Helicobacter pylori HP0518 affects flagellin glycosylation to alter bacterial motility
Author Contributions: Conceived and designed the experiments: HA, YC, TFM. Performed the experiments: HA, BB, JPB, NH, HJM. Analysed the data: HA, JPB, NH, PRJ, VB. Contributed reagents, materials, analysis tools: BB, SB, JPB. Wrote the paper: HA, YC, TFM.
Summary
Helicobacter pylori is a human gastric pathogen associated with gastric and duodenal ulcers as well as gastric cancer. Mounting evidence suggests this pathogen's motility is prerequisite for successful colonization of human gastric tissues. Here, we isolated an H. pylori G27 HP0518 mutant exhibiting altered motility in comparison to its parental strain. We show that the mutant's modulated motility is linked to increased levels of O-linked glycosylation on flagellin A (FlaA) protein. Recombinant HP0518 protein decreased glycosylation levels of H. pylori flagellin in vitro, indicating that HP0518 functions in deglycosylation of FlaA protein. Furthermore, mass spectrometric analysis revealed increased glycosylation of HP0518 FlaA was due to a change in pseudaminic acid (Pse) levels on FlaA; HP0518 mutant-derived flagellin contained approximately threefold more Pse than the parental strain. Further phenotypic and molecular characterization demonstrated that the hyper-motile HP0518 mutant exhibits superior colonization capabilities and subsequently triggers enhanced CagA phosphorylation and NF-κB activation in AGS cells. Our study shows that HP0518 is involved in the deglycosylation of flagellin, thereby regulating pathogen motility. These findings corroborate the prominent function of H. pylori flagella in pathogen–host cell interactions and modulation of host cell responses, likely influencing the pathogenesis process.
Introduction
The human gastric pathogen Helicobacter pylori infects approximately 50% of the population worldwide. Infections can persist throughout the lifetime of the host and can ultimately lead to gastritis, duodenal ulcers and certain gastric cancers (Parsonnet et al., 1991; Parsonnet, 1994; Kusters et al., 2006). Recent work has also linked H. pylori with mucosa-associated lymphoid tissue (MALT) and B-cell MALT lymphomas (Andersen et al., 2007; Mbulaiteye et al., 2009). A growing body of evidence suggests motility is a key factor in this pathogen's virulence. Indeed, successful colonization in vivo seems to rely upon the bacterium's ability to move within the gastric mucus (Eaton et al., 1996; Andrutis et al., 1997; Terry et al., 2005).
Helicobacter species possess a motility organelle, the flagella, which consists of three main structures: the basal body, the hook/universal joint and the filament (Chevance and Hughes, 2008). The H. pylori filament is composed of two flagellin proteins, FlaA and FlaB (Suerbaum et al., 1993). FlaA comprises the majority of the filament, whereas FlaB is found only in the base (Kostrzynska et al., 1991). Bacterial flagellar components are synthesized intracellularly, together with most post-translational modifications, and exported by the flagellar type III protein export apparatus (Minamino et al., 2008). H. pylori flagellar motility is a prerequisite for colonization both in vivo and in vitro (Eaton et al., 1996; Ottemann and Lowenthal, 2002). The annotated genomic sequences of H. pylori 26695 contain at least 40 genes likely to be involved in flagellar function and/or assembly (Tomb et al., 1997).
Helicobacter pylori flagellin filaments are post-translationally modified by glycosylation with a nine-carbon pseudaminic acid (Pse) sugar derivative that resembles sialic acid, which is typically found on mammalian cell surfaces (Schirm et al., 2003; Logan, 2006). Deletion of genes responsible for the glycosylation process leads to a loss of late flagellar structures (hook and filaments) and a loss of motility (Josenhans et al., 2002; Schirm et al., 2003). Studies on a number of other pathogens have shown flagellin glycosylation is not only important for flagellar assembly but also for virulence; for instance, glycan composition on the flagellin of Camplyobacter spp. affects autoagglutination and microcolony formation on intestinal epithelial cells, traits associated with disease in animal models (Guerry, 2007). Despite the genetic pathways delineating Pse biosynthesis in H. pylori being well defined (Josenhans et al., 2002; Schirm et al., 2003), the role of flagellar glycosylation in this bacterium's virulence is currently unknown. Thus, pursuing analyses of the function of flagellin glycosylation in H. pylori could reveal interesting insights.
In this study, we isolated an H. pylori transposon mutant deficient in the HP0518 gene that exhibited altered motility in comparison to its parental strain. Subsequent phenotypic and molecular characterization revealed that this altered motility was due to increased flagellar glycosylation. In addition, we examined the effects of the HP0518 mutation on H. pylori-induced changes in human gastric epithelial cells to gain insight into the function of flagellar glycosylation in pathogenesis.
