Mutants for photosystem I subunit D of Arabidopsis thaliana: effects on photosynthesis, photosystem I stability and expression of nuclear genes for chloroplast functions
Summary
In Arabidopsis thaliana, the D-subunit of photosystem I (PSI-D) is encoded by two functional genes, PsaD1 and PsaD2, which are highly homologous. Knock-out alleles for each of the loci have been identified by a combination of forward and reverse genetics. The double mutant psad1-1 psad2-1 is seedling-lethal, high-chlorophyll-fluorescent and deficient for all tested PSI subunits, indicating that PSI-D is essential for photosynthesis. In addition, psad1-1 psad2-1 plants show a defect in the accumulation of thylakoid multiprotein complexes other than PSI. Of the single-gene mutations, psad2 plants behave like wild-type (WT) plants, whereas psad1-1 markedly affects the accumulation of PsaD mRNA and protein, and photosynthetic electron flow. Additional effects of the psad1-1 mutation include a decrease in growth rate under greenhouse conditions and downregulation of the mRNA expression of most genes involved in the light phase of photosynthesis. In the same mutant, a marked decrease in the levels of PSI and PSII polypeptides is evident, as well as a light-green leaf coloration and increased photosensitivity. Increased dosage of PsaD2 in the psad1-1 background restores the WT phenotype, indicating that PSI-D1 and PSI-D2 have redundant functions.
Introduction
Photosystem I (PSI) mediates light-driven electron transport across the thylakoid membrane. The PSI complexes of cyanobacteria and plants are functionally and structurally similar, and the crystal structure of cyanobacterial PSI has recently been established (Jordan et al., 2001). Plant PSI is slightly larger than its cyanobacterial pendant (Kitmitto et al., 1997), and trimer formation has been observed only in cyanobacteria (Chitnis and Chitnis, 1993). In plants, the PSI core is associated with the light-harvesting complex I (LHCI), which consists of four different pigment-binding polypeptides (Jansson, 1999). In addition, of the 14 polypeptides that form the core of PSI in flowering plants (PSI-A to -L and PSI-N to -O; Knoetzel et al., 2002; Scheller et al., 2001), four (G, H, N and O) have no counterpart in cyanobacteria. Conversely, no homologue of the cyanobacterial PSI-M has yet been discovered in flowering plants.
The three subunits PSI-A, -B and -C, which bind the electron acceptors, are crucial for PSI function. In Arabidopsis, these proteins, together with PSI-I and -J, are encoded by plastid DNA, whereas all other subunits are encoded by the nuclear genome. Two gene copies each for PSI-D, -E and -H exist. Downregulation of individual PSI subunits by antisense or co-suppression strategies, combined with the identification of insertion mutants, has provided the basis for the functional analysis of almost all nucleus-encoded PSI polypeptides (Haldrup et al., 1999, 2000; Jensen et al., 2000, 2002; Lunde et al., 2000; Naver et al., 1999; Varotto et al., 2000a, 2002a).
Ferredoxin – and in cyanobacteria and some algae, also flavodoxin – is reduced at the stromal face of PSI by the extrinsic subunits C, D and E. These three polypeptides form a compact interconnected structure, the so-called stromal ridge (Jordan et al., 2001; Klukas et al., 1999; Kruip et al., 1997). In cyanobacteria, loss-of-function mutants for each of the corresponding genes have been characterized. PSI-C is necessary for the stable association of PSI-D, -E and -L (Mannan et al., 1994; Yu et al., 1995). Cells that lack PSI-D are affected in photoautotrophic growth and in flavodoxin-mediated photoreduction of NADP+, and moreover, they show no ferredoxin-mediated NADP+ photoreduction at all (Chitnis et al., 1989; Xu et al., 1994). Lines in which PSI-E is missing are impaired in NADP+ photoreduction, but exhibit normal photoautotrophic growth (Xu et al., 1994; Zhao et al., 1993).
The eukaryotic PSI-D and -E subunits are larger than their cyanobacterial counterparts, containing N-terminal extensions of 25–30 amino acids. In the psae1-1 mutant of Arabidopsis thaliana, knocked-out for one of the two genes coding for PSI-E, the level of PSI-E and concomitantly those of PSI-C and -D, are significantly reduced. The oxidation state of PSI, photosynthetic state transitions and photoautotrophic growth are also altered in this mutant (Pesaresi et al., 2002; Varotto et al., 2000a).
Recently, the analysis of antisense lines with 5–60% of PSI-D showed that downregulation of PSI-D de-stabilizes PSI, results in increased photosensitivity and in an altered thiol disulphide redox state of the stroma (Haldrup et al., 2003). Here, we characterize the effects of complete lack of PSI-D, as well as the impact of the two PsaD genes on PSI-D function. Disruption of the PsaD1 gene, but not of PsaD2, affects the composition of PSI, alters the redox state of the photosynthetic apparatus, and impairs plant growth in the greenhouse. Analysis of the expression of nuclear genes for chloroplast proteins reveals further physiological effects of the psad1-1 mutation. Overexpression analysis indicates that the products of the two PsaD genes have redundant functions. The absence of both functional PsaD genes is incompatible with the accumulation of functional PSI, affects the accumulation of all other thylakoid multiprotein complexes and is lethal at the seedling stage.
Results
Identification and phenotype of PSI-D mutants
Screening of a collection of Arabidopsis lines carrying independent insertions of the dSpm transposon (the Sainsbury Laboratory Arabidopsis Transposants (SLAT) collection; Tissier et al., 1999) for alterations in the effective quantum yield of PSII (ΦII; Varotto et al., 2000b) resulted in the identification of the mutant line photosynthesis-affected mutant 62 (pam62). The photosynthetic lesion was inherited as a recessive trait, and RFLP analysis of plants segregating for the pam62 mutation with a dSpm-specific probe revealed the presence of only one transposon copy that co-segregated with the mutant allele (data not shown). Isolation of genomic sequences flanking the termini of the dSpm insertion was achieved by inverse PCR as described by Tissier et al. (1999), and the insertion site was identified. The dSpm transposon was inserted in the unique exon of the PsaD1 gene (At4g02770) coding for PSI-D (Figure 1a), and the mutant pam62 was accordingly designated psad1-1.
