progeny from crosses between transgenic, silenced tester stocks and lettuce accessions carrying other resistance genes previously mapped to the RGC2 locus indicated that two additional resistance specificities to B. lactucae, Dm14 and Dm16, as well as resistance to lettuce root aphid (Pemphigus bursarius L.), Ra, are encoded by RGC2 family members.
Introduction
Classical genetic analyses have shown that disease resistance in plants is often determined by clustered dominant genes, each conferring resistance to a specific pathotype of the pathogen (Pryor, 1987; Hulbert et al., 2001). During the past two decades many of these resistance (R) genes have been cloned and the majority of them encode nucleotide binding site–leucine-rich repeat (NBS–LRR) proteins (van’t Slot and Knogge, 2002; Xiao, 2006). Their structures and patterns of evolution are consistent with roles in direct or indirect recognition of pathogens and triggering of the plant’s defense responses (Jones and Jones, 1997; Michelmore and Meyers, 1998; Wei et al., 2002; Jones and Dangl, 2006).
The genes that encode NBS–LRR proteins are members of one of the most diverse and largest families within the plant kingdom (Meyers et al., 2003; Monosi et al., 2004; McHale et al., 2006). There are approximately 150 NBS–LRR-encoding genes in Arabidopsis ecotype Columbia-0 (Col-0) and approximately 400 in rice (Oryza sativa ssp. japonica). The conserved NBS motif is required for ATP binding and acts as a molecular switch for downstream signaling (Tameling et al., 2002; Takken et al., 2006). Leucine-rich repeat domains contain various numbers of tandemly repeated, leucine-rich motifs, each approximately 26 amino acids long and capable of forming a series of β-sheets that model into a horseshoe-like structure providing a potential binding surface for intra- and intermolecular interactions (Jones and Jones, 1997; Hwang et al., 2000; Moffett et al., 2002; Enkhbajar et al., 2003). Some NBS–LRR-encoding genes are present in plant genomes as single-copy sequences; others are members of multigene families (McHale et al., 2006). Members of two or more distinct families of NBS–LRR-encoding genes may also be juxtaposed within one chromosomal region (Halterman et al., 2001; Meyers et al., 2003).
In several species, separate members of an NBS–LRR-encoding gene family are known to encode different race-specific resistances to a particular pathogen. The Mla locus of barley (Hordeum vulgare) harbors at least four members of the RGH1 gene family that determine distinct resistance specificities against the biotrophic fungus Blumeria graminis (Zhou et al., 2001; Halterman et al., 2001; Halterman and Wise, 2004). Three homologs in the RPP1 gene cluster and two in the RPP4/5 cluster in Arabidopsis each code for resistance to different isolates of Hyaloperonospora parasitica (Parker et al., 1997; van der Biezen et al., 2002; Botella et al., 1998). An allelic series at the L locus in flax is responsible for resistances to Melampsora lini (Ellis et al., 1999; Luck et al., 2000; Dodds et al., 2006). Similarly, different alleles of the RPP13 gene in Arabidopsis encode race-specific resistances to H. parasitica (Bittner-Eddy et al., 2000). In contrast, a single RPM1 gene in Arabidopsis is responsible for recognition of the activity of two distinct effectors from Pseudomonas syringae, AvrRpm1 and AvrB (Bisgrove et al., 1994; Grant et al., 1995).
There are also a few examples in which homologs within one family of NBS–LRR-encoding genes code for resistance to different, taxonomically distinct pests and pathogens. For example, two paralogs in Arabidopsis, HRT and RPP8, encode resistances to turnip crinkle virus and H. parasitica, respectively (Cooley et al., 2000). Similarly, the Rx and Gpa2 genes in potato (Solanum tuberosum) encode resistances to potato virus X and cyst nematode, respectively (van der Vossen et al., 2000). The Mi gene in tomato (Solanum lycopersicum) not only encodes resistance to three different pests, root-knot nematode (several species), potato aphid, and white fly (Kaloshian et al., 1998; Milligan et al., 1998; Rossi et al., 1998; Vos et al., 1998; Nombela et al., 2003), but also has a close homolog within the syntenic region in potato, Rpi-blb2, that confers resistance to late blight (Van der Vossen et al., 2005).
The Resistance Gene Candidate 2 (RGC2) family in lettuce (L. sativa) is the largest family of NBS–LRR-encoding genes that have been characterized to date. One family member, Dm3, determines resistance to isolates of the oomycete pathogen Bremia lactucae, carrying the corresponding avirulence gene, Avr3 (Meyers et al., 1998a; Shen et al., 2002). Depending on the genotype, the RGC2 family contains from about 14 to more than 40 paralogous sequences spread over several megabase pairs on chromosome 2 (Meyers et al., 1998a; Kuang et al., 2004). Most RGC2 family members are expressed, but only at low levels (Shen et al., 2002). In addition to Dm3, at least eight other resistance specificities that confer resistance to downy mildew in a gene-for-gene manner are genetically linked to the RGC2 locus (Crute and Dunn, 1980; Hulbert and Michelmore, 1985; Farrara et al., 1987; Maisonneuve et al., 1994), but only single specificities have usually been detected in individual haplotypes. Of these additional resistance specificities, Dm18 (Maisonneuve et al., 1994) has been widely used in lettuce breeding programs as many isolates of B. lactucae carry the corresponding avirulence gene, Avr18, and are therefore avirulent on cultivars carrying Dm18. The determinant of resistance to an insect pest, lettuce root aphid (Pemphigus bursarius L.), has also been linked to the same locus (Crute and Dunn, 1980; Ellis et al., 1994). The RGC2 family is accompanied by at least two other NBS–LRR-encoding genes that are distinct from RGC2 but co-located in the same region on chromosome 2 (L. McHale and RWM, U.C. Davis, CA, USA, unpublished).
The RGC2 family in lettuce has been the subject of extensive study over the past 10 years (Meyers et al., 1998a; Sicard et al., 1999; Chin et al., 2001; Kuang et al., 2004, 2006). Point mutations, gene conversions and frequent sequence exchanges between RGC2 paralogs have resulted in hundreds, possibly thousands, of sequences that are distributed among different lettuce accessions (Kuang et al., 2004, 2006). Patterns of nucleotide variation indicate that diversifying selection is acting on the highly variable residues of the LRR domain of RGC2 genes (Meyers et al., 1998b; Kuang et al., 2004). Further sequence analysis indicated that RGC2 genes exist as two groups: Type I homologs that evolve rapidly because of high rates of sequence exchange between paralogs and relatively invariant Type II groups of orthologs which undergo purifying selection (Kuang et al., 2004). Dm3 is a Type I gene that is very rare in natural lettuce populations (Kuang et al., 2006). The molecular bases of other resistance specificities await characterization.
Post-transcriptional gene-silencing (PTGS) has become a powerful tool for assigning function to genes through reverse genetics (Napoli et al., 1990; Zamore, 2001; Baulcombe, 2004). Double-stranded RNA (dsRNA) homologous to the gene targeted for knock-out can be an efficient agent (trigger) for inducing PTGS in plants and animals (Fire et al., 1998; Meister and Tuschl, 2004); dsRNA can be produced through the expression of two complementary DNA sequences, one transcribed in the sense and the other in the antisense orientation (Waterhouse et al., 1998). Transformation vectors have been designed such that two complementary DNA fragments, separated by a third linker fragment, will be transcribed as one molecule capable of self-annealing into a hairpin structure referred to as interfering hairpin RNA (ihpRNA; Wesley et al., 2001). The presence of the linker fragment not only stabilizes plasmid vectors but may also enhance the efficiency of PTGS (Smith et al., 2000). Two or more genes can be silenced simultaneously using one ihpRNA-producing chimeric gene containing sequences identical with multiple targets (Helliwell et al., 2002; Lu et al., 2003; Miki et al., 2005).