Results
Inactivation of HP0518 alters H. pylori G27 motility
The H. pylori transposon (Tn) mutant library was used to select mutants exhibiting increased motility in a GC soft agar assay. Sequencing of the Tn-flanking regions of eight randomly selected clones revealed all were deficient in the locus HP0518. Transposon insertions were located at positions either +765 bp or +803 bp (4 clones each) from the 5′ terminus (Fig. 1A, arrows). To confirm that the absence of HP0518 was associated with the altered motility, we constructed a HP0518 deletion mutant of H. plyori strain G27 deleted in HP0518. HP0518 deletion increased swimming motility by 20–25% in comparison to both the complemented HP0518 and the wild-type (WT) strains (Fig. 1B). Interestingly, bacterial tracking assays showed that the HP0518 mutant exhibited a more directed circular motility pattern than the parental strain (Fig. 1C–E). The flaA mutant strain (Fig. S1), used as a control, was non-motile (Fig. 1C). To further define the behavioural phenotype, bacterial velocity and number of stops (every 3 s) were recorded by single-cell tracking (Schweinitzer et al., 2008). Although the velocities of both the WT and HP0518 mutant strains were almost identical, the HP0518 mutant displayed significantly fewer stops than the WT strain (WT versus 518− = 6.3 ± 3.7 versus 2.28 ± 3.7, P < 0.01) (Fig. 1F), indicating that the mutant strain changes direction less often. Taken together, these data show that inactivation of HP0518 alters the pattern of motility, with increasing directionality.
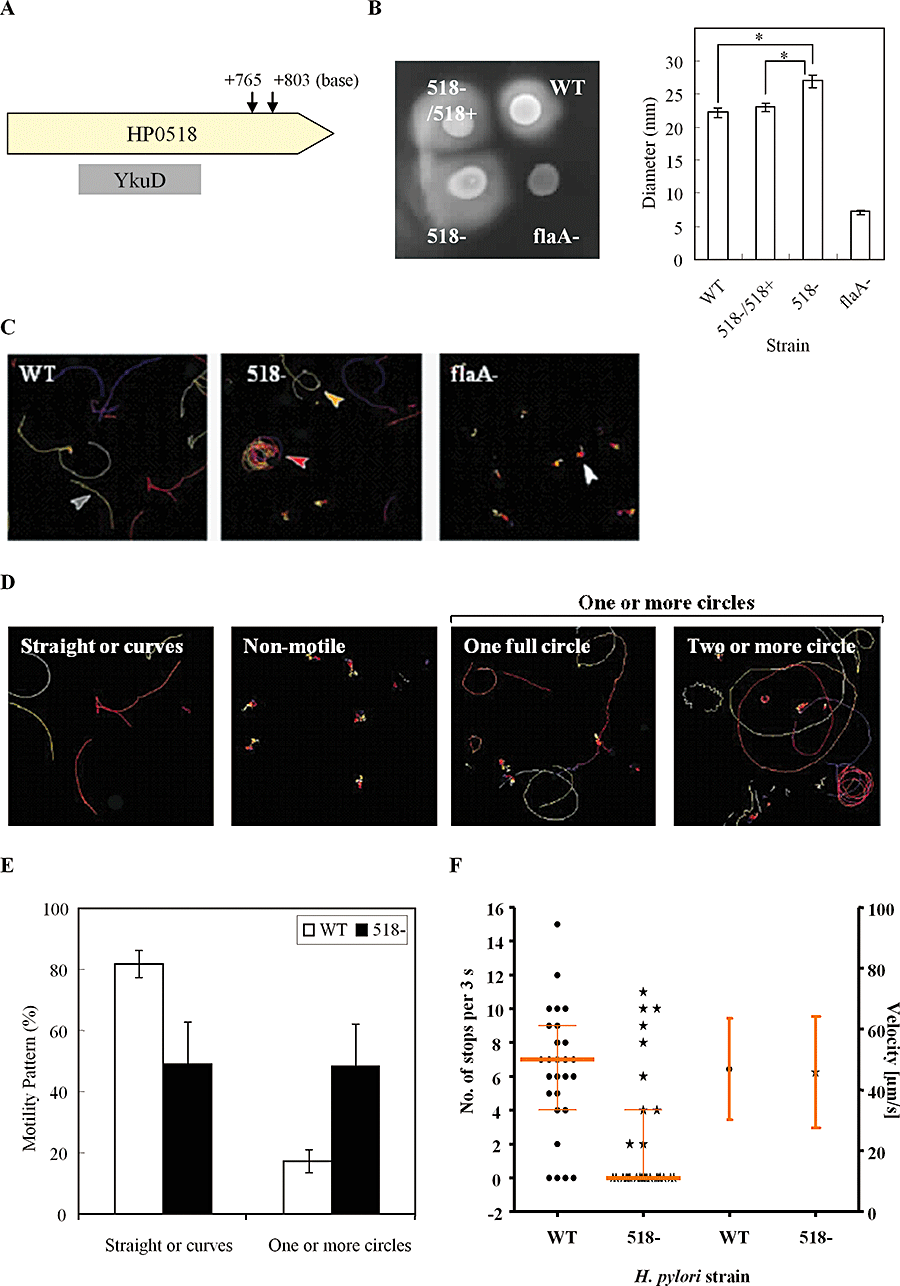
Increased motility of H. pylori G27 following HP0518 inactivation. A. Arrow indicates the transposon insertion locus in the HP0518 gene. The central region of HP0518 is homologous to YkuD. B. Colony swarming diameters were measured on soft agar. Data are expressed as the mean ± SD of three independent experiments. The non-motile H. pylori flaA mutant strain was used as a negative control. Asterisks indicate statistical significance (P < 0.01). C–F. Disruption of HP0518 altered the bacterial motility pattern. (C) Representative tracking images of WT and mutant strains of H. pylori. FlaA mutant exhibited no motility. (D) Bacterial motility