Effects of altered α- and β-branch carotenoid biosynthesis on photoprotection and whole-plant acclimation of Arabidopsis to photo-oxidative stress
ABSTRACT
Functions of α- and β-branch carotenoids in whole-plant acclimation to photo-oxidative stress were studied in Arabidopsis thaliana wild-type (wt) and carotenoid mutants, lutein deficient (lut2, lut5), non-photochemical quenching1 (npq1) and suppressor of zeaxanthin-less1 (szl1) npq1 double mutant. Photo-oxidative stress was applied by exposing plants to sunflecks. The sunflecks caused reduction of chlorophyll content in all plants, but more severely in those having high α- to β-branch carotenoid composition (α/β-ratio) (lut5, szl1npq1). While this did not alter carotenoid composition in wt or lut2, which accumulates only β-branch carotenoids, increased xanthophyll levels were found in the mutants with high α/β-ratios (lut5, szl1npq1) or without xanthophyll-cycle operation (npq1, szl1npq1). The PsbS protein content increased in all sunfleck plants but lut2. These changes were accompanied by no change (npq1, szl1npq1) or enhanced capacity (wt, lut5) of NPQ. Leaf mass per area increased in lut2, but decreased in wt and lut5 that showed increased NPQ. The sunflecks decelerated primary root growth in wt and npq1 having normal α/β-ratios, but suppressed lateral root formation in lut5 and szl1npq1 having high α/β-ratios. The results highlight the importance of proper regulation of the α- and β-branch carotenoid pathways for whole-plant acclimation, not only leaf photoprotection, under photo-oxidative stress.
INTRODUCTION
Carotenoids play important roles in photosynthetic membranes (thylakoids), such as folding and stabilization of pigment-binding proteins, regulation of light harvesting and photoprotection (Yamamoto & Bassi 1996). Typically, leaves accumulate β-carotene (β-C), lutein (L), violaxanthin (V) and neoxanthin (N). In addition, part of V is converted to antheraxanthin (A) and zeaxanthin (Z) by the enzyme V de-epoxidase, which is activated in excess light conditions. Upon return to low light (LL), that is, without concurrent activity of V de-epoxidase, epoxidation by Z epoxidase restores the V pool in the so-called ‘xanthophyll cycle’ (or V cycle). The Z enhances scavenging of reactive oxygen species (ROS) and thermal energy dissipation [commonly measured as non-photochemical quenching (NPQ) of fluorescence] in the thylakoids, thus playing central roles in protection against photo-oxidative damage (Havaux & Niyogi 1999; Müller, Li & Niyogi 2001; Havaux, Dall'Osto & Bassi 2007).
The leaf carotenoid composition has been conserved throughout evolution of higher plants, which suggests distinct functions for each carotenoid species. Interestingly, two additional carotenoids, α-carotene (α-C) and L epoxide (Lx), can accumulate in large amounts in leaves of certain taxa, especially in shade environments (García-Plazaola, Matsubara & Osmond 2007; Matsubara et al. 2009) where they replace, respectively, β-C and L or the V-cycle pigments (V, A and Z) in pigment-protein complexes of photosystem II (PSII) and photosystem I (PSI) (Matsubara et al. 2007). Based on its predominant occurrence in shade leaves (Krause et al. 2001; Matsubara et al. 2009), an adaptive advantage of having α-C under LL conditions has been proposed. Accumulation of Lx can result in operation of a second light-dependent xanthophyll cycle between Lx and L (Lx cycle), which presumably is catalyzed by the enzymes V de-epoxidase and Z (or L) epoxidase (García-Plazaola et al. 2007). Similar to V-to-Z de-epoxidation, Lx-to-L de-epoxidation results in faster induction of NPQ upon illumination, suggesting involvement of both Z and L in regulation of light harvesting and NPQ (Matsubara et al. 2008, 2011; Esteban et al. 2010; Förster, Pogson & Osmond 2011).
Carotenoids are synthesized in the carotenoid biosynthetic pathway (DellaPenna & Pogson 2006) which is split into a β-branch (with two β-ionone rings) and an α-branch (with a β-ionone and an ε-ionone ring) by the activities of lycopene β- and ε-cyclase (Fig. 1). The first product of the β-branch, β-C, binds to the photosynthetic core complexes. The β-ring hydroxylation of β-C produces Z, which is followed by its epoxidation to A and V by Z epoxidase and further modification to N. In the α-branch, ε-cyclase and ε-hydroxylases are also needed to produce L, the most abundant carotenoid in photosynthetic tissues. In many plants α-C and Lx, the precursor and the epoxidation product of L, respectively, do not accumulate in leaves in significant amounts. In species in which these additional α-branch carotenoids occur, their pronounced accumulation in shade leaves leads to a high α-branch to β-branch carotenoid composition (α/β-ratio) that is reversed in sun leaves (Matsubara et al. 2009). The β-branch xanthophylls are essential for photoprotection (Dall'Osto et al. 2007b), but they cannot fully functionally replace the α-branch xanthophyll L, as shown in mutants of Arabidopsis thaliana (L.) Heynh. with altered carotenoid compositions (Dall'Osto et al. 2007b; Kalituho, Rech & Jahns 2007).

Two branches of the carotenoid biosynthetic pathway in higher plants. The enzyme activities impaired in lut2, lut5, szl1npq1 and npq1 mutants are also indicated.
Compared with the increasing knowledge about the roles of different carotenoids in photoprotection revealed by studies using Arabidopsis carotenoid mutants and combining genetics, biochemistry, biophysics and physiology (e.g. Niyogi, Grossman & Björkman 1998; Havaux & Niyogi 1999; Dall'Osto et al. 2007b; Havaux et al. 2007; Johnson et al. 2007, 2009; Kalituho et al. 2007; Li et al. 2009; Cazzaniga et al. 2012), little attention has been paid to the importance of carotenoid biosynthesis in whole-plant acclimation to photo-oxidative stress. Leaf acclimation to irradiance involves marked modifications of leaf biochemistry as well as leaf thickness, structure and morphology (Evans & Poorter 2001; Terashima et al. 2006). Plant stress acclimation can also entail resource allocation between different organs or between young and old tissues of the same organ. A good example for such acclimation is enhanced (or less impaired) growth of roots compared with leaves in the face of nutrient limitation, resulting in altered root-shoot ratios (Poorter et al. 2012), or the well-known symptom of shade avoidance manifested by strong elongation of stems and petioles (Franklin 2008). Because changes in carotenoid composition are a hallmark of leaf photoacclimation, and furthermore, the β-branch pathway is upstream of the biosynthesis of abscisic acid (ABA) and strigolactones (SL), two important plant hormones that regulate shoot and root growth (Koornneef, Bentsink & Hilhorst 2002; Horvath et al. 2003; Kozuka et al. 2005; De Smet et al. 2006; Gomez-Roldan et al. 2008; Umehara et al. 2008; Koltai 2011), the carotenoid biosynthesis may play a key role in plant acclimation to light stress on molecular as well as leaf and whole-plant levels.