Any genes sharing sequence similarity to the dsRNA trigger may be affected by PTGS (Qiu et al., 2005) but the parameters determining ‘off target’ effects are largely unknown. Subsets within a larger family of genes (He et al., 2004), or even individual members within a family (Miki et al., 2005), have been specifically silenced in plants when triggers similar exclusively to a subset of sequences were used. When dsRNA sharing sequence similarity to multiple members of gene family was used as a trigger, many members were silenced simultaneously (Miki et al., 2005). The minimum amount of identity between a trigger and target sequences sufficient to induce PTGS in plants was 23 bp in virus-induced gene silencing (Thomas et al., 2001) and 19 bp when short interfering RNA was used (Vanitharani et al., 2003).
In this paper we characterize the use of ihpRNA to induce PTGS of RGC2B and other members of the RGC2 family in lettuce. This large, weakly expressed family has previously been recalcitrant to silencing using other methods. We measured the simultaneous silencing of the GUS reporter and RGC2 genes as well as describe the use of transient GUS expression assays to assess gene silencing in transgenic plants. We also achieved knock-outs of some but not all resistance specificities that mapped to the RGC2 locus using primary transgenics as well as crosses to silenced tester stocks. This identified four resistance specificities, in addition to Dm3, that are encoded by RGC2 family members.
Results
Simultaneous silencing of GUS and Dm3 in transgenic lettuce plants
A series of chimeric ihpRNA constructs were designed to silence the Dm3 gene (Shen et al., 2002) and simultaneously monitor the effectiveness of PTGS using the GUS reporter gene (Jefferson et al., 1987). All of these constructs contained, in both an antisense and a sense orientation, a 459-bp fragment corresponding to the LRR-encoding region of the RGC2B gene (Dm3;Shen et al., 2002) fused to a 451-bp fragment of the GUS reporter gene (Figure 1). The two RGC2B-GUS chimeras in each construct comprised the arms of an inverted repeat structure separated by the 788-bp intron-3 from the gene encoding pyruvate orthophosphate dikinase (pdk) from Flaveria trinervia (Rosche et al., 1994; Wesley et al., 2001). Four constructs were made that differed in the order and orientation of the RGC2B and GUS fragments (Figure 1). Each chimeric ihpRNA gene was constitutively expressed using the CaMV-35S promoter (Ow et al., 1987).
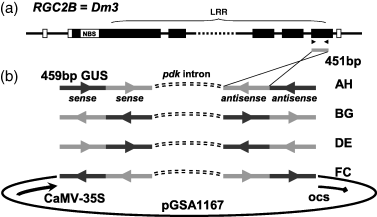
Chimeric gene constructions used for silencing RGC2 gene family members in cultivars Diana and Mariska.(a) Structure of the RGC2B gene encoding Dm3-associated resistance in cv. Diana. The gray bar corresponds to the fragment of the gene coding sequence within the LRR motif used to induce PTGS.(b) A 451-bp DNA fragment of the RGC2B gene (gray) was fused to a 459-bp fragment of the GUS gene (black) and used to produce chimeric ihpRNA constructs in the binary vector plasmid pGS1165 for plant transformation. The four constructs: AH, BG, DE and FC varied in the orientation and position of RGC2B and GUS fragments. In each construct the two arms comprising the inverted repeat were separated by Intron-3 of the pdk gene. In transgenic plants expression of the inverted repeat resulted in the production of dsRNA identical to both the GUS and RGC2B genes. (CaMV-35S, promoter from the 35S gene of cauliflower mosaic virus; ocs, terminator from gene encoding octopine synthase).
All four constructs were used to transform lettuce cv. Diana containing Dm3 (Table 1) and the resulting transformants were evaluated for silencing related to expression of the ihpRNA transgene. Multiple, independently transformed plants were obtained from transformations carried out with each of the four gene constructs. All transgenic T1 plants were initially analyzed to determine whether the GUS reporter gene had been silenced using a transient expression system for lettuce and the plasmid pDraGON-G:GFP as described previously (Wroblewski et al., 2005). The plasmid pDraGON-G:GFP contains two reporter genes, GUS and the gene encoding green fluorescent protein (GFP; Chalfie et al., 1994), each under the control of the CaMV-35S promoter. Infiltration of wild-type lettuce leaves with Agrobacterium tumefaciens harboring pDraGON-G:GFP resulted in the transient expression of both genes (Figure 2). In transgenic plants expressing the silencing trigger, transient expression of GUS was inhibited while expression of GFP remained at wild-type levels and served as a positive control for the effectiveness of transient expression. In 14 of the 31 transgenic T1 plants analyzed, transient expression of GUS was not detectable or was greatly reduced compared with non-transgenic plants, whereas expression of GFP was high and similar in all plants analyzed (Figure 2). Therefore GUS was silenced in almost half of the transformed plants.
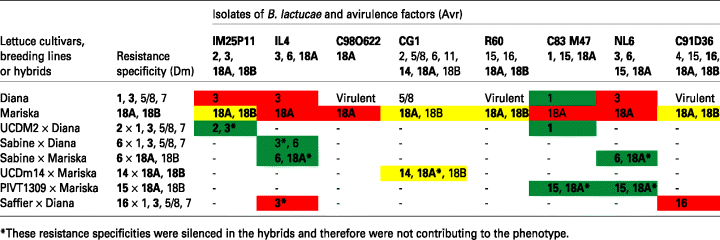
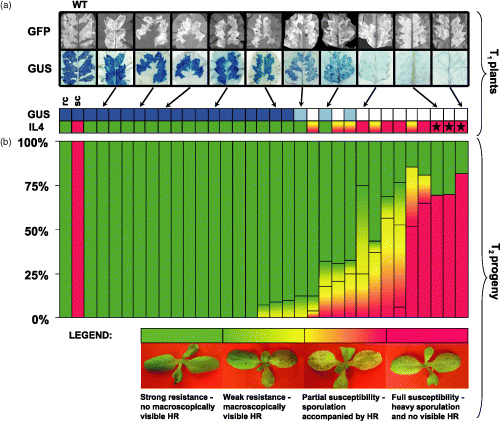
Simultaneous silencing of GUS and Dm3 in T1 transgenic cv. Diana lettuce plants and their T2 progenies.(a) Transient expression of GUS and GFP in the leaves of T1 transgenic plants and the reaction to Bremia lactucae isolate IL4 carrying Avr3 (Table 1). Colors and color combinations represent levels of resistance or susceptibility. Red, fully susceptible; red-yellow, partially susceptible; green, resistant; wt, wild type; sc, susceptible control, cv. Cobham Green; rc, resistant control, cv. Diana. Stars indicate plants used for qPCR and genetic crosses.(b) Loss of Dm3-encoded resistance among derived T2 progenies challenged with isolate IL4. Reactions among the seedlings (25–30 per progeny) were evaluated based on four categories that reflected the levels of resistance; the color combinations and photographs at the bottom of the figure represent these four categories. In the most susceptible progenies approximately 75% of seedlings were fully susceptible. Within progenies weakly silenced only 25% or less lost the Dm3 resistance.