patterns: straight or curve (shown as grey arrow in C); one full circle (yellow arrow); two or more circles (red arrow); non-motile (white arrows). (E) Percentages of differential motility patterns of WT and 518− mutant strains. (F) Velocity and number of stops exhibited by H. pylori WT (circles) and 518− mutant (stars) strains were analysed. Velocity remained unaltered, whereas 518− showed a significantly decreased stopping frequency (P < 0.01). Stopping frequencies (left y-axis) of each strain (25 bacteria per strain) are shown in scatter dot blots and the respective median and quartiles are depicted. Average velocities (right y-axis) of each strain (20 bacteria per strain) ± SD are given.
HP0518 mutant exhibits increased flagellin glycosylation
To assess the flagellar composition and possible protein modifications, we extracted the sodium lauroyl sarcosine (SLS)-insoluble proteins from H. pylori. Both sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2A) and Western blotting (Fig. 2B; top panel) showed that the FlaA protein isolated from the WT and HP0518 mutant strains (FlaA, marked in dotted box, identified by MALDI-MS) possessed different molecular masses. This altered motility suggests that FlaA is post-translationally modified in the HP0518 mutant. DNA sequencing and RT-PCR confirmed that the flaA gene from the WT and HP0518 mutant strains exhibited no sequence or expression differences (data not shown). Because H. pylori FlaA is O-linked glycosylated with Pse (Schirm et al., 2003; Logan, 2006), we examined whether glycosylation of this protein is altered in the HP0518 mutant. As expected, FlaA glycosylation in the HP0518 mutant was enhanced in comparison to the WT and the complemented strains (Fig. 2B, bottom panel). In addition, chemical deglycosylation with trifluoromethanesulphonic acid (TfOH) resulted in a marked decrease in the apparent molecular mass and glycostain intensity of FlaA in all strains tested (Fig. 2B), demonstrating that FlaA of the HP0518 mutant exhibited increased glycosylation levels. Enzymatic deglycosylation assays revealed that O-glycosidases synchronized the mobility of the FlaA protein from the WT and the HP0518 mutant, whereas N-glycosidase F had no effect (Fig. 2C), suggesting O-glycosylation increases the molecular mass of FlaA in the HP0518 mutant.
