Silencing of G proteins uncovers diversified plant responses when challenged by three elicitors in Nicotiana benthamiana
Huajian Zhang's present address is Department of Plant Pathology, Anhui Agricultural University, Hefei, Anhui 230026, China.
ABSTRACT
Signalling through heterotrimeric G protein composed of α-, β- and γ-subunits is essential in numerous physiological processes. Here we show that this prototypical G protein complex acts mechanistically by controlling elicitor sensitivity towards hypersensitive response (HR) and stomatal closure in Nicotiana benthamiana. Gα-, Gβ1-, and Gβ2-silenced plants were generated using virus-induced gene silencing. All silenced plants were treated with Xanthomonas oryzae harpin, Magnaporthe oryzae Nep1 and Phytophthora boehmeriae boehmerin, respectively. HR was dramatically impaired in Gα- and Gβ2-silenced plants treated with harpin, indicating that harpin-, rather than Nep1- or boehmerin-triggered HR, is Gα- and Gβ2-dependent. Moreover, all Gα-, Gβ1- and Gβ2-silenced plants significantly impaired elicitor-induced stomatal closure, elicitor-promoted nitric oxide (NO) production and active oxygen species accumulation in guard cells. To our knowledge, this is the first report of Gα and Gβ subunits involvement in stomatal closure in response to elicitors. Furthermore, silencing of Gα, Gβ1 and Gβ2 has an effect on the transcription of plant defence-related genes when challenged by three elicitors. In conclusion, silencing of G protein subunits results in many interesting plant cell responses, revealing that plant immunity systems employ both conserved and distinct G protein pathways to sense elicitors from distinct phytopathogens formed during plant-microbe evolution.
INTRODUCTION
In the course of their development, plants have to face a wide range of potential pathogens, including viral, bacterial, fungal and oomycete pathogens. Plants have developed a complex immune system to generate systemic signals emanating from the infection site. The first active layer of the plant immune system is referred to as pathogen-associated molecular patterns (PAMPs)-triggered immunity (PTI), which is induced by PAMPs (also called microbe-associated molecular patterns: MAMPs) and elicitors (Zhao, Davis & Verpoorte 2005; Garcia-Brugger et al. 2006; Hématy, Cherk & Somerville 2009). However, some pathogens deliver virulence proteins that target host protein to overcome the plant immunity response. Most plants have evolved the corresponding resistance (R) protein to recognize effector activity, which will trigger plant resistance through effector-triggered immunity (ETI) (Chisholm et al. 2006). Natural selection drives evolution of new pathogen effector proteins and plant R proteins. This tug of war between plants and pathogens is represented as a zig-zag-zig model (Jones & Dangl 2006; Hok, Attard & Keller 2010; Nishimura & Dangl 2010). Both PTI and ETI can induce plant defence response, which includes stomatal closure and hypersensitive response (HR), a programmed host cell death (PCD) to limit pathogen development (Jones & Dangl 2006; Zeng, Melotto & He 2010). Many key players involved in HR have been identified (del Pozo, Pedley & Martin 2004; Gabriëls et al. 2006; Gan et al. 2009). However, how signalling pathways lead to local HR but not whole-plant cell death, and how death occurs remains unclear.
Regulation of the stomatal aperture in plants controls photosynthesis and the water status of the plant (Fan, Zhao & Assmann 2004; Nadeau 2008). As mature guard cells lack plasmodesmata, all solute uptake and efflux must occur via the plasma membrane and vacuole (Pandey, Zhang & Assmann 2007). Historically, stomata were considered as a passive portal for the entry of pathogenic bacteria (Pandey et al. 2007), but recent studies have suggested that stomata play an active role in plant innate immune system (Melotto et al. 2006; Melotto, Underwood & He 2008; Zhang, He & Assmann 2008; Zeng & He 2010). Stomatal closure restricts bacterial invasion, although plant pathogenic bacteria can secrete specific virulence factors to reopen stomata effectively, which is an important pathogenic strategy (Melotto et al. 2006). Moreover, some fungal phytopathogens, in addition to bacteria, force entry through closed stomata, whereas others require open stomata to successfully penetrate plants (Allègre et al. 2009). Some fungi and oomycetes are able to manipulate stomata to facilitate sporulation (Guimarães & Stotz 2004; Allègre et al. 2007). Therefore, it is necessary to study the signalling mechanism of stomatal closure responses to PAMPs and elicitors, in order to further clarify phytopathogenic pathogenesis, disease epidemiology and phyllosphere microbiology.
The understanding of elicitor signalling has recently advanced, but whether and how heterotrimeric G protein is involved in the elicitor-induced hypersensitive response and stomatal closure remains unclear. Heterotrimeric G protein signalling is conserved in all eukaryotic organisms. The canonical heterotrimer consists of three different subunits, α, β and γ, which are organized in a highly conserved structure and typically bound to specific G protein-coupled receptors (GPCRs). The Gα subunit undergoes conformational changes due to GDP to GTP exchange and dissociates from the Gβγ complex (Gilman 1987; Oldham & Hamm 2008). The activated Gα returns to the GDP bound state upon GTP hydrolysis, and further signalling is blocked by its reassociation with the Gβγ dimer (Schmidt et al. 1992; Clapham & Neer 1993; Gautam et al. 1998; McCudden et al. 2005; Oldham & Hamm 2008). While interaction between Gα and Gβγ dimer is dependent on the conformational status of Gα, interaction between Gβ and Gγ is typically non-dissociable (Gautam et al. 1998). It was initially thought that signalling in animals only occoured via the GTP-Gα subunit, while Gβγ either function to inhibit the action of Gα by reforming the inactive heterotrimer, or guide Gα recognition of the receptor for reactivation. However, it is now established that both the GTP-Gα subunit and Gβγ can interact with specific downstream effector molecules to initiate unique intracellular signalling responses (Adjobo-Hermans, Goedhart & Gadella 2006; Digby et al. 2006; Fan et al. 2008; Oldham & Hamm 2008).
In animals, the repertoire of heterotrimeric G protein complexes is extremely redundant; however, it is specific to the unique function and structure of individual components. In contrast to animals, plants have a greatly simplified repertoire of G protein signalling elements. For example, the higher plant Arabidopsis has only one canonical G protein α-subunit (Gα) (Ma, Yanofsky & Meyerowitz 1990; Ma 1994), one β-subunit (Gβ) (Weiss et al. 1994) and two γ-subunits (Gγ) (Mason & Botella 2000, 2001). The same number of G protein subunits was found in the monocot species rice (Ishikawa et al. 1995; Ishikawa, Iwasaki & Asahi 1996; Kato et al. 2004). However, two Gα subunits were reported for legume species (Kim et al. 1995; Gotor et al. 1996; Marsh & Kaufman 1999). Similarly, based on sequence information of Nicotiana tabacum, there is a single candidate Gα gene and possibly two Gβ-subunit genes. Mutational and pharmacological studies have implicated that plant heterotrimeric G protein subunits may be involved in numerous physiological processes, including auxin and gibberellin signalling, K+ channel regulation, Ca2+ regulation, cell division, stomatal function and plant defence against necrotrophic fungi (Aharon et al. 1998; Jones et al. 1998; Murga et al. 1998; Lee & Assmann 1999; Saalbach et al. 1999; Ullah et al. 2001; Wang et al. 2001; Siekhaus & Drubin 2003; Llorente et al. 2005; Trusov et al. 2006, 2008; Warpeha et al. 2006, 2007).