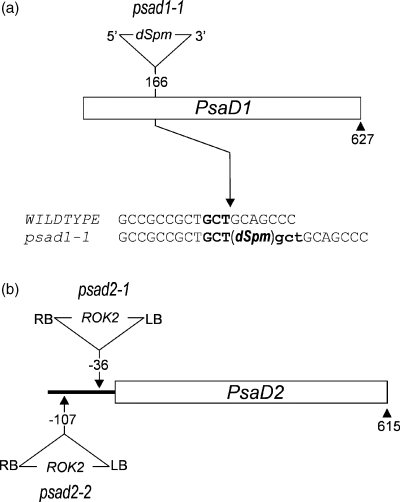
Tagging of the PsaD1 and PsaD2 genes.
(a) In psad1-1, the PsaD1 gene (At4g02770) is disrupted by an insertion of the non-autonomous dSpm element (Tissier et al., 1999). Upper case letters indicate plant DNA sequences flanking the dSpm element, bold uppercase letters indicate the transposon target site in WT and the duplicated target site is indicated by bold lowercase letters.
(b) The psad2-1 and psad2-2 mutants carry insertions of the 5.2-kbp pROK2 T-DNA (http://www.signal.salk.edu) in the promoter region of the PsaD2 gene (At1g03130).The dSpm and T-DNA insertions are not drawn to scale.
In the A. thaliana genome, a second PsaD gene (PsaD2, At1g03130) exists, which codes for a protein highly homologous to the PsaD1 gene product (96/95% similarity/identity; Figure 2). After cleavage of predicted chloroplast transit peptides (domain I in Figure 2), the proteins PSI-D1 and -D2 differ only in the highly variable plant-specific N-terminal extension (domain II). To establish the photosynthetic function of PsaD2, the database signal (http://signal.salk.edu/cgi-bin/tdnaexpress; Alonso et al., 2003) was searched for insertions in the gene. A line was identified that carries a copy of the 5.2-kbp ROK2 T-DNA inserted 36 bp 5′ to the ATG of the PsaD2 gene, and this mutant line was designated psad2-1 (Figure 1b). A second psad2 mutant allele was identified when DNA pools of the SALK collection of T-DNA lines were screened by PCR specific for insertions at the PsaD2 locus. Out of the pool CS61661, the psad2-2 line was isolated, which had a copy of the ROK2 T-DNA inserted 107 bp 5′ to the ATG of the PsaD2 gene (Figure 1b).

Comparison of PSI-D protein sequences from higher plants, algae and prokaryotes.
The amino acid sequences of the Arabidopsis PSI-D1 and -D2 proteins (Accession numbers: At4g02770 and At1g03130) were compared with those of PSI-D from spinach (GI:19855891), tomato (GI:82100), barley (GI:478404), rice (GI:29367391) and with PSI-D sequences from the green alga Chlamydomonas reinhardtii (GI:498824), the red alga Porphyra purpurea (GI:2147918), the cryptophyte alga Guillardia theta (GI:3603032), the chlorophyll a/c containing alga Odontella sinensis (GI:7443141) and from four cyanobacterial species: Nostoc sp. PCC 8009 (GI:5052771), Synechocystis sp. PCC 6803 (GI:16329280), Synechococcus sp. (GI:47576) and Mastigocladus laminosus PCC 7605 (GI:2160762). The three-part alignment shows the N-terminal chloroplast transit peptide of higher plant and Chlamydomonas PSI-D (I), which was either experimentally determined (spinach, tomato, barley, Chlamydomonas) or predicted by targetp (Arabidopsis, rice); the N-terminal extension of mature PSI-D specific to higher plants (II); and the C-terminal domain of PSI-D from higher plants that is highly similar to the corresponding segment of algal and cyanobacterial PSI-D proteins (III). Note that the N-terminal extension of the higher-plant proteins is rich in alanine and proline and highly diversified. The two mature Arabidopsis PSI-D proteins show amino acid exchanges at six positions of the N-terminal extension, but none in the C-terminal domain. Black boxes indicate strictly conserved amino acids, shaded boxes closely related amino acids. The symbol ‘+’ refers to amino acid residues involved in the binding of ferredoxin (Bottin et al., 2001; Hanley et al., 1996; Lagoutte et al., 2001); circles highlight lysine residues of the basic domain, which are important for the interaction of PSI-D with other PSI subunits (Chitnis et al., 1997).
Under greenhouse conditions, psad1-1 plants were smaller than the wild type (WT) and had light-green leaves; in contrast, both psad2 lines showed WT-like growth and leaf coloration (Figure 3a). When the growth rates of the two single-gene mutants psad1-1 and psad2-1 were compared to that of WT plants (Leister et al., 1999), growth of psad1-1 plants was found to be substantially reduced compared to the mutants psad2-1 and psad2-2 (data not shown), and WT plants (Figure 3b). Crosses were carried out between psad1-1 and psad2-1, and homozygous F2 double-mutant plants were identified. The psad1-1 psad2-1 double mutants did not survive when grown on soil, but could be propagated in axenic culture on medium supplemented with sucrose. Heterotrophically grown double mutants had yellowish leaves, remained very small and exhibited a high-chlorophyll-fluorescence (hcf) phenotype, similar to the mutant phenotypes previously observed by Meurer et al. (1996). This indicates that the absence of PSI-D causes a block in photosynthetic electron flow (Figure 3c). In addition, double mutants were highly photosensitive and could only be propagated under low-light conditions (photon flux density, PFD = 15 µmol photons m−2 sec−1).

Phenotypes of psad1-1, psad2-1, psad2-2 and the double mutant psad1-1 psad2-1.
(a) WT, psad1-1, psad2-1 and psad2-2 plants (4 weeks old) were grown in the greenhouse under long-day conditions.
(b) Growth kinetics of psad1-1 and psad2-1 mutants compared to WT. Thirty-six plants of each genotype were measured during the period from 8 to 20 days after germination. Mean values ± SDs (bars) are shown.
(c) WT and psad1-1 psad2-1 double mutant plants (d1 d2) grown on sucrose-containing MS medium and illuminated with white light (top) or UV light (bottom).
PsaD expression
Northern analysis revealed a marked decrease in the level of PsaD mRNA in psad1-1, and a slighter decrease in psad2-1 (Figure 4a). Reverse-transcription PCR (RT-PCR) designed to discriminate between PsaD1 and PsaD2 transcripts indicated that in psad1-1, PsaD1 transcript accumulation was completely suppressed, and that in psad2-1 and psad2-2, the transcript of PsaD2 was not detectable (Figure 4b). In the psad1-1 psad2-1 double mutant, the accumulation of both forms of PsaD transcripts was completely suppressed (Figure 4c).