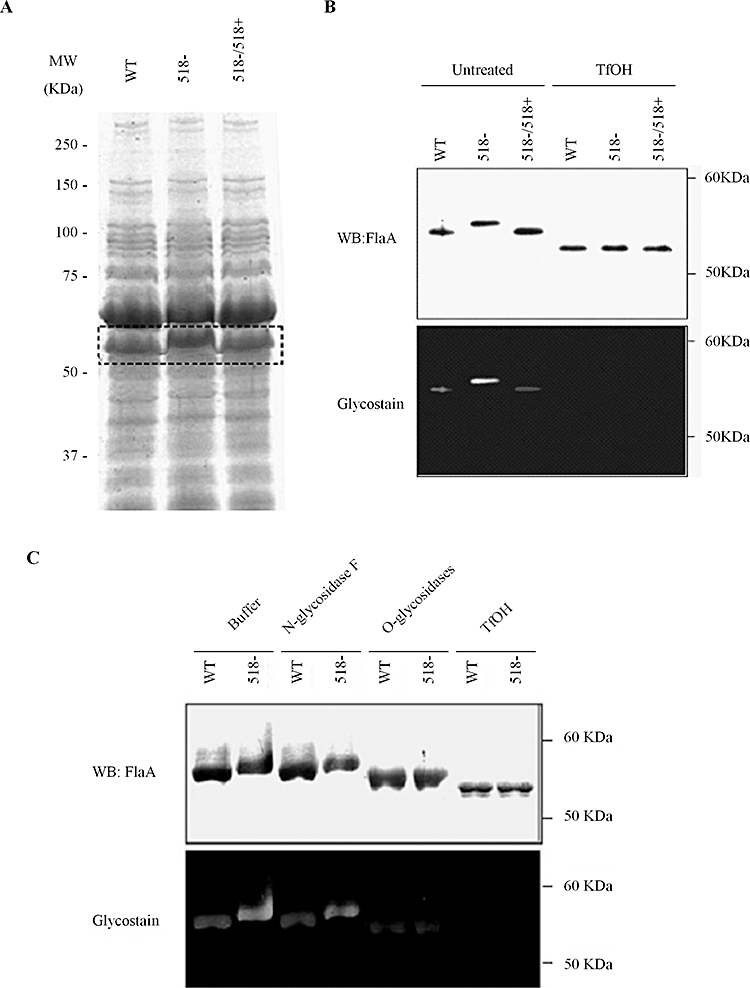
Deletion of HP0518 gene modulates O-linked glycosylation of H. pylori FlaA protein. A. Fractionation of H. pylori sodium lauroyl sarcosine (SLS)-insoluble (mainly outer membrane and flagellar-associated) proteins. FlaA proteins (indicated by the dashed box) from H. pylori G27 WT, its isogenic HP0518 mutant (518−) and the complemented mutant (518−/518+) were identified by MALDI-TOF/MS. B. Western blot and glycostain analysis of FlaA proteins in the SLS-insoluble proteins from H. pylori strains. Chemical deglycosylation with TfOH resulted in equalization of mass of FlaA protein among H. pylori strains tested. C. Enzymatic deglycosylation of FlaA. SLS-insoluble proteins were incubated for 24 h with either N- or O-glycosidases under denaturing conditions followed by glycostain and Western blot analysis. Images in A–C are representative of three independent experiments.
HP0518 is involved in the deglycosylation of FlaA
To elucidate the biological function of HP0518, we produced recombinant HP0518 protein (rHP0518) in E. coli. Co-incubation of the purified rHP0518 with SLS-insoluble proteins (containing FlaA) decreased the molecular mass and glycostain intensity of FlaA from the WT and HP0518 mutant strains (Fig. 3A), whereas incubation of whole H. pylori bacteria with rHP0518 had no effect (Fig. 3B), suggesting that HP0518 might exert activity in the cytoplasm. Accordingly, the molecular mass of FlaA from both the WT and the HP0518 mutant decreased when treated with water-soluble extracts from the WT but not from the HP0518 mutant (Fig. 3C). Our previous observations (Bumann et al., 2002; see also 2D-PAGE Database, http://web.mpiib-berlin.mpg.de/cgi-bin/pdbs/2d-page/extern/index.cgi) and bioinformatic analysis using the protein localization prediction tool SOSUIGramN (Imai et al., 2008) also suggested that the HP0518 protein is located intracellularly. To determine the actual location of HP0518, we constructed an H. pylori G27 strain producing HA-tagged HP0518 protein. Detection of HA by Western blot revealed that the HP0518 protein was located in the cytoplasm (Fig. S2). Interestingly, the