The transformants of cv. Diana were then assayed for Dm3-encoded resistance. Detached leaves of the T1 transgenic plants, as well as T2 seedlings obtained from each of these plants, were challenged with three isolates of B. lactucae, IM25P11, IL4 and NL6, each expressing the Avr3 avirulence gene (Table 1). Sporulation occurred on 12 of the 31 T1 plants indicating that the resistance encoded by Dm3 had been lost (Figure 2a). All 12 of these plants also lacked GUS expression as determined above. Dm3-encoded resistance was also lost in the T2 progenies of 17 of the 31 T1 plants; however, the degree of susceptibility varied widely. In the T2 progenies of five T1 plants, profuse sporulation indicating the complete loss of Dm3-encoded resistance occurred in approximately three-quarters of the seedlings consistent with segregation of a transgene(s) inserted at a single locus (and on one chromosome, i.e. hemizygous) in the original T1 plants. All of these progenies originated from T1 plants that exhibited complete silencing of GUS and these families were considered to be silenced to the greatest extent. In the T2 progenies originating from another seven T1 plants, the loss of resistance was less profound; 25–75% of these seedlings supported mild sporulation and exhibited a macroscopically visible hypersensitive response (HR) indicating that they partially recognized and responded to the pathogen (Figure 2b). In this group, silencing of Dm3 was not detected in one of the seven T1 progenitors (Figure 2a). Finally, among the T2 progenies of the remaining five T1 plants, fewer than a quarter of the seedlings supported any sporulation and resistance was manifested as a macroscopically visible HR. Three of these five transgenic families originated from T1 plants in which silencing of GUS or Dm3 had not been detected (Figure 2).
Silencing of both GUS and Dm3 was achieved in transgenic plants generated with each of the four ihpRNA constructs; no substantial differences between constructs were observed. Therefore there was no evidence that the orientation or order of the trigger fragments had a major effect on the frequency or level of silencing obtained.
We also tested whether the resistance specificity encoded by Dm1 had been silenced along with Dm3 in these plants. In addition to Dm3, lettuce cv. Diana carries three other genes for resistance to downy mildew, Dm1, Dm5/8 and Dm7 (Table 1). Of these, Dm1 is within about 10 cM of the RGC2 cluster and the other two are unlinked (Hulbert and Michelmore, 1985). T2 seedlings from the five T1 plants most silenced for Dm3 were challenged with isolate C83 M47 of B. lactucae, which expresses the Avr1 avirulence gene. All the T2 seedlings tested were fully resistant to isolate C83 M47 (Table 1). Therefore the Dm1 gene had not been silenced along with Dm3; this is consistent with Dm1 not being encoded by a close homolog of Dm3.
Silencing of GUS provided a reliable indication that Dm3-encoded resistance had also been silenced. There was no case where a lack of GUS expression failed to coincide with a loss of the Dm3-encoded resistance in a transgenic T1 plant or at least some of its progeny. Therefore transient GUS expression was used in subsequent experiments to assay for the silencing of Dm3 in all other transgenic plants and their hybrids.
Transcript levels of other members of the RGC2 family were reduced in addition to mRNA of RGC2B
We performed a series of RT-PCR experiments to assess the steady-state mRNA levels of the RGC2 paralogs in silenced plants. Initially, we used microsatellite E6 (MSATE6) to assay the mRNA present in transgenic plants. Microsatellite E6 is a hypervariable, complex trinucleotide repeat within the 3’ LRR-encoding region of RGC2 paralogs and more than 20 distinct, differentially sized fragments representing RGC2 family members can be amplified from cDNA of wild-type cv. Diana (Kuang et al., 2004; Shen et al., 2002). Complete silencing would be expected to result in a lack of one or more of these fragments after amplification. However, no qualitative differences were observed among the MSATE6 profiles amplified from any of the transgenic plants tested (data not presented). Therefore the gene responsible for the lost resistance had probably not been completely silenced and at least some mRNA from all the RGC2 family members was present in the silenced plants.
We then performed quantitative PCR (qPCR) in order to determine how many members of the RGC2 family had reduced transcript levels in our transgenic plants. Sequence similarity among paralogs varied within the region co-linear to the 459-bp region of RGC2B which was used to trigger silencing (Figure 3), although all 27 members of the RGC2 family sequenced from cv. Diana (Kuang et al., 2004) contained at least 19 bp of continuous identity to the RGC2B trigger fragment, making them all potential targets for PTGS. We designed primers specific to each of seven paralogs that sampled the diversity within the RGC2 family (Supplementary Table S1). In preliminary qPCR analyses of T1 plants, the levels of RGC2B (Dm3) transcript were approximately five- to sixfold lower in the transgenic, silenced T1 plants than in non-transgenic cv. Diana (data not presented). Quantitative PCR was subsequently carried out on T2 seedlings from three T1 plants (indicated in Figure 2) for which the loss or presence of Dm3-encoded resistance and the presence or lack of the transgene had been determined. Expression of the seven paralogous RGC2 genes assayed was on average at least two to three orders of magnitude lower in wild-type plants than expression of the three house-keeping genes used as controls. The levels of RGC2B mRNA in the T2 seedlings lacking the transgene (the azygous T2 segregants) and resistant to IL4 did not differ from wild-type levels (data not presented). The average level of RGC2B transcript in the silenced transgenic plants was reduced by 84% compared with its level in non-silenced controls (sevenfold difference; Figure 3). The total regions of continuous identity that the seven amplified RGC2 paralogs shared with RGC2B correlated with the extent to which the mRNA for each gene was reduced (r = 0.79; Supplementary Figure S1). The mRNA level for the most similar RGC2B paralog analyzed, RGC2A, was reduced by 3.7-fold. The mRNA levels of four other paralogs, RGC2S, RGC2O, TDF and RGC2 K, was decreased in the silenced plants by 2.7- to 3.5-fold. Expression of the two most divergent paralogs assayed, TDL and TDM, was reduced by only 2.0- and 1.6-fold (Figure 3). Therefore most (possibly all) members of the RGC2 family had been partially but not completely silenced and to varying extents.
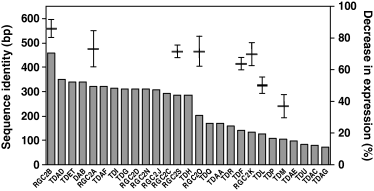
Continuous sequence identity with in the segment used for silencing between different members of the RGC2 gene family and the percentage decrease in expression of eight family members.Bars correspond to the summarized length of continuous sequence identity. Hash marks with the error bars correspond to the percentage decrease of expression in specific genes in the silenced cv. Diana plants.
A component of Dm18 resistance is encoded by an RGC2 family member
We used PTGS to investigate whether Dm18 resistance that is genetically linked to the Dm3 gene (Maisonneuve et al., 1994), is encoded by a member of the RGC2 family. The RGC2 family in cv. Mariska contains at least 14 homologs but none of these 14 genes had previously been shown to be a functional R gene (Kuang et al., 2004). We used the same four ihpRNA constructs (Figure 1) to transform cv. Mariska. Transgenic T1 plants were assayed for transient expression of GUS and Dm18-encoded resistance. Clear silencing of GUS occurred in 9 out of 17 T1 transgenic plants (Supplementary Figure S2). Leaves of these nine T1 plants were also susceptible to isolate C98O622 of B. lactucae (Supplementary Figure S2) and partially susceptible to isolates CG1 and R60, each expressing Avr18. Sporulation of CG1 and R60 occurred later than sporulation of C98O622, at 14 versus 8 days post-inoculation (dpi), respectively, and was also accompanied by macroscopic necrosis, indicating that isolates CG1 and R60 were recognized by and only partially virulent on these silenced T1 plants.