Thus, in order to study the impact of altered α- and β-branch carotenoid composition and biosynthesis on photoprotection and whole-plant stress acclimation, we examined the responses of four different Arabidopsis carotenoid mutants, lut2, lut5, npq1 and szl1npq1 (all in the Columbia-0 background), to photo-oxidative stress. The lut2 lacks ε-cyclase (Fig. 1), which blocks the α-branch pathway and results in strong accumulation of the V-cycle pigments replacing L (Pogson et al. 1996). The lut5 does not have a cytochrome P450 that catalyzes β-ring hydroxylation of α-C, leading to pronounced accumulation of α-C at the expense of β-C and the V-cycle pigments (Fiore et al. 2006; Kim & DellaPenna 2006). The npq1 is deficient in V de-epoxidase and thus cannot employ Z-dependent photoprotective mechanisms under high-light (HL) stress (Niyogi et al. 1998). The szl1npq1 is a double mutant which, besides lacking Z, has reduced amounts of β-branch carotenoids and remarkably high levels of L and α-C due to a point mutation in β-cyclase (Li et al. 2009). The pronounced accumulation of L in szl1npq1 partly rescues the NPQ deficiency phenotype of npq1, confirming the ability of L to compensate, at least in part, for the function of Z in regulating light harvesting and NPQ (Li et al. 2009). It is important to note that none of these four Arabidopsis carotenoid mutants shows an altered or visibly impaired growth phenotype under non-stressful or LL conditions, indicating that their hormone synthesis is not strongly affected under these conditions. This allows us to investigate roles of carotenoids and their biosynthesis in stress-induced acclimatory responses.
Photo-oxidative stress was applied by exposing the LL-grown Arabidopsis plants (60 µmol photons m−2 s−1) to repetitive short ‘sunflecks’ (1000 µmol photons m−2 s−1, lasting ca. 20 s) every 6 min under the background illumination of 60 µmol photons m−2 s−1 during the daytime. In highly variable natural light environments, sunflecks can expose shade-acclimated leaves to sudden and strong increase in irradiance. While they provide the major fraction of light energy available in shade environments and may thus improve carbon gain in some plants (Pearcy 1990), they can also be a source of photo-oxidative stress due to temporal excess of light (Logan et al. 1997; Watling et al. 1997; Adams et al. 1999). In Arabidopsis, short and strong sunflecks primarily induce photoprotective acclimation, such as decrease in leaf Chl content, up-regulation of NPQ, increase in the pool size of the V-cycle pigments and enhanced activity of superoxide dismutase (Alter et al. 2012). Hence, sunfleck treatments can be used to study photo-oxidative stress in Arabidopsis plants without concomitant up-regulation of photosynthesis, carbon gain and growth as is often found in leaves and plants under continuous HL.
The roles of α- and β-branch carotenoids in photoprotective acclimation were studied by monitoring changes in pigment composition, PSII efficiency and NPQ in fully expanded, mature leaves of Arabidopsis wild-type (wt) Columbia-0 and the four carotenoid mutants during the sunfleck treatment over 7 d. The impact of altered α- and β-branch carotenoid biosynthesis on stress responses was also evaluated from a whole-plant perspective by analysing leaf and root growth as well as seed production under the same conditions.
MATERIALS AND METHODS
Plant material and growth conditions
The four mutants of A. thaliana (L.) Heynh. used in this study are all in the Columbia-0 background: lut2 (Pogson et al. 1996), lut5 (Fiore et al. 2006; Kim & DellaPenna 2006), npq1 (Niyogi et al. 1998) and szl1npq1 (Li et al. 2009). The carotenogenic enzymes impaired in the mutants are shown in Fig. 1. Seeds were stratified in moist soil at 4 °C in the dark for 4 to 5 d before transferring to a climate chamber at 23 °C/18 °C (day/night) air temperature, 60% constant relative air humidity and a 12 h/12 h photoperiod. Photosynthetically active radiation of approx. 60 µmol photons m−2 s−1 was provided by Osram Fluora Typ L36 W/77 fluorescent tubes (München, Germany) during the day period. Plants were grown in pots (7 × 7 × 8 cm, one plant per pot) containing soil (ED 73 Einheitserde; Balster Einheitserdewerk, Fröndenberg, Germany).
After 3 or 4 weeks from germination, plants of each genotype were divided into two populations (day 0): the first population (‘control’ plants) was kept in the growth light condition of 60 µmol photons m−2 s−1 while the second population (‘sunfleck’ plants) was transferred to a fluctuating light condition where 20 s pulses of HL (1000 µmol photons m−2 s−1) were applied with three halogen lamps (Haloline; Osram) every 6 min under the background light intensity of 60 µmol photons m−2 s−1 during the day. The sunfleck treatment was performed by using an automated set-up described elsewhere (Alter et al. 2012).
Pigment analysis
Following 7 d of the control or sunfleck treatment (day 7), leaf discs (50 mm2) were removed from mature leaves that had been fully expanded prior to the starting of the light treatments. All samples were collected in a dark-adapted state at the end of the night period and immediately frozen in liquid nitrogen. They were stored at −80 °C until pigment extraction. For each treatment, five replicates were analysed for wt plants and four for the mutants. Leaf discs were ground in a small amount of liquid nitrogen by using a mortar and a pestle. Pigments were extracted twice in chilled acetone and the final volume of the extract was adjusted to 1 mL. Then the extracts were centrifuged at 15 700 ×g for 5 min and syringe filtered prior to the high-performance liquid chromatography (HPLC) analysis.
Photosynthetic pigments were separated by an Allsphere ODS-1 column (5 µm, Alltech Associates, Inc., Deerfield, IL, USA) by using the solvents and protocols modified from Gilmore & Yamamoto (1991). Pigments were identified by retention times and absorption spectra monitored by a Waters 996 photodiode array detector (Waters Corporation, Milford, MA, USA). Peak area was integrated at 440 nm and data were analysed with Waters Empower software.
Chlorophyll a fluorescence analysis
Chlorophyll a fluorescence measurements were performed on mature leaves of dark-adapted plants (wt, n = 8; mutants, n = 4) at the end of the night period of days 0, 1, 3 and 7 by using a portable fluorometer (PAM-2100; Walz, Effeltrich, Germany). Following a measurement of the maximal PSII efficiency, Fv/Fm = (Fm – Fo) / Fm, leaves were exposed to actinic light of 600–800 µmol photons m−2 s−1 for 5 min followed by 2 min of darkness. We noticed that the intensity of the built-in actinic light of PAM-2100 slowly increased from about 600 to 800 µmol photons m−2 s−1 during the experiment. The maximal intensities of the actinic light reached during the 5 min light induction were: 750–800 µmol photons m−2 s−1 for wt, lut2 and lut5, and ca. 700 µmol photons m−2 s−1 for szl1npq1 and npq1. These intensities were saturating for the LL grown plants used in this study. NPQ = (Fm – Fm')/Fm' was determined by applying a train of saturation pulses every 30 s during actinic light illumination and subsequent dark relaxation. The Fm value of the initial Fv/Fm measurement was used for NPQ calculation for each plant. Fluorescence nomenclature is according to van Kooten & Snel (1990).
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis
For analysis of PsbS protein content, leaf discs (50 mm2) were taken from mature leaves of control and sunfleck plants of each genotype on day 7 and immediately frozen in liquid nitrogen. Samples were stored at −80 °C until protein extraction.
Discs were homogenized in an extraction buffer containing 50 mm Tris-HCl (pH 7.6), 7 m urea, 5% SDS, 5% β-mercaptoethanol and centrifuged at 15 700 ×g for 10 min at 4 °C. An aliquot of the supernatant, containing 2 µg Chl, was loaded on SDS-PAGE. Three replicates were analysed for each genotype and treatment. Together with the samples from the experiments, a reference sample was also loaded on every SDS-PAGE gel. Proteins were blotted onto a nitrocellulose membrane (Roche, Indianapolis, IN, USA) and the PsbS protein was detected with PsbS antiserum (Bonente et al. 2008) and alkaline phosphatase-conjugated secondary antibody (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Band intensity of each sample was quantified by using the ImageJ software (http://rsbweb.nih.gov/ij) and normalized to the intensity of the reference sample of the same membrane.