related bacterium Campylobacter jejuni strain 81-176 has more glycosylation sites on its FlaA protein (19 motifs) than H. pylori FlaA (7 motifs) (Schirm et al., 2003; Ewing et al., 2009). However, the rHP0518 protein did not affect the glycosylation level of FlaA from C. jejuni 81-176 strain (Fig. S3). Together, these data suggest that HP0518 plays a critical role in H. pylori FlaA deglycosylation.
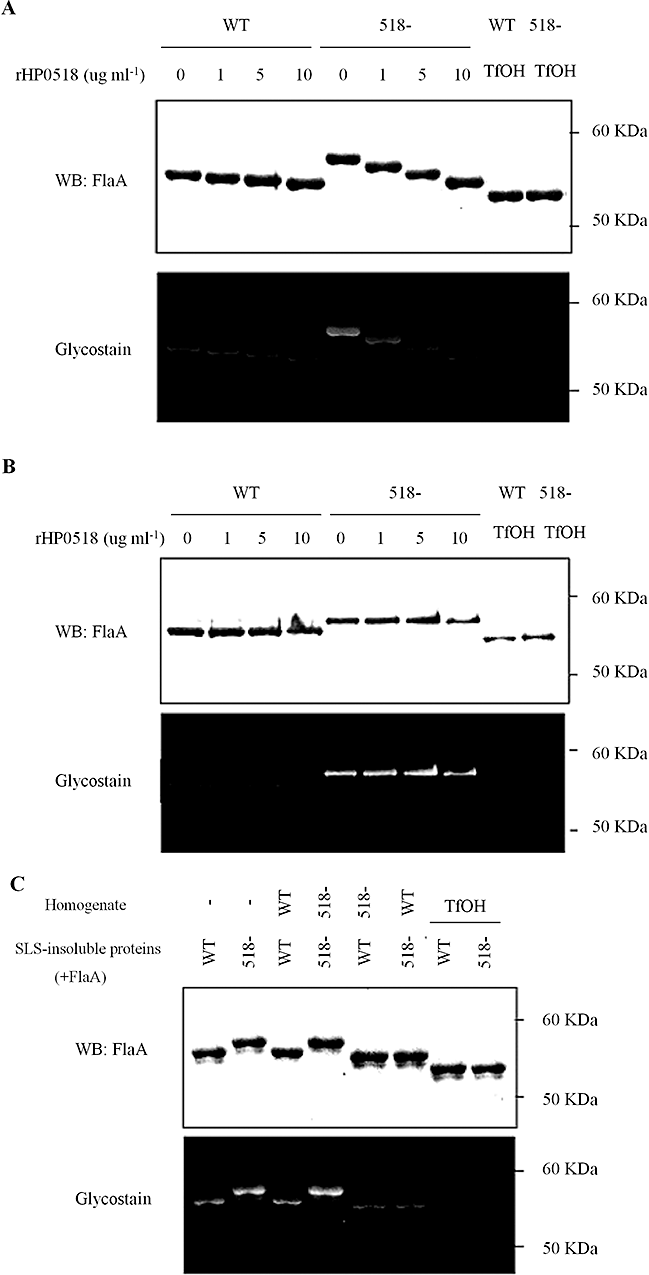
HP0518 protein is involved in the deglycosylation of FlaA in H. pylori.A and B. SLS-insoluble proteins from the H. pylori HP0518 mutant (containing FlaA) were incubated for 6 h with His-tagged recombinant HP0518 protein (rHP0518) at a concentration of 0, 1, 5, 10 µg (A) or with whole H. pylori bacteria (B), followed by glycostain and Western blot for the detection of FlaA. rHP0518 deglycosylates H. pylori FlaA, but only when it is not sheathed. C. H. pylori G27 WT homogenates deglycosylate FlaA protein from H. pylori. The purified SLS-insoluble membrane fractions (contain FlaA) from both the WT and HP0518 mutant were treated with water-soluble cell extracts (containing no flagellins) from the two strains. After overnight incubation, the SLS-insoluble proteins were collected, separated by SDS-PAGE and the mass of FlaA was determined by Western blot with glycostain analysis. TfOH was used as a control. Images in A–C are representative of three independent experiments.
HP0518 negatively controls the amounts of Pse on FlaA
Previous work has shown at least six genes (HP0840, HP0366, HP0178, HP0326A, HP0326B, HP0114) are associated with Pse biosynthesis-mediated flagellar glycosylation (Schoenhofen et al., 2006). Examination of the role of HP0518 gene inactivation in H. pylori gene expression using microarrays revealed no significant differences in the expression of these six genes in the HP0518 mutant and H. pylori WT (GEO Accession No. GSE14752). However, quantitative analysis of Pse in the SLS-insoluble protein fraction by LC-MS/MS revealed that HP0518-derived SLS-insoluble proteins contain approximately threefold more Pse than WT strain proteins (Fig. 4A and B; Figs S4–S6). The H. pylori neuA mutant (HP0326A) is also reported to express unglycosylated flagellin inside the bacteria