Isolates C98O622, CG1 and R60 were then used to test T2 seedlings. With C98O622 the reactions were very similar to those observed in the progenies of the transgenic cv. Diana challenged with isolate IL4 (Figure 2 and Supplementary Figure S2). Close to 75% of seedlings derived from T1 plants that exhibited negligible GUS expression were fully susceptible to isolate C98O622 (Supplementary Figure S2). However, these same silenced T2 progenies were nearly completely resistant to isolates CG1 and R60. Two weeks after infection with the CG1 and R60 isolates, sparse sporulation was occasionally observed accompanied by extensive macroscopic necrosis (Figure 4). When T2 seedlings susceptible to C98O622 were grown to obtain larger plants, their mature leaves were still fully susceptible to C98O622 and only partially susceptible to CG1 and R60; again, sparse sporulation of these latter two isolates was accompanied by necrosis. Furthermore, two other isolates, IM25P11 and C91D36, that also carry Avr18 were only partially virulent on these silenced T2 transgenic plants. However, a majority of seedlings from these silenced T2 progenies were fully susceptible to three other isolates, C83 M47, NL6 and IL4, all carrying Avr18 (Table 1).
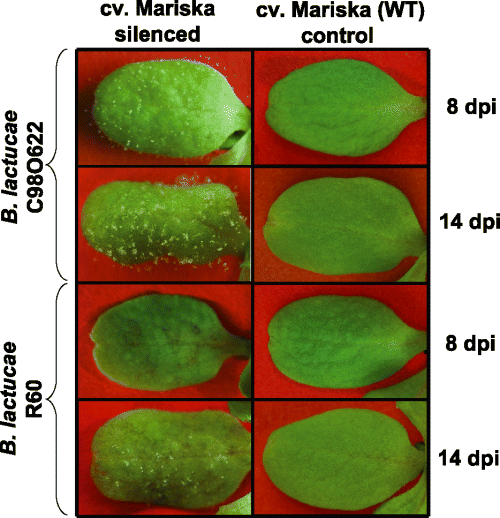
Loss of the resistance to two isolates of Bremia lactucae C98O622 and R60, both carrying Avr18 in transgenic and silenced cv. Mariska lettuce plants.Silenced cv. Mariska plants challenged with C98O622 show complete loss of the resistance manifested as sparse sporulation 8 days post-inoculation (dpi) and profuse sporulation 14 dpi. In contrast, seedlings infected with R60 show some macroscopically visible necrosis 8 dpi and only sparse sporulation 14 dpi. Necrosis observed after infection with R60 indicates only partial virulence of this isolate on the silenced plants, which were, however, clearly less resistant in comparison with the cv. Mariska (wild type).
From these data we concluded that the Dm18-encoded resistance phenotype is probably mediated through the recognition of two avirulence determinants by one or more resistance genes at the RGC2 locus: Avr18A present in isolates C98O622, C83 M47, NL6 or IL4 and Avr18B present in isolates CG1, R60, IM25P11 or C91D36. The determinant encoded by Avr18A is recognized by a member of the RGC2 gene family. The avirulence determinant encoded by Avr18B may also be recognized by the same Dm18 gene but, because of incomplete PTGS, the response to that determinant was not fully compromised in silenced plants. Alternatively, recognition of the determinant encoded by Avr18B could be mediated by a second Dm18 gene which has not been silenced in these transgenic plants. If the latter alternative were the case, these two plant genes (Dm18A and Dm18B) must be closely linked because resistance to B. lactucae isolate R60, which expresses both Avr18A and Avr18B, maps to a single locus linked to Dm3 (Maisonneuve et al., 1994).
Several other resistance specificities to B. lactucae are encoded by RGC2B homologs
In order to determine whether other resistance specificities to downy mildew are encoded by RGC2B homologs we made crosses to combine the ihpRNA transgene with various resistance genes. In additional to Dm1 and Dm18, five other genes determining resistance to lettuce downy mildew, Dm2, Dm6, Dm14, Dm15 and Dm16, have been genetically linked to Dm3 (Hulbert and Michelmore, 1985; Farrara et al., 1987; Table 1). UCDM2, a genetic stock containing Dm2, Sabine, a cultivar containing Dm6, UCDM14, a genetic stock carrying Dm14, PIVT1309, an accession of Lactuca serriola containing Dm15, and Saffier, a cultivar containing Dm16, were crossed with the transgenic cv. Diana and cv. Mariska plants that had been most effectively silenced (indicated in Figure 2b and Supplementary Figure S2); the transgenic plants were used as the pollen parents.
The F1 plants were challenged with different diagnostic isolates of B. lactucae and subsequently evaluated for the presence of the T-DNA and silencing of GUS (Figure 5). In all cases the presence of the transgene correlated with strongly reduced or a complete lack of GUS expression in the transient assay. Among 13 transgenic UCDM2 F1 hybrids, all were resistant to isolate IM25P11, which carries Avr2 (Table 1, Figure 5b). Among 16 transgenic cv. Sabine F1 hybrids, all were resistant to isolate IL4, which carries Avr6. Twelve of these 16 seedlings, for which cv. Mariska plants had served as the pollen parent (the other four had transgenic cv. Diana as the pollen donor) were also resistant to isolate NL6 that expresses Avr6. Among 12 transgenic F1 hybrids between UCDM14 and cv. Mariska, all were partially susceptible to isolate CG1 containing Avr14, Avr18A and Avr18B. The phenotypes of these hybrids were similar to the cv. Mariska T2 seedlings in which Avr18A had been silenced (Table 1) and so we concluded that only Dm18B was being expressed in these plants. Among seven transgenic F1 hybrids resulting from crosses between PIVT1309 and transgenic cv. Mariska, all the plants were resistant to isolates C83 M47 and NL6, both of which contain Avr15. Finally, among eight transgenic Saffier F1 hybrids, all were fully susceptible to isolate C91D36 containing Avr16; these hybrids exhibited no HR and profuse sporulation (Table 1, Figure 5c). Non-transgenic progeny of cv. Saffier and transgenic cv. Diana showed partial resistance, manifested as macroscopically visible HR and sparse sporulation 2 weeks post-inoculation as compared with wild-type cv. Saffier (Figure 5), indicative of a dosage effect involving the Dm16 gene. No dosage effect was observed in the other F1 heterozygotes. Therefore among the five additional resistance specificities tested, silencing of only Dm14 and Dm16, but not of Dm2, Dm6 or Dm15, was triggered by the RCG2B ihpRNA fragment.
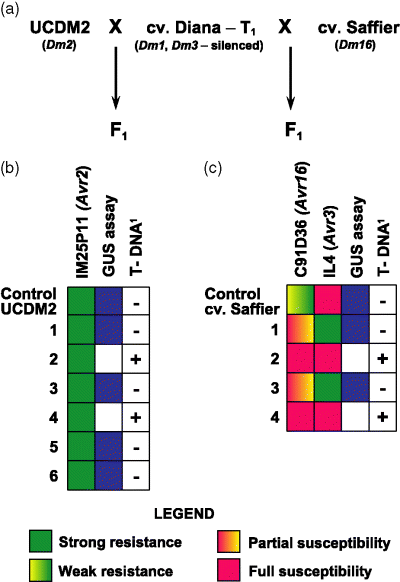
Schematic depiction of genetic crosses and tests to determine whether Dm2 and Dm16 became silenced in plants transformed with the ihpRNA chimeric gene constructs.(a) Genetic crosses made with silenced T1 plants. Clearly silenced T1 plants of cv. Diana (indicated with stars on Figure 2) were used as donors of pollen in crosses with UCDM2 (carrying Dm2) and cv. Saffier (carrying Dm16).(b) Analysis of the UCDM2 offspring. Seedling offspring (indicated with numbers) were tested for silencing of GUS and the presence of T-DNA. Seedlings were challenged with Bremia lactucae isolate IM25P11 (carrying Avr2) to determine whether any of them had lost their resistance. The hybrids that had received the transgene from their hemizygous pollen parent (2 and 4) were just as resistant to IM25P11 as those that had not, indicating that Dm2 had not been silenced.(c) Analysis of the cv. Saffier offspring. Seedling offspring (indicated with numbers) were tested for silencing of GUS, the presence of T-DNA, and were challenged with B. lactucae isolate IL4 (carrying Avr3) and C91D36 (carrying Avr16). The F1 hybrids lacking the T-DNA (1 and 3) exhibited partial susceptibility to C91D36. Hybrids containing the transgene (2 and 4) were fully susceptible to both C91D36 and IL4 and therefore silenced at both Dm16 and Dm3.1Indicates that presence of the T-DNA was confirmed by PCR.
