Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation
Summary
The chromosome of Mycobacterium tuberculosis encodes five type VII secretion systems (ESX-1–ESX-5). While the role of the ESX-1 and ESX-3 systems in M. tuberculosis has been elucidated, predictions for the function of the ESX-5 system came from data obtained in Mycobacterium marinum, where it transports PPE and PE_PGRS proteins and modulates innate immune responses. To define the role of the ESX-5 system in M. tuberculosis, in this study, we have constructed five M. tuberculosis H37Rv ESX-5 knockout/deletion mutants, inactivating eccA5, eccD5, rv1794 and esxM genes or the ppe25-pe19 region. Whereas the Mtbrv1794ko displayed no obvious phenotype, the other four mutants showed defects in secretion of the ESX-5-encoded EsxN and PPE41, a representative member of the large PPE protein family. Strikingly, the MtbeccD5ko mutant also showed enhanced sensitivity to detergents and hydrophilic antibiotics. When the virulence of the five mutants was evaluated, the MtbeccD5ko and MtbΔppe25-pe19 mutants were found attenuated both in macrophages and in the severe combined immune-deficient mouse infection model. Altogether these findings indicate an essential role of ESX-5 for transport of PPE proteins, cell wall integrity and full virulence of M. tuberculosis, thereby opening interesting new perspectives for the study of this human pathogen.
Introduction
Mycobacterium tuberculosis, the etiological agent of human tuberculosis, employs distinct pathways that mediate protein transport across the complex mycobacterial cell envelope (Bitter et al., 2009): (i) the SecA1-operated pathway, responsible for the secretion of proteins carrying typical N-terminal signal sequences, and an alternative SecA2-operated pathway for proteins lacking such sequences; (ii) the twin arginine translocation (TAT) system, devoted to the secretion of proteins with atypical N-terminal signal sequences containing two arginine residues; and (iii) the recently described type VII secretion pathway, known to secrete Esx proteins with characteristic WXG100 motifs (Pallen, 2002).
The genome of M. tuberculosis harbours five gene clusters coding for type VII secretion systems, designated ESX-1–ESX-5 (Cole et al., 1998; Tekaia et al., 1999). ESX-1 is the most extensively studied, due to its crucial implication in virulence of pathogenic mycobacteria mediated by secretion of effector molecules such as EsxA (6 kDa Early Secreted Antigenic Target, ESAT-6), its protein partner EsxB (10 kDa Culture Filtrate Protein, CFP-10), as well as EspA and other associated proteins (Pym et al., 2003; Fortune et al., 2005; MacGurn et al., 2005; McLaughlin et al., 2007; Frigui et al., 2008; Gordon et al., 2009; Simeone et al., 2009; Garces et al., 2010; Sani et al., 2010; Bottai et al., 2011).
In the present study we focused on the ESX-5 system, which represents the most recently evolved ESX system (Gey van Pittius et al., 2001), encoded in the genomes of various slow growing mycobacteria, including the human pathogens Mycobacterium leprae and Mycobacterium ulcerans and the fish pathogen Mycobacterium marinum, but lacking from phylogenetically more distant saprophytic species such as Mycobacterium smegmatis. Similarly to other ESX clusters, the ESX-5 locus consists of a pair of esx genes (Fig. 1A), coding for EsxM and EsxN proteins, immunodominant antigens, which induce strong CD4+ T-cell responses both in humans and in different animal models (Alderson et al., 2000; Jones et al., 2010). Immediately upstream of esxM/esxN the ESX-5 locus harbours the ppe25-pe19 gene cluster, coding for members of mycobacteria-specific protein families, named after the conserved N-terminal proline-glutamic acid (PE) or proline-proline-glutamic acid (PPE) motifs (Cole et al., 1998; Tekaia et al., 1999). The ppe-pe-esx genes are flanked by blocks of ecc (esx conserved components) genes that encode membrane proteins and ATP-binding proteins, predicted to be components of an ATP-powered secretion machine involved in the export of the corresponding Esx proteins (Brodin et al., 2004; Bitter et al., 2009). First insights into the role of ESX-5 were gained in the last few years by using M. marinum as model (Abdallah et al., 2006; 2009; Daleke et al., 2011; Weerdenburg et al., 2012). Analysis of secretomes of two M. marinum ESX-5 transposon mutants revealed that a functional ESX-5 system is required for secretion/transport of various PPE and PE proteins, such as the heterogeneously expressed PPE41 protein from M. tuberculosis (Abdallah et al., 2006) or PPE and PE proteins belonging to the PPE_MPTR and PE_PGRS subgroups (Abdallah et al., 2009). PPE_MPTR and PE_PGRS, characterized by C-terminal domains containing Major Polymorphic Tandem Repeats (PPE_MPTR) or a Polymorphic GC-Rich Sequence (PE_PGRS), are the phylogenetically most recent subclasses of PE and PPE proteins, whose massive expansion is closely associated with the ESX-5 cluster (Gey van Pittius et al., 2006). Recently, also the M. tuberculosis LipY lipase (LipYMt) and its orthologous protein in M. marinum (LipYMm) were found to be targeted to the cell surface of M. marinum in an ESX-5-dependent manner (Daleke et al., 2011). Due to its role in the transport of PE and PPE proteins in M. marinum, ESX-5 is predicted to play a major role in the interaction of pathogenic mycobacteria with the host. Although the exact function of most of PPE and PE proteins is still unknown, several of these proteins have been proposed to be virulence factors (Ramakrishnan et al., 2000; Brennan et al., 2001; Li et al., 2005). Because of their abundance (pe and ppe genes account for 7.1% of the coding capacity of the M. tuberculosis genome) (Cole et al., 1998), their surface localization (Sampson et al., 2001; Banu et al., 2002; Cascioferro et al., 2007; Song et al., 2008; Chaturvedi et al., 2010) and the highly polymorphic nature of the C-terminal domain, PPE and PE proteins are thought to be implicated in antigenic variation of mycobacterial strains (Delogu and Brennan, 2001; Bottai and Brosch, 2009).
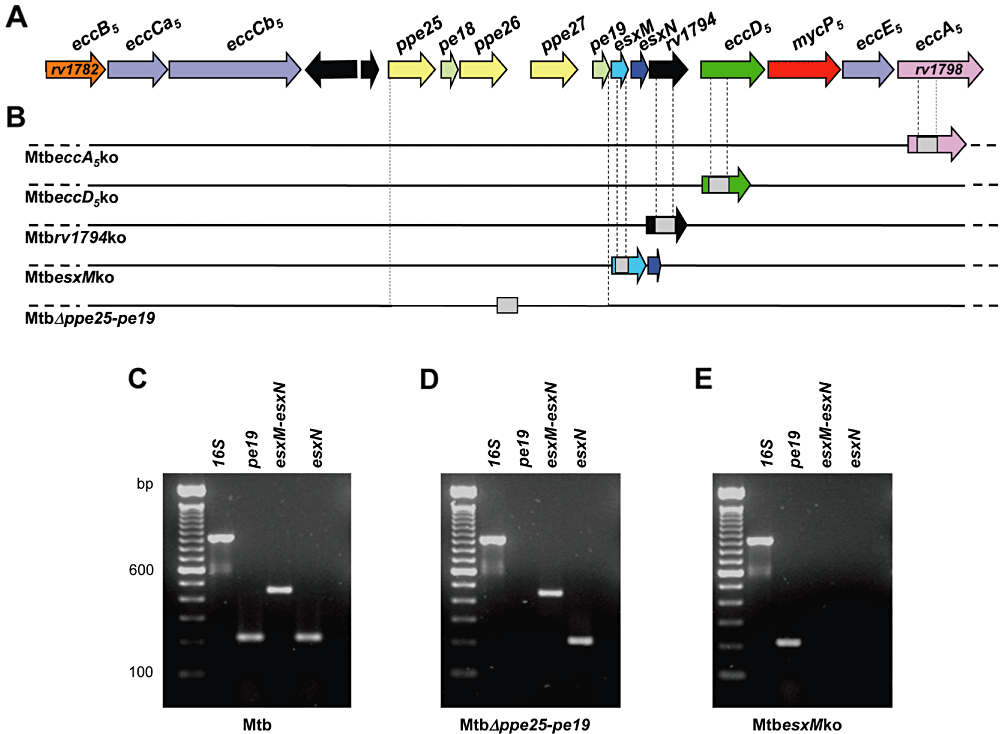
Selective inactivation of ESX-5 genes in M. tuberculosis H37Rv. A. Genomic organization of the ESX-5 locus in M. tuberculosis H37Rv. The various ESX-5 genes encoding proteins belonging to different protein families are represented by coloured arrows (Gey van Pittius et al., 2006; Bitter et al., 2009): orange, amino-terminal transmembrane protein; violet, amino-terminal transmembrane ATPase; yellow, PPE family; light green, PE family; light blue, CFP-10 (EsxB)-homologue; blue, ESAT-6 (EsxA)-homologue; black, Rv1794 (orthologous to the M. marinum ESX-5-associated MMAR_2676 gene); green, integral membrane protein; red, mycosin (subtilisin-like serine protease); pink, AAA+ ATPase. Black arrows indicate region-associated genes coding for proteins not thought to be involved in the ESX-5 secretion machinery. B. Schematic representation of genomic organization of ESX-5 mutants. In each mutant strain, only the arrow representing the mutated gene is depicted. The grey boxes represent the kanamycin resistance cassettes used to inactivate the various ESX-5 genes. C–E. Analysis of esxM–esxN expression in WT M. tuberculosis (C), MtbΔppe25-pe19 (D) and MtbesxMko (E) mutants by RT-PCR using various combinations of primers specific for esxM and esxN. As control, RT-PCR was performed with primers specific for rRNA 16S or pe19.