Analysis of leaf growth with GROWSCREEN FLUORO
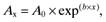 (1)
(1)Analysis of root growth with GROWSCREEN-ROOT
Root system architecture was analysed by using GROWSCREEN-ROOT (Nagel et al. 2009). For the analysis of roots, seeds were surface sterilized with sodium hypochlorite solution and sown on sterile agarose (1%, w/w) in Petri dishes (120 × 120 × 17 mm). The medium contained 1/3 modified Hoagland solution [stock solution: 1 m KNO3, 1 m Ca(NO3)2, 1 m MgSO4, 1 m KH2PO4, trace elements]. In the experiment to examine the effect of osmotic shock on root system architecture, 100 mm sorbitol was also added to the medium. Seeds were pushed slightly into the agarose through holes (diameter 2 mm, four holes for four seeds per Petri dish) on the upper side of the closed Petri dishes sealed with fabric tape (Micropore, 3 M Health Care, Neuss, Germany) and placed vertically in a plastic box to avoid light exposure of roots. During germination, the holes were covered with a film (Parafilm, Pechiney Plastic Packaging, Menasha, WI, USA) to keep the seeds moist. This way, the shoot can grow out of the Petri dish (and the plastic box) through the hole, while roots grow in the agarose in the dark (Nagel, Schurr & Walter 2006). The seeds had been stratified in the Petri dishes at 4 °C in the dark for 5 d before they were placed vertically in the plastic box and transferred to the climate chamber with the conditions described previously.
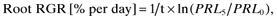 (2)
(2) (3)
(3)The number of replicates differed between genotypes and treatments (11–13 for wt; 6–8 for lut2; 9–19 for lut5; 7–13 for npq1; and 7–17 for szl1npq1) because of the susceptibility of sorbitol-containing agarose medium (used for the osmotic stress experiment) to fungi contamination in the climate chamber.
Determination of leaf dry mass
On the last day of the leaf growth analysis (day 10), the above-ground part of plants were harvested for each genotype and treatment (n = 12 for wt and n = 6 for mutants). Samples were dried in an oven at 70 °C until a constant mass was reached. The dry weight was measured by using an analytical balance (Explorer, Ohaus, Pine Brook, NJ, USA). Leaf mass per area (g per m2) was calculated from the dry weight and the projected total leaf area determined for each plant.
Seed harvesting
Following the experiment of leaf growth analysis, some plants were left under the control and sunfleck conditions. After bolting, inflorescence stems were covered with white paper bags so that only rosette leaves, but not inflorescence and cauline leaves, were directly exposed to the sunflecks. After ca. 20 more weeks to complete flowering and senescence under the corresponding light conditions, plants were moved to LL (20 µmol photons m−2 s−1) in the same climate chamber and watering was stopped. When they were completely dried, seeds were harvested for each plant separately and weighed with an analytical balance (Explorer, Ohaus). The number of plants used for seed harvesting was: 6 and 5 (control and sunfleck, respectively) for wt, 6 and 3 for lut2, 10 and 4 for lut5, 7 and 7 for szl1npq1, and 7 and 7 for npq1. Some plants of lut2 and lut5 prematurely died under the sunfleck condition so that fewer plants were available for seed harvesting.
Statistical data analysis
All data were statistically tested by using SigmaStat (SYSTAT Software GmbH, Erkrath, Germany). A two-way analysis of variance (Tukey test) was used to test differences among genotypes and treatments.
RESULTS
Pigment composition
Four-week-old plants of wt, lut2, lut5, szl1npq1 and npq1 were placed under the control or sunfleck condition for 7 d. Figure 2 shows the carotenoid content (relative to Chl) in dark-adapted leaves taken at the end of the night on day 7; these leaves were fully expanded when the treatments were started on day 0. The pigment profiles previously described for these mutants were confirmed in the control plants: lack of L together with increased levels of β-C and V + A + Z in lut2 (Pogson et al. 1996); pronounced accumulation of α-C at the expense of all xanthophylls and β-C (with more than twice as much α-C as β-C, Fig. 2b) in lut5 (Fiore et al. 2006; Kim & DellaPenna 2006); and over-accumulation of L accompanied by appearance of α-C and strong reduction of all β-branch carotenoids in szl1npq1 (Li et al. 2009). Under the control condition, the carotenoid composition of npq1 was very similar to that of wt. Only lut2 leaves contained A (but not Z) in the control condition even after a 12 h dark period, as indicated by the de-epoxidation state (DPS) of the V-cycle pigments calculated as (A + Z)/(V + A + Z) (Fig. 2d).
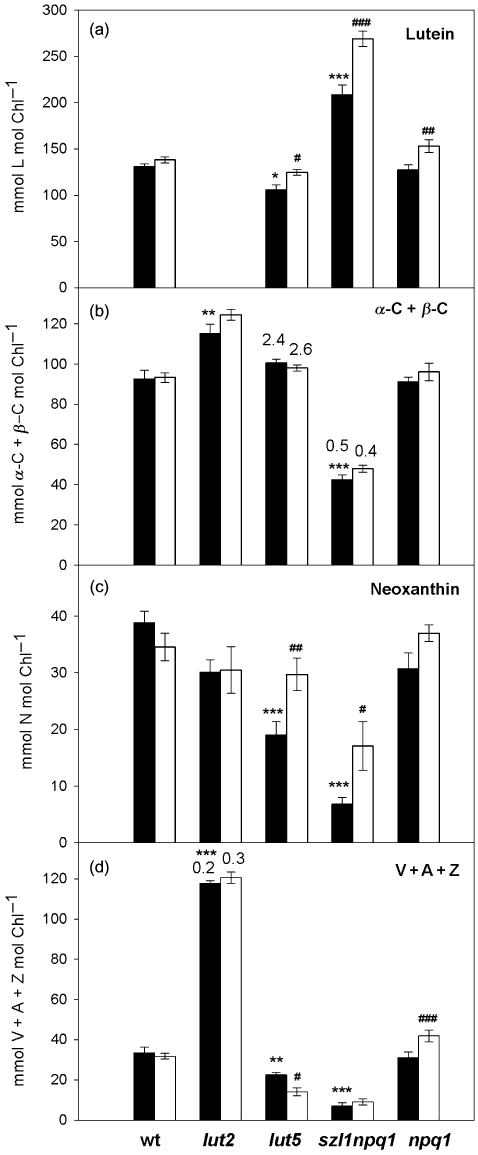
Carotenoid composition in dark-adapted leaves of wild type, lut2, lut5, szl1npq1 and npq1 plants. Samples were harvested at the end of the night period after 7 d of exposure to the growth light environment (control; ca. 60 µmol photons m−2 s−1; closed bars) or the growth light with high-light pulses (sunfleck; ca. 1000 µmol photons m−2 s−1 for 20 s every 6 min; open bars). (a) Lutein. (b) Sum of α-C and β-C. Only lut5 and szl1npq1 accumulated more than a trace of α-C in leaves. The numbers above the bars of lut5 and szl1npq1 indicate the ratio of α-C to β-C. (c) Neoxanthin. (d) Sum of the V-cycle pigments (V + A + Z). None of the samples contained Z. Antheraxanthin was detected only in lut2 plants, signifying sustained de-epoxidation of the V-cycle pigments; the numbers above the bars of lut2 show the de-epoxidation state defined here as (A + Z)/(V + A + Z). Carotenoid contents are given on a Chl basis (mmol mol Chl−1). Data are means ± SE (n = 5 for wild type and n = 4 for the mutants). Significant differences (Tukey test of the two-way anova) between the control and sunfleck treatments are marked with ‘#’ for each plant; significant differences between the control plants of wild type and the mutants are marked with ‘*’ (*P ≤ 0.05, #P ≤ 0.05; **P ≤ 0.01, ##P ≤ 0.01; ***P ≤ 0.001, ###P ≤ 0.001).