Resistance to lettuce root aphid is also encoded by a RGC2 family member
Resistance to root aphids, Ra, had been genetically linked to the Dm6 gene and Dm6, in turn, had been mapped to the Dm3 locus (Crute and Dunn, 1980; Hulbert and Michelmore, 1985; Ellis et al., 1994). However, resistance to root aphid is not a qualitative all-or-nothing phenotype. Therefore, in preparation for experiments to determine whether Ra is also an RGC2 family member, we challenged cvs Sabine, Diana and Mariska with locally collected lettuce root aphids (P. bursarius L.) to check for resistance similar to that detected previously using an aphid population from Wellsbourne, UK (Ellis et al., 2002). As expected, aphids colonized all three cultivars; however, the number of insects on the infected roots of cvs Diana and Mariska 3 to 4 weeks after infection was consistently higher (one to five aphids per cm2) than the number on cv. Sabine (less than one aphid per cm2), a cultivar characterized as resistant to root aphid (Ellis et al., 2002). In addition to severe colonization by aphids, cvs Diana and Mariska showed obvious wilting followed by death 4 and 7 weeks, respectively, after infection. In contrast, plants of cv. Sabine remained alive and seemingly healthy even 3 months after infection, although their growth was somewhat reduced as compared with unchallenged controls.
Pollen from transgenic Diana and Mariska plants (the T1 individuals indicated on Figure 2 and Supplementary Figure S2, respectively) was used to fertilize flowers of cv. Sabine and 74 of the resulting seedlings were tested for resistance to root aphids. These seedlings comprised inadvertently selfed cv. Sabine offspring as well as F1 hybrids that either contained or lacked the T-DNA carrying the ihpRNA construct (Figure 6). Of these 74 seedlings, 24 showed severe infection, consisting of more than five aphids per cm2 of the root surface and wilting. All of these 24 plants contained the T-DNA and had been silenced for GUS expression (Figure 6b). Furthermore, among the 74 individuals analyzed only one plant carrying the transgene and GUS-silenced did not show severe symptoms of infection. Several plants that lacked the transgene exhibited a phenotype intermediate between the susceptibility exhibited in cv. Diana and the resistance of cv. Sabine, mild wilting 5–6 weeks after infection; these plants were confirmed as hybrids between cvs Sabine and Diana and we concluded that the intermediate phenotype was a consequence of a dosage effect because of heterozygosity of the Ra gene.
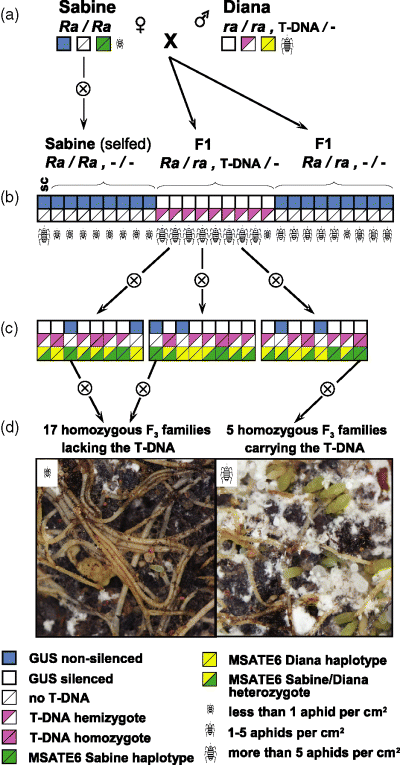
Schematic depiction of genetic crosses and tests carried out to determine whether resistance to root aphid (Ra) is encoded by an RGC2 family member.(a) Schematic depiction of the initial cv. Sabine and T1 cv. Diana genetic crosses. The pollen of T1 cv. Diana plants was used to fertilize flowers of cv. Sabine. Genotypes and phenotypes of the parental plants are indicated.(b) Schematic representation of 27 out of 74 F1 offspring from the genetic cross depicted in panel A. Genotypes and phenotypes of individual offspring are indicated. All but one of the GUS-silenced hybrids were also more susceptible to root aphids. sc, susceptible control, cv. Diana.(c) Schematic representation of eight plants each from three of 22 F2 families. Genotypes, including the haplotype of the co-dominant marker MSATE6 (green/yellow), and phenotypes of individual offspring are indicated. Each of the eight F2 plants derived from silenced F1 hybrids was examined for silencing of GUS, presence of T-DNA and the haplotype of the RGC2 locus using the co-dominant marker MSATE6.(d) Roots of representative plants of the F3 families showing different levels of colonization by root aphids.
All but two of the 24 F1 plants showing severe infection were successfully rescued from the infection and grown to produce F2 seeds (Figure 6c). To provide a more thorough analysis we analyzed seedlings of 176 F3 families obtained after self-pollination of eight F2 individuals produced from each of these 22 F1 hybrids. Using MSATE6 we identified 17 non-transgenic T3 families that were homozygous for the MSATE6 haplotype identical to cv. Sabine and therefore likely homozygous for the Ra gene. We also identified five T3 families with the same homozygous haplotype that were additionally homozygous for the T-DNA and silenced for GUS expression (Figure 6d). Ninety F3 plants from each of these 22 families were then challenged with root aphids. All individuals from the five families homozygous for the transgene were strongly colonized with more than five aphids per cm2 and died by 5 weeks after the infection. All plants lacking the transgene displayed only sparse colonization by the fifth week (less than one aphid per cm2; Figure 6) and grew similarly to the wild-type cv. Sabine. The susceptibility to root aphids of every family containing the transgene implies that Ra is one of the many genes in the RGC2 cluster.
Discussion
We used ihpRNA derived from the LRR-encoding region of the Dm3 gene for resistance to downy mildew to induce PTGS of RGC2B and other paralogs. This involved the development of approaches for the efficient application of PTGS for silencing large, low-expressed multigene families and provided insights into the diversity of resistance conferred by this extensive NBS–LRR-encoding gene family.
Chimeric ihpRNA constructs provided efficient simultaneous silencing of Dm3 and GUS
Although PTGS is a powerful tool for reverse genetics, it has been more successfully applied to some genes than others; the reasons for this are still being elucidated. The successful silencing of Dm3 and its paralogs reported here contrasts with our earlier unsuccessful attempts to silence RGC2 genes in lettuce. We had previously tried to silence RGC2 family members using several approaches including over-expression, in the sense or antisense orientation, of regions encoding the N-terminus, NBS domain or LRR regions of RGC2B as well as crosses between plants expressing these fragments in the sense and antisense orientation. None of these attempts were successful. Subsequently, the GUS reporter fragment was fused to the RGC2B trigger sequence in our chimeric ihpRNA constructs in part to enable us to monitor PTGS for trouble-shooting purposes. The LRR-encoding region of RGC2B that we utilized in our previous studies was expressed from the same promoter and overlapped with the 459-bp fragment used for the experiments reported here. Therefore the successful silencing of RGC2 genes using ihpRNA as described in this paper is probably a consequence of the increased efficiency of generating dsRNA through intramolecular association of the inverted repeats.
Silencing was incomplete at the RNA level; complete silencing was not observed for any RGC2 family member. This may be related to the especially low levels at which the endogenous RGC2 genes are expressed or suggests that complete silencing was lethal. However, the latter possibility is unlikely because deletion mutants of lettuce lacking most of the RGC2 genes are phenotypically normal (Meyers et al., 1998a). The decreases in RGC2B mRNA we observed were at least 10 times lower than those observed in similar experiments carried out on the OsRac gene family in rice (Miki et al., 2005). RGC2B mRNA was reduced by an average of 84%, whereas the levels of mRNA identical to the gene-silencing trigger used to silence OsRac genes were reduced by more than 99%. A few other members of the OsRac gene family sharing only 72–82% identity with the trigger were still silenced by more than 90%. Miki et al. (2005) proposed that highly expressed genes may be easier to silence than genes with lower levels of expression because of better access to short interfering RNA (siRNA; Hamilton and Baulcombe, 1999) and the RNA-induced silencing complex (RISC; Hammond et al., 2000; Elbashir et al., 2001). Target mRNA transcribed from highly expressed genes may also participate more readily in the production of secondary siRNA via transitive silencing, thereby amplifying the original pool of siRNA originating from the trigger. In contrast, the majority of the siRNA for genes expressed at low levels would be derived from ihpRNA expression. The mRNA levels of RGC2 family members detected in wild-type plants were at least two to three orders of magnitude lower than those of several house-keeping genes and are consistent with previous RNA gel blot analyses of RGC2 genes (Shen et al., 2002). These low levels of expression may explain the incomplete levels of PTGS we obtained as well as the previous difficulties we experienced in attempting to silence RGC2 family members using other approaches.
The transient GUS expression assays provided a highly reliable indication of successful PTGS. The technique was simple to perform, reproducible and therefore suitable for high-throughput experiments in which multiple plants, from multiple generations and genetic crosses, could be analyzed. This was particularly important for the generation of the homozygous T3 families required for demonstrating silencing of the Ra gene. In addition, it allowed the selection of transgenic plants exhibiting high levels of silencing and their use as defined tester stocks in crosses with other resistant genotypes. This resulted in the efficient transfer of the gene-silencing T-DNA to additional lettuce genotypes through straightforward genetic hybridizations and avoided the need for multiple transformation experiments.
Incomplete PTGS was sufficient for silencing at the phenotypic level
Despite the lack of complete silencing of RGC2 genes at the mRNA level, silencing was highly effective at the phenotypic level for several genes. Reduction of RGC2B mRNA by 84% was sufficient to cause the complete loss of Dm3-encoded resistance to B. lactucae in transgenic cv. Diana plants. F1 hybrids between plants transformed with the ihpRNA constructs and lettuce cultivars carrying Dm14 and Dm16 also became fully susceptible to downy mildew isolates expressing the cognate avirulence genes. Hybrids between transgenic tester stocks and cv. Sabine carrying the Ra gene became clearly susceptible to infection with root aphids. Therefore the level of reduction of mRNA was sufficient to eliminate each of these resistance specificities in heterozygous plants. This silencing of Dm3, Dm14, Dm16, Dm18A and Ra at the phenotypic level despite being incomplete at the mRNA level indicates that expression of these genes in wild-type plants is at a level minimally adequate to induce resistance. This conclusion is supported by the observation that non-transgenic plants heterozygous for Dm16 or Ra exhibited intermediate resistance phenotypes. Whether this is a consequence of the metabolic cost of supra-sufficient levels of expression of large numbers of RGC2 genes or of supra-optimal levels of RGC2 protein resulting in a fitness penalty is unclear. However, overexpression of RGC2B from the 35S promoter results in normal, viable transgenic plants (K. Shen and RWM, U.C. Davis, CA, USA, unpublished); therefore, overexpression of at least one member of this gene family is not deleterious.
The RGC2 family encodes diverse resistance specificities
These PTGS experiments indicated that the RGC2 family encodes at least 4 of the 16 Dm genes known to date, plus Ra. Germplasm screens have identified many additional sources of resistance and sequence analysis suggests that there are probably thousands of different RGC2 sequences present in Lactuca spp. (Bonnier et al., 1992; Kuang et al., 2004, 2006; Beharav et al., 2006). It is likely that the RGC2 family encodes numerous other functional resistance genes. Crosses to the PTGS tester stocks will be highly informative in indicating which accessions harbor additional resistances encoded by RGC2 genes.
The phenotypes associated with expression of specific RGC2 genes can vary. The resistance associated with Dm3 and Dm14 is strong and complete, for example; recognition of B. lactucae isolates carrying the cognate avirulence genes takes place rapidly in lettuce cultivars carrying these genes. The hypersensitive reaction (HR) occurs in the first cells that the pathogen comes in contact with, and is consequently macroscopically invisible. In contrast, resistance encoded by Dm16 is weaker. The response to pathogen isolates containing the corresponding Avr16 gene is delayed; haustoria penetrate the tissue beyond the cells of initial infection before the HR occurs and, consequently, HR is macroscopically visible in incompatible Dm16-mediated interactions. Whether this is a consequence of the Dm16 protein, the Avr16 protein, or both is not known. Characterization of the molecular determinants of plant-pathogen specificity as well as of components that affect the strength or speed with which a defensive response occurs are required to determine the basis of these phenotypic differences.
Similarly to the tomato Mi gene, that among others confers resistance to the potato aphid (Rossi et al., 1998), lettuce Ra belongs to the NBS–LRR-encoding genes. Interestingly Mi has a homolog in potato that encodes resistance to late blight caused by oomycete pathogens (Van der Vossen et al., 2005). In our case members of the RGC2 gene family provide resistances to both oomycetes and the aphid, but in one species, namely lettuce. In both cases it is unclear whether the proteins encoded by these gene families are detecting different ligands from each pest or pathogen, or maybe they monitor the status of the plant target that is common to different effectors. The latter would most readily explain the recognition of such diverse pathogens and pests by the same gene or gene family.
The reductions in transcript levels we observed for all seven of the members of the RGC2 gene family we tested in cv. Diana along with RGC2B suggests that all of the RGC2-related genes in the lettuce accessions we examined were silenced to some degree. Assuming Dm1, Dm2, Dm6 and Dm15 are also expressed at minimal levels, our data provide no evidence that these resistance specificities are not encoded by RGC2 family members. Dm1, Dm2, Dm6 and Dm15 may be divergent members of the RGC2 family or other NBS–LRR-encoding genes present near the RGC2 locus.
Genetically defined Dm18 represents multiple functional resistance specificities
Previous genetic analysis indicated that Dm18 was a single gene (Maisonneuve et al., 1994). However, our data indicate that the situation is more complex and help explain a variety of observations concerning Dm18. Isolates of B. lactucae previously determined to contain Avr18 produced different responses in the same silenced plants, indicating the existence of at least two avirulence factors, Avr18A and Avr18B. The observed variation suggests either differential recognition of these two avirulence factors by the product of a single resistance gene or recognition by products of two distinct Dm18 genes (Dm18A and Dm18B) in cv. Mariska. The existence of two Dm18 genes is consistent with observations made during the breeding of Dm18-mediated resistance into lettuce cultivars. Dm18 has been widely used in lettuce breeding programs, partly because it was considered potentially more durable based on the longevity of resistance in early cultivars with Dm18. However, after introgression of Dm18 into several cultivars, resistance was often overcome (O. Ochoa and RWM, U.C. Davis, CA, USA, unpublished). Also, the virulence phenotypes produced by isolates expressing Avr18 were heterogeneous across lettuce cultivars reported to contain Dm18. This heterogeneity and the reduced durability itself can be explained by the breakage of linkage between two resistance genes and the consequent introgression of only one of the two genes into subsequent generations of a particular lettuce cultivar. A single gene is also likely to be less durable than two. In addition, as part of a molecular cloning strategy we attempted to generate deletion mutants of Dm18 to aid in assigning this resistance to a particular RGC2 gene, similar to the approach successfully used to identify RGC2B as Dm3 (Anderson et al., 1996; Meyers et al., 1998a; Shen et al., 2002). No mutants of cv. Mariska were identified that were susceptible to isolates CG1, R60 and C91D36 despite screening large M2 populations (L. McHale and RWM, U.C. Davis, CA, USA, unpublished). The existence of two resistance genes, thereby requiring a double knock-out to produce the anticipated mutant phenotype, is consistent with this lack of success. Both breakage of the linkage during introgression and lack of recovery of deletion mutants following fast neutron irradiation suggest that Dm18A and Dm18B are close but not extremely tightly linked and that Dm18B is located outside the main cluster of RGC2 sequences.
Future directions
An increasing number of resistance gene candidates (RGCs) are being identified using PCR amplification and searches through the growing EST and genomic sequence databases (McHale et al., 2006). Libraries of transgenic plants silenced for representatives for each of these candidate sequences would be highly informative for assigning resistance phenotypes to RGC gene family members. The simultaneous silencing of multiple family members will significantly reduce the number of ihpRNA constructs and transgenic plants necessary to perform such analyses. Utilization of a GUS reporter gene fragment and the transient expression assay described here, which is feasible for several crop plants (Wroblewski et al., 2005), will significantly reduce the effort necessary to identify the most efficiently silenced trangenics and to follow the silencing transgene in their offspring. Defined silencing lines can be used as tester stocks in crosses to numerous resistant accessions. Functional assignments to RGCs will provide excellent molecular markers for breeding for disease resistance (Michelmore, 2003) as well as focus cloning efforts on specific families of sequences.
Most of the resistance genes cloned to date have been isolated by positional cloning which is laborious, particularly for plants with large genomes. Post-transcriptional gene-silencing narrows novel resistance specificities to families of RGC sequences facilitating subsequent cloning. For example, RGC2B sequences can now be used as probes for screening genomic libraries of relevant genotypes to select candidates for Dm14, Dm16, Dm18A and Ra that can then be used in functional complementation experiments. Although there will be more than one candidate sequence for each of these specificities, all these resistance genes certainly contain sequences similar to the RGC2B-derived trigger of PTGS. In addition, if silencing could be refined further to target individual family members, this would greatly increase the efficiency of cloning numerous resistance genes.
Experimental procedures
Production of ihpRNA constructs and plant transformations
All constructs were produced using the binary vector pGSA1165 (a pCAMBIA1200 derivative; Cambia, http://www.cambia.org/) using a two-step cloning protocol as described previously (http://www.chromdb.org/). The pGSA1165 vector was first modified by deleting the 372-bp fragment of the GUS gene located between the restriction enzyme cleavage sites SwaI and BamHI and replacing it with a 788-bp long fragment containing intron-3 of the pdk gene of F. trinervia (Rosche et al., 1994). The DNA fragment used to produce the two arms of the inverted repeat in the ihpRNA constructs was designed to contain inner restriction enzyme sites, AscI and SwaI, and outer restriction enzyme sites, BamHI and SpeI, at its ends. These inverted repeat regions were then assembled in the binary vector via a two-step cloning process utilizing the introduced restriction enzyme sites. In the first cloning step, the inverted repeat fragment was cleaved at the inner restriction sites, AscI and SwaI, and ligated to the cleaved AscI and SwaI sites within the modified pGSA1165 vector. For the second cloning step the same fragment was cleaved at the outer restriction enzyme sites, BamHI and SpeI, and incorporated into the BamHI- and SpeI-cleaved vector plasmid resulting from the first cloning step. Four different inverted repeat fragments were made and inserted into the vector resulting in four different constructs AH, BG, DE and FC (Figure 1).
The inverted repeat fragments consisted of fusions between a 451-bp fragment of the GUS gene (U12639, bases 2644 to 3095) and a 459-bp fragment of the RGC2B gene (AF113948, bases 5169 to 5628) in four different orders and orientations (Figure 1). During the same PCR amplifications in which the inner and outer restriction sites mentioned above were introduced, an SfiI restriction enzyme cleavage site was also introduced on one end of the GUS and one end of the RGC2B DNA fragments (see Supplementary Table S2 for primer sequences). The GUS and RGC2B fragments were then cleaved with SfiI and ligated together at the SfiI junction point. All restriction enzymes used were obtained from New England Biolabs (http://www.neb.com/). T4 DNA ligase was obtained from Invitrogen (http://www.invitrogen.com/).
For lettuce transformation all four constructs (Figure 1) were introduced into the LBA4404 strain of A. tumefaciens (Hoekema et al., 1983). Transgenic plants were produced using explants from cotyledons as previously reported (Michelmore et al., 1987) at the Ralph Pearson Transformation Facility at U.C. Davis (http://ucdptf.ucdavis.edu/).
Plant material and genetic hybridizations
All lettuce cultivars and genetic stocks used in these experiments originated from the collection at U.C. Davis and have been used previously in genetic studies of downy mildew (Farrara et al., 1987; Ilott et al., 1987; Maisonneuve et al., 1994). Diana, Mariska, Sabine and Saffier are butterhead cultivars. UCDM2, UCDM14 and PIVT1309 are homozygous inbred lines. Hybridizations of lettuce were carried out according to the method described by Jones (1927). Hybrids were distinguished from selfed offspring of the female parents based on morphological characteristics and, in the case of hybrids with cv. Diana, the presence of additional Dm genes (Dm1, Dm5/8 or Dm7) (Figure 5).
Detection of T-DNA
The presence T-DNA was detected by analyzing plants for the presence of the neomycin phosphotransferase gene (NPTII) using PCR. DNA was isolated from leaves using a modified cetyltrimethylammonium bromide (CTAB) procedure (Bernatzky and Tanksley, 1986). Two primers, 5’-GAGGCTATTCGGCTATGACTG-3’ and 5’-ATCGGGAGCGGCGATACCGTA-3’, were then used to assay for a 699-bp fragment diagnostic of the NPTII gene.
Microsatellite E6 (MSATE6) analysis
T2 plants homozygous for the RGC2 cluster were identified by determining plant haplotype in terms of the microsatellite MSATE6 (a complex trinucleotide repeat within the LRR-encoding region of RGC2 genes; Shen et al., 2002). Genomic DNA was used as template in PCR amplifications carried out according to Shen et al. (2002). The primers used were: 5’-G(G/C)AATGAAAGTGAT(A/T)GTGAAG-3’ and 5’-GGGAGCTGTGGACCCACCAG-3’and reactions were carried out for 30 cycles of: 30 s at 94° C, 30 s at 52° C, 30 s at 72° C.
Transient expression assays
Agroinfiltration (Schob et al., 1997) was used to monitor PTGS in transgenic plants and the offspring of crosses made with them. Simultaneous, transient expression of GFP and GUS was induced in the leaves of T1 plants, their progenies and various hybrids using A. tumefaciens strain C58C1 harboring plasmid pDraGON-G:GFP as described previously (Wroblewski et al., 2005). Cultures of A. tumefaciens were maintained using liquid YEP medium (Bacto-Pepton 10 g l−1, yeast extract 10 g l−1, NaCl 5 g l−1, pH = 7) supplemented with 50 mg l−1 of kanamycin sulfate (Invitrogen). Bacteria were then harvested after 15 min of centrifugation at 1000 g and resuspended in water. Bacterial density was adjusted to OD600 = 0.4–0.5 and used for infiltrations within 2 h of harvesting. Infiltrations were performed as described by Schob et al. (1997) by infusing the bacterial suspension under pressure from a syringe without a needle held against the lower side of the leaf lamina. Transient expression of GFP was used as an internal, positive control for transient gene expression. Transient expression of GUS was used as an indicator of PTGS. Expression of GFP and GUS was evaluated 3 dpi. Expression of GFP was monitored using FluorImagerSI (Molecular Dynamics, http://www5.gelifesciences.com) and GUS expression assays were carried out on the same leaves and according to Jefferson et al. (1987). Assays were repeated at least three times on each plant; the phenotypes of plants were highly reproducible.
Quantitative polymerase chain reactions
Levels of gene expression were determined using qPCR. Total RNA was extracted from approximately 50 mg of young leaf tissue or 12 cotyledons using Qiagen RNeasy Plant Miniprep kits (Qiagen; http://www.qiagen.com/). The RNA was subsequently subjected to DNase treatment (Roche, http://roche-applied-science.com). First-strand cDNA was synthesized using 1.5 μg of total RNA, an oligo(dT) 18-mer and SuperScriptIII Reverse Transcriptase (Invitrogen) in a 20-μl volume according to the manufacturer’s protocol. Before amplification RNA was removed by using an RNaseH (Invitrogen) treatment. Samples to which no reverse transcriptase was added were processed simultaneously as controls for contaminating genomic DNA. Quantitative PCR amplifications were carried out in volumes of 25 μl using 1 μl of a 5× dilution of the cDNA produced during the first-strand synthesis reaction. SYBR Green PCR mix (Applied Biosystems; http://www.appliedbiosystems.com/) was used according to the manufacturer’s protocol. The reactions were carried out using two gene-specific primers (200 μm of each; Supplementary Table S1) and 0.2 u of AmpliTaq Gold Polymerase (Applied Biosystems).
Primers were designed and the annealing temperature optimized to provide selective amplification of individual members of the RGC2 multigene family. The products of amplification from both silenced and non-silenced plants were sequenced to confirm the specificity of amplification. Amplifications of three house-keeping genes, Actin, Tubulin and Ubiquitin, were performed as internal controls. Expression of RGC2B was evaluated using two pairs of primers (Supplementary Table S1). To amplify RGC2B (BFor and BRev primers, Supplementary Table S1) and RGC2 K the reactions were carried out for one cycle of 8 min. at 95° C and 40 cycles of 20 s at 94° C, 30 s at 63° C and 10 s at 72° C. To amplify RGC2B (B’For and B’Rev primers, Supplementary Table S1), RGC2A, RGC2 F, RGC2 L, RGC2 M and RGC2S the annealing temperature was lowered to 59° C and for RGC2O to 57° C. The decrease of expression (Figure 3 and Supplementary Figure S1) was calculated as 100%– 100%/1.8N where N was the number of cycles differentiating silenced from non-silenced plants and necessary to reach a threshold of fluorescence 20 times higher than the background. The actin, tubulin and ubiquitin gene fragments were amplified at all three annealing temperatures. The reactions were carried out using an OPTICON®2 real-time PCR machine (Bio-Rad; http://www.bio-rad.com/). Data were analyzed using MJOPTICON MonitorTM (Bio-Rad), microsoft excel and sas (SAS Institute Inc.; http://www.sas.com/) software.
Assays for resistance to downy mildew
Isolates of downy mildew (B. lactucae L.) were accessed from the collection of isolates at U.C. Davis (Table 1). Cultures were maintained on seedlings of the susceptible cultivar Cobham Green as described previously (Hulbert and Michelmore, 1985). Seven-day-old seedlings or leaf disks obtained from older plants were challenged by spraying them with suspensions containing 5 × 105 conidia ml−1. After inoculation seedlings or disks were incubated in a growth room at 15° C and illuminated with 150 μE/m2/s fluorescent light for 14 h per day. Interactions on seedlings were scored 8 and 14 days after infection as one of four categories (Figure 2b): (i) no sporulation or macroscopically visible necrosis – full resistance; (ii) no sporulation with macroscopically visible necrosis – partial resistance; (iii) sparse sporulation accompanied by macroscopically visible necrosis – partial susceptibility; and (iv) profuse sporulation and no macroscopically visible necrosis – full susceptibility. For leaf disks only three categories were distinguishable (Figure 2a): (i) no sporulation, (ii) sparse sporulation accompanied by necrosis and (iii) sporulation without visible necrosis. Each assay was repeated twice for leaf disks collected from T1 plants. At least 25 seedlings were evaluated from each T2 progeny. Interaction phenotypes of F1 hybrids (Figure 2) were determined using at least seven seedlings identified as hybrids between each particular accession and at least two independent transgenic T1 plants and verified as containing the silencing T-DNA.
Assays for resistance to lettuce root aphid
Lettuce root aphids (P. bursarius L.) were collected from naturally infected, greenhouse-grown lettuce plants (Davis, CA) and used to establish a population on the susceptible cultivar Diana in a controlled environment chamber (16° C/14° C, 60% relative humidity, and strong light, approximately 300 μE/m2/s, produced by high-pressure sodium lamps with a 12 h photoperiod). The 74 plants resulting from crosses between cv. Sabine and transgenic (T1) cv. Diana or cv. Mariska were grown to the three- to four-leaf stage (about 3 weeks old); three to four aphids were then transferred to each plant. This was repeated four times at 2-day intervals to provide reliable inoculations. Scoring commenced 3 weeks after the initial infection; aphid colonization on the surface of the root ball and the progress of wilting were assessed for each plant. Plants were considered resistant if colonization had reached an average density of less than one aphid cm−2 by 4 weeks after the initial infection. Plants were considered susceptible if colonization density was more than five aphids cm−2. Colonization densities of between one and five aphids cm−2 were considered an intermediate phenotype. Susceptible plants were rescued from the infection by washing the roots and transplanting to fresh soil. Seedlings from each of 22 F3 families were also evaluated for their susceptibility to root aphids. Approximately 90 seedlings (three replicas of 30) from each of the 22 families, plus control plants from the susceptible (Diana) and resistant (Sabine) cultivars, were grown in partitioned trays. Plants were infected at the one-leaf stage (about 14 days old). Inoculations were again made four times with three to four aphids per family each time.
Acknowledgement
We thank Pauline Sanders and Rommel Alfonso for greenhouse assistance, Maria Jose-Truco for help with statistical analysis, members of the Michelmore Lab for discussion and Belinda Martineau for editing the manuscript. pGSA1165 (a binary vector derived from pCAMBIA1200; Cambia, Canberra, Australia) was kindly provided by Natalie Doetsch and Richard Jorgensen, the Chromatin Functional Genomics Consortium (Tucson, AZ, USA). Intron-3 of the pdk gene from F. trinervia was helpfully provided by P. Westhoff and U. Gowik (Heinrich-Heine Universitaet, Duesseldorf, Germany). This research was supported by an award from NSF Plant Genome Program, no. 0211923.