In contrast to M. marinum, the biological function of the ESX-5 system in M. tuberculosis, as well as the impact of this system in virulence and host-pathogen interaction remain largely unknown. In the present study, in order to identify the elements constituting the M. tuberculosis ESX-5 secretion machinery, and to evaluate their involvement in PPE/PE transport and virulence, various genes from the ESX-5 locus were inactivated or deleted in M. tuberculosis H37Rv. The mutants were then tested in vitro for their ability to secrete EsxN and export various PPE and PE proteins, such as PPE41 and PE_PGRS proteins. Moreover, to investigate the impact of different ESX-5 genes on virulence of M. tuberculosis, the growth properties of all ESX-5 mutants were analysed in ex vivo/in vivo models, i.e. murine macrophages and severe combined immune-deficient (SCID) mice. This approach allowed us to characterize the ESX-5 system of M. tuberculosis H37Rv and demonstrate the importance of this system for the transport of particular ESX and PPE proteins and full virulence of the pathogen.
Results
Identification of ESX-5 genes required for EsxN secretion in M. tuberculosis
Five M. tuberculosis H37Rv knockout (KO) mutants were constructed, in which selected ESX-5 genes were inactivated by insertion of a kanamycin resistance cassette, or from which a defined genomic segment of the ESX-5 locus was deleted (Fig. 1B). MtbeccA5ko and MtbeccD5ko are inactivated for eccA5Mt (rv1798) or eccD5Mt (rv1795), which code for an AAA+ ATPase (EccA5) or a putative transmembrane protein (EccD5) of the ESX-5 secretion machinery respectively. In the Mtbrv1794ko strain, gene rv1794 is interrupted, whose orthologue in M. marinum (MMAR_2676) encodes a protein with unknown function involved in ESX-5-linked secretion (Abdallah et al., 2009). In MtbesxMko, gene esxM (rv1792) is inactivated, which is encoding a WXG-100 member in the ESX-5 region. Finally, MtbΔppe25-pe19 is lacking the genomic segment containing the ESX-5-associated ppe25-pe19 (rv1787–rv1791) genes. For each mutant, a complemented derivative was constructed, in which the cosmid I104, carrying the entire ESX-5 cluster, was stably integrated into the chromosome via the attB site, following a strategy that has previously allowed to successfully complement ESX-related mutant strains (Pym et al., 2003; Brodin et al., 2010; Bottai et al., 2011). Southern hybridization patterns displayed by M. tuberculosis wild-type (WT), KO constructs and complemented strains confirmed the partial replacement of the selected ESX-5 genes by a kanamycin cassette in the different mutants as well as the presence of an intact ESX-5 cluster in complemented strains (Fig. S1).
Expression studies by reverse-transcription PCR (RT-PCR) on RNAs obtained from WT and ESX-5 mutant strains (Fig. 1C–E) demonstrated that the esxM gene is co-transcribed with the paired gene esxN (Fig. 1C). As expected, inactivation of other ESX-5 genes, such as rv1794, eccD5Mt and eccA5Mt, did not affect expression of esxM/N (data not shown). The esxM/N genes were still transcribed in the MtbΔppe25-pe19 strain (Fig. 1D), indicating that ESX-5-associated pe and ppe genes are not required for the expression of downstream esxM/N genes. In contrast, the disruption of esxM also abolished the esxN transcription (Fig. 1E), thus indicating that the MtbesxMko strain is actually a double KO strain for the two esx genes encoded by the ESX-5 locus, similar as what was observed for the esxB/A couple of the ESX-1 system (Brodin et al., 2006).
To investigate whether selected M. tuberculosis ESX-5 KO mutants displayed a defect in EsxN secretion, culture filtrates as well as total lysates from WT, ESX-5 KO and complemented strains were tested in Western blot analyses using an anti-EsxN polyclonal serum. As shown in Fig. 2A, large amounts of EsxN were detected in the culture filtrates as well as in total lysates of M. tuberculosis H37Rv WT and the Mtbrv1794ko mutant. Lysis controls using antibodies against GroEL2 demonstrated that these samples were not contaminated with cytosolic or membrane associated proteins, thus indicating that in contrast to its M. marinum orthologue, Rv1794 is not directly involved in EsxN secretion in M. tuberculosis. Conversely, no EsxN was detected in the culture supernatants from MtbeccD5ko and MtbeccA5ko mutants, despite a 10 kDa specific band corresponding to EsxN observed in the total lysates from both these strains (Fig. 2A). These results indicate that eccD5Mt and eccA5Mt are required for an efficient translocation of EsxN. Interestingly, no EsxN specific band was detected in the culture supernatants from the MtbΔppe25-pe19 strain, suggesting that ESX-5-associated -PPE and -PE proteins also play a role in the export of this protein. In contrast to expression studies, showing a tight link between esxM and esxN expression (Fig. 1), a 10 kDa band was recognized by the anti-EsxN polyclonal serum in the total lysates from the MtbesxMko mutant. The M. tuberculosis genome harbours four other esxN-like genes not associated to any ESX cluster (Cole et al., 1998; Uplekar et al., 2011), which are co-transcribed with their paired esxM-like genes during the in vitro growth (data not shown). EsxN and the four EsxN-like proteins, collectively known as Mtb9.9 family (Alderson et al., 2000), share highly similar protein sequence, differing only by a few amino acids. When immunoblot analysis was performed on culture supernatants subjected to 2D gel electrophoresis, four different spots were detected in the culture supernatants from the WT strain as well as from the Mtbrv1794ko mutant (Fig. 2C). The absence of specific spots recognized by the anti-EsxN serum in the culture filtrates from the KO mutants suggests that ESX-5 is involved in the secretion of all Mtb9.9 proteins and that secretion of EsxN and/or EsxM is required for secretion of other Mtb9.9 family members. However, the exact identification by mass spectrometry of the four spots recognized by the anti-EsxN serum was unsuccessful, due to low amount of the proteins. For this reason, it cannot be excluded that some spots might represent different isoforms of EsxN, resulting from post-transcriptional modifications. As depicted in Fig. 2B and C, secretion of EsxN was restored in all complemented strains.