In this study, we used virus-induced gene silencing (VIGS) to investigate the role of Gα, Gβ1 and Gβ2 in Nicotiana benthamiana responses to elicitors from fungi, bacteria, and oomycetes. We have found that plant immunity systems employ both conserved and distinct G protein pathways to sense elicitors from distinct phytopathogens formed in plant-microbe evolution.
MATERIALS AND METHODS
Plant materials, elicitors and treatment protocol
N. benthamiana plants were grown in a growth chamber under a 16:8 h light : dark cycle at 25 °C. A needle-less syringe was used to inject 25 µL of an elicitor (50 nm) into tiny cuts on the underside of the leaf, thereby flooding the apoplastic space. To prepare Phytophthora boehmeriae boehmerin and Magnaporthe oryzae Nep1, overnight cultures of Escherichia coli BL21 cells carrying pET32b harbouring the boehmerin (GenBank Accession No. AY196607) or nep1 (GenBank Accession No. MGG_08454.5) genes were diluted (1:100) in Luria–Bertani (LB) medium containing ampicillin (50 mg mL−1) and incubated at 37 °C. To prepare E. coli-expressed harpin, overnight cultures of E. coli BL21 cells carrying pET30a harbouring the hrf1 gene (GenBank Accession No. AY875714) were diluted (1:100) in LB medium containing kanamycin (50 mg mL−1) and incubated at 37 °C. When the OD600 of the cultures reached 0.6, secretion of boehmerin, harpin or Nep1 into the culture medium was induced via the addition of 0.4 mm isopropyl-beta-D-thiogalactopyranoside for 6 h. Boehmerin, harpin and Nep1 were expressed as a His tag fusion protein. Proteins were examined by SDS-polyacrylamide gel electrophoresis. Protein purification was performed with the Ni-nitrilotriacetic acid resin (QIAGEN, Valencia, CA, USA), and the purified proteins were dialyzed against a phosphate-buffered saline (PBS) buffer (pH 7.4) and stored at −20 °C prior to use. Protein concentrations were determined using the Bradford reagent (Qutob et al. 2006) and concentrated stock solutions (500 nm) were prepared.
DNA constructs and seedling infection for virus-induced gene silencing
Total RNA from N. benthamiana leaves was reverse-transcribed using an oligo (dT) 18 primer (TaKaRa, Dalian, China). Using this cDNA as a template, a cDNA containing the entire Gα open reading frame (ORF) was amplified by PCR with the primers listed in Supporting Information Table S1 (NtGα-F and NtGα-R), designed from the DNA sequence of Gα from N. tabacum (GenBank Accession No. Y08154). The PCR-amplified product (1155 bp) was ligated to the PMD19 T-Vector (TaKaRa) to generate T-Gα. Similarly, cDNAs containing the entire Gβ1 and Gβ2 ORF were amplified using the primers listed in Supporting Information Table S1 (NtGβ1-F and NtGβ1-R, NtGβ2-F and NtGβ2-R, respectively), based on the cDNA sequences of Gβ1 (GenBank Accession No. Z84820) and Gβ2 (GenBank Accession No. Z84821). The PCR products (981 bp and 1143 bp) were separately cloned into the PMD19 T-Vector to generate T-Gβ1 and T-Gβ2, respectively. Virus-induced gene silencing for the Gα, Gβ1, and Gβ2 genes in N. benthamiana was performed using potato virus X (PVX), as described by Sharma et al. (2003). Then, the fragments of Gα (255-bp), Gβ1 (216-bp) and Gβ2 (235 bp) were separately amplified from T-Gα, T-Gβ1, T-Gβ2 plasmids. The 255 bp fragment of Gα was amplified with the forward primer NbGα-U/SalI (5′-CGGTCGACGAAGCTCTTCGGAAAGATCC-3′; SalI site underlined) and the reverse primer NbGα-R/ClaI (5′-CCATCGATATTTCTCTGACCTCCAACATC-3′; ClaI site underlined). The 216 bp fragment of Gβ1 was amplified with the forward primer NbGβ1-U/SalI (5′-CGGTCGACCATTAGAACTGGACACCAGC-3′; SalI site underlined) and the reverse primer NbGβ2-R/ClaI (5′-CCATCGATCCAAGACAACTAATCCGACC-3′; ClaI site underlined). The 235 bp fragment of Gβ2 was amplified with the forward primer NbGβ2-U/SalI (5′-CGGTCGACCCTTGAGACCTTGACAAGTG-3′; SalI site underlined) and the reverse primer NbGβ2-R/ClaI (5′-CCATCGATCCACCTCTGAGTAACAACTC-3′; ClaI site underlined). After that, the PCR products of Gα, Gβ1 and Gβ2 were digested with SalI and ClaI, and ligated into the corresponding sites in the PVX vector pgR107, to generate PVX.Gα, PVX.Gβ1 and PVX.Gβ2, respectively. The constructs containing the inserts were transformed into Agrobacterium tumefaciens strain GV3101. Bacterial suspensions were applied to the undersides of N. benthamiana leaves using a 1 mL needle-less syringe. Plants exhibited mild mosaic symptoms 3 weeks after inoculation. The third or fourth leaf above the inoculated leaf, where silencing was most consistently established, was used for further analyses.
Yeast two-hybrid assay
The Saccharomyces cerevisiae strain AH109 and vectors were supplied with the Matchmaker yeast two-hybrid kit (BD Biosciences Clontech, Oxford, UK). The ORF region encoding Gβ1 and Gβ2 was amplified by PCR using the primers listed in Supporting Information Table S1 and cloned into pGBKT7 to produce in-frame translational fusions to the GAL4 binding domain. The coding regions for full-length Gα were amplified by PCR using the primers listed in Supporting Information Table S1 and cloned into pGADT7 to produce in-frame translational fusions to the GAL4 activation domain. Following the Matchmaker Two-Hybrid System 3 User's Manual (BD Biosciences Clontech) and Yeast Protocols Handbook (BD Biosciences Clontech), bait and prey plasmids were co-transformed into the yeast strain AH109. Co-transformants were maintained on SD medium (a defined minimal medium lacking Leu and Trp) and putative interacting clones were obtained by selecting transformants in SD/-Ade/-His/-Leu/-Trp and checked for α-galactosidase expression by the X-α-Gal assay. The specificity of the interaction was determined using pGADT7-RecT and pGBKT7-Lam as negative controls, and pGADT7-RecT and pGBKT7-53 as positive controls. Experiments to investigate interactions and protein expression were repeated at least three times with different isolates.
Diaminobenzidine (DAB) staining
According to Lamb & Dixon (1997) and Zhang et al. (2009), leaves were harvested 6 h after elicitor treatment. Following the methods of Gan et al. (2009), samples were vacuum infiltrated for 20 min with PBS (pH 7.4) containing 0.5% (w/v) DAB. The leaves were placed in light for 10 h and then boiled for 20 min in 80% ethanol. The intensity and pattern of DAB staining was assessed visually. Quantitative scoring of hydrogen peroxide (H2O2) staining in leaves was analysed using Quantity One software (Leica DMR, Wetzlar, Germany).