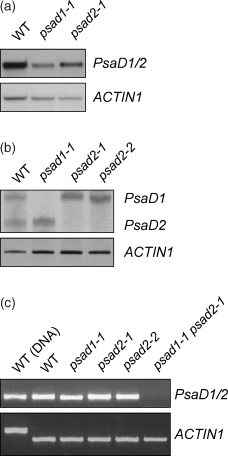
Expression of PsaD mRNA in mutant and WT plants.
(a) Northern analysis of PsaD transcripts. Aliquots (20 µg) of total RNA were hybridized with a mixture of PsaD1 and PsaD2 cDNA fragments. To control for variation in loading, the blots were probed with a cDNA fragment derived from the ACTIN1 gene.
(b) Detection of PsaD1 and PsaD2 transcripts by RT-PCR. Products obtained after PCR for 35 cycles with PsaD1/PsaD2 specific primers (PsaD1/2-22/22s and PsaD1/2-230/218as) and control primers for the ACTIN1 gene in the same reactions were analysed on a 4.5% (w/v) polyacrylamide gel. The products derived from transcripts of the two PsaD genes differ in size by 12 bp and were visualized by silver staining.
(c) Detection of PsaD transcripts by RT-PCR. Products obtained after PCR for 35 cycles with primers recognizing both PsaD transcripts (PsaD1/2-22/22s and PsaD1/2-230/218as) and control primers for the ACTIN1 gene were analysed on a 2.0% agarose gel. As a control, PCR with genomic WT-DNA was performed. The difference in size of the ACTIN1‘WT (DNA)’ band is because of the presence of two introns in the genomic amplicon.
Western analyses demonstrated that the psad1-1 mutant had 40% of WT amounts of PSI-D in thylakoids, while in psad2-1 plants PSI-D was reduced by only 10% (Figure 5a; Table 1). In the double mutant psad1-1 psad2-1, no PSI-D was detected (Figure 5b; Table 1). Closer inspection of the D-specific immunoblot signals in the two single-gene mutants revealed that the gene products of PsaD1 and PsaD2 differed slightly in molecular mass, as one would expect from the predicted difference in the lengths of the mature proteins (PSI-D1, 164 amino acids; PSI-D2, 161 amino acids). In agreement with the transcription data, in psad1-1, only PSI-D2, and in psad2-1, only PSI-D1 were detectable. As residual PSI-D level in the two single-gene psad mutants added up to more than WT levels, it was concluded that – at least in psad2-1– the effects of the single-gene mutations were partially compensated by increased accumulation of the alternative PSI-D form.
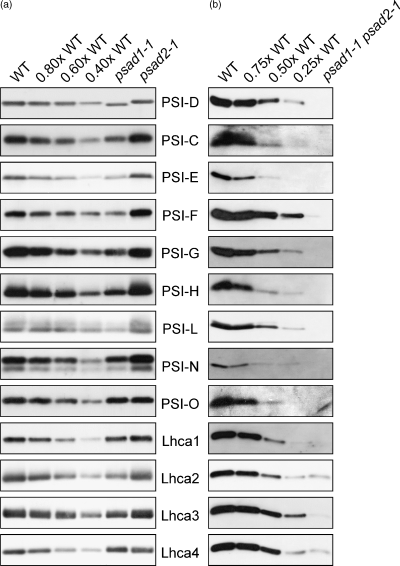
Levels of PSI polypeptides in mutant and WT plants.
Aliquots of thylakoid proteins corresponding to 5 µg of chlorophyll (psad1-1, psad2-1 and WT plants; panel (a)), or 40 µg of total protein (psad1-1 psad2-1 and WT plants; panel (b)) were loaded in each lane, and decreasing amounts of WT proteins were added to lanes 0.8×, 0.6× and 0.4× WT in panel (a), or to lanes 0.75×, 0.5× and 0.25× WT in panel (b). Replicate filters were immunolabelled with antibodies raised against individual PSI or LHCI polypeptides. Three independent experiments were performed, and representative results are shown. Signals obtained in three independent experiments were quantified using lumi analyst 3.0 (Boehringer Mannheim/Roche). In the case of the psad1-1 mutant, because of its reduced chlorophyll content, relatively more proteins were loaded. Relative values for the mutant genotypes, reflecting the ratio of expression between the mutant and WT (see Table 1), were normalized on the basis of their chlorophyll content (see Table 2). The PSI-D-specific immunoblot signals in psad1-1 and psad2-1 slightly differ in their molecular mass, as expected from the different predicted lengths of the mature proteins (PSI-D1, 164 amino acids; PSI-D2, 161 amino acids). When a mix of psad1-1 and psad2-1, or of psad1-1 and WT, or of psad2-1 and WT thylakoid proteins was analysed, WT-like bands were detected (data not shown). This indicates that the two PSI-D forms can be discriminated only in the corresponding mutant backgrounds.
| PSI subunit | psad1-1 | psad2-1 | psad1-1 psad2-1 | psae1-1 | ΔF |
|---|---|---|---|---|---|
| A/B | nd | nd | nd | 100 | 120 |
| C | 50 | 100 | nd | 30 | 60 |
| D | 40 | 90 | 0 | 30 | 40 |
| E | 40 | 100 | 0 | 30 | 40 |
| F | 20 | 100 | 2 | 100 | 40 |
| G | 40 | 90 | 0 | 100 | nd |
| H | 50 | 100 | 0 | 60 | 100 |
| K | nd | nd | nd | nd | 20 |
| L | 20 | 100 | 0 | 15 | nd |
| N | 50 | 100 | 0 | 100 | 50 |
| O | 70 | 100 | 0 | nd | nd |
| Lhca1 | 70 | 100 | 1 | 100 | 100 |
| Lhca2 | 50 | 100 | 30 | 100 | 100 |
| Lhca3 | 50 | 100 | 5 | 100 | 100 |
| Lhca4 | 70 | 100 | 20 | 100 | 120 |
| Rieske | 120 | 110 | 50 | nd | nd |
| ATPase (α + β) | 100 | 100 | 70 | nd | nd |
| ATPase (α + β)* | 20 | nd | nd | nd | nd |
| PSII core* | 70 | nd | nd | nd | nd |
| OEC* | 70 | nd | nd | nd | nd |
| LHCII* | 70 | nd | nd | nd | nd |
| PSI* | 40 | nd | nd | nd | nd |
- psae1-1 and ΔF data are from Haldrup et al. (2000), Pesaresi et al. (2002, 2003b), and Varotto et al. (2000a). psad1-1, psad2-1 and psad1-1 psad2-1 signals from three independent experiments were quantified using lumi analyst 3 (Boehringer Mannheim/Roche). Quantifications of proteins based on 2-D gel analysis are indicated by an asterisk (*). Relative values for the single mutant genotypes, reflecting the ratio of expression between the mutant and WT, were normalized on the basis of total chlorophyll content (see Table 2). Standard deviations were all within ±10%. Relative accumulation of proteins in the double mutant were not quantified after 2-D gel analysis (Figure 6d), because of non-linearity of silver staining. nd = data not determined.