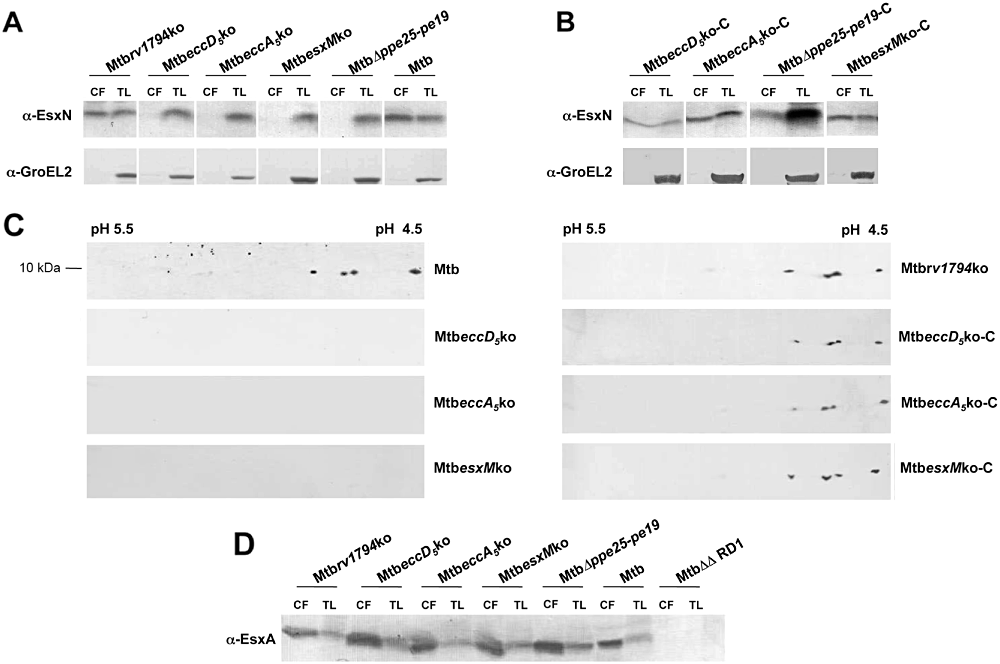
In vitro expression and secretion of EsxN in M. tuberculosis ESX-5 mutants. A and B. Fifteen micrograms of culture filtrate (CF) or total cell lysate (TL) proteins from ESX-5 mutants (A) and their complemented derivatives (B) were subjected to SDS-PAGE and tested in Western blotting by using a rabbit anti-EsxN polyclonal serum. Mouse anti-GroEL2 antibody was used for lysis control. C. Two-dimensional analysis and immunoblot of CF from M. tuberculosis WT, ESX-5 KO and complemented strains. Fifty micrograms of culture supernatants were separated by isofocusing using a pH 4.5–5.5 gradient and 4–20% SDS-PAGE, in the first and second dimension respectively. Protein detection was carried out by immunoblot using the anti-EsxN polyclonal serum as previously described. For simplicity, only the region of the filter containing the anti-EsxN-recognized spots is shown. D. Expression and secretion of EsxA in ESX-5 mutant strains. CF and TL proteins from WT M. tuberculosis and ESX-5 mutants were subjected to SDS-PAGE and tested in Western blot using the anti-ESAT-6 Hyb76-8 monoclonal antibody. As depicted in A, culture filtrates were negative for GroEL2. Preparations from the MtbΔΔRD1 strain were included in the immunoblotting analysis as negative control.
Preliminary experiments in M. marinum suggested a potential cross-interaction between ESX-1 and ESX-5 (A. Abdallah and W. Bitter, unpubl. obs.). To investigate whether the inactivation of ESX-5 could affect the secretion of ESX-1 substrates, culture filtrates from all ESX-5 mutants were tested in Western blotting for the presence of EsxA (ESAT-6). Whereas no EsxA was observed in culture filtrates from the negative control strain MtbΔΔRD1 (Bottai et al., 2011) (Fig. 2D), similar amounts of the protein were detected in samples from various ESX-5 mutants and WT M. tuberculosis (Fig. 2D), suggesting that the inactivation of different components of the ESX-5 secretion apparatus has no relevant impact on EsxA production and secretion.
PPE41 and PE_PGRS secretion/transport in ESX-5 mutant strains
Previous findings in M. marinum showed that various PPE and PE proteins, such as PPE41 and PE_PGRS, are exported in an ESX-5-dependent manner (Abdallah et al., 2006; 2009). In order to investigate whether these proteins were secreted/transported via the ESX-5 system also in M. tuberculosis, all ESX-5 mutants, their complemented derivatives and the WT strain were tested for their ability to secrete PPE41 and PE_PGRS proteins in the same growth conditions that have been previously described for M. marinum ESX-5 mutants using Middlebrook 7H9 medium (Abdallah et al., 2006; 2009). As shown in Fig. 3A, a specific band of 23 kDa corresponding to PPE41 was observed in culture supernatants from the M. tuberculosis control strain as well as in preparations from Mtbrv1794ko and MtbesxMko strains. PPE41 was still detected in the culture supernatants from the MtbeccA5ko mutant, although the amounts were substantially lower than those detected in samples from the WT strain. In contrast, a clear defect in PPE41 secretion was observed for MtbeccD5ko: no specific band corresponding to PPE41 was indeed detected in the culture supernatants from this strain, despite the presence of a PPE41-specific band in the whole cell lysates. Similarly, no PPE41 was detected in culture filtrates from MtbΔppe25-pe19, further suggesting that ESX-5 encoded PE and PPE proteins might be involved in the ESX-5 secretion machinery. Secretion of PPE41 in culture supernatants was restored in MtbeccD5ko-C and MtbΔppe25-pe19-C complemented strains (Fig. 3B).
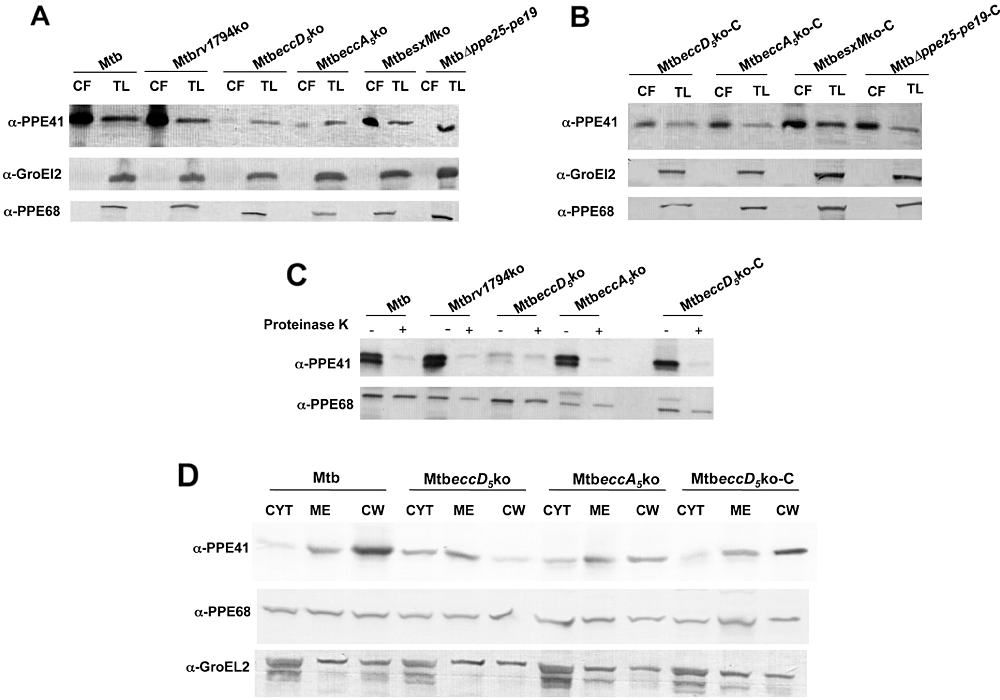
Effect of ESX-5 inactivation on PPE41 secretion and transport. A and B. Culture supernatants and total lysates from WT M. tuberculosis and ESX-5 mutants (A) or complemented strains (B) were tested in Western blot with anti-PPE41 rabbit polyclonal serum. As control, samples were tested with a mouse anti-GroEL2 antibody or a rabbit polyclonal serum anti-PPE68. C. Proteinase K sensitivity assay performed on WT M. tuberculosis, Mtbrv1794ko, MtbeccD5ko and MtbeccA5ko. Live bacteria were incubated with (+) or without (−) proteinase K, and the whole cell lysates obtained from treated and untreated bacteria were separated by SDS-PAGE. PPE41 and PPE68 detection was carried out by immunoblotting as previously reported. D. Ten micrograms of cytoplasm (CYT), membrane (ME) and cell wall (CW) proteins from WT M. tuberculosis, MtbeccD5ko and MtbeccA5ko strains were tested in Western blot using anti-PPE41 and anti-PPE68 polyclonal sera.