The sunfleck treatment did not significantly alter the carotenoid composition in mature leaves of wt and lut2 plants; only the extent of dark-sustained DPS increased in lut2 from 0.2 to 0.3 (Fig. 2d), which was attributable to A and not Z. In all other mutants, levels of L significantly increased under the sunfleck condition (Fig. 2a). This increase in L was accompanied by an increase in N, although the change in npq1 was not statistically significant (Fig. 2c). Generally, carotenes changed little in response to the sunfleck treatment (Fig. 2b). An increase in the V-cycle pigments was found in npq1 (+35%) while the content of V + A + Z decreased in lut5 (−37%) under the same condition (Fig. 2d). Following several hours of sunfleck exposure, a trace of Z and somewhat increased amounts of A were detected in leaves of lut2 (DPS ca. 0.5, data not shown), indicating operation of the V cycle under the sunfleck condition. The V-cycle operation was not seen in other plants under the same condition.
The Chl a and Chl b contents per unit leaf area uniformly declined in mature leaves of the five genotypes during the sunfleck treatment (Fig. 3); the decrease was statistically significant for both Chl a and Chl b in all plants but lut2, which had lower Chl contents than wt even under the control condition. Following the 7 d sunfleck treatment, the largest reduction in the total Chl content was found in lut5 and szl1npq1; in these plants Chl a declined by nearly 25% and Chl b by 30 to 35% compared with the levels found in the corresponding plants in the control condition. The reduction in the Chl content did not significantly alter the ratio of Chl a to Chl b (Chl a/b), although the values tended to increase in the mutants under the sunfleck condition. The Chl a/b values measured in the control plants on day 7 were 4.3 (±0.2), 3.9 (±0.4), 4.3 (±0.2), 4.5 (±0.2) and 4.5 (±0.3) for wt, lut2, lut5, szl1npq1 and npq1, respectively. The corresponding values in the sunfleck plants (in the same order) were 4.0 (±0.1), 4.2 (±0.4), 4.6 (±0.2), 5.3 (±0.5) and 4.9 (±0.2).
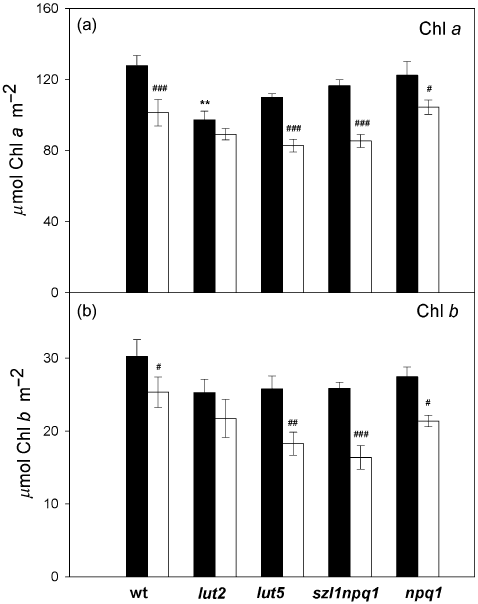
Chlorophyll content of dark-adapted leaves of wild type and the mutants on day 7. Closed bars, control; open bars, sunfleck. For descriptions of the treatments, see legend to Fig. 1. (a) Chlorophyll a. (b) Chlorophyll b. Chlorophyll concentrations are expressed on a leaf area basis (µmol m−2). Data are means ± SE (n = 5 for wild type and n = 4 for the mutants). Significant differences (Tukey test of the two-way anova) between the two treatments are marked with ‘#’ for each plant; significant differences between the control plants of wild type and the mutants are marked with ‘*’ (#P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001, **P ≤ 0.01).
The maximal PSII efficiency and NPQ
The maximal PSII efficiency (Fv/Fm) was measured in mature leaves at the end of the night during the 7 d experiment (Fig. 4). None of the plants exhibited a significant decline in Fv/Fm in response to the sunfleck treatment; the values remained nearly unchanged under both conditions. The szl1npq1 always had lower Fv/Fm of around 0.72, indicating constitutively reduced PSII efficiency.
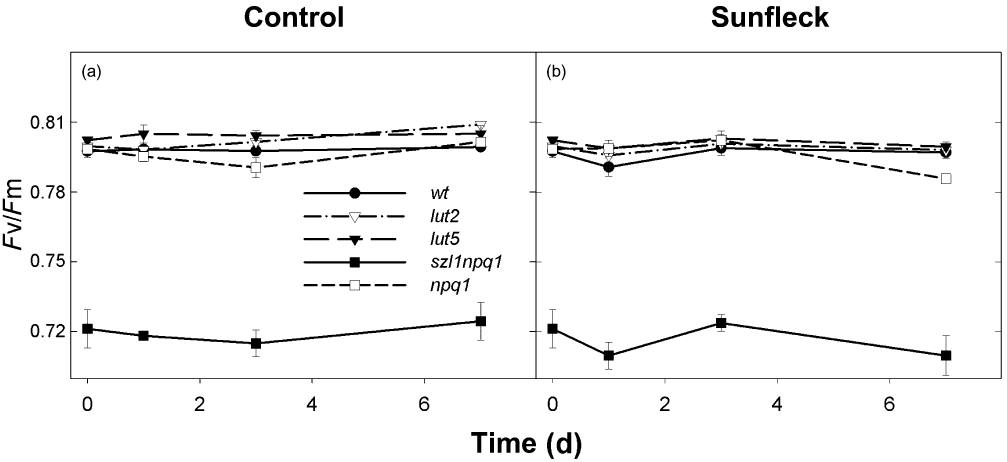
Maximal photosystem II efficiency (Fv/Fm) of dark-adapted leaves of wild type and the mutants during 7 d exposure to the control (a) or sunfleck (b) condition. Measurements were performed at the end of the night period on days 0, 1, 3 and 7. Data are means ± SE (n = 8 for wild type and n = 4 for the mutants).
Following the Fv/Fm measurements, light induction and dark relaxation of NPQ were analysed in the same leaf spots. In the control plants of wt, NPQ rapidly developed to reach 0.6–0.8 in the first 30 s of HL illumination and the maximum of 1.2–1.5 was reached within 5 min (Fig. 5a). Subsequent darkening quickly diminished NPQ to about 0.6 in 30 s, which was followed by a slower decrease to 0.4. The NPQ induction patterns of the control plants of the four mutants (Fig. 5b–e) were comparable with the previous reports. The plants of lut2 exhibited slower induction of NPQ (Pogson et al. 1998) despite retention of a large amount of A (Fig. 2d), reaching no more than 0.2–0.5 in the first 30 s of illumination (Fig. 5b). Likewise, low initial NPQ values were measured in lut5 which also attained much lower maximal NPQ levels than wt (−50%, Fig. 5c; Dall'Osto et al. 2007b). In contrast, the NPQ induction of szl1npq1 was characterized by a fast rise (Li et al. 2009) to ca. 0.8 within 30 s (Fig. 5d); thereafter NPQ did not increase in szl1npq1, or even decreased down to 0.4–0.6, during the illumination. The HL exposure induced NPQ of about 0.5 in npq1 in which the value remained at this level during the subsequent dark period (Fig. 5e; Niyogi et al. 1998). Apart from npq1, the control plants of lut5 and szl1npq1 retained the highest and the lowest NPQ, respectively, at the end of the dark relaxation.