RNA isolation and quantitative real-time PCR
Total RNA was extracted following the Trizol extraction protocol (Invitrogen, Carlsbad, CA, USA) and treated with RNAse-free DNAse I (TaKaRa). First-strand cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) following the manufacturer's directions. Quantitative real-time PCR was performed using the cDNA and gene-specific primers. Each cDNA was amplified by quantitative PCR using SYBR® PrimeScript™ RT-PCR Kit (TaKaRa) and the ABI 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). N. benthamiana EF-1α expression was used to normalize the expression value in each sample, and relative expression values were determined against buffer or PVX-infected plants using the comparative Ct method (2-ΔΔCt).
Primers of quantitative real-time PCR are described in Supporting Information Table S1.
Stomatal aperture measurements
Stomatal apertures were measured as described by Chen et al. (2004) and Zhang et al. 2009, 2010a). Leaves were derived from various silenced plants (for Gα, Gβ1 and Gβ2, respectively) and controls. After a 3 week inoculation, leaf samples were harvested from the third and fourth leaves above the inoculation site. Abaxial (lower) epidermis was peeled off and floated in 5 mm KCl, 50 mm CaCl2 and 10 mm MES-Tris (pH 6.15) in light at least for 2 h to open the stomata fully before experimentation to minimize the effects of other factors in stomatal response, such as the mesophyll signals that can also significantly influence stomatal behaviour. When used, nitric oxide (NO) modulator [2-Phenyl-4,4,5,5-tetramethyl imidazoline-1-oxyl 3-oxide (cPTIO), NO scavenger] was added after the 3 h light periods, followed by elicitor treatment after 10 min. Incubation of epidermal strips was then continued for another 3 h in the same light, before measuring the stomatal apertures. The images of stomatal aperture in the peer strips were captured by a digital camera under a microscope. The maximum diameter of stomata was measured under an optical microscope. At least 50 apertures in each treatment were obtained. The experiments were repeated three times.
NO measurement in guard cells
NO accumulation was determined using fluorophore 4,5-diaminofluorescein diacetate (DAF-2DA, Sigma-Aldrich, St Louis, MO, USA) according to Ali et al. (2007). Epidermal strips were prepared from control and gene-silenced plants, respectively, and incubated in 5 mm KCl and 10 mm MES-Tris (pH 6.15) in the light for 2 h, followed by incubation in 20 µm DAF-2DA for 1 h in the dark at 25 °C, and finally rinsed three times with 10 mm Tris-HCl (pH 7.4) to wash off excess fluorophore. The dye-loaded strips were kept in the incubation medium. When used, cPTIO was added after the 3 h light periods, followed by elicitor treatment after 10 min. Images of guard cells were obtained 3 h after elicitor treatment under a fluorescence microscope (excitation wavelength, 470 nm; emission wavelength, 515 nm). Fluorescence emission from guard cells was analysed using Quantity One software.
Active oxygen species (AOS) measurement in guard cells
Dihydrorhodamine 123 (DHR; Merck, Whitehouse Station, NJ, USA) was used to analyse elicitor-induced AOS production in guard cells. Epidermal strips were incubated in 20 µm DHR for 2 h in the dark at 37 °C, and then rinsed three times with PBS (pH 7.4) to remove excess fluorophore. Subsequently, 3 h after elicitor treatment, guard cell images were obtained using Adobe Photoshop 5.5 (Mountain View, CA, USA) during a 2 s short ultraviolet (UV) exposure (one exposure per sample) under a fluorescence microscope equipped with a digital camera. Fluorescence emission of guard cells was analysed using Quantity One software.
RESULTS
Isolation and confirmation of Gα, Gβ1 and Gβ2 cDNAs from N. benthamiana
Based on the cDNA sequences of Gα, Gβ1 and Gβ2 of N. tabacum, the full-length cDNAs containing Gα (GenBank Accession No. GQ223793), Gβ1 (GenBank Accession No. GQ223794) and Gβ2 (GenBank Accession No. GQ223795) were isolated from N. benthamiana leaves by RT-PCR. The deduced amino acid sequence identity between N. benthamiana Gα and N. tabacum Gα was 97%, and that between N. benthamiana Gβ1 and N. tabacum Gβ1, N. benthamiana Gβ2 and N. tabacum Gβ2 were 99 and 91%, respectively. To better elucidate the function of Gα, Gβ1 and Gβ2, we utilized the yeast two-hybrid assay to examine whether Gα physically interacts with Gβ1 and Gβ2. As shown in Fig. 1, yeast cells transformed with both Gα and the full-length Gβ1 or Gβ2 were able to grow on the permissive medium and the selective medium. In contrast, yeast expressing Gα, Gβ1 or Gβ2 failed to grow on the selective medium. Control strains expressing the strongly interacting pGADT7-RecT and pGBKT7-53, or the non-interacting pGADT7-RecT and pGBKT7-Lam, were included as controls. Thus, Gα was fully capable of interacting with Gβ1 and Gβ2.
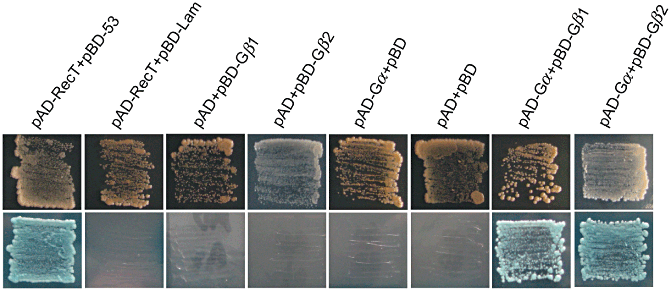
Yeast two-hybrid interaction of Gα with Gβ1 and Gβ2. Gα was co-expressed with Gβ1 and Gβ2 in yeast strain AH109 and grown on SD medium lacking Leu and Trp (upper) or SD medium lacking Ade, His, Leu and Trp (lower). Further details are given in Materials and methods.
Gα and Gβ2 silencing interfere with harpin-triggered HR
To examine the biological function of Gα, Gβ1 and Gβ2 in response to three elicitors (boehmerin, harpin and Nep1), we employed VIGS using PVX to specifically silence these genes in N. benthamiana (Baulcombe 1999). The use of PVX for VIGS in N. benthamiana has been demonstrated previously in respiratory burst oxidase homologs (RBOH) and vacuolar processing enzyme (VPE) (Zhang et al. 2009, 2010a). The levels of Gα, Gβ1 and Gβ2 mRNAs in the agro-infiltrated plants were determined by quantitative real-time RT-PCR (qRT-PCR). The transcript level of Gα in the gene-silenced plants was at least 10-fold lower than in non-silenced control plants, indicating that Gα was efficiently silenced in N. benthamiana by VIGS. Similarly, qRT-PCR with Gβ1 showed an appropriately eightfold lower level of transcripts in Gβ1-silenced line than in the PVX control, and a sevenfold lower level of Gβ2 transcripts in Gβ2-silenced plants compared with control plants. These results confirmed silencing of the endogenous gene in the gene-silenced plants (Fig. 2).