Increased dosage of the PsaD2 gene can complement the psad1-1 mutation
The almost identical sequence of the two mature PSI-D proteins suggested that PSI-D1 and -D2 have redundant functions. An experiment was carried out to complement the psad1-1 mutation by introducing into the mutant background either the PsaD1 or the PsaD2 gene under transcriptional control of the 35S promoter. Increased dosage of either PsaD1 or PsaD2 in psad1-1 could, in fact, restore the WT phenotype. In particular, the effect of the psad1-1 mutation on effective quantum yield of PSII (WT, 0.76 ± 0.01; psad1-1, 0.52 ± 0.03; 35S::PsaD1 psad1-1, 0.76 ± 0.03; 35S::PsaD2 psad1-1, 0.77 ± 0.02), growth and leaf coloration could be fully reversed, demonstrating that PsaD1 and PsaD2 encode proteins with redundant functions. An explanation for the decrease of PSI-D in the psad1-1 mutant is that the promoter of the PsaD2 gene cannot drive sufficient expression of PsaD2 to compensate the absence of PsaD1 mRNA. In consequence, much less PsaD transcripts accumulate (see Figure 4a), and fewer PSI-D is synthesized (see Figure 5a).
PSI composition, accumulation of other thylakoid proteins and leaf pigments
The decrease in the amount of PSI-D in the psad1-1 mutant was paralleled by a decrease in the levels of several PSI polypeptides, particularly of PSI-F, -H and -L, as well as of the four LHCI proteins (Figure 5a; Table 1). In the double mutant psad1-1 psad2–1, with the exception of traces of PSI-F, no accumulation of PSI core proteins was detectable. LHCI proteins were present but to a much lesser extent than in the WT (Figure 5b; Table 1), which is consistent with previous studies of barley (Hordeum vulgare) mutants that accumulate LHCI but no PSI core proteins (Hoyer-Hansen et al., 1988; Nielsen et al., 1996).
To reveal whether also the accumulation of other thylakoid proteins was affected, 2-D PAGE analyses were performed (Figure 6) and the intensity of signals was quantified (Table 1). In psad1-1, the level of PSI proteins was decreased, whereas PSII dimers accumulated to higher levels than in the WT (PSIID band in Figure 6a; spot 12 in Figure 6b). This accumulation was at the cost of monomeric forms (spots 2–4 in Figure 6b), suggesting that such dimeric forms are more stable in the mutant, possibly because of an altered pigment composition (see below). In psad1-1 psad2-1 plants, no bands indicative for PSI complexes or for PSII dimers could be observed (Figure 6c,d), indicating a drastic reduction of PSII and pointing again to the absence of PSI complexes in this genotype.
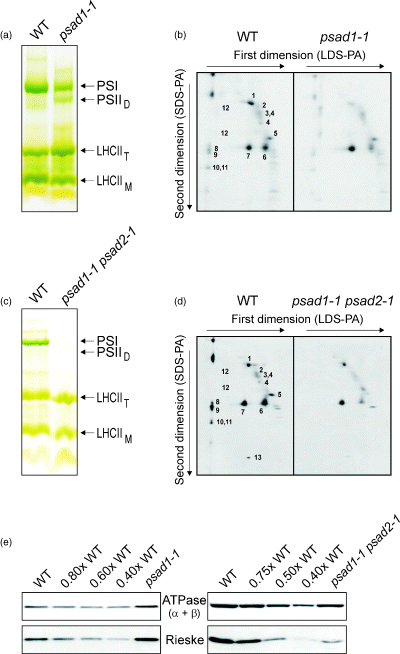
Protein composition of thylakoid membranes in mutant and WT plants.
(a, c) Thylakoid membranes corresponding to 30 µg of chlorophyll from WT and psad1-1 (grown in the greenhouse) (a), or corresponding to 20 µg of chlorophyll from WT and psad1-1 psad2-1 (grown in sterile culture) (c), were fractionated by electrophoresis on a LDS-PA gel. The bands were assigned to PSI, photosystem II dimers (PSIID), LHCII trimers (LHCIIT) and monomers (LHCIIM).
(b, d) Thylakoid proteins separated by LDS–PAGE (see (a) and (c)) were fractionated on a denaturing SDS-PA gel. Positions of WT thylakoid proteins previously identified by Western analyses with appropriate antibodies are indicated by numbers to the right of the corresponding spots: 1, α- and β-subunits of the ATPase complex; 2, D1–D2; 3, CP47; 4, CP43; 5, oxygen-evolving complex (OEC); 6, LHCII monomer; 7, LHCII trimer; 8, PSI-D; 9, PSI-F; 10, PSI-C; 11, PSI-H; 12, PSII dimers; 13, cyt b559. Considering the different leaf chlorophyll contents of the two genotypes, quantification (Table 1) was performed as described in the legend of Figure 5 and listed in Table 1. Relative accumulation of proteins in the double mutant was not quantified because of non-linearity of silver staining.
(e) Aliquots of thylakoid proteins corresponding to 5 µg of chlorophyll (psad1-1 and WT plants; left panel), or 40 µg total protein (psad1-1 psad2-1 and WT plants; right panel) were analysed as in Figure 5. Immunolabelling was performed with antibodies raised against the Rieske protein and the chloroplast ATPase (α- and β-subunits). Note that in the electrophoresis conditions used, the α- and β-subunits of the chloroplast ATPase could not be discriminated.
Immunoblot analyses of proteins separated by denaturing 1-D PAGE (Figure 6e) revealed that in psad1-1 thylakoids the accumulation of the Rieske protein, a subunit of the cyt b6/f complex, was increased. The concentration of the α- and β-subunits of the ATPase complex appeared unchanged in psad1-1 plants. This was in contrast to the results of 2-D PAGE analysis (Figure 6b), where a marked reduction of the α- and β-subunits of the chloroplast ATPase was detected. This discrepancy between the results of immunoblot analysis and 2-D gel analysis was reported before for another photosynthetic mutant (Varotto et al., 2002b), and was interpreted as the result of a decreased stability of mutant ATPase during 2-D PAGE. In contrast to the psad1-1 mutant, in the double mutant psad1-1 psad2-1 the accumulation of the Rieske protein and of the ATPase (α- and β-subunits) was decreased (Figure 6e).
Effects of PSI-D mutations on leaf pigment composition were studied by HPLC (Table 2). WT levels of leaf pigments were detected in the psad2-1 mutant. In contrast, in psad1-1 a disproportionate increase in the abundance of xanthophylls violaxanthin, antheraxanthin and zeaxanthin (VAZ) with respect to neoxanthin and lutein likely was observed. Chlorophyll content (Chl a + b) decreased by about 25% in psad1-1, which is consistent with the reduced amount of PSI and – to a less extent – of PSII complexes. The psad1-1 psad2-1 double mutation resulted in a dramatic drop of chlorophyll (Chl a + b) and β-carotene content. Together with the lower chlorophyll a/b ratio, the data highlight a drastic reduction of PSII and, in particular, of PSI complexes. The relative increase of the VAZ pool size support an extreme photosensitivity of the double mutant.
| Leaf pigments | psad1-1 | psad2-1 | WT | psad1-1 psad2-1 * | WT* |
|---|---|---|---|---|---|
| Nx | 36 ± 1 | 33 ± 1 | 33 ± 1 | 58 ± 1 | 40 ± 1 |
| VAZ | 80 ± 2 | 39 ± 0 | 36 ± 3 | 57 ± 3 | 24 ± 1 |
| Lu | 121 ± 2 | 116 ± 1 | 111 ± 2 | 239 ± 43 | 130 ± 1 |
| β-Car | 67 ± 1 | 76 ± 2 | 79 ± 3 | 25 ± 3 | 86 ± 1 |
| Chl a/b | 3.41 ± 0.03 | 3.46 ± 0.06 | 3.50 ± 0.06 | 2.38 ± 0.03 | 3.05 ± 0.02 |
| Chl a + b | 681 ± 121 | 883 ± 24 | 907 ± 115 | 223 ± 71 | 1797 ± 275 |
- Pigment content was determined by HPLC of three plants for each genotype. The carotenoid content is given in mmol per mol Chl (a + b), and the Chl content is expressed as nmol Chl (a + b) per g FW. Mean values ± SD are shown. Nx, neoxanthin; VAZ, xanthophyll cycle pigments (violaxanthin + antheraxanthin + zeaxanthin); Lu, lutein; β-Car, β-carotene. The asterisk indicates genotypes grown on MS medium supplemented with sucrose.
Photosynthetic electron flow
Photosynthetic electron flow was characterized by measuring parameters of chlorophyll fluorescence and of P700+ absorbance (Table 3). In psad1-1 plants, the maximum quantum yield of PSII (FV/FM) was reduced when compared to psad2-1 and WT plants. Moreover, the effective quantum yield of PSII (ΦII) was substantially decreased in psad1-1, but not in psad2-1 plants. Similarly, psad2-1 showed normal photochemical (qP) and non-photochemical (qN) quenching. In contrast, in psad1-1 the fraction of QA– the primary electron acceptor of PSII – present in the reduced state (1 − qP), was increased by about sixfold, indicating a partial block in the electron transfer steps downstream of QA. Also, qN slightly increased, supporting the conclusion that, as a consequence of the perturbation in photosynthetic electron flow, the thermal dissipation of excitation energy in the antenna of PSII was higher in psad1-1 than in WT. Only negligible alterations in the reduction rate of P700 were found in the two single-gene mutants: t1/2ox was not altered in psad2-1, but a pronounced delay in P700 oxidation was noted for psad1-1, suggesting an impairment of electron transfer from PSI to ferredoxin.
| Parameter | psad1-1 | psad2-1 | WT | psad1-1 psad2-1 * | WT* |
|---|---|---|---|---|---|
| F v/Fm | 0.77 ± 0.02 | 0.82 ± 0.01 | 0.83 ± 0.01 | 0.40 ± 0.07 | 0.80 ± 0.01 |
| Φ II | 0.52 ± 0.03 | 0.75 ± 0.02 | 0.76 ± 0.01 | 0.07 ± 0.01 | 0.76 ± 0.01 |
| 1 − qP | 0.30 ± 0.05 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.78 ± 0.03 | 0.03 ± 0.01 |
| qN | 0.23 ± 0.03 | 0.14 ± 0.01 | 0.17 ± 0.01 | 0.22 ± 0.08 | 0.05 ± 0.02 |
| qT | 0.03 ± 0.02 | 0.09 ± 0.03 | 0.12 ± 0.01 | nd | nd |
| t ½red (msec) | 63 ± 4 | 59 ± 2 | 57 ± 2 | nd | nd |
| t ½ox (sec) | 1.43 ± 0.16 | 0.54 ± 0.06 | 0.47 ± 0.06 | nd | nd |
- Mean values for five plants (±SD) are shown. t1/2red and t1/2ox were calculated from the recorded kinetics of P700 reduction and re-oxidation. For greenhouse-grown plants, an actinic light intensity of 65 µmol photons m−2 sec−1 was used to drive electron transport before measuring. Because of its high photosensitivity, the double mutant and WT control plants were grown under low-light conditions (15 µmol photons m−2 sec−1) on MS + sucrose (indicated by an asterisk (*)), and an actinic light intensity of 15 µmol photons m−2 sec−1 was applied to drive electron transport before measuring. nd: not determined.
State transition quenching were followed by measuring maximum PSII fluorescence signals in states 1 (FM1) and 2 (FM2), after irradiating plants at wavelengths that target PSII and PSI, respectively, and normalizing the values to the maximum PSII fluorescence of dark-adapted leaves (FM). In the WT, FM1/FM and FM2/FM differed significantly (0.84 ± 0.02 versus 0.75 ± 0.02), while in the psad1-1 mutant, the two values were essentially the same (0.76 ± 0.02 versus 0.74 ± 0.02). This corresponds to a reduction of 70% in state transition quenching (qT) in the psad1-1 mutant (Table 3), indicating a severe impairment in the re-distribution of excitation energy between the photosystems. In psad2-1, state transition quenching was similar to that in the WT (FM1/FM versus FM2/FM: 0.82 ± 0.02 versus 0.75 ± 0.01).
In psad1-1 psad2-1 plants, photosynthetic electron flow was severely perturbed (Table 3). FV/FM was substantially decreased, as expected from a drastic reduction in the amount of active PSII centres. Moreover, the strong reduction in ΦII was consistent with the high photosensitivity of the double mutant. The dramatically increased fraction of reduced QA (1 − qP) suggested that electron flow through PSII still occurred, while a block existed at a later electron transfer step. In general, psad1-1 psad2-1 exhibited parameters of chlorophyll fluorescence induction similar to the ones of the plastocyanin-less mutant pete1 pete2 (Weigel et al., 2003) and the Rieske knock-out mutant petc-2 (Maiwald et al., 2003), both exhibiting a block in the linear electron flow.
In summary, only psad1-1 seemed to be altered in photosynthetic electron flow. Interestingly, the chlorophyll fluorescence parameters of psad1-1, summarized above, were similar to those noted for psae1-1 (Pesaresi et al., 2002; Varotto et al., 2000a), supporting the view that a reduction in the amount of either of these stromal proteins – which interact directly (Klukas et al., 1999) – has similar effects on PSI function. Complete lack of PSI-D in psad1-1 psad2-1 abolished photosynthetic electron flow and resulted in a dramatically increased photosensitivity.
Expression of mRNAs for nucleus-encoded chloroplast proteins in psad1-1
To test for further effects of the absence of PSI-D on photosynthesis and other chloroplast functions, the expression of nuclear genes contributing chloroplast functions was determined at the mRNA level by DNA array analysis. This was carried out for the psad1-1 mutant, using a set of 3292 nuclear genes spotted on nylon membranes, 81% of which coding for chloroplast-targeted proteins, and the mRNA expression pattern observed was compared to that of the WT as described by Kurth et al. (2002), Pesaresi et al. (2003a) and Richly et al. (2003). Differential gene expression values (psad1-1 versus WT) were determined by comparing hybridization signals. Among the 1101 genes that resulted differentially expressed, 574 were up- and 527 downregulated. The differentially expressed genes could be grouped into 13 major functional categories, including photosynthesis (dark or light reaction), metabolism, secondary metabolism, transcription, protein synthesis, protein phosphorylation, protein modification and fate, sensing, signalling and communication, stress response and transport (Figure 7). In general, genes for secondary metabolism, protein modification and fate, as well as for stress response, were upregulated more than others, supporting the conclusion that the impaired function of the thylakoid electron transport chain has profound effects on plant functions not directly related to photosynthesis. Preferentially, genes for the light reaction of photosynthesis and transcription were downregulated, implying that a response of the plant exists, aiming to limit and/or compensate the perturbation in photosynthetic electron flow by downregulating both chloroplast- and nucleus-encoded photosynthetic genes.
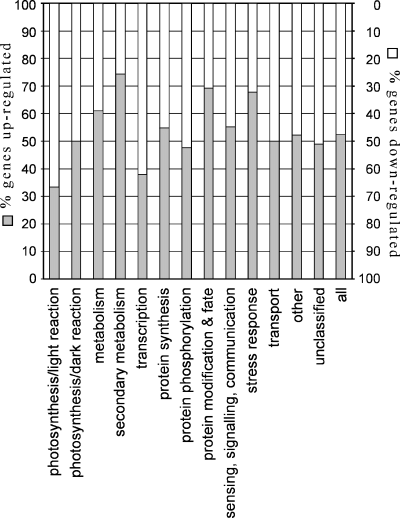
Effects of the psad1-1 mutation on the accumulation of nuclear transcripts encoding chloroplasts proteins.
The fraction of up- and downregulated genes in 13 major functional categories is shown. A complete list of significantly differentially expressed genes is available at GEO (http://www.ncbi.nlm.nih.gov/geo/), Accession number GSM11018.
Discussion
Photosystem I subunit D is a hydrophilic subunit of PSI exposed on the stromal face and known to interact with ferredoxin in both eukaryotes and cyanobacteria (Andersen et al., 1992; Merati and Zanetti, 1987; Zilber and Malkin, 1988). Together with PSI-C and -E, PSI-D forms a compact interconnected structure, the so-called stromal ridge (Jordan et al., 2001; Klukas et al., 1999; Kruip et al., 1997). The characterization of psad1-1 and psad1-1 psad2-1 indicates that PSI-D is necessary for the stability of PSI in Arabidopsis, whereas Synechocystis PSI without PSI-D can still reduce flavodoxin (Xu et al., 1994). In Arabidopsis, complete lack of the D subunit leads to seedling lethality under photoautotrophic conditions. The instability of Arabidopsis PSI without PSI-D can be explained either by degradation of the incomplete PSI complex or by downregulation of the synthesis of PSI subunits.
In comparison to its cyanobacterial counterpart, the mature PSI-D from higher plants has an N-terminal extension of 20–30 amino acid residues. The N-terminal extension of PSI–D stabilizes the interaction of PSI-C with the rest of the PSI core (Naver et al., 1995). Moreover, cross-linking experiments carried out in barley have suggested that PSI-D exerts its stabilizing effect via an interaction with PSI-H, an integral membrane protein located near PSI-I and PSI-L (Naver et al., 1995). This suggestion is supported by the analysis of transgenic Arabidopsis lines co-suppressed for PSI-H, in which PSI-C is not as tightly bound to the PSI core as in the WT (Naver et al., 1999). Our data are compatible with the hypothesis that PSI-D is in physical contact with PSI-H: in the psad1-1 and psad1-1 psad2-1 mutants, the decrease of PSI-D is, in fact, associated with a concomitant reduction in the level of PSI-H.
The changes of PSI polypeptide levels in psad1-1 are reminiscent of those observed in the psae1-1 mutant (Pesaresi et al., 2002; Varotto et al., 2000a) and in PsaF antisense plants (ΔF; Haldrup et al., 2000; listed in Table 1). Considering that PSI-D and -E form, together with PSI-C, the stromal face of PSI, available results indicate that alterations in the expression of any of the peripheral stromal subunits de-stabilizes the entire stromal domain of PSI. In this context, it is not a surprise that the mRNA expression pattern of 3292 nuclear genes, most of them coding for chloroplast proteins, is very similar for the psad1-1 and psae1-1 mutants (Maiwald et al., 2003). This supports the conclusion that a decreased accumulation of PSI-E or -D induce similar changes in the physiological state of the chloroplast. In this context, it is now clear that the coordinated partial loss of stromal polypeptides in the single-gene mutants psae1-1 (Pesaresi et al., 2002; Varotto et al., 2000a), or psad1-1 (this study), complicates the functional analysis of the role of individual stromal PSI polypeptides. As a starting point for future in planta studies of the function of the D- and E-subunits, double mutant lines like psad1 psad2 and psae1 psae2 should be transformed with mutagenized functionally inactive versions of the concerned proteins, which retain the ability to stabilize the stromal domain of PSI.