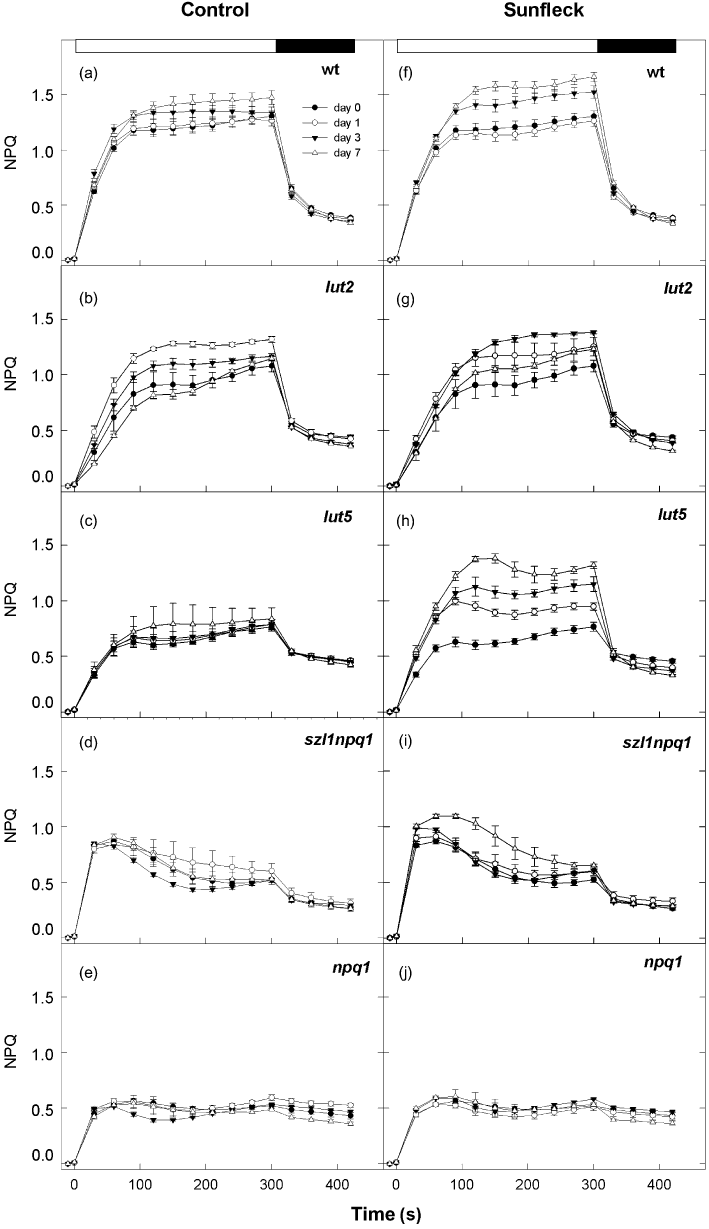
Light induction and dark relaxation of non-photochemical quenching (NPQ) in wild type (a and f), lut2 (b and g), lut5 (c and h), szl1npq1 (d and i) and npq1 (e and j) plants during 7 d exposure to the control (a–e) or sunfleck (f–j) condition. Light-induction measurements were started immediately after the Fv/Fm measurements shown in Fig. 4. NPQ was measured during 5 min illumination at saturating light intensities of up to 750–800 µmol photons m−2 s−1 for wt, lut2 and lut5, and ca. 700 µmol photons m−2 s−1 for szl1npq1 and npq1 (indicated by white bars at the top of the panels a and f) followed by 2 min dark relaxation (indicated by black bars). Data are means ± SE (n = 8 for wild type and n = 4 for the mutants).
The NPQ capacity increased progressively in the wt plants during the 7 d sunfleck treatment (Fig. 5f). The enhanced NPQ in the sunfleck plants was rapidly reversible upon darkening, indicating that the sunfleck-induced acclimatory NPQ up-regulation was attributable to rapidly inducible and reversible component of NPQ (termed qE). The maximal NPQ levels of lut2 varied considerably from day to day under both conditions (Fig. 5g) so that effects of the sunfleck treatment could not be evaluated for these plants. The sunfleck plants of lut5 strongly up-regulated the NPQ capacity already on day 1, followed by a continuous increase until day 7 (Fig. 5h). However, the subsequent darkening always decreased the NPQ to the same low levels within 30 s, suggesting NPQ up-regulation by qE enhancement also in these plants. No or only minor increase in NPQ, respectively, was detected in the sunfleck plants of npq1 (Fig. 5j) and szl1npq1 (Fig. 5i) even though the treatment resulted in a large and significant increase in L in both plants (Fig. 2a).
PsbS protein level
The levels of PsbS protein, which is essential for qE in higher plants (Li et al. 2000), were also analysed in mature leaves of wt and the carotenoid mutants after 7 d in the control or sunfleck condition (Fig. 6). Except in lut2 which exhibited large variations between individual plants, the PsbS protein content (relative to Chl) increased in all plants under the sunfleck condition; the increase was statistically significant for wt and npq1 (+40%).
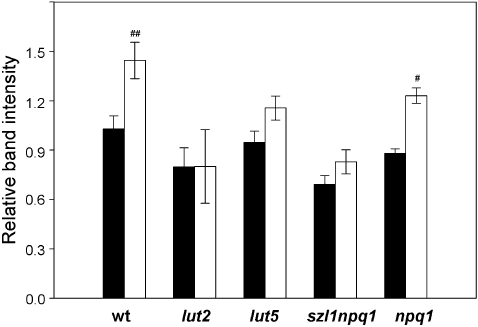
Levels of PsbS protein in leaves of wild type and the mutants. Leaves were collected after 7 d exposure to the control (closed bars) or sunfleck (open bars) condition. Protein samples corresponding to 2 µg total chlorophyll were loaded and separated by SDS-PAGE. PsbS content was determined by Western blot analysis and the band intensity of each sample was normalized to a reference sample. Data are means ± SE (n = 3). Significant differences (Tukey test of the two-way anova) between the two treatments are marked with ‘#’ for each plant (#P ≤ 0.05, ##P ≤ 0.01). The minor differences between the control plants of wild type and the mutants are not statistically significant.
Growth analyses
Leaf and root growth was analysed during 10 d (leaf growth) or 5 d (root growth) of the control or sunfleck treatment (7, 8). The RGR calculated from the projected total leaf area was comparable in all plants under both conditions, ranging between 19 and 22% per day (Fig. 7a). A small decrease of leaf RGR observed in wt and lut2 during the sunfleck treatment, or a minor increase in szl1npq1, was statistically not significant; we note that these changes in RGR are most likely underestimated (or overestimated for szl1npq1). As the sunfleck treatment induced leaf flattening (i.e. from a convex lamina in the control condition to a flat lamina in the sunfleck condition) in all genotypes, as has been reported by Alter et al. (2012), our leaf growth analysis based on projected leaf area results in an underestimation for the control plants, but not for the sunfleck plants. The plants had different leaf dry mass per area (LMA) under the control condition, with the largest LMA found in wt and the lowest in szl1npq1 and npq1 (Fig. 7b). While this parameter remained unchanged in the latter two, it decreased in the sunfleck plants of wt and lut5. Only lut2 showed a significant increase in LMA under the sunfleck condition.
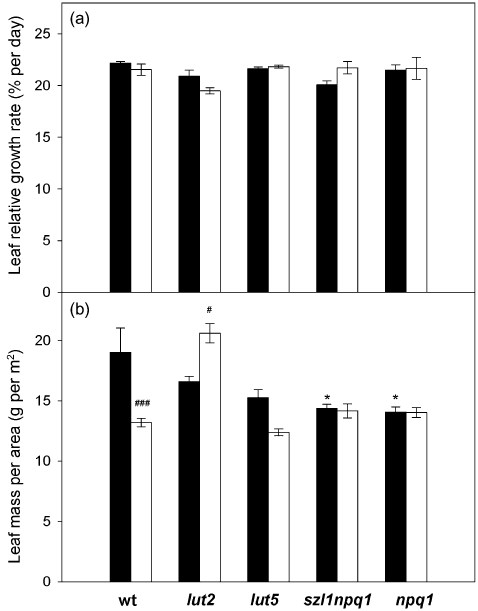
Leaf relative growth rate (RGR) and leaf dry mass per area (LMA) of wild type and the mutants under the control (closed bars) or sunfleck (open bars) condition for 10 d. (a) Leaf RGR. The leaf RGR was obtained by fitting data of the total projected leaf area collected every other day to an exponential growth curve. (b) LMA. LMA was calculated from leaf dry weight and the total projected leaf area measured for each sample on day 10. Values are means ± SE (n = 15–37 for leaf RGR analysis and n = 6–12 for dry weight measurements). There was no significant difference between the two treatments or genotypes for leaf RGR. For LMA, significant differences (Tukey test of the two-way anova) between the control and sunfleck treatments are marked with ‘#’ for each plant; significant differences between the control plants of wild type and the mutants are marked with ‘*’ (*P ≤ 0.05, #P ≤ 0.05, ###P ≤ 0.001).
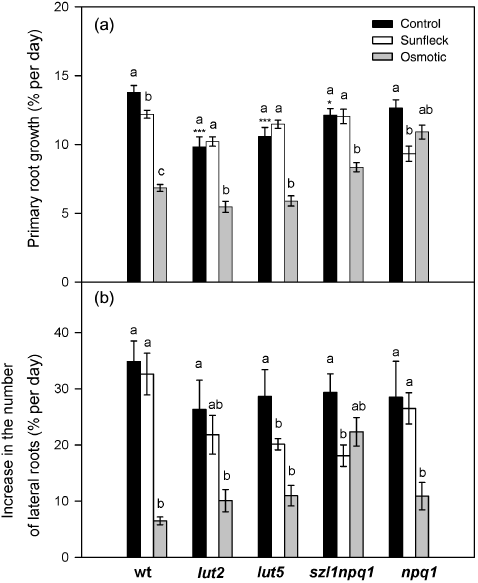
Root growth analysis in wild type and the mutants during the 5 d exposure to the control and sunfleck condition or 17 d cultivation in high-osmorality medium containing 100 mm sorbitol. Relative growth rates of the primary root (a) and lateral roots (b) were calculated from the length of the primary root and the number of lateral roots measured in control (closed bars), sunfleck (open bars) or osmotic stress (grey bars) plants at the beginning (day 0, corresponding to the 12th day of cultivation in the osmotic stress) and at the end of the experiment (day 5, corresponding to the 17th day in the osmotic stress). Values are means ± SE (n = 6–19). Significant differences (Tukey test of the two-way anova) between the three treatments are marked with different letters for each plant; significant differences between the control plants of wild type and the mutants are marked with ‘*’ (*P ≤ 0.05, ***P ≤ 0.001).
For root growth analysis, plants were grown in Petri dishes filled with agarose (Fig. 8). The experiment was stopped after 5 d due to the susceptibility of this cultivation system to fungi attack in non-sterile conditions. Under the control condition, wt and the mutants showed similar rates of lateral root formation (Fig. 8b), whereas the RGR of the primary root was somewhat lower in lut2, lut5 and szl1npq1 mutants (Fig. 8a). The sunfleck treatment resulted in a significant decrease in the primary root growth of wt and especially npq1, while it suppressed formation of lateral roots in lut5 and szl1npq1.
In order to check whether these root growth responses observed under the sunfleck condition are similar to the responses induced by drought or osmotic stress, an additional treatment with 100 mm sorbitol was included in the root growth experiment. The osmotic stress strongly inhibited both primary and lateral root growth in all plants (Fig. 8) although the primary root growth of npq1 and lateral root formation of szl1npq1 were affected much less. Overall, the patterns of root growth responses to the osmotic stress were different from the patterns found in the sunfleck plants, suggesting distinct effects and signals of the two treatments.
Seed production
Following the leaf growth analysis, the effects of the sunfleck-induced photo-oxidative stress on seed production were examined under the same conditions (Fig. 9). After bolting, inflorescence stems were covered with paper bags so that only rosette leaves and basal cauline leaves were directly exposed to the sunflecks (or the control light). In the control condition, szl1npq1 produced about 60% less seeds (in weight) than other genotypes. The sunfleck treatment reduced seed yield of all plants, with the largest decrease found in npq1 (Fig. 9). Note that seed production was analysed after many weeks of exposure to the two conditions. Earlier onset of flowering and rosette leaf senescence observed in all sunfleck plants most likely contributed to the strong reduction of seed yield.
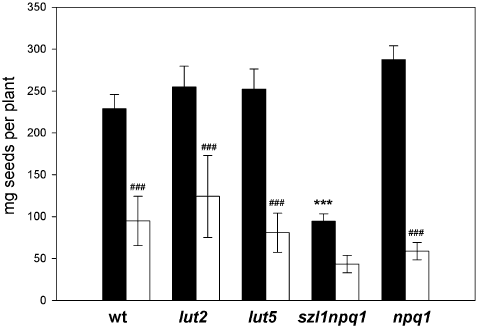
Seed harvest of wild type and mutant plants grown under the control (closed bars) or sunfleck (open bars) condition. Data are means ± SE (n = 3–10). Significant differences (Tukey test of the two-way anova) between the control and sunfleck treatments are marked with ‘#’ for each plant; significant differences between wild type and mutants under the control condition are marked with ‘*’ (###P ≤ 0.001, ***P ≤ 0.001).
DISCUSSION
The previous studies have reported the unique pigment phenotypes and photoprotective characteristics of the four mutants (e.g. Pogson et al. 1996; Niyogi et al. 1998; Dall'Osto et al. 2007b; Kalituho et al. 2007; Johnson et al. 2009; Li et al. 2009; Cazzaniga et al. 2012). In the present study, we focused on the effects of α- and β-branch carotenoids and their biosynthesis on photoprotective (NPQ) acclimation and whole-plant growth responses to photo-oxidative stress. Table 1 summarizes the acclimatory responses observed in wt and the carotenoid mutants of Arabidopsis during the sunfleck treatment.
| wt | lut2 | lut5 | szl1npq1 | npq1 | |
|---|---|---|---|---|---|
| Pigments | |||||
| L | No change | – | Increase | Increase | Increase |
| V + A + Z | No change | No change | Decrease | No change | Increase |
| N | No change | No change | Increase | Increase | No change |
| α-C + β-C | No change | No change | No change | No change | No change |
| Chl | Decrease | Decrease | Decrease | Decrease | Decrease |
| PSII | |||||
| Fv/Fm | No change | No change | No change | No change | No change |
| NPQ capacity | Increase | Variable | Increase | Little change | No change |
| PsbS | Increase | Variable | Increase | Increase | Increase |
| Growth | |||||
| Leaf RGR | No change | No change | No change | No change | No change |
| LMA | Decrease | Increase | Decrease | No change | No change |
| Root RGR | Decrease in primary root | No change | Decrease in lateral roots | Decrease in lateral roots | Decrease in primary root |
| Seed harvest | Decrease | Decrease | Decrease | Decrease | Decrease |
- Responses in the mutant genotypes that were different from wt are highlighted.
- L, lutein; V, violaxanthin; A, antheraxanthin; Z, zeaxanthin; N, neoxanthin; α-C, α-carotene; β-C, β-carotene; PSII, photosystem II; NPQ, non-photochemical quenching; RGR, relative growth rate; LMA, leaf dry mass per area.
Acclimatory adjustment of pigment composition
A marked response of Arabidopsis to strong sunflecks is the decrease in leaf Chl content (Fig. 3; Yin & Johnson 2000; Alter et al. 2012). Unlike in the study by Alter et al. (2012), in which more severe sunflecks resulted in an increase in the V-cycle pigments in wt, Chl-based carotenoid contents did not change in mature leaves of wt in the present work (Fig. 2), indicating net degradation of Chl and all carotenoids under the photo-oxidative condition. In contrast, exposure to HL and low temperature did not enhance xanthophyll degradation in Arabidopsis leaves (Ramel et al. 2012a); only the β-C oxidation increased, which was interpreted as chemical scavenging of PSII-derived 1O2 by β-C. Whether the sunfleck-induced acclimatory decline in Chl and carotenoid contents is a result of chemical reactions with ROS or enzymatic degradation is a question for future investigations.
Unlike in wt, the decrease in Chl was accompanied by an increase in L and N in lut5 and szl1npq1 or L and V in npq1 (Fig. 2). The lut5 and szl1 alleles are characterized by enhanced synthesis of α-branch carotenoids (Fig. 2; Fiore et al. 2006; Kim & DellaPenna 2006; Li et al. 2009) and accumulation of α-C. This kind of pigment phenotype is found in leaves of tropical plants under shade environments (e.g. Thayer & Björkman 1990; Logan et al. 1997; Matsubara et al. 2009). The sunfleck plants of lut5 and szl1npq1 showed the largest decrease in Chl contents (Fig. 3), suggesting that high α/β-ratios may exacerbate photo-oxidative stress (Dall'Osto et al. 2007b). Higher turnover of α-C than β-C in light (Beisel et al. 2010) could also increase the sensitivity of these plants to HL (Kim et al. 2009). Increased L and N accumulation observed in the sunfleck plants of lut5 and szl1npq1 (Fig. 2) may be an acclimatory response to compensate in part for the low availability of V + A + Z. Both L and N are able to contribute to photoprotection via quenching of singlet or triplet Chl (L) (Dall'Osto et al. 2006; Matsubara et al. 2008, 2011; Li et al. 2009) and detoxification of ROS (L and N) (Peng et al. 2006; Dall'Osto et al. 2007a).
The control plants of npq1 had a near-wt carotenoid composition (Fig. 2). The major difference between wt and npq1 resides in the inability of the latter to form Z through light-induced de-epoxidation of V (Niyogi et al. 1998). Thus, wt and npq1 would display similar phenotypes unless V de-epoxidation occurs in wt. The distinct pigment changes found in these plants (Fig. 2) suggest that the sunfleck treatment induced V de-epoxidation in wt, although neither A nor Z was detected in leaves after several hours of sunfleck exposure (data not shown). A small amount of A + Z formed during the 20 s sunflecks may have been converted back to V in the sunfleck intervals. The lack of the V-cycle operation and Z-associated photoprotection in npq1 probably intensified the photo-oxidative stress (Havaux & Niyogi 1999) and promoted synthesis of V and L (Fig. 2). A HL-induced parallel increase in V + A + Z and L has been seen in leaves of different species, including Arabidopsis (Beisel et al. 2010) and Persea americana Mill. (Förster, Osmond & Pogson 2009).
The extent of the sunfleck-induced increase in α- and β-branch xanthophylls in the different mutants corresponds by and large to the genotype-specific balance between α- and β-branch carotenoids (Fig. 2), which is determined by the activities of cyclases and hydroxylases. This suggests the importance of these enzymes in controlling stress-induced acclimatory adjustment of leaf xanthophyll composition. Indeed, HL induces up-regulation of a gene encoding non-haeme di-iron β-ring hydroxylase in Arabidopsis plants (Cuttriss et al. 2007), which may represent an early step to trigger a stress-induced increase in V + A + Z.
While most of the xanthophylls are bound in the antenna complexes, carotenes are in the core complex of PSII and PSI (Yamamoto & Bassi 1996). The lut2 mutant had somewhat more β-C per Chl than wt, whereas szl1npq1 had very low carotene contents (Fig. 2b). Interestingly, some of the carotene binding sites of the PSII and PSI core complexes are empty in the szl1 mutant (Cazzaniga et al. 2012), which may be related to the low Fv/Fm of szl1npq1 (Fig. 4). Because the sunflecks did not significantly affect the carotene profile of wt and the mutants (Fig. 2b), Chl and carotenes must have decreased to similar extents during acclimation, regardless of the carotenoid compositions (Fig. 2) and changes in NPQ (Fig. 5). When α-C naturally occurs in a large amount in leaves, the balance between α-C and β-C shifts during acclimation to light environments (e.g. Thayer & Björkman 1990; Logan et al. 1997; Matsubara et al. 2009). No such shift was found in lut5 and szl1npq1 (Fig. 2b), demonstrating their inability to adjust carotene balance.
Together, α- and β-branch carotenoid composition seems to determine the sensitivity of Arabidopsis leaves to photo-oxidative stress, which in turn determines the extent of acclimatory pigment degradation and feedback up-regulation of xanthophyll biosynthesis.
Acclimatory up-regulation of NPQ
Dynamic photoprotection by thermal energy dissipation, which is regulated by formation of a pH gradient across the thylakoid membrane (ΔpH), plays a crucial role in plants exposed to sunflecks (Logan et al. 1997; Watling et al. 1997; Adams et al. 1999). During the 7 d sunfleck treatment, the NPQ capacity increased in wt (Fig. 5f), concomitant with a significant increase in the PsbS protein content relative to Chl (Fig. 6); the PsbS protein is needed for the ΔpH-dependent, qE component of NPQ in higher plants (Li et al. 2000), and its absence reduces fitness of Arabidopsis plants under fluctuating light (Külheim, Ågren & Jansson 2002; Logan, Terry & Niyogi 2008). An increase in PsbS was also found in the carotenoid mutants following the sunfleck treatment, with an exception of lut2 showing large variations in PsbS level (Fig. 6). The fact that a 40% increase in PsbS did not allow NPQ enhancement in npq1 (Fig. 5j) underscores the involvement of both PsbS and Z in NPQ.
The control plants of lut2, lut5 and npq1 had lower NPQ than wt (Fig. 5a–c,e), in agreement with the NPQ characteristics previously described in these mutants (Niyogi et al. 1998; Pogson et al. 1998; Dall'Osto et al. 2007b). Conversely, szl1npq1 achieved somewhat higher NPQ in the initial induction phase (Fig. 5d), which has been attributed to an extra pool of L (Li et al. 2009). Rapid NPQ induction with additional L has also been documented in Arabidopsis plants overexpressing ε-cyclase (Pogson & Rissler 2000) or in species having the Lx cycle (Matsubara et al. 2008, 2011; Esteban et al. 2010; Förster et al. 2011). The lack of L in lut2 (Fig. 2a), on the other hand, slows down the NPQ induction (Pogson et al. 1998) even in the presence of A (Fig. 2d), and seems to cause erratic behaviour of NPQ (Fig. 5b,g) presumably through destabilization of trimeric LHCII (Lokstein et al. 2002) and variable PsbS level (Fig. 6).
The most pronounced acclimatory qE enhancement was found in the sunfleck plants of lut5 (Fig. 5h) which had a reduced NPQ capacity under the control condition (Fig. 5c; Dall'Osto et al. 2007b). The NPQ in lut5 may be limited by its small V + A + Z content (Fig. 2d) as the PsbS levels were comparable in lut5 and wt (Fig. 6; Dall'Osto et al. 2007b). The marked qE up-regulation, despite diminishing V + A + Z found in the sunfleck plants of lut5, may be explained by the increased accumulation of L, N and/or PsbS (2, 6), although a significant increase in L and N as well as PsbS had little or no influence on acclimatory up-regulation of NPQ in szl1npq1 or npq1 (Fig. 5i,j). The lack of NPQ increase despite the substantial accumulation of additional L (+25 mmol mol Chl−1) is at variance with rescuing of the qE-deficient npq1 phenotype in szl1npq1 (Li et al. 2009). Unlike Z, the quenching effect of L is not unequivocal; for example, substitution of V by L (or Lx by L) in one of the xanthophyll binding sites results in fluorescence quenching in monomeric recombinant antenna complexes of PSII (Formaggio, Cinque & Bassi 2001; Matsubara et al. 2007; Li et al. 2009), but not in recombinant Lhcb1 or native LHCII trimers in the case of Lx-L substitution (Matsubara et al. 2007). Multiple pools of L, having different localization and hence different functions, are probably responsible for these seemingly contradictory observations concerning the quenching effect of L.
Notably, the plants of szl1npq1 were unable to maintain high NPQ following rapid induction; NPQ started to decline after 1 min in HL and only half of the initial level remained at the end of the 5 min illumination (Fig. 5d,i). This peculiar pattern of NPQ induction, observed not only in dark-adapted plants but also in light-adapted plants under different light intensities except for very strong light (Matsubara & Osmond, unpublished data), contrasts with the typical NPQ induction curve previously reported in this mutant (Li et al. 2009). In our experimental conditions, the unique pigment phenotype of szl1npq1, that is, extremely rich in L and poor in all other xanthophylls and carotenes (Fig. 2), and/or a 50% decrease in the two minor antenna complexes CP29 and CP26 (Li et al. 2009), may have interfered with qE stabilization while allowing rapid ΔpH and qE formation (Fig. 5d,i). The qE component of NPQ involves PsbS- and Z-dependent protein conformational changes which supposedly result in dissociation of PSII antenna supercomplexes consisting of CP29, CP24 and LHCII (Betterle et al. 2009). The szl1npq1 mutant offers an interesting system to study the mechanism of qE stabilization and roles of Z and L therein.
Based on these observations, we conclude that the sunfleck-induced acclimatory up-regulation of NPQ by qE enhancement necessitates the ability to synthesize Z by de-epoxidation of V; neither in szl1npq1 nor in npq1 can additional L molecules replace Z in this acclimatory function. Thus, the composition of de-epoxidized α- and β-branch xanthophylls affects not only the intensity and kinetics of NPQ but also the ability and capacity of plants to adjust the maximal NPQ level during acclimation to stress conditions.
Acclimation via growth
In a wide range of plant species, LMA is strongly correlated with the daily total irradiance received by leaves (Poorter et al. 2009); sun-exposed leaves usually have higher LMA than leaves growing in the shade (e.g. Matsubara et al. 2009). The decrease in LMA found in the sunfleck plants of wt and possibly also lut5 (Fig. 7b), despite the greater daily total irradiance under the sunfleck condition than the control condition, may be due to the enhanced NPQ in these plants (Fig. 5f,h), resulting in lower light energy utilization for photosynthesis. Only lut2 plants, having no α-branch carotenoids and extremely large amounts of V + A + Z which were always partially de-epoxidized (Fig. 2), exhibited an increase in LMA (Fig. 7b), indicating that their leaves became thicker and/or had higher dry mass density under the sunfleck condition. Although reduced leaf area growth has been reported previously for lut2 compared with wt and npq1 (Kalituho et al. 2007), we found comparable leaf RGR values in these three plants (as well as lut5 and szl1npq1) under both control and sunfleck conditions (Fig. 7a).
Compared with chloroplasts and leaves, much less is known about physiological functions and stress responses of the carotenoid biosynthetic pathway for non-photosynthetic organs (Howitt & Pogson 2006; Cazzonelli 2011). The plants of szl1npq1 produced far less seeds than the others in the control condition (Fig. 9) even though leaf and root growth did not show such inhibition (7, 8). This may indicate a direct effect of the altered carotenoid biosynthesis on the reproductive organ of szl1npq1. The long-term sunfleck treatment further diminished the seed yield of szl1npq1, as seen also in other genotypes, demonstrating a strong negative impact of photo-oxidative stress. As for roots, our root growth analysis revealed different sensitivity to the sunflecks among the five genotypes, as well as between the primary and lateral roots within a genotype (Fig. 8). The primary root growth decreased in the sunfleck plants having normal α/β-ratios in leaves (wt and npq1), whereas those having high α/β-ratios (lut5 and szl1npq1) responded by reducing lateral root formation (2, 8). Were these distinct responses of root growth caused by different sensitivity (of leaves) to photo-oxidative stress and/or by altered carotenoid metabolism?
Two plant hormones are known to be derived from β-branch carotenoids via the activities of carotenoid cleavage dioxygenases: ABA from 9′-cis N or 9′-cis V (Tan et al. 1997; Nambara & Marion-Poll 2005), and SL from β-C (Schwartz, Qin & Loewen 2004; Gomez-Roldan et al. 2008; Umehara et al. 2008). In addition to controlling aboveground growth, for example, bud dormancy/outgrowth, leaf expansion and shoot branching (Koornneef et al. 2002; Horvath et al. 2003; Kozuka et al. 2005; Gomez-Roldan et al. 2008; Umehara et al. 2008), both ABA and SL can suppress lateral root formation under drought and osmotic stress (ABA) or phosphate deficiency (SL) (De Smet et al. 2006; Koltai 2011). Alterations in carotenoid biosynthesis can affect apocarotenoid levels, as exemplified by the aba1 (npq2) mutant lacking Z epoxidase and thus also epoxycarotenoids and ABA (Koornneef et al. 1982), or the β-hydroxylase-deficient mutants having less ABA in leaves (Tian, DellaPenna & Zeevaart 2004).
Conceivably, the α- and β-branch activities may affect plant growth behaviour via apocarotenoid hormones. For the acclimatory responses of LMA and root growth, however, there seems to be an interaction between carotenoid metabolism and photo-oxidative stress because the phenotypic differences in LMA and root growth emerged only in the sunfleck plants, not in the control plants (7, 8). As short sunflecks primarily cause photo-oxidative stress in Arabidopsis leaves (Alter et al. 2012), stress signals may travel from leaves to roots. The signals of sunflecks are different from those of osmotic stress, judging by the distinct patterns of root growth under these conditions (Fig. 8). As growing roots quickly respond to carbon supply from the shoot (Nagel et al. 2006; Yazdanbakhsh & Fisahn 2010), the sunfleck treatment may have affected the root growth by decreasing leaf carbohydrate status. Furthermore, oxidative signals involving ROS (Karpinski et al. 1999; Rossel et al. 2007) are implicated in long-distance signalling in systemic HL acclimation. As α- and β-branch xanthophylls have different ROS-scavenging capacities in leaves (Dall'Osto et al. 2007b; Havaux et al. 2007; Johnson et al. 2007), altered balance between the two branches could influence long-distance stress signalling indirectly via its effect on ROS detoxification. Moreover, a recent study by Ramel et al. (2012b), which has shown reprogramming of gene transcription by volatile oxidation products of β-C (especially β-cyclocitral), suggests a direct role of β-C as a precursor of messenger molecules in 1O2-induced systemic acclimation. The distinct changes in LMA and root growth found in the carotenoid mutants during the sunfleck treatment (7, 8) provide a further support to carotenoid functions in whole-plant acclimation to photo-oxidative stress.
CONCLUSIONS
Alterations in the balance between the α- and β-branch of the carotenoid biosynthetic pathway can affect not only acclimatory adjustment of pigment composition and photoprotection in leaves but also photo-oxidative stress responses of leaf and root growth in Arabidopsis plants. Except for the very low seed yield of szl1npq1, however, large variations in carotenoid biosynthesis did not seriously penalize any of the mutants under our experimental conditions, demonstrating the ability of Arabidopsis to cope, to certain extent, with perturbations in the pathway through acclimation. Nevertheless, the conserved leaf pigment complement suggests strong selection pressure favouring the normal carotenoid composition and acclimatory regulation at least in chloroplasts. Further investigations are needed to elucidate the roles of α- and β-branch carotenoid metabolism in whole-plant acclimation to variable and adverse environments.
ACKNOWLEDGMENTS
We thank Beate Uhlig and Marcel Schneider for seed harvesting; Thomas Hombach for maintenance of the ‘sunfleck machine’; and Sebastian Samer for helping the root growth analysis. R.C. acknowledges the support of her PhD thesis at the Heinrich-Heine-Universität Düsseldorf. Z.L. and K.K.N. were supported by a grant from the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (FWP number 449B).