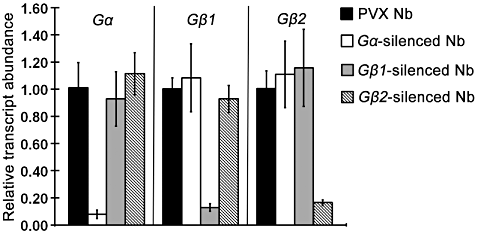
Virus-induced gene silencing mediates degradation of specific RNA transcripts in Nicotiana benthamiana. PVX.Gα, PVX.Gβ1 and PVX.Gβ2 specifically silence their respective target genes. Relative expression of Gα, Gβ1, and Gβ2 determined by qRT-PCR in Nicotiana benthamiana plants 3 weeks after Agrobacterium inoculation. The transcripts levels of Gα, Gβ1 and Gβ2 in control and N. benthamiana silenced with PVX.Gα, PVX.Gβ1 or PVX.Gβ2 are shown. RNA was extracted from leaves infected with PVX empty vector or the gene-specific PVX constructs. The error bars were derived from three technical replicates. PVX, potato virus X; qRT-PCR, quantitative real-time RT-PCR.
A study has reported that 23 nucleotides (nt) or a longer stretch of perfect sequence identity is necessary to target a gene for silencing (Thomas et al. 2001). BLAST analysis indicated that Gα, Gβ1 and Gβ2 showed no significant homology with any expressed sequence tags (ESTs) in the N. benthamiana database (http://compbio.dfci.harvard.edu/cgi-bin/tgi/gimain.pl?gudb=n_benthamiana) other than Gα-, Gβ1- and Gβ2-related sequences, and Gβ1 did not share more than a 10-nt stretch of 100% identity with Gβ2. qRT-PCR indicated that each of the Gα and Gβ subunits was specifically silenced by the respective PVX construct, and no co-silencing occurred (Fig. 2). These results verify a high degree of target specificity using VIGS, thus the three gene-silenced plants were deemed appropriate for further analysis.
Treatment with a cellulase elicitor is known to activate the plant G protein signal pathway (Assmann 2005; Ma 2008; Zhang et al. 2008). Boehmerin, harpin and Nep1 are elicitors that cause HR and stomatal closure in N. benthamiana (Zhang et al. 2009, 2010a; Zhang, Zheng & Zhang 2010b). We therefore studied the responses of Gα-, Gβ1- and Gβ2-silenced N. benthamiana plants to boehmerin, harpin and Nep1, and compared them with those of control plants. As shown in Fig. 3, these elicitors induced an obvious HR cell death in control PVX-infected leaves. None of the Gα, Gβ1 and Gβ2 silencing interfered with the ability of boehmerin and Nep1 to activate HR cell death. Both Gα and Gβ2 silencing was able to suppress harpin-induced HR in N. benthamiana leaves (Fig. 3).
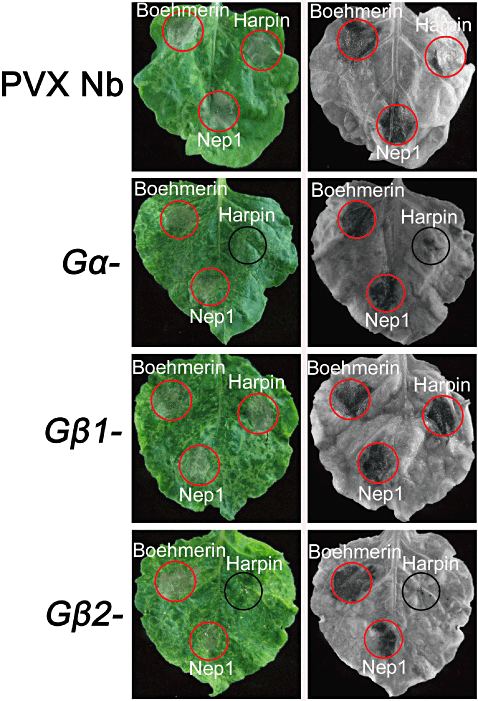
Local induction of hypersensitivity responses with boehmerin (50 nm), harpin (50 nm), and Nep1 (50 nm). Leaves (representative of three replicate treatments) from control PVX, Gα-, Gβ1- and Gβ2-silenced Nicotiana benthamiana were simultaneously infiltrated with the elicitors. The red and black circles indicate cell death and no cell death, respectively. Leaves were removed from plants after 3 d of treatment (left panels) and bleached in ethanol (right panels). The experiment was repeated four times with similar results, and six silenced plants were used in each independent experiment. PVX, potato virus X.
Gα and Gβ2 silencing attenuate elicitor-induced H2O2 accumulation
It has been reported that the accumulation of H2O2 is characteristic of the HR in plant tissues and functions as a second signal that mediates plant-programmed cell death during HR (Alvarez et al. 1998; Li et al. 2006; Gan et al. 2009). In order to evaluate whether elicitor-triggered H2O2 accumulation is dependent on the G protein, we examined the contribution of Gα, Gβ1 and Gβ2 to H2O2 accumulation in response to elicitors. DAB polymerizes on contact with H2O2 in a reaction requiring peroxidase; thus, H2O2 can be visualized in situ as a reddish-brown precipitate (Thordal-Christensen et al. 1997). We observed heavy staining in control plants 6 h after boehmerin, harpin or Nep1 treatment (Fig. 4a), which is consistent with the late peak of oxidative compound production during incompatible plant–pathogen interactions (Baker & Orlandi 1995; Allan & Fluhr 1997; Lamb & Dixon 1997). Light staining was observed after injecting PBS. We conducted similar analyses with Gα-, Gβ1- and Gβ2-silenced plants. The brown precipitate decreased, with lighter colouring and lower distribution in Gα- and Gβ2-silenced leaves after infiltration with various elicitors. No change in DAB staining intensity was observed in Gβ1-silenced plants compared with control plants after boehmerin, harpin or Nep1 infiltration (Fig. 4b). These data suggest that Gα and Gβ2 contribute to elicitor-induced H2O2 accumulation.
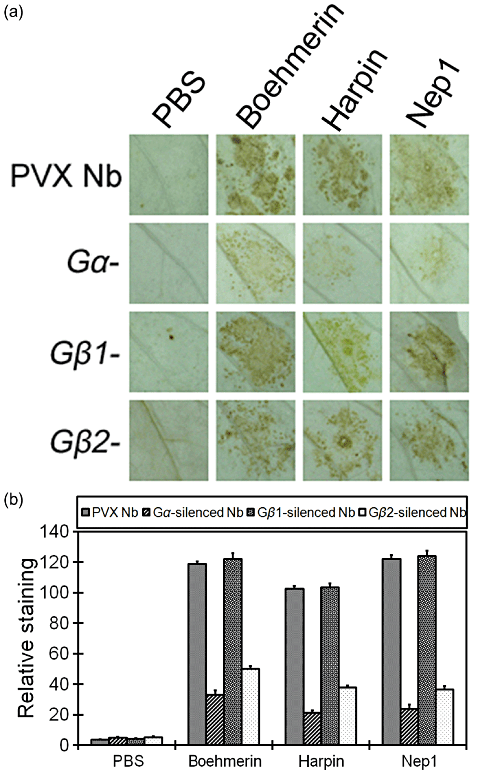
In situ detection of H2O2 using DAB staining on Gα-, Gβ1-, and Gβ2-infected Nicotiana benthamiana leaves in response to elicitors. (a) Photographs of representative leaves that were ethanol-bleached from control and Gα-, Gβ1-, and Gβ2-silenced plants 6 h after PBS (10 mm), boehmerin (50 nm), harpin (50 nm), or Nep1 (50 nm) treatment. (b) Quantitative scoring of staining in leaves of the control and Gα-, Gβ1-, and Gβ2-silenced plants with elicitor treatment using the Quantity One software. The analysis was repeated for three sets of independently silenced plants in each experiment; the values shown are the means ± SD of duplicate assays. DAB, diaminobenzidine; H2O2, hydrogen peroxide; PBS, phosphate-buffered saline; PVX, potato virus X.
Gα-, Gβ1- and Gβ2-silenced plants show impaired elicitor-activated stomatal closure
Besides HR, stomatal closure has been considered as another active plant defence response to resist pathogen attack. We have previously reported that elicitors, including boehmerin, harpin and Nep1, can induce stomatal closure in N. benthamiana (Zhang et al. 2009, 2010a; Zhang, Zheng & Zhang 2010b). As the heterotrimeric G protein α-subunit is the classic target of abscisic acid (ABA), sphingosine-1-phosphate (S1P) and extracellular calmodulin (ExtCaM) in stomatal closure in Arabidopsis (Wang et al. 2001; Coursol et al. 2003; Chen et al. 2004), silencing of G protein signalling may interfere with its function in elicitor-activated stomatal closure. To test this hypothesis, we examined changes of stomatal aperture in response to elicitors in the control PVX-infected and Gα-, Gβ1- and Gβ2-silenced N. benthamiana leaves. Boehmerin, harpin and Nep1 induced stomatal closure in control leaves, but elicitor promotion of stomatal closure was attenuated in the Gα-silenced plants. Identical attenuation was also seen in Gβ1- and Gβ2-silenced plants (Fig. 5a). We consequently performed elicitor-induced stomatal aperture measurement with Gα-, Gβ1- and Gβ2-silenced plants, and found that boehmerin-induced stomatal closure was clearly impaired compared with that of control plants. Similarly, silenced plants showed a significantly reduced response to harpin and Nep1 (Fig. 5b). Gα-, Gβ1- and Gβ2-silenced plants showed no alteration in stomatal aperture after treatment with PBS compared with control PVX-infected plants. These results suggest that Gα, Gβ1 and Gβ2 silencing compromised elicitor-induced stomatal closure.
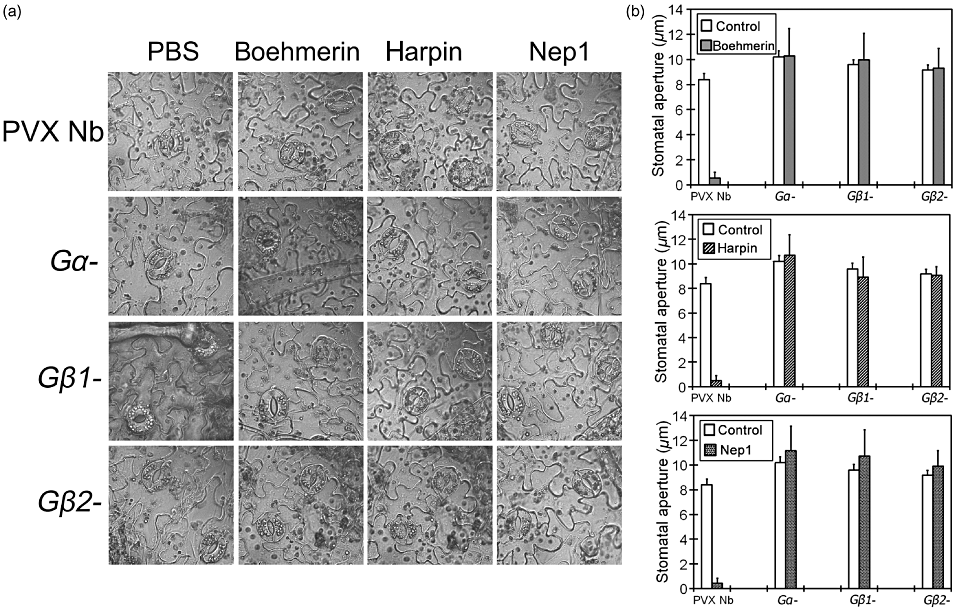
Elicitor-induced promotion of stomatal closure is impaired in Gα-, Gβ1- and Gβ2-silenced plants. (a) Photograph of leaf epidermal peels from control and Gα-, Gβ1- and Gβ2-silenced plants were taken after 3 h of incubation in PBS (10 mm), boehmerin (50 nm), harpin (50 nm), or Nep1 (50 nm). The experiment was repeated four times with similar results, and six silenced plants were used in each independent experiment. Representative results from one of these experiments are shown. (b) The stomatal aperture was measured after incubation in PBS (10 mm), boehmerin (50 nm), harpin (50 nm) or Nep1 (50 nm). Values represent the means ± SE from three independent experiments; n = 50 apertures per experiment. Data were compared using Student's t-test at the 95% significance level. PBS, phosphate-buffered saline; PVX, potato virus X.
Gα, Gβ1 and Gβ2 silencing decreases NO production in guard cells in response elicitors
It has been reported that NO is a key signalling molecule mediating stomatal closure induced by ABA, ExtCaM and chitosan (Desikan et al. 2002; Neill et al. 2002; Li et al. 2009; Srivastava et al. 2009). We further investigated whether G-protein is involved in elicitor-induced stomatal closure through regulating NO production. The NO modulator cPTIO (NO scavenger) was applied to study its effect on stomatal closure. cPTIO alone did not have any direct effect on stomatal closure, but affected the boehmerin-induced stomatal closure. Similarly, cPTIO also prevented the stomatal closure induced by harpin and Nep1 (Fig. 6a). The level of NO in guard cells was monitored by cell permeable fluorophore, DAF-2DA. Elicitor induced a marked rise in production of NO in guard cells. cPTIO restricted the rise of NO induced by boehmerin. Similarly, cPTIO prevented NO production induced by harpin and Nep1. A quantitative evaluation of fluorescence images demonstrated clearly that the elicitor-induced NO production in guard cells decreased significantly upon cPTIO treatment (Fig. 6b). These results show that stomatal closure by boehmerin, harpin or Nep1 is mediated by increase in level of NO. In light of these results, G protein might be required for NO bursts in elicitor-activated stomatal closure. To test this hypothesis, we examined NO generation in guard cells isolated from control and Gα-, Gβ1- and Gβ2 -silenced N. benthamiana plants 3 h after treatment with boehmerin, harpin or Nep1. Elicitor treatment evoked NO generation in the guard cells of PVX-control plants (Fig. 7a), but this response was inhibited in Gα-, Gβ1- and Gβ2-silenced plants. PBS-treated guard cells showed almost no fluorescence in PVX-control and gene-silenced plants. Quantification of NO fluorescence with Quantity One software demonstrated that Gα-, Gβ1- and Gβ2-silenced plants had levels of fluorescence comparable with those of PVX-control plants after boehmerin, harpin or Nep1 treatment. NO generation in Gα-, Gβ1- and Gβ2-silenced plants decreased markedly after elicitor treatment compared with the PVX-control plants (Fig. 7b). Collectively, these data suggest that elicitor-induced activation of NO production in guard cells is associated with G protein α- and β-subunits.
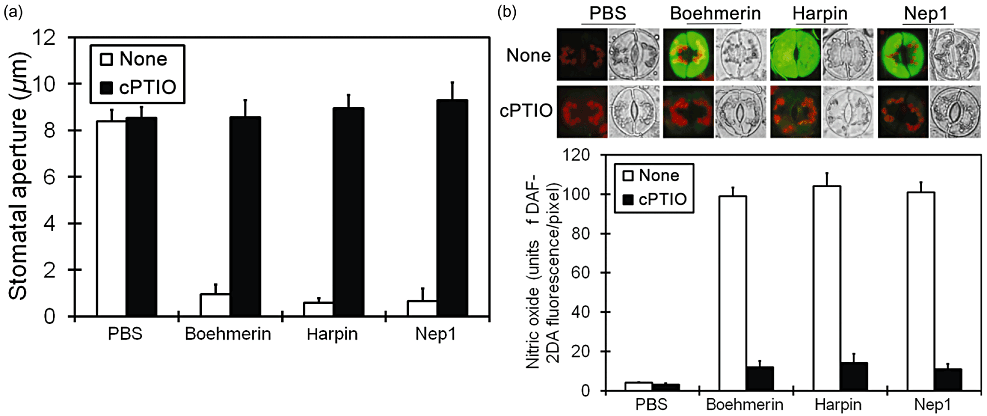
cPTIO affects elicitor-induced stomatal closure and NO production in guard cells of Nicotiana benthamiana. (a) Values represent the means ± SE from three independent experiments; n = 50 apertures per experiment. Data were compared using Student's t-test at the 95% significance level. (b) The level of NO is monitored by the fluorescence of DAF-2DA. For each treatment, fluorescence and bright-field images are shown. Results from several experiments are compiled in this figure. Experiments were repeated at least three times, and quantitative analysis of in vivo NO generation is shown. Results are presented as the mean (n ≥ 3) fluorescence intensity per pixel. cPTIO, 2-Phenyl-4,4,5,5-tetramethyl imidazoline-1-oxyl 3-oxide; DAF-2DA, 4,5-diaminofluorescein diacetate; NO, nitric oxide; PBS, phosphate-buffered saline.

Elicitor activation of NO is reduced in guard cells of Gα-, Gβ1- and Gβ2-silenced plants. (a) In all cases, the NO-sensitive dye DAF-2DA was loaded into cells of the epidermal peels, and fluorescence was measured after addition of PBS (10 mm), boehmerin (50 nm), harpin (50 nm) or Nep1 (50 nm). For each treatment, fluorescence and bright-field images are shown. Results from several experiments are compiled in this figure. Experiments were repeated at least three times, and representative images are shown. (b) Quantitative analysis of in vivo NO generation monitored using DAF-2DA fluorescence as shown in (a). Results are presented as the mean (n ≥ 3) fluorescence intensity per pixel. DAF-2DA, 4,5-diaminofluorescein diacetate; NO, nitric oxide; PBS, phosphate-buffered saline; PVX, potato virus X.
Gα, Gβ1 and Gβ2 regulate elicitor-induced AOS accumulation in guard cells
In eukaryotic organisms, AOS acts as a signal in a multitude of physiological processes, including HR and stomatal closure (Lamb & Dixon 1997; Gechev & Hille 2005; Li et al. 2006; Gan et al. 2009). The INF1 elicitor is able to induce a massive accumulation of AOS in guard cells in N. benthamiana (Asai, Ohta & Yoshioka 2008). Therefore, it is necessary to further evaluate whether Gα, Gβ1, and Gβ2 silencing affects the generation of other AOS in elicitor signalling. We analysed peroxide and peroxynitrite levels via incubation with DHR, which is oxidized to form fluorochrome rhodamine 123 in the presence of AOS (Schulz, Weller & Klochgeter 1996). Neither control nor gene-silenced plants showed AOS fluorescence after PBS treatment. As shown in Fig. 8a, treating guard cells with one of the indicated elicitors resulted in obvious AOS fluorescence. The fluorescence density decreased sharply, with lighter colouring, in the guard cells of all the gene-silenced plants after boehmerin, harpin or Nep1 treatment (Fig. 8b). These results suggest that G protein signalling positively regulates AOS accumulation in guard cells in response to different elicitors.

Elicitor-induced AOS increase in guard cells of the control and Gα-, Gβ1- and Gβ2-silenced plants. (a) In all cases, the AOS dye DHR was loaded into cells of epidermal peels, and fluorescence was detected after incubation in PBS (10 mm), boehmerin (50 nm), harpin (50 nm) or Nep1 (50 nm). For each treatment, fluorescence and bright-field images are shown. Results from several experiments are compiled in this figure. Experiments were repeated at least three times, and representative images are shown. (b) Quantitative analysis of in vivo AOS generation monitored using DHR fluorescence as shown in (a). Results are presented as the mean (n ≥ 3) fluorescence intensity per pixel. AOS, active oxygen species; DHR, dihydrorhodamine 123; PBS, phosphate-buffered saline; PVX, potato virus X.
Gα-, Gβ1- and Gβ2-silenced plants alter gene expression related to AOS homeostasis and transcription
Compromised cell death in Gα- and Gβ2-silenced plants after harpin treatment, and differences in the accumulation of H2O2, NO and AOS between control and silenced plants, might have an effect on the expression of defence-related genes. To address this possibility, the kinetics of the expression of selected genes (PR2b, EDS1, NbrbohA, NbrbohB, NIA, NOA and NR) after elicitor infiltration was examined by qRT-PCR in gene-silenced and control plants (Fig. 9). The transcript level of PR2b (X53600) showed no significant difference (P > 0.05) in Gα-, Gβ1- and Gβ2-silenced and control plants with PBS treatment, but it was 3.1- to 6.3-fold lower in gene-silenced plants than in control plants upon elicitor treatment. In Gα-, Gβ1- and Gβ2-silenced and control plants, the expression of a disease resistance protein (EDS1, AF479625) was induced by elicitors compared with PBS treatment and was down-regulated 2.3- to 3.5-fold in gene-silenced plants compared with control plants upon elicitor treatment. These results show that G protein may have an effect on the transcripts of defence-related genes in elicitor signalling. Compared with PBS treatment, the expression of NbrbohA (encoding an AOS-inducible NADPH oxidase, AB079498) was induced by elicitor in Gα-, Gβ1- and Gβ2-silenced and control plants, and was down-regulated 4- to 5.2-fold in gene-silenced plants compared with control plants upon elicitor treatment. Compared with the control plants, NbrbohB (AB079499) expression showed no obvious difference in Gα-, Gβ1- and Gβ2-silenced plants with PBS treatment, but was appropriately 6.7- to 29.8-fold lower after elicitor treatment in gene-silenced plants than in control plants. The experimental data suggest NbrbohA and NbrbohB may contribute to elicitor-induced AOS production. In comparison with the control plants, nitric reductase (NR, AB245431) expression showed no obvious difference in Gα-, Gβ1- and Gβ2-silenced plants with PBS and Nep1 treatment. It was approximately 2.2- to 3.2-fold lower in Gα-, Gβ1- and Gβ2-silenced plants than in control plants after boehmerin and harpin treatment. The transcript level of NOA1 (associated with NO, AB303300) was detected no obvious difference with PBS and harpin treatment; however, it was induced by boehmerin and Nep1, and was appropriately 2.7 to 4.5-fold lower in Gα-, Gβ1- and Gβ2-silenced plants than in control plants in response to boehmerin and Nep1. Compared with PBS, boehmerin and harpin treatment, the expression of NIA (responsible for NO production, X14058) was detected to be induced by Nep1 and down-regulated 4- to 5.2-fold lower in Gα-, Gβ1- and Gβ2-silenced plants than in control plants after Nep1 treatment.
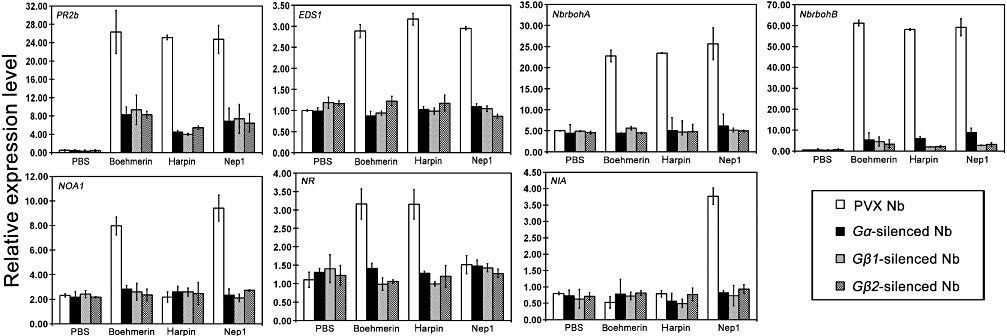
Expression analysis of genes associated with redox-control and transcription in Gα-, Gβ1- and Gβ2-silenced plants by qRT-PCR. 6 hours after treatment with or without boehmerin (50 nm), harpin (50 nm) and Nep1 (50 nm), leaf samples were harvested from the inoculation site, the lower and the upper leaves; bars represent mean (three biological replicates) ±standard deviation. PBS, phosphate-buffered saline; PVX, potato virus X; qRT-PCR, quantitative real-time RT-PCR.
DISCUSSION
A primary goal of the present study is to determine the roles of N. benthamiana Gα and Gβ subunits (Gβ1 and Gβ2) in the elicitor signalling and to determine whether there is evidence that the heterotrimeric state of N. benthamiana G protein complex plays a regulatory role. From our study, in N. benthamiana, Gα and Gβ2 silencing compromises harpin-induced HR. Additionally, Gα, Gβ1 and Gβ2 silencing causes identical hyposensitivity to elicitor promotion of stomatal closure.
Yeast hybrid assay of Gα and Gβ from N. benthamiana (Fig. 1) supports previous observations of their biochemical evidence for interaction in Arabidopsis, rice and pea (Kato et al. 2004; Misra et al. 2007; Fan et al. 2008). Interaction between Gβ and Gγ is essentially non-dissociable (Gautam et al. 1998). Collectively, these data support the notion that the plant α, β and γ subunits form heterotrimers. It suggests that silencing either Gα or Gβ subunit is sufficient to cause elicitor hyposensitivity, and elicitor regulation of stomatal closure is mediated by the G protein heterotrimer.
Harpin-, rather than boehmerin- or Nep1-induced HR, is Gα- and Gβ-dependent
In comparison with the activation of gene-for-gene-mediated plant cultivar-specific resistance, little is known about the signalling pathways mediating non-cultivar-specific plant resistance. In this paper, harpin, Nep1 and boehmerin are elicitors from fungi, bacteria and oomycetes, respectively. Our findings suggest that at least in the canonical heterotrimeric protein in N. benthamiana, both the Gα and Gβ2 subunits are related to H2O2 accumulation triggered by elicitors. Neither boehmerin nor Nep1-induced hypersensitive response requires a functional heterotrimeric G protein. Only harpin-triggered HR is compromised in Gα- and Gβ2-silenced plants, suggesting that Gα and Gβ2 contribute to harpin-induced HR. It suggests that H2O2 plays different roles in cell death induced by various elicitors. Although not all G protein subunits are involved in elicitor-induced cell death, all the gene-silenced plants show decreased transcripts of PR2b, EDS1, NbrbohA, NbrbohB, indicating that G protein positively regulated defence-related genes in plant defence signalling. Both the Gα and Gβ subunits are necessary for the rapid initial component of the biphasic oxidative burst, but only the Gα protein is required for O3-induced cell death in Arabidopsis (Joo et al. 2005). The Arabidopsis Gβ is involved in unfolded protein response-associated cell death (Wang, Narendra & Fedoroff 2007). Our results are consistent with these reports stating that Gα and Gβ2 are involved in harpin-induced cell death, but Gβ1 silencing does not.
Heterotrimeric G protein is involved in elicitor-induced stomatal closure
One explanation for the similarity of stomatal aperture phenotypes in Gα-, Gβ1- and Gβ2-silenced plants would be that Gβ1 and Gβ2 silencing affects Gα expression. However, qRT-PCR analyses indicate that Gβ1 and Gβ2 silencing have no effect on expression of Gα, and Gα silencing does not affect Gβ1 and Gβ2 expression as well (Fig. 2).
Genetic data from null mutants for GPA1 (α-subunit) and the G protein-coupled receptors, GCR1 and GCR2, support that participation of heterotrimeric G protein plays important roles in guard cell signalling (Wang et al. 2001; Pandey & Assmann 2004; Liu et al. 2007; Zhang et al. 2008). In this study, Gα, Gβ1 and Gβ2 silencing inhibits elicitor-induced stomatal closure, suggesting that both the Gα and Gβ subunits are involved in stomatal closure triggered by elicitors. To our knowledge, this is the first report that G protein Gα and Gβ subunits participate in elicitor-induced stomatal closure. Recent studies indicate that Gβ-deficient mutants show the same alterations in guard cell ABA response in stomatal closure as observed in the Gα-deficient mutants (Fan et al. 2008). Therefore, G protein regulation of stomatal closure may be in the same manner in response to elicitors and ABA. The G protein-dependent ion channel has been reported to regulate the ABA-triggered stomatal response (Viehweger et al. 2006; Fan et al. 2008). In plants, data from patch-clamp experiments demonstrate that G protein-mediated ABA activation of stomatal closure implicates regulation of slow anion channel other than Kout channel (Fan et al. 2008). Alternatively, the elicitor hyposensitive phenotypes in stomatal closure reported here may be accounted for by disruption of signalling via a non-dissociated heterotrimer. However, what kind of channel in guard cells the G protein might stimulate in the elicitor promotion of stomatal closure still needs to be determined.
Other studies have shown that membrane-associated proteins are involved in guard cell signal transduction (Hosy et al. 2003; Negi et al. 2008; Vahisalu et al. 2008). The NtrbohD protein (Kwak et al. 2003), GPCR (Pandey & Assmann 2004), fast vacuolar (FV) channels, K+-selective vacuolar (VK) cation channels (Allen & Sanders 1995) and slow vacuolar (SV) channels are localized on the membrane and function during ABA responses in guard cells (MacRobbie 2006). G protein signalling begins with binding to GPCR, which contains seven transmembrane-spanning domains. Thus, it is suggested that G protein silencing may affect membrane signal and subsequently inhibit elicitor-induced stomatal closure.
Modulation of NO generation by G proteins in elicitor-induced stomatal closure
NO, as a signalling molecule, is a key regulator of plant responses to a range of endogenous signals and stimuli, such as auxin, ABA and elicitors (Zaninotto et al. 2006; Srivastava et al. 2009). It is a key mediator of ABA-induced stomatal closure in peas (Neill et al. 2002), Vicia faba (Garcia-Mata & Lamattina 2002) and Arabidopsis (Bright et al. 2006). The importance of NO during elicitor-induced stomatal closure was demonstrated here by multiple observations: significant rise in NO levels in guard cells, prevention of stomatal closure along with a decrease in NO levels by cPTIO (Fig. 6). Thus, the effects of elicitors with different origin were quite similar to that of auxin and ABA. Our results show that G protein signalling is required to activate the intracellular sources of NO, and contributes to the elicitor-induced stomatal closure in N. benthamiana. We make this conclusion from the observation that NO accumulation is highly attenuated or absent in the guard cells of Gα-, Gβ1- and Gβ2-silenced plants. Hence, the rapid activation of intracellular NO production in guard cells requires signalling through the heterotrimeric G protein. To our knowledge, this is the first study to demonstrate that the Gα and Gβ subunits are involved in NO-mediated stomatal closure triggered by elicitors. Modulation of AOS and NO by G proteins was found both in ExtCaM-induced stomatal closure and in ozone stress responses (Chen et al. 2004; Joo et al. 2005; Li et al. 2009). G proteins may play a central mediating role in guard cell signalling transduction. These results suggest that elicitors, similar to ABA, activate the Gα and Gβ subunits pathway upstream of NO generation in guard cell signalling.
NO can be synthesized in plant via reduction in nitrite by nitrate reductase, oxidation of Arg to citrulline by NO synthase (NOS) and a non-enzymatic NO generation system (Crawford 2006). NOA1 was initially isolated as a NOS in Arabidopsis, but no NOS activity (Zemojtel et al. 2004). NOA1 Arabidopsis mutant shows impaired stomatal closure and less NO accumulation in response to ABA, salt and ExtCaM (Guo, Okamoto & Crawford 2003; Zhao, Tian & Zhang 2007; Li et al. 2009). NR and NIA have been demonstrated to be enzymes that mediate NO synthesis in plants. NR-mediated NO generation has also been suggested to regulate ABA-induced stomatal closure (Desikan et al. 2002; Bright et al. 2006). Here, qRT-PCR analyses of NOA, NIA and NR in gene-silenced plants after elicitor treatment indicate that Gα, Gβ1 and Gβ2 silencing appears to inhibit the expression of NOA1 after boehmerin and Nep1 treatment, the expression of NIA after Nep1 treatment, and the expression of NR upon boehmerin and harpin treatment. Together, these results suggest that G protein-mediated stomatal closure induced by boehmerin may be related with NOA1- and NR-dependent NO accumulation; G protein-mediated stomatal closure induced by harpin may associate with NR-dependent NO accumulation; and G protein-mediated stomatal closure induced by Nep1 may regulate by NOA1- and NIA-dependent NO accumulation. The further identities of these genes involved in response to boehmerin, harpin or Nep1 during G protein-mediated stomatal closure need to be characterized. As shown here, plant stomatal closure induced by elicitors is commonly associated with NO. It is stimulated not only upon boehmerin, oomycete elicitor, but also in response to harpin, Nep1, bacterial and fungal elicitors, respectively. However, the transcript analyses of gene associated with NO have suggested that the major routes of signalling pathways to NO production in response to boehmerin, harpin or Nep1 are with some differences, indicating that G protein and NO are the common signalling molecules during elicitor-induced stomatal closure. They may interconnect and specify the plant signalling transduction through transcriptional changes in response to different pathogen elicitors, and the pattern of NO accumulation involved in plant-microbe interactions has diversified throughout evolution.
The complexity of G protein-mediated signalling in plants
Heterotrimeric G proteins in animals and plants are structurally similar with a conserved mechanism of action. However, plant G proteins lack the multiplicity of genes encoding each of the subunits, as in animals (Temple & Jones 2007). The existence of two different Gβ subunits provides functional diversity to the entire heterotrimer for effector activation and receptor specificity. The similarities of the phenotypes displayed in Gα- and Gβ-silenced plants provide a functional association between the Gα subunit and each of the Gβ subunits in plants. HR is dramatically impaired in Gα- and Gβ2-silenced plants treated with harpin. However, all gene-silenced plants developed normal HRs after boehmerin and Nep1 treatment, indicating that harpin-induced, rather than Nep1- or boehmerin-triggered, HR is Gα- and Gβ2-dependent, and that Gβ1 and Gβ2 act separately in elicitor-triggering HR signalling. Our results further indicate that the Gα and Gβ subunits in the N. benthamiana heterotrimeric G protein act positively in activating different intracellular signalling. A rapid AOS increase in control plants, a sharp AOS decrease, and the down-regulation of NbrbohA and NbrbohB in silenced plants in response to elicitors suggest that heterotrimeric G proteins act positively in regulating AOS accumulation in guard cells in response to different elicitor stimuli. Note that all the Gα-, Gβ1- and Gβ2-silenced plants show significantly impaired elicitor-induced stomatal closure and elicitor-promoted NO and AOS production in guard cells. These results imply that NO and AOS bursts are directly triggered by G protein-mediated signalling and that the G protein signals may act synergistically to trigger NO and AOS bursts.
In this paper, harpin, Nep1 and boehmerin were selected as representative bacterial, fungal and oomycete elicitors, respectively, to compare the patterns recognized by plants. The studies on elicitor/plant interactions revealed common and specific signalling pathway that can be induced by elicitors from different pathogen species throughout evolution. During HR, plants can activate distinct defence pathways involving different regulators depending on the types of pathogen elicitors. DAB staining and the transcript analyses of defence-related genes have shown that a conserved defence-related signalling system can be induced by different pathogen elicitors. The signalling events, such as mobilizing or generating diverse signalling molecules directly/indirectly [such as free calcium (Zhang et al. 2009), H2O2, NO and AOS], can regulate many processes. They might interconnect branch pathways and specify the plant response through transcriptional changes. During stomatal closure, G protein signalling is required to activate the intracellular sources of NO, and contributes to the stomatal closure induced by different pathogen elicitors. It has been suggested that cross-talk between the elicitor signalling pathways exist, enabling the plant to adapt the response depending on the types of pathogen elicitors. Thus, the study here using VIGS in N. benthamiana help to identify G protein involved in signalling pathways induced by elicitors with different origin and how they may be interconnected. As many components of the transduction pathway are still unknown, it is required to investigate the signalling events induced by elicitors with different origin to determine the common and unique steps in each pathway.
In conclusion, silencing of G protein subunits results in many interesting plant cell responses, which reveals that plant immune systems employ both conserved and distinct G protein pathways to sense elicitors from distinct phytopathogens formed in plant-microbe evolution.
ACKNOWLEDGMENTS
This research was supported in part by the National Natural Science Foundation of China (Grant no. 30871605 and 31071645), and the Fundamental Research Funds for the Central Universities (KYZ201105). We thank David Baulcombe (Sainsbury Laboratory, John Innes Centre, Norwich, UK) for the gift of PVX vector and Agrobacterium strains, and Xueping Zhou (College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, China) for the gift of Nicotiana benthamiana seeds.