When samples were tested with the anti-PGRS antibody, a few PE_PGRS proteins were observed in the culture filtrates of M. tuberculosis H37Rv. However, although some differences in the amounts of PE_PGRS proteins were detected in culture filtrates from various ESX-5 KO and complemented strains (Fig. S2), variations in the protein pattern recognized by the anti-PGRS antibody appeared to be weaker than differences previously observed in ESX-5 M. marinum mutants (Abdallah et al., 2009). No significant difference was detected among the strains when the analysis of PE_PGRS secretion was performed on culture supernatants obtained from bacteria grown in Sauton medium (data not shown). The lack of GroEL2 and PPE68 in culture supernatants from all tested strains confirmed the absence of contamination of these samples with cell-associated proteins (Fig. 3A and B).
To investigate more in-depth the impact of eccD5Mt inactivation on transport of PPE41 and PE_PGRS, the localization of these proteins was determined both in WT and MtbeccD5ko strains as well as in Mtbrv1794ko and MtbeccA5ko mutants by proteinase K assays and Western blot analysis of subcellular fractions. As control, the localization of PPE68 was analysed. The proteinase K treatment removed the majority of PPE41 in M. tuberculosis WT, Mtbrv1794ko and MtbeccA5ko (Fig. 3C), indicating that in these strains the protein is exposed on the bacterial surface. In contrast, the protein was (partially) resistant to the protease treatment in the MtbeccD5ko mutant, suggesting that PPE41 is not exposed on the cell surface of this strain. Western blot analysis of cytosol, membrane and cell wall fractions with the anti-PPE41 antibody demonstrated that the protein was mainly associated to membrane and cell wall in the WT M. tuberculosis strain and MtbeccA5ko mutant. In contrast, the protein accumulated in the cytoplasm and membrane fractions in MtbeccD5ko (Fig. 3D), further confirming an involvement of EccD5 in the transport of PPE41. These analyses revealed substantially lower amounts of PPE41 in the MtbeccD5ko mutant as compared to the control strain. It cannot be excluded that the protein might have a reduced stability as consequence of its altered localization. Sensitivity to proteinase K treatment and translocation to cell wall were restored in the MtbeccD5ko-C complemented derivative (Fig. 3C and D).
Western blot analysis of proteinase-K-treated sample from WT M. tuberculosis H37Rv with the anti-PGRS monoclonal antibody revealed that some PE_PGRS proteins were sensitive to protease digestion and thus exposed to the mycobacterial surface (data not shown). In agreement with data from secretion analyses, PE_PGRS protein patterns from proteinase K-treated WT M. tuberculosis H37Rv and ESX-5 mutants revealed no appreciable differences between WT and mutant strains (data not shown). Similarly as what was observed in samples from WT M. tuberculosis, PPE68 was resistant to protease treatment and efficiently translocated to membrane and cell wall also in the MtbeccD5ko mutant (Fig. 3C and D), thus suggesting that this ESX-1-associated protein is not exposed on the mycobacterial surface and is transported to the cell wall in an ESX-5-independent manner. Together, these results indicate that in M. tuberculosis, a functional ESX-5 system is required for an efficient translocation and a correct localization of PPE41.
Effect of ESX-5 inactivation on cell wall integrity
To investigate the impact of ESX-5 inactivation on cell wall integrity/stability, the survival of the MtbeccD5ko and WT M. tuberculosis after exposure to detergents was compared. Bacteria in logarithmic growth phase were exposed to 0.1% SDS treatment and after 6 h viable bacteria were counted. Although the addition of the detergent caused a rapid decrease in the cfu numbers recovered for all the tested strains (Fig. 4A), the MtbeccD5ko mutant showed an enhanced sensitivity to SDS as compared to control strains: the percentage survival values were 1% for the MtbeccD5ko mutant and 10% for the WT or the complemented MtbeccD5ko-C strains respectively (Fig. 4B). The greater sensitivity of the MtbeccD5ko mutant to SDS was confirmed when bacterial survival was assessed after 24 and 72 h of incubation with SDS (data not shown). The sensitivity of the other ESX-5 KO strains was comparable to that of the parental strain (Fig. 4).
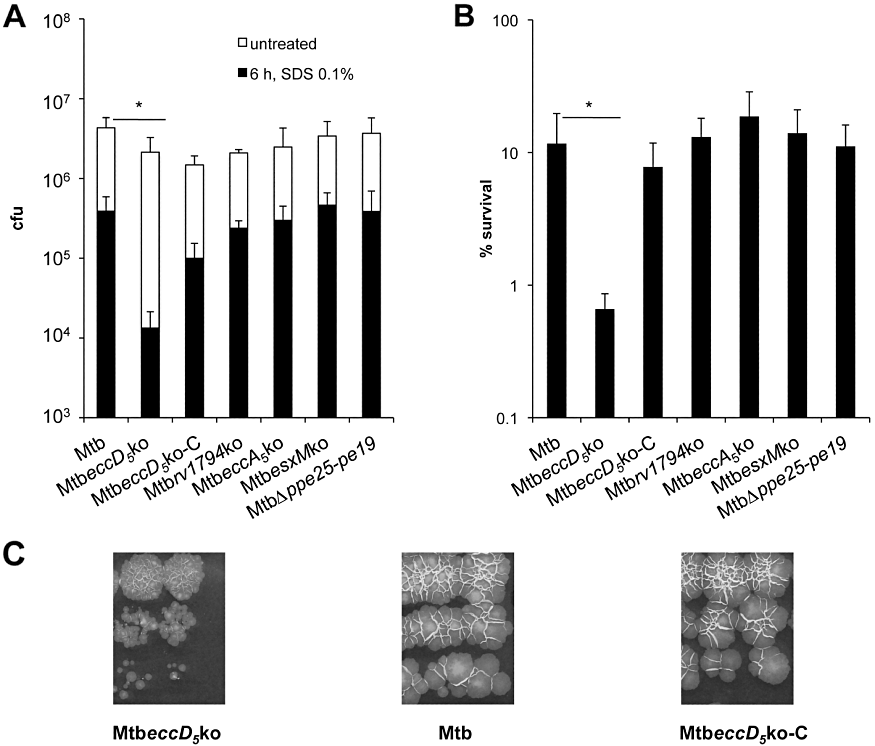
Effect of EccD5 disruption on cell wall integrity of M. tuberculosis. A and B. Sensitivity of MtbeccD5ko and other ESX-5 mutants to SDS. Bacterial strains were exposed to SDS 0.1% for 6 h and the number of cfu (A) and the survival percentages (B) were determined. The means and the standard deviations of cfu values and survival percentages obtained in four independent experiments are shown. The significant difference in cfu and survival percentage values between H37Rv and MtbeccD5ko strain was determined by one-way anova test, followed by Bonferroni post-hoc test (*P < 0.05). C. Growth of MtbeccD5ko on solid medium. Three-week-old cultures of MtbeccD5ko, M. tuberculosis WT and the MtbeccD5ko-C strain grown on Middlebrook 7H11 medium. Bacterial cultures were washed, resuspended and serially 10-fold diluted in PBS: a sample of 20 µl of each dilution was spotted onto agar plates.