without flagellin secretion (Josenhans et al., 2002; Schirm et al., 2003). HP0326A is one of the putative regulatory genes of Pse biosynthesis in C. jejuni (McNally et al., 2006). Accordingly, the neuA mutant failed to form flagella (Fig. S1) and no Pse was detected in the SLS-insoluble proteins from this strain (Fig. 4A). Thus, the FlaA protein from the HP0518 mutant can bind greater amounts of Pse than the WT.
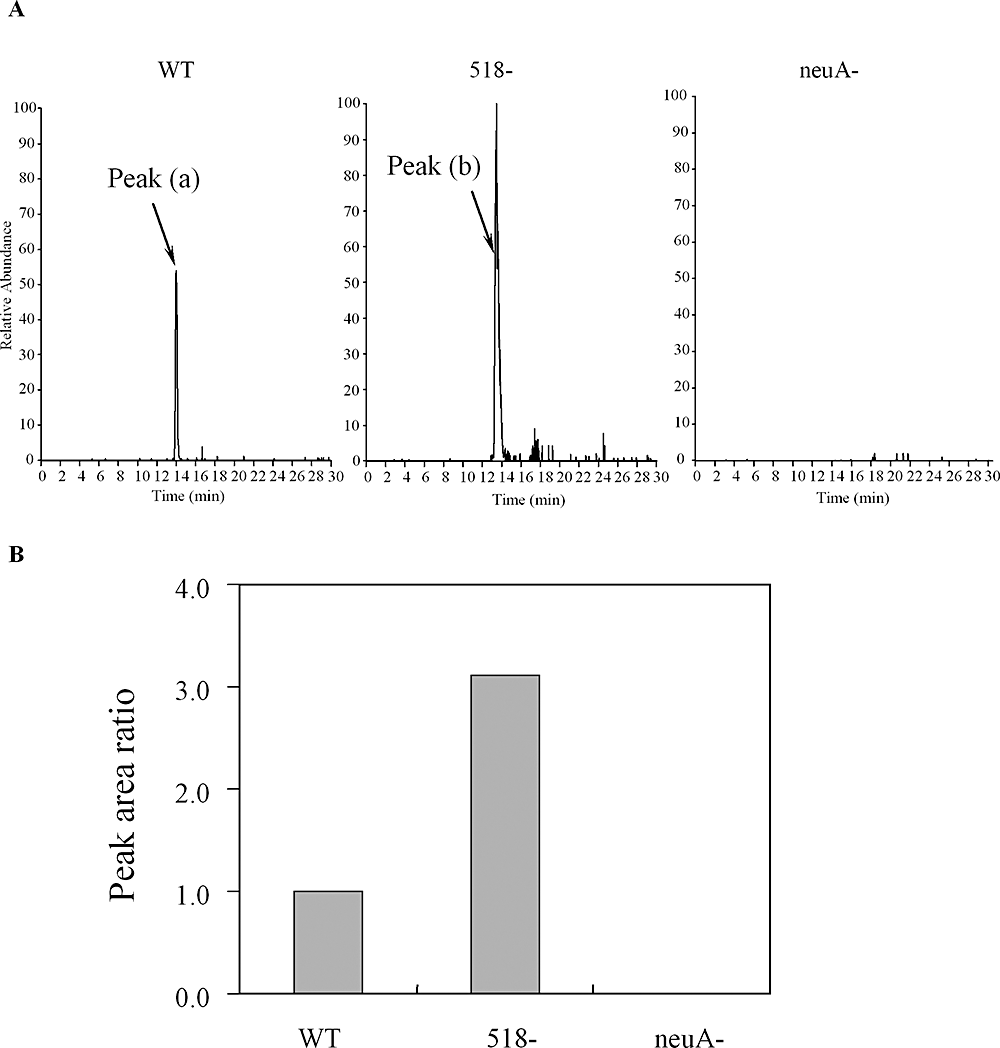
Quantitative analysis of DMB-labelled pseudaminic acid (Pse) from SLS- insoluble proteins of H. pylori G27 WT, its isogenic HP518 mutant and neuA mutant strains by LC/MS. The neuA mutant produced unglycosylated FlaA and thus was used as negative control. A. Extracted ion chromatogram at m/z 450.7–451.7 obtained by SIM of DMB-labelled Pse from G27 WT, its isogenic HP518 mutant and neuA mutant strains. Each ion signal was expressed as a relative percentage of the HP0518 mutant-derived sample (set at 100%). B. Peak area ratio of DMB-labelled Pse from WT, HP518 mutant and neuA mutant.
Deletion of HP0518 facilitates host cell interaction, causing enhanced CagA phosphorylation
Since flagellar motility is required for colonization both in vivo (Eaton et al., 1996; Ottemann and Lowenthal, 2002; Terry et al., 2005) and in vitro (Clyne et al., 2000), we expected that the altered motility associated with the HP0518 mutant could influence H. pylori colonization efficiency. Infection of AGS cells with H. pylori WT and mutant strains (including the flaA mutant as a motility-negative control) resulted in a time-dependent increase in adherence of all strains (Fig. 5A). However, the HP0518 mutant interacted with AGS cells most efficiently, with colonization rates at early infection times [2 h, multiplicity of infection (moi) 100] almost double that of the parental strain (Fig. 5A). Centrifugation of WT bacteria (WT-SD) onto cells increased numbers of cell-associated bacteria by approximately threefold in comparison to normal infection conditions (Fig. 5A). Moreover, the flaA mutant strain exhibited significantly reduced adherence as compared with WT bacteria (Fig. 5A). These results clearly indicate a prominent role of flagellar motility in the promotion of host cell interactions.