Unlike the psae1-1 mutant (Varotto et al., 2000a), psad1-1 plants show a reduction in the level of PSI-F and -N proteins (Table 1); a similar behaviour has been reported for ΔF lines (Haldrup et al., 2000), supporting the observation that PSI-N co-varies with PSI-F (Pesaresi et al., 2003b). PSI-H levels also decrease in psad1-1 (this study) and in psae1-1 (Pesaresi et al., 2002) but not in ΔF lines (Haldrup et al., 2000; Table 1), allowing to conclude that the effects of the absence of particular PSI polypeptides on the overall composition of PSI are not additive.
A marked decrease in the level of state transition quenching was noted in psad1-1. Whereas in psae1-1, a stable LHCII-PSI aggregate was responsible for the suppression of state transitions (Pesaresi et al., 2002), no such aggregate exists in psad1-1 (data not shown), pointing to a further difference in the consequences of the two mutations. Most likely, the suppression of state transitions in psad1-1 is associated with the decrease in the abundance of PSI-H, the presumed docking site of LHCII (Lunde et al., 2000).
The complementation of the psad1-1 mutant by increased dosage of the PsaD2 gene indicates that the two corresponding proteins are functionally redundant, and that the psad1-1 phenotype is the result of a dosage effect of the two genes encoding the D subunit. The presence of two functional Arabidopsis PsaD genes raises the question of why these and other PSI genes are duplicated in the nuclear genome of A. thaliana. In A. thaliana, all of the gene pairs for PSI-E, -H and -D appear to have originated from relatively recent segmental duplications (between 24 and 40 million years ago; Blanc et al., 2003), and the persistence of two functional genes for the same protein might have been under positive selection because it might have enabled responses of the same subunit to different signal transduction pathways. This would allow the plant cell to react more flexibly to environmental stimuli, as it seems to be the case for the PsaD gene family in Nicotiana sylvestris, where the PsaD1 and PsaD2 genes are differentially expressed during leaf development (Yamamoto et al., 1993).
Experimental procedures
Plant lines, propagation and growth measurement
The screen for photosynthetic mutants was performed on 4-week-old, greenhouse-grown plants. Methods for propagation of A. thaliana plants under greenhouse conditions and for the measurement of their growth have been described by Leister et al. (1999).
The psad1-1 mutant was identified among a collection of dSpm-mutagenized A. thaliana (ecotype Col-0) lines (SLAT collection; Tissier et al., 1999) on the basis of a decrease in the effective quantum yield of PSII. The mutant psad2-1 was identified in the SALK collection (http://signal.salk.edu/; Alonso et al., 2003) which is made up of flank-tagged ROK2 T-DNA lines (ecotype Col-0) by searching the insertion flanking database signal (http://signal.salk.edu/cgi-bin/tdnaexpress). The psad2-2 mutant was identified in the SALK collection by PCR-screening hierarchically pooled plant DNA.
For the analysis of psad1-1 psad2-2 double mutants, WT and double mutant plants were grown on Murashige and Skoog medium containing 2% Sucrose in a culture chamber on a 16-h-day period (PFD = 15 µmol photons m−2 sec−1) at 22°C.
Analysis of nucleic acids
Arabidopsis DNA was isolated as described by Varotto et al. (2000a). Total leaf RNA was extracted using the RNeasy Plant System (Qiagen, Hilden, Germany). RNA gel blot analysis was performed under stringent conditions (Sambrook et al., 1989) using a 32P-labelled PsaD1/PsaD2-specific probe. Signals were quantified by using a phosphorimager (Storm 860; Molecular Dynamics, Sunnyvale, CA, USA) and the program image quant for macintosh (version 1.2; Molecular Dynamics).
For RT-PCR analysis, first-strand cDNA was synthesized using the SuperScript Pre-amplification System (Invitrogen, Karlsruhe, Germany). Products obtained after PCR for 35 cycles with PsaD1/PsaD2-specific primers and control primers for the ACTIN1 gene in the same reactions were analysed on either 4.5% (w/v) polyacrylamide gel or 2% agarose gel. The products derived from transcripts of the two PsaD genes differ in size by 12 bp and were visualized by silver-stained polyacrylamide gel.
Sequence analysis
Sequence data were analysed with the Wisconsin Package Version 10.0, Genetics Computer Group, Madison, Wisconsin (GCG; Devereux et al., 1984) and amino acid sequences were aligned using clustalw (Thompson et al., 1994). Chloroplast import sequence predictions were carried out using the targetp program (Emanuelsson et al., 2000).
Complementation of the psad1-1 mutant
The PsaD2 gene was ligated into the plant expression vector pJAN33 under the control of the Cauliflower Mosaic Virus 35S promoter. As a control 35S::PsaD1 constructs were generated. Flowers of psad1-1 mutant plants were transformed according to Clough and Bent (1998). Plants were transferred into the greenhouse, and seeds were collected after 3 weeks. Thirty-two independent transgenic plants for each 35S::PsaD2 and 35S::PsaD1 were selected on the basis of their resistance to kanamycin. Successful complementation was confirmed by measurements of chlorophyll fluorescence and growth. In addition, presence and overexpression of the transgene in the complemented mutant plants was confirmed by PCR and RT-PCR.
Chlorophyll fluorescence measurements
The procedure used to identify mutants that showed a change in ΦII, the effective quantum yield of PSII (ΦII = (FM′ − F0′)/FM′), has been described before by Varotto et al. (2000a,b). In vivo Chl a fluorescence of single leaves was measured using PAM 101/103 (Walz, Effeltrich, Germany) as described by Varotto et al. (2000a). To determine the maximum fluorescence (FM) and the (FM − F0)/FM ratio (=FV/FM) 0.8-sec pulses of white light (6000 µmol photons m−2 sec−1) were used. A 15-min illumination with actinic light (65 µmol photons m−2 sec−1 for single-gene mutants and WT grown on soil; 15 µmol photons m−2 sec−1 for double mutants and WT grown in sterile culture) was used to drive electron transport between PSII and PSI before measuring ΦII, qN (non-photochemical quenching = 1 − (FM′ − F0′)/(FM − F0)), and qP (photochemical quenching = (FM′ − FS)/(FM′ − F0)).
State transitions were measured with a PAM 101/103 fluorometer (Walz, Effeltrich, Germany). After a 30-min incubation in the dark, the maximum fluorescence (FM) of leaves was measured by using a saturating light pulse (0.8 sec, 6000 µmol photons m−2 sec−1). Leaves were subsequently illuminated for 20 min with blue light (80 µmol photons m−2 sec−1) from a Schott KL-1500 lamp equipped with a Walz BG39 filter. The maximum fluorescence in state 2 (FM2), was then measured. Next, state 1 was induced by switching to far-red light (Walz 102-FR; peak emission 730 nm, 90 µmol photons m−2 sec−1), and FM1 was recorded 20 min later. qT was calculated according to the equation: qT = (FM1 − FM2)/FM2 (Jensen et al., 2000).
A dual-wavelength pulse-modulation system (ED-P700DW; Walz, Effeltrich, Germany) was used to record changes in the absorbance of P700+. Leaves were illuminated with background far-red light, and immediately after full oxidation of P700, a saturating blue-light pulse (50 msec) was applied (XMT-103; Walz, Effeltrich, Germany) to reduce P700+. t1/2red and t1/2ox were calculated from the recorded kinetics of P700 reduction and re-oxidation.
Western and 2-D PAGE analysis of proteins
Leaves from 4-week-old plants were harvested in the middle of the light period. Thylakoids were prepared as described by Bassi et al. (1985), and total proteins were isolated as described by Jensen et al. (2000). For 1-D PAGE analysis, protein amounts equivalent to 5 µg of chlorophyll (for psad single-gene mutants, see Figure 5a), or 40 µg of total proteins (for psad1 psad2, see Figure 5b) were loaded for each genotype. Decreasing amounts of WT proteins were loaded in parallel lanes (0.8× WT, 0.6× WT and 0.4× WT for psad1-1 and psad2-1; 0.75× WT, 0.5× WT and 0.25× WT for psad1-1 psad2-1). For immunoblot analyses, proteins were transferred to Immobilon-P membranes (Millipore, Eschborn, Germany) and incubated with antibodies specific for individual polypeptides of PSI and LHCI. Signals were detected using the Enhanced Chemiluminescence Western Blotting Kit (Amersham Biosciences, Sunnyvale, CA, USA) and quantified using the lumi analyst 3.0 (Boehringer Mannheim/Roche, Basel, Switzerland).
For 2-D gel analysis of WT and psad1-1 plants, thylakoid membrane samples corresponding to 30 µg of chlorophyll were first fractionated on non-denaturing lithium dodecyl sulfate polyacrylamide (LDS-PA) gradient gels, and in the second dimension in a denaturing SDS-PA gradient (10–16%) gel, as described by Pesaresi et al. (2001, 2002). Proteins were visualized by Coomassie staining, and densitometric analyses of the protein gels were performed by using the lumi analyst 3.0 (Boehringer Mannheim/Roche). Because of the limited amount of psad1-1 psad2-1 samples, 2-D gel analysis was performed by using thylakoid membrane samples corresponding to 20 µg of chlorophyll. Proteins were visualized by silver staining.
Oligonucleotides (5′−3′ orientation) and isolation of insertion-flanking sequences
The regions flanking dSpm insertions were isolated by inverse PCR as described by Tissier et al. (1999). PsaD1- or PsaD2-specific sequences were amplified from WT and mutant plants using the primer pairs PsaD1-1s (ATGGCAACTCAAGCCGCCGG) and PsaD1-999as (TATGGTTTTGGATCGGAGACT) or PsaD2-226s (GTGGCATGTGGGAAACATATCC) and PsaD2-185as (CACGTAAAATTCCTCTACTTGTGCT); to amplify regions flanking the dSpm or pROK2 insertions, the primer pairs dspm1 and dspm11 (Tissier et al., 1999), LBa1 (http://signal.salk.edu/tdna_FAQs.html) and pROK2/pBIN19-460as (GTGCCCAGTCATAGCCGAATAGC) were used.
Primers that anneal to both PsaD genes were used for amplification of the PsaD1/PsaD2-specific Northern probe (PsaD1/2-22/22s, ATCTTCA(A/G)C(C/T)CCGCCATAACAACC; and PsaD1/2-441/429-as, CACTCTGTAAAACTGGTAAGTGATC); and for RT-PCR (PsaD1/2-22/22s and PsaD1/2-230/218as, GGTGTGTTTGGGTCTAGCTGCGG).
The ACTIN1-specific primers were ACTIN1-33s (TGCGACAATGGAACTGGAATG), ACTIN1-994as (GGATAGCATGTGGAAGTGCATACC).
Pigment analysis
Pigments were analysed by reversed-phase HPLC as described previously by Färber et al. (1997). For pigment extraction, leaf discs were frozen in liquid nitrogen and disrupted in a mortar in the presence of acetone. After a short centrifugation, pigment extracts were filtered through a 0.2-µm membrane filter and either used directly for HPLC analysis or stored for up to 2 days at −20°C.
Expression profiling
The 3292-GST array, representing genes known or predicted to code for proteins featuring a chloroplast transit peptide (cTP), has been described previously by Richly et al. (2003). At least three experiments with different filters and independent cDNA probes derived from plant material corresponding to pools of at least 50 individuals were performed for each condition or genotype tested, thus minimizing variation between individual plants, filters or probes. cDNA probes were synthesized by using as primer a mixture of oligonucleotides matching the 3292 genes in antisense orientation, and hybridized to the GST array as described by Kurth et al. (2002) and Richly et al. (2003). Images were read using the Storm phosphorimager (Molecular Dynamics).
Hybridization images were imported into the arrayvision program (version 6; Imaging Research Inc.), where artefacts were removed, background correction was performed and resulting values were normalized with reference to intensity of all spots on the array (Kurth et al., 2002; Richly et al., 2003). In the next step, those data were imported into the arraystat program (version 1.0 Rev. 2.0; Imaging Research Inc.) and a z-test (nominal α set to 0.05) was performed to identify statistically significant differential expression values as described by Pesaresi et al. (2003a). Only differential expression values fulfilling the criteria of the z-test are listed in the Supplementary Material.
Accession of mRNA expression profiling data
Primary data of the mRNA profile of the psad1-1 mutant are available at the Gene Expression Omnibus (GEO) site (http://www.ncbi.nlm.nih.gov/geo/), Accession number GSM11018.
Acknowledgements
We thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants, as well as the Sainsbury Laboratory for making dSpm-tagged lines publicly available. Grateful acknowledgements are extended to Paul Hardy for critical reading of the manuscript. This work was supported by the European Community's Human Potential Programme (contract no. HPRN-CT-2002-00248 (PSI-CO)), and by the Deutsche Forschungsgemeinschaft (DL 1265-1 and -8, and III GK – GRK 306/1).
Supplementary Material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/TPJ/TPJ2011/TPJ2011sm.htm
Table S1 List of significantly differentially expressed genes in psad1-1 (compared to the WT) and their classification