The mycobacterial cell envelope, which represents an effective barrier to both hydrophobic and hydrophilic solutes, is considered a major determinant of the intrinsic resistance of M. tuberculosis to different antibiotics (Brennan and Nikaido, 1995). Thus, alterations in cell wall integrity may also be detected by measuring the susceptibility of mycobacteria to various antibiotics. To further evaluate the effects of eccD5Mt inactivation on cell wall properties, the susceptibility of MtbeccD5ko, WT M. tuberculosis and other ESX-5 mutants (e.g. MtbΔppe25-pe19) to various classes of antibiotics with diverse chemical structure and mechanism of action to which mycobacteria are naturally resistant was compared (Jackson et al., 1999). These included antibiotics targeting several steps in the cell wall biosynthetic pathway, such as ampicillin, bacitracin and vancomycin; antibiotics targeting DNA replication such as ofloxacin and norfloxacin; antibiotics targeting transcription, such as rifampicin, which is also a front-line anti-tuberculosis drug. Isoniazid and ethambutol, two antibiotics affecting the cell wall biosynthesis, to which mycobacteria are susceptible, were included in the analysis for control purposes. As shown in Table 1, while all strains displayed comparable sensitivity to isoniazid and ethambutol, the MtbeccD5ko mutant was highly sensitive to ampicillin, vancomycin and bacitracin. The MIC values for these antibiotics were indeed 32- or 64-fold lower for the MtbeccD5ko mutant than those of the WT strain (Table 1) or the MtbΔppe25-pe19 mutant (data not shown). The enhanced sensitivity of the MtbeccD5ko strain to hydrophilic antibiotics was confirmed when the susceptibility was tested by using the microplate-based nitrate reductase assay in broth (Kumar et al., 2005). In this case, the MIC values for ampicillin, vancomycin and bacitracin were 0.25, 0.5 and 8 µg ml−1 for MtbeccD5ko and 100, 8 and 256 µg ml−1 for the WT M. tuberculosis H37Rv or MtbΔppe25-pe19 respectively. These results thus suggest that the inactivation of the ESX-5 system via the disruption of EccD5 strongly affects the mutant's cell wall stability. This effect was also visible by inspection of the MtbeccD5ko mutant on Middlebrook 7H11 plates, which was characterized by a small colony phenotype that was reverted upon complementation with the cosmid encoding a functional ESX-5 system (Fig. 4B). It is noteworthy that the MtbeccD5ko strain also displayed smaller colonies than the M. tuberculosis H37Rv control on Middlebrook 7H9 agar plates without malachite green (data not shown), thus suggesting that the small colony morphotype of the EccD5 mutant is not due to the presence of this inhibitory agent in the medium. The finding that the MtbeccD5ko mutant showed no difference in growth as compared to the WT strain in liquid medium (Fig. S3) further emphasizes the importance of EccD5 and ESX-5 in general for a fully functional cell envelope that determines the contact with its immediate environment.
| MtbeccD5ko | Mtb | MtbeccD5ko-C | |
|---|---|---|---|
| Ampicillin (µg ml−1) | 12.5 | 400 | n.a. |
| Bacitracin (µg ml−1) | 6.25 | 400 | 400 |
| Vancomycin (µg ml−1) | 1.25 | >40 | 10 |
| Norfloxacin (µg ml−1) | 1.25 | 2.5 | 2.5 |
| Ofloxacin (µg ml−1) | 0.125 | 1 | 0.5 |
| Rifampicin (µg ml−1) | 0.03 | 0.06 | 0.06 |
| Isoniazid (µg ml−1) | 0.5 | 0.5 | 0.5 |
| Ethambutol (µg ml−1) | 3.2 | 3.2 | 3.2 |
- MIC values were determined as the lowest concentration of antibiotic which reduced the bacterial viability by at least 99% on solid medium. n.a.: not applicable due to the presence of an ampicillin resistance gene in the cosmid used for the construction of the complemented strain.
Growth of the ESX-5 mutant strains in macrophages
To investigate the effect of ESX-5 inactivation on intracellular growth characteristics of M. tuberculosis, the ex vivo growth of the ESX-5 mutants and the WT strain were compared in bone marrow-derived macrophages (BMDM). Cells were infected with the various strains at a multiplicity of infection (moi) of 1:1, and the number of intracellular bacteria was determined at 4 h, and 1, 4 and 7 days after infection. The ESX-5 mutants were taken up by BMDM with similar efficiency as the M. tuberculosis WT (data not shown), indicating that inactivation of different components of the ESX-5 system has no relevant impact on the ability of M. tuberculosis to be engulfed by host macrophages.
In contrast, when the ex vivo growth kinetics of the various ESX-5 KO strains were analysed, differences in their intracellular growth abilities were observed (Fig. 5). The Mtbrv1794ko, MtbesxMko and MtbeccA5ko mutants showed intracellular growth kinetics similar to that of the M. tuberculosis WT, resulting in 1 log10 increase in the cfu number over a 7 day period. It should be mentioned, however, that the MtbeccA5ko showed impaired growth as compared to the WT M. tuberculosis H37Rv in THP-1-derived macrophages and type II pneumocyte cell line (A549) (Fig. S4). An attenuated phenotype in murine macrophages was observed for MtbΔppe25-pe19 and MtbeccD5ko mutants. Seven days after infection, only a 0.5 log10 increase was indeed observed in the cfu numbers recovered from macrophages infected with this mutant strain MtbΔppe25-pe19. An even stronger attenuation phenotype was displayed by the MtbeccD5ko mutant for which no growth was observed in murine macrophages over the same period. Complementation of MtbΔppe25-pe19 and MtbeccD5ko strains with an entire ESX-5 cluster increased the intracellular growth ability of the mutants, resulting in a 0.8 log10 increase in the cfu numbers recovered from infected cells after 7 days from infection.
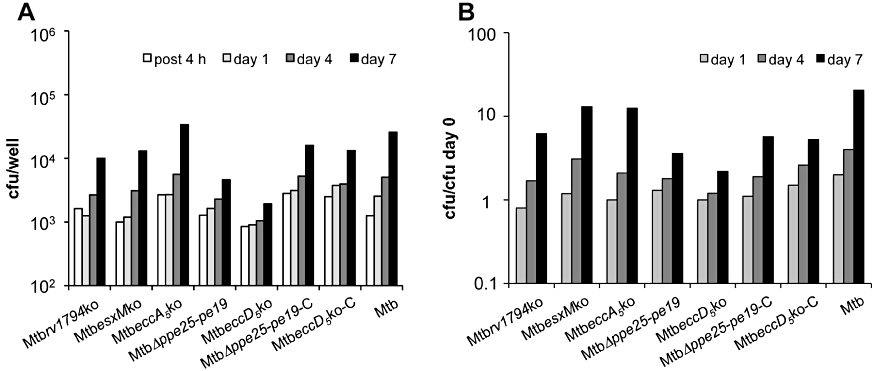
Intracellular growth of ESX-5 mutant strains in BMDM. BMDM (1 × 104 cells per well) obtained from C57BL/6 mice were infected with various ESX-5 mutants, their corresponding complemented derivatives, and the WT M. tuberculosis H37Rv strain using an moi of 1:1. cfu numbers of intracellular bacteria were determined 4 h, and 1, 4 and 7 days after infection. The figure shows the average of cfu numbers (A) and fold growth values (cfu/cfu at day 0) (B) obtained in one experiment performed in triplicate.
Virulence of the ESX-5 mutants in SCID mice
The impact of ESX-5 inactivation on virulence of M. tuberculosis was assessed by evaluating the in vivo growth of ESX-5 mutants in SCID mice. In agreement with results obtained from murine macrophage infection studies, the MtbeccA5ko, MtbesxMko and Mtbrv1794ko mutants displayed a rapid growth both in spleen and lungs of infected mice (Fig. 6A–D), as indicated by high bacterial load in the target organs (108–109 cfu) and severe splenomegaly (data not shown), comparable to those observed for the M. tuberculosis WT.
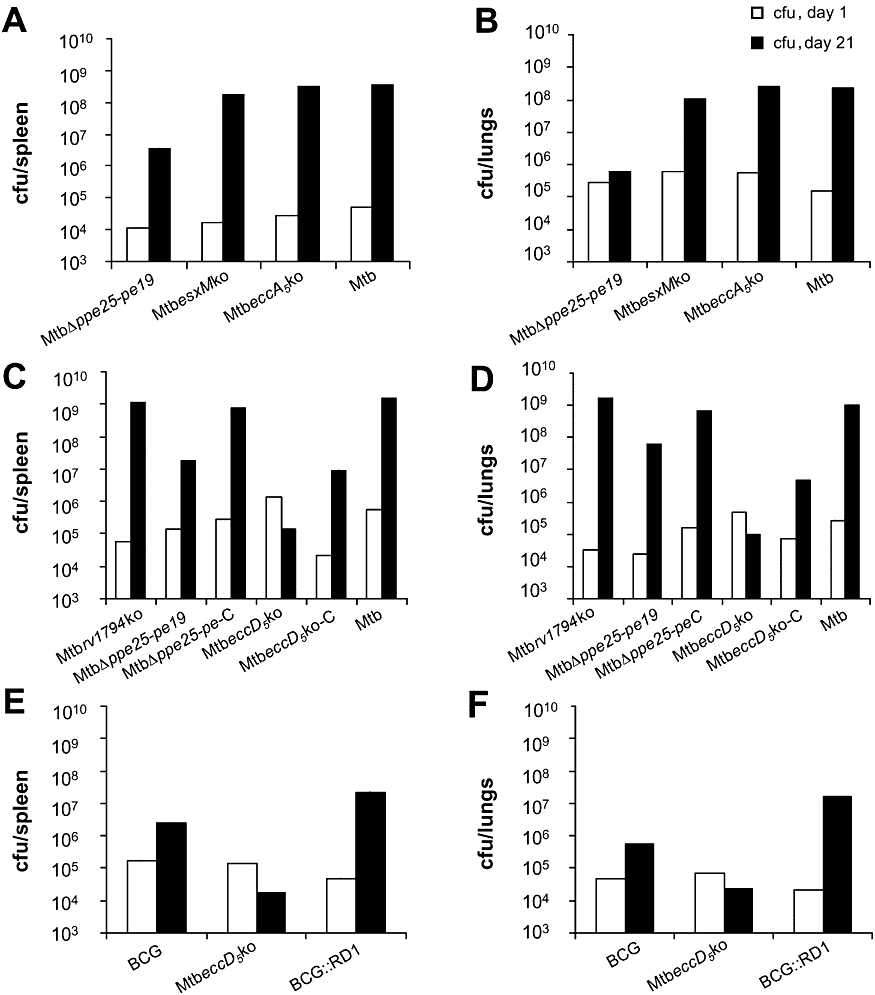
Virulence of different ESX-5 mutant strains in a SCID mouse infection model. SCID mice were infected intravenously with 1 × 106 cfu per mouse of various ESX-5 mutant and control strains. A, C and E depict the average cfu numbers recovered from the spleen of groups of three to four mice in three independent experiments at day 1 and day 21 post infection. B, D and F show the average cfu numbers in the lungs of the same groups of mice.
Conversely, the MtbΔppe25-pe19 mutant showed a strongly attenuated phenotype. Three weeks after infection, a 3- and 2-log10 reduction was observed in the numbers of cfu recovered from spleen and lungs of MtbΔppe25-pe19-infected mice relative to the M. tuberculosis H37Rv-infected controls (Fig. 6A–D). Levels of virulence similar to those of the M. tuberculosis control strain were restored in the ESX-5 complemented MtbΔppe25-pe19-C derivative (Fig. 6C and D). The MtbeccD5ko strain showed an even stronger attenuation: inactivation of eccD5Mt gene resulted indeed in no growth either in spleen or lungs of infected mice over a 21 day period (Fig. 6C and D), and reduced splenomegaly (data not shown). Complementation of MtbeccD5ko with a functional ESX-5 system increased the in vivo growth of the strain, resulting in a 3 log10 increase in numbers of cfu recovered in spleen and lungs, although it did not restore the virulence to the level of the WT strain.
The severe attenuation of the MtbeccD5ko mutant was further confirmed when the in vivo growth of this strain was compared to that of the vaccine strain Mycobacterium bovis BCG (BCG) and the partially virulent BCG::RD1 (Brodin et al., 2006). As depicted in Fig. 6E and F, no growth was observed for the MtbeccD5ko mutant, whereas 1- and 2-log10 increase in cfu number was observed in spleen and lungs of mice infected with BCG and BCG::RD1 strains respectively.
Discussion
The role of the ESX-5 system in the export of selected PPE and PE proteins in M. marinum, as well as the involvement of this system in modulation of cytokine responses by M. marinum-infected human macrophages are well established (Abdallah et al., 2006; 2008; 2009; Daleke et al., 2011). In contrast, nothing is known about the function of the ESX-5 system in M. tuberculosis. The present study was thus focused on the characterization of the ESX-5 system of M. tuberculosis on the basis of ESX-5 KO and deletion constructs.
As a first conclusion drawn from the results obtained, the characteristics of different mutants and the positions of the corresponding inactivated genes argue against the possibility that the phenotypes displayed by the various ESX-5 mutants (with exception of the EsxM/N couple, for which a downstream effect of esxM disruption on esxN was observed) were due to polar effects on the expression of downstream genes. Screening of ESX-5 mutants for their ability to secrete EsxN showed that eccA5Mt and eccD5Mt are required for secretion of EsxN. These genes are homologous to eccA1Mt (rv3868) and eccD1Mt (rv3877), both encoding for components of the ESX-1 secretion apparatus involved in transport of ESAT-6 and CFP-10 (Brodin et al., 2006). In contrast to the ESX-1-encoded pe35 gene that is implicated in the expression of esxB/esxA (Brodin et al., 2006), the ESX-5-associated pe and ppe genes are dispensable for the expression of esxM/N but required for secretion of EsxN. Finally, rv1794, the orthologue of MMAR_2676, which in M. marinum was reported to affect EsxN secretion and export of various PE and PPE proteins (Abdallah et al., 2009), is not implicated in the secretion of EsxN in M. tuberculosis H37Rv. These findings suggest that in spite of high amino acid similarity (96% identity), Rv1794 and MMAR_2676 might have different functions in the ESX-5 secretion machinery that have evolved as a consequence of the adaptation of these two mycobacterial species to different hosts. Substantial differences in function of proteins encoded by ESX clusters from different mycobacterial species have been previously described for the ESX-1 secretion-associated protein EspG1 (Bottai et al., 2011), which shows weak similarity with Rv1794. While EspG1Mm is required for EsxA secretion and virulence in M. marinum (Gao et al., 2006), the EspG1Mt is not directly involved in EsxA secretion, although it plays a relevant role in virulence of M. tuberculosis (Bottai et al., 2011). Alternatively, the function of Rv1794 could be redundant in M. tuberculosis.
PPE41 secretion and localization studies in M. tuberculosis ESX-5 mutant strains confirmed previous findings in M. marinum showing a major role of the ESX-5 secretion system in PPE export. However, the various components of the ESX-5 secretion apparatus seem to have a different impact on this process. Whereas only minor reduction of PPE41 transport was observed after disruption of the eccA5Mt gene, the inactivation of eccD5Mt abolished the secretion of PPE41, and also resulted in a profound reduction of the amounts of PPE41 transported to the bacterial envelope. It is plausible that the different phenotypes exhibited by EccD5 and EccA5 mutants might be caused by a deviating involvement of the proteins in the stability of the ESX-5 secretion machinery. Recently, biochemical characterization of ESX-5 components in M. marinum and BCG suggested that EccD5 is part of a four-membered protein complex forming the core of the ESX-5 secretion apparatus, whereas EccA5 is not part of this complex (E. Houben, pers. comm.). Thus, disruption of a core component, such as EccD5, likely abolishes the stability of the entire complex. In support of this hypothesis, preliminary immunoblotting experiments identified much lower amounts of EccB5, another ESX-5 core component, in membrane and cell wall fractions of MtbeccD5ko relative to WT and MtbeccA5ko strains (Fig. S5). These results suggest that EccD5 and EccB5 are indeed part of the same core complex, which does not include EccA5 (Fig. S5) or ESX-5-associated PE and PPE proteins (data not shown). In addition, the minor effect of eccA5 disruption in M. tuberculosis on PPE41 secretion compared to M. marinum (Abdallah et al., 2006) might also be due to a possible redundancy of eccA5 with homologues eccA genes from other ESX systems such as ESX-2, which is not present in the genome of M. marinum (Stinear et al., 2008). However, as deletion of the ESX-2 cluster in the MtbeccA5ko genetic background did not interrupt the PPE41 secretion in the double mutant (data not shown), this does not seem to be the case. The example of eccA5, like for rv1794 and espG1, shows again that in spite of the high conservation of ESX loci between M. marinum and M. tuberculosis certain genes might not have the same impact in one species than in the other. Finally, it cannot be excluded that other AAA+ ATPases specifically present in M. tuberculosis might complement for the loss of EccA5Mt function.
No obvious effect on PE_PGRS proteins exposed on the cell surface or released in the culture supernatants was detected after inactivation of different components of the ESX-5 secretion machinery in M. tuberculosis H37Rv. These results are in contrast with previous observations in M. marinum, where several M. marinum specific PE_PGRS proteins are secreted in an ESX-5-dependent manner (Abdallah et al., 2009). A number of studies suggest that some PE_PGRS and PPE proteins are associated with the cell envelope. It is possible that the impact of ESX-5 inactivation on release of PE_PGRS proteins is also affected by the composition of the cell wall. For example, cell wall of M. marinum contains a group of glycolipids, designated lipooligosaccharides (LOSs), which are absent from the cell wall of M. tuberculosis H37Rv and several M. tuberculosis clinical isolates (Ren et al., 2007). As the number and composition of PE_PGRS proteins as well as their expression profiles vary considerably among different M. tuberculosis strains (Voskuil et al., 2004), strain specific differences in PE_PGRS secretion patterns might as well occur.
Although the growth of M. tuberculosis in liquid medium is not affected by disruption of individual components of the ESX-5 secretion machinery (Fig. S3), the virulence properties of M. tuberculosis are strictly dependent on a functional ESX-5 system (Fig. 6). Disruption of eccD5 indeed results in the loss of the ability of M. tuberculosis to replicate in SCID mice. Differently from ESX-1 mutants, whose attenuation is due to defects in EsxA/B expression or secretion (Brodin et al., 2006), the attenuation of the MtbeccD5ko mutant is not related to the loss of EsxN secretion. Indeed, the characterization of the ex vivo and in vivo growth properties of the MtbesxMko mutant demonstrated that inactivation of esxM/esxN genes does not result in attenuation of virulence of M. tuberculosis. The profound attenuation of virulence of M. tuberculosis resulting from inactivation of eccD5 could be in part due to the alteration of the integrity of the mycobacterial cell wall. Alteration of cell wall structure may cause attenuation of virulence by disrupting the bacterial interface with the host cell, undermining specific virulence functions, or increasing the susceptibility of the microorganism to host antimicrobial defences (Barry, 2001). Such a scenario is in good agreement with the increased sensitivity of the MtbeccD5ko strain to detergents and antibiotics to which mycobacteria are naturally resistant. Apart from the direct implication of the EccD5-containing multi-membered protein complex in cell envelope stability (see above), some of the observed effects might also be linked to a defect in transport of PPE proteins and/or other mycobacterial cell wall components in ESX-5 mutants. To date, the function and localization of ESX-5-associated PPE and PE proteins are unknown. However, as the virulence of the MtbΔppe25-pe19 mutant was also found to be attenuated, it seems likely that these proteins, as well as their transport across the cytoplasmic membrane, might play a role for a fully functional cell envelope. Because of their high homology in primary amino acid sequence, it cannot be excluded that these PE and PPE proteins might play a redundant role in M. tuberculosis virulence. Homologous recombination events between ppe25 and ppe27 genes resulting in deletion of ppe25, pe18 and ppe26 genes have been reported in some Tunisian clinical isolates of M. tuberculosis (Karboul et al., 2008). As these clinical isolates apparently conserved their virulence and infectivity, it is plausible that not all of the five PE and PPE proteins in the ESX-5 locus, deleted in the attenuated MtbΔppe25-pe19 strain, are involved in virulence functions. However, it seems likely that at least one PE/PPE pair from the ESX-5 region is. A similar situation was observed in M. avium, where a PPE25 homologue is required for growth in macrophages and in mice (Li et al., 2005).
In agreement with the above mentioned minor effects on PPE transport and resistance to cell wall stresses, no defect in the virulence properties of M. tuberculosis in SCID mice was observed after inactivation of eccA5. However, it should be mentioned that the eccA5 gene was recently identified as required for cholesterol catabolism in M. tuberculosis (Griffin et al., 2011). As cholesterol uptake and catabolism are not required for growth during the acute phase of infection but for mycobacterial persistence during the chronic phase (Pandey and Sassetti, 2008), it cannot be excluded that a more evident growth defect might be observed for the MtbeccA5ko mutant during long-term infection experiments. In this respect, it is also noteworthy that eccA5 was suggested as virulence factor in the guinea-pig infection model (Jain et al., 2007). The impact of EccA5 on in vivo growth of M. tuberculosis might thus vary with the type of infected host, which is also relevant to our observation that the MtbeccA5ko mutant strain has an impaired intracellular growth in human THP-1 and A549 cell lines (Fig. S4).
In conclusion, the results obtained in this study demonstrate that the ESX-5 system of M. tuberculosis has an important function for the cell wall integrity and virulence of M. tuberculosis. It is clear that the ESX-5 system in M. tuberculosis shares certain basic features with the ESX-5 system in M. marinum that can now be evaluated also in other M. tuberculosis lineages. However, several differences have also been highlighted, which seem to be specific for the system in M. tuberculosis. After the ESX-1 and the ESX-3 systems of M. tuberculosis, which were subject of numerous publications from various research groups, our data allow new insights to be made into the ESX-5 system of M. tuberculosis. From comparison of the three ESX systems studied so far in M. tuberculosis, it is obvious that despite their different functions, each of them plays an important role in the biology and pathogenicity of the etiological agent of human tuberculosis, which makes them interesting and important targets for potential new prevention and control strategies.
Experimental procedures
Plasmids, bacterial strains and growth conditions
Plasmid pPR27, used for construction of KO strains, is a thermosensitive vector, carrying the sacB counterselectable marker (Pelicic et al., 1997); plasmid pUC4K (Amersham Biosciences) contains the kanamycin resistance cassette (aph); cosmid I104, used for construction of complemented strains, is an integrative pYUB412 vector (Bange et al., 1999) containing a 41 kb fragment (2005–2046 kb) of the M. tuberculosis H37Rv genome including the entire ESX-5 locus. For cloning procedures and plasmid amplification, Escherichia coli DH10B (Invitrogen) was grown either in LB liquid or solid medium, added when required with 20 µg ml−1 of gentamicin, 100 µg ml−1 of hygromycin or 100 µg ml−1 of ampicillin. For the construction of mycobacterial KO mutants, M. tuberculosis H37Rv (stocks held at the Institut Pasteur) (Cole et al., 1998) was used as the reference strain. M. tuberculosis strains were grown in Middlebrook 7H9 medium (Becton-Dickinson) supplemented with albumin-dextrose-catalase (ADC, Becton-Dickinson) or on Middlebrook 7H11 medium (Becton-Dickinson) supplemented with oleic acid-albumin-dextrose-catalase (OADC). When required, the media were added with 2% sucrose or the following concentrations of antibiotics: 20 µg ml−1 of kanamycin and/or 50 µg ml−1 of hygromycin and 25 µg ml−1 of gentamicin.
Construction of KO mutant and complemented strains
Knockout mutants were constructed by allelic exchange using the Ts/sacB method (Pelicic et al., 1997). Fragments bearing the genes of interest, 1000–1500 bp of flanking regions, and the aph gene were synthesized by PCR (see Table S1 for primer sequences). Amplicons corresponding to 5′ or 3′ flanking regions and kanamycin resistance cassette were digested with the appropriate restriction endonuclease (Table S2) and fragments obtained were cloned into BamHI-NotI or SpeI-NotI digested pPR27 vector. The resulting constructs were electroporated into M. tuberculosis for allelic replacement experiments. To obtain complemented strains, the cosmid I104 was used. The cosmid was electroporated into mutant strains and transformants were selected on Middlebrook 7H11 medium supplemented with 50 µg ml−1 hygromycin. Resistant colonies were analysed for the presence of the integrated ESX-5 locus by PCR and/or Southern blot on genomic DNA.
RNA extraction and RT-PCR reactions
RNA was extracted from bacteria in exponential growth phase as previously described (Bottai et al., 2011) and used in RT-PCR reactions using the SS-One Step RT-PCR kit (Invitrogen) in accordance with the manufacturer's instructions. Sequences of primers used are listed in Table S1.