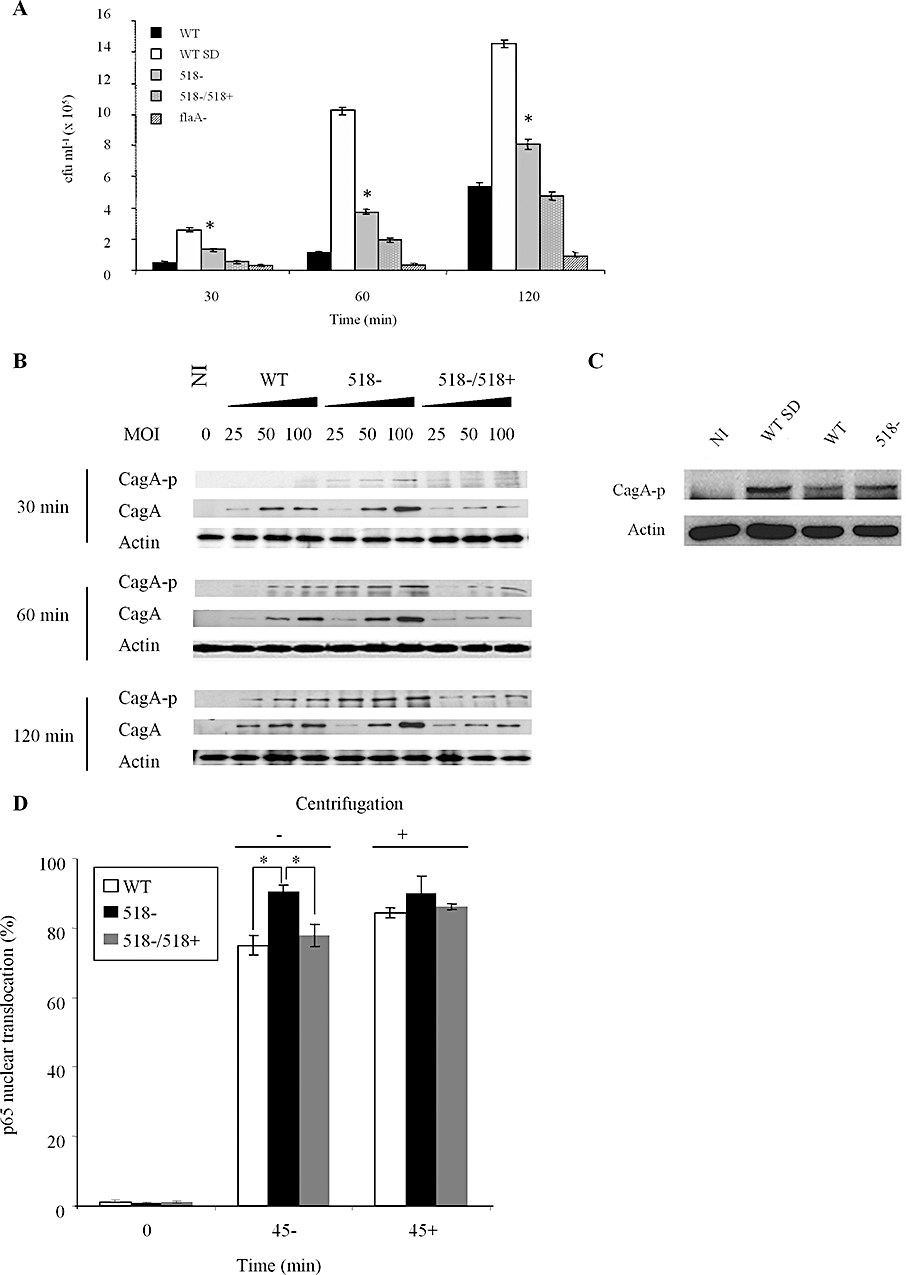
Mutation of HP0518 enhances cell interaction capabilities and increases CagA phosphorylation and NF-κB activation in host cells. AGS cells were infected with (A) H. pylori WT, spun-down WT (WT-SD), the HP0518 mutant (518−), the HP0518 mutant complemented with the HP0518 gene (518−/518+) or the flaA mutant, respectively, at moi 100 for 30, 60 or 120 min. Numbers of adhered bacteria were enumerated by determining colony-forming units (cfu). Data are expressed as mean ± SD number of adhered bacteria per well of three independent experiments. Adherence is significantly different (*t-test; P < 0.01) between the WT and 518−, and 518− and the complemented strain. B. Western blot detection of CagA translocation/phosphorylation in AGS cells infected with H. pylori. For each time point examined, each lane for the respective H. pylori groups (WT, 518− and 518−/518+) corresponds to one of the three moi tested. C. Western blot shows H. pylori-induced CagA phosphorylation is enhanced in upon centrifugation (WT-SD). D. NF-κB activation during H. pylori infection. AGS-SIBO2 cells, which stably express GFP-p65, were infected with either H. pylori WT or the HP0518 mutant strain with or without centrifugation (500 r.p.m., 3 min). At 45 min post infection, cells were fixed and stained with Hoechst 33342 to facilitate enumeration of p65 translocation into the nucleus by tracing GFP signals. Data show mean percentage of cells with p65 nuclear translocation ± SD per well (n = 8) (*t-test; P < 0.01). Blots in (B) and (C) are representative of at least two independent experiments.
Upon interaction with its host cell, H. pylori translocates CagA into epithelial cells via a type IV secretion system (T4SS). CagA translocated into host cells is phosphorylated via Src and Abl kinases (Backert et al., 2000; Backert and Selbach, 2008), leading to cell scattering (hummingbird phenotype) in AGS cells (Segal et al., 1999). Following infection with the HP0518 mutant, CagA phosphorylation occurred more efficiently than in the parental and the complemented strain (Fig. 5B). This process was accompanied by a rapid onset of the hummingbird phenotype as early as 2 h after infection (Fig. S7). Consistent with the host cell interaction data, centrifugation also enhanced CagA phosphorylation (Fig. 5C). This demonstrates that the HP0518 mutant interacts more efficiently with AGS cells, causing enhanced CagA phosphorylation and CagA-dependent host cell responses.