Recombinant EsxN purification and production of EsxN antisera
The esxN coding sequence was amplified by PCR from H37Rv genomic DNA (see Table S1 for primer details) and cloned into pET303/CT-His vector (Invitrogen). Recombinant C-terminal 6His-tagged-EsxN (EsxN-6His) was expressed in E. coli BL21 (Invitrogen) and purified by affinity chromatography on Ni2+-NTA affinity columns (Invitrogen) under denaturing conditions. Briefly, binding of the protein to the column was performed in 20 mM sodium-phosphate buffer containing 6 M guanidine hydrochloride, 500 mM NaCl, pH 7.8. Elution of EsxN-6His was carried out in 20 mM sodium-phosphate buffer, 8 M urea, 500 mM NaCl, pH 4. Removal of contaminant proteins co-purified with EsxN-6His was obtained by electroelution of recombinant EsxN from polyacrylamide gel. To generate anti-EsxN polyclonal serum the protein preparation obtained was used for rabbit immunization using the mineral oil-based adjuvant Stimmune (Prionics) as recommended by the manufacturer.
Preparation of culture filtrates and total lysates from M. tuberculosis strains and immunoblotting
Preparation of mycobacterial culture filtrates was performed as previously described (Fortune et al., 2005; Abdallah et al., 2009). For analysis of EsxN secretion, M. tuberculosis strains were grown for 6 days in Sauton medium supplemented with 0.05% Tween 80. Mid-log phase bacteria were then resuspended in fresh Sauton medium and cultured for 4 days: cultures were harvested by centrifugation and culture filtrates were recovered after filtration through 0.22-µm-pore-size filters (Millipore MillexR GP), followed by concentration using Centricon filters with a 3 kDa cut-off (Millipore). Alternatively, following experimental protocols described elsewhere (Abdallah et al., 2006; 2009), mycobacterial strains were grown to mid- or late logarithmic growth phase in Middlebrook 7H9 medium supplemented with 0.05% Tween 80. Pre-cultures were then diluted in fresh Middlebrook 7H9 medium supplemented with 0.1% ADC. After 6 days, culture supernatants were recovered and proteins were precipitated with 10% TCA. To obtain total lysates, mycobacterial pellets were washed twice and resuspended in 20 mM Tris buffer (pH 7.5) and cells were broken by shaking with 106 µm acid washed-glass beads (Sigma-Aldrich) for 8 min in a Tissue Lyser apparatus (Qiagen). The supernatant fraction recovered after centrifugation at 5000 r.p.m. for 30 min represented the whole-cell lysate. Cell wall, cytosolic and membrane fractions were prepared from total lysates by 60 min centrifugation at 14 000 r.p.m. (CW), followed by ultracentrifugation at 55 000 r.p.m. for 120 min (CYT and ME). Total protein concentration of samples obtained from mycobacterial cultures in Sauton medium was determined by using a Bradford protein assay (Sigma-Aldrich).
Immunoblot analyses were carried out with rabbit anti-PPE41 polyclonal serum (Abdallah et al., 2006), rabbit anti-EsxN, a mouse monoclonal antibody raised against the PGRS domain of PE_PGRS33 (Abdallah et al., 2009) or anti-EsxA (Hyb76-8, Antibodyshop, Statens Serum Institut). As control, culture supernatants were also analysed by Western blot for the presence of GroEL2 (anti-GroEL2 monoclonal antibody, Colorado State University, NIH, NIAID contract NO AI75320), and PPE68, a PPE protein encoded by the ESX-1 locus, reported to be associated to the mycobacterial cell envelope (Demangel et al., 2004).
Two dimensional gel electrophoresis
Culture filtrates from M. tuberculosis strains grown in Sauton medium were concentrated in presence of EDTA-free protease inhibitor (Roche). Proteins were desalted and concentrated using the 2-D-Clean Up kit (GE Healthcare) and resuspended in 2D buffer (7 M urea, 4% CHAPS, 5 mM DTT, 2% ampholytes pH 4-6). Forty micrograms of protein was subjected to 2D gel electrophoresis using pH 4.5–5.5 immobilized gradient strips for the first dimension (15 min at 200 V, 20 min at 450 V, 20 min at 750 V and 105 min at 2000 V), and 4–20% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) for separation in the second dimension (120 min at 200 V). EsxN detection was performed by immunoblotting as mentioned above.
Proteinase K sensitivity assay
Proteinase-K treatment was performed as previously reported (Cascioferro et al., 2007). Briefly, mycobacterial strains were grown in Middlebrook 7H9 medium for 6 days. Cells were washed in TBS buffer (150 mM NaCl, 3 mM KCl, 20 mM Tris-HCl, pH 7.5) and resuspended in 1 ml of the same buffer. Each sample was divided in two identical aliquots, one of which was added to proteinase K (Sigma-Aldrich) 100 µg ml−1. Treated and untreated samples were incubated for 30 min at room temperature. The reaction was stopped adding 1× complete EDTA-free protease inhibitor (Roche). Samples were washed once in TBS and resuspended in 1× loading buffer (Sucrose 10% w/v, SDS 2% w/v, Tris-HCl 1.36 mM, bromophenol blue 0.01% w/v, β-mercaptoethanol 1% v/v). Equal amount of treated and untreated samples were loaded on polyacrylamide gel and tested in Western blotting as mentioned above.
SDS and drug sensitivity tests
Mutant, WT and complemented strains were grown to mid-logarithmic phase in Middlebrook 7H9 medium supplemented with 0.05% Tween 80. For detergent resistance/survival assay, bacterial cultures were diluted at a final OD600 of 0.1, and SDS was added to a final concentration of 0.1%. After 6, 24 and 72 h of incubation, the number of viable bacteria was determined.
Drug sensitivity assays were performed as previously described (Jackson et al., 1999). Bacterial suspensions were plated on Middlebrook 7H11 medium containing twofold serial dilutions of ampicillin (3.125–6400 µg ml−1), norfloxacin (0.3125–40 µg ml−1), ofloxacin (0.03–4 µg ml−1), bacitracin (1.56–800 µg ml−1), vancomycin (0.3125–40 µg ml−1), rifampicin (0.0075–0.24 µg ml−1), isoniazid (0.125–2 µg ml−1), ethambutol (0.8–6.4 µg ml−1). As control, bacterial suspensions were plated on Middlebrook 7H11 medium without antibiotics. The number of viable bacteria was determined after incubation of plates at 37°C for 3 weeks. For each drug tested, the MIC value was defined as the lowest concentration of antibiotic that reduced the bacterial viability by at least 99% on solid medium. Microplate-based nitrate reductase assay was performed on mycobacterial cultures in exponential growth phase, as previously reported (Kumar et al., 2005).
Macrophage infection assays
Bone marrow-derived macrophages were obtained from 8-week-old C57BL/6 mice. Cells were seeded in a 96-well plate at a density of 4 × 104 cells per well and differentiated into macrophages by culturing them for 7 days in RPMI medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum, 10% l-cell conditioned medium, and 2 mM l-glutamine. Macrophages were infected with bacterial suspensions of M. tuberculosis H37Rv WT, KO and complemented strains at an moi of 1:1 (bacteria : cell). At 4 h, or 1, 4 and 7 days after infection, infected cells were lysed by the addition of 0.1% Triton X-100 (Fluka) in PBS. The number of intracellular bacteria was determined by plating serial dilutions of cell lysates on solid medium.
Mouse infection studies
Six-week-old CB17/Ico SCID mice (Charles River) were infected intravenously with 1 × 106 cfu per mouse of various mycobacterial strains. Three weeks after infection mice were sacrificed, and spleens and lungs were homogenized using an MM300 apparatus (Qiagen) and 2.5-mm-diameter glass beads. Serial fivefold dilutions of organ homogenates were plated on solid medium, and the cfu were counted after 3–4 weeks of incubation at 37°C. All animal studies were approved by the Institut Pasteur Safety Committee (Protocol 11.245; experimentation authorization number 75-1469), in accordance with European and French guidelines (Directive 86/609/CEE and Decree 87–848 of 19 October 1987).
Acknowledgements
We are grateful to Eddie Maranghi and Karim Sébastien for expert assistance in animal care in A3 facilities. FS is a recipient of a PhD fellowship from the University of Damascus, Syria. This work received funding from the European Community's Seventh Framework Programme [(FP7/2007–2013)] under grant agreement n°201762.