Altered motility of the H. pylori HP0518 mutant promotes rapid NF-κB activation
Besides inducing dramatic phenotypic changes in epithelial cells, H. pylori infection also triggers vigorous inflammatory responses in host cells (Naumann, 2001). NF-κB is known to play a central role in H. pylori-mediated inflammatory responses in gastric epithelia by regulating neutrophil infiltration into the gastric mucosa (Nardone and Morgner, 2003). NF-κB activation upon H. pylori infection is mainly mediated by the T4SS after cell attachment (Brandt et al., 2005; Backert and Selbach, 2008). Therefore, we compared NF-κB activation levels upon infection with H. pylori WT, the HP0518 mutant and its complemented strain, using an AGS reporter cell line expressing a GFP-p65 fusion protein (Bartfeld et al., 2010). These studies were carried out in parallel to the host cell interaction and CagA phosphorylation studies described above. We found that nuclear translocation of p65 was more pronounced in AGS cells infected with the HP0518 mutant as compared with cells infected with either the WT or the complemented strain (Figs 5D and S8), demonstrating a link between motility and NF-κB activation in host cells. Moreover, synchronization of infection by centrifugation gave rise to identical patterns of NF-κB activation between strains (Fig. 5D). Thus, our results demonstrate that increased motility is the major cause of rapid NF-κB activation following interaction of the H. pylori HP0518 mutant with host AGS cells.
Deletion of HP0518 appears to increase colonization of H. pylori in vivo
Because the HP0518 mutant exhibited altered motility in association with faster swimming to the cell surface in vitro, we tested its colonization ability in C57BL/6 mice (Baldwin et al., 2007). After 7 days of infection, the HP0518 mutant (1.47 ± 0.73 × 107 g−1) exhibited an enhanced ability, albeit statistically not significant, to colonize the gastric tissues of mice in comparison to the parental strain (5.08 ± 2.99 × 106 g−1), as determined by quantifying colony-forming units (Fig. 6). However, in accordance with our in vitro data, two of the five animals infected with the HP0518 mutant displayed superior colonization abilities (Fig. 6), suggesting that deletion of the HP0518 gene increases the potential of H. pylori to colonize in vivo.
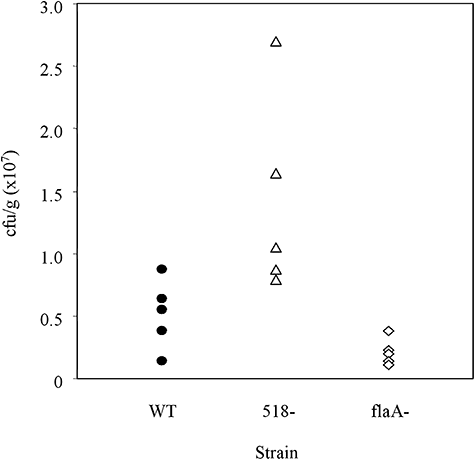
Deletion of HP0518 increases H. pylori colonization in vivo. C57BL/6 mice (n = 5) were inoculated twice with approximately 3.0 × 108 cells of H. pylori G27 WT (circle), HP0518 mutant (triangle) or flaA mutant (diamond) respectively. Seven days after infection, numbers of viable bacteria in gastric tissues (aseptically removed and homogenized) were determined. Colonization levels are expressed as colony-forming units per gram of tissue (cfu g−1).
Discussion
We have identified an H. pylori HP0518 mutant strain exhibiting an altered pattern of motility in comparison to the parental G27 strain. Phenotypic and molecular characterization revealed that the mutant's motility was a consequence of elevated levels of O-linked FlaA glycosylation. We also demonstrated that the flagellar hyper-glycosylation promotes enhanced interaction between the bacteria and AGS cells, accelerating cellular responses. CagA phosphorylation and NF-κB activation occurred more efficiently following infection of AGS cells with the HP0518 mutant than with the WT strain.
Over the last decade, numerous studies have reported the presence of glycoproteins on bacterial cell surface structures such as pilins and flagella (Szymanski and Wren, 2005). For example, the flagella of H. pylori, C. jejuni, Pseudomonas aeruginosa and Listeria monocytogenes have been shown to be O-glycosylated (Thibault et al., 2001; Schirm et al., 2003; 2004). It is known that H. pylori flagellin is modified with Pse (Schirm et al., 2003). Here we found that the H. pylori protein HP0518 modulates flagellin glycosylation. Our data suggest a direct enzymatic function of HP0518: Chemical and enzymatic deglycosylation assays as well as mass spectrometric analysis data clearly showed that the FlaA protein of the HP0518 mutant was strongly O-glycoside-modified. Recombinant HP0518 protein was capable of deglycosylating purified H. pylori FlaA protein, whereas the FlaA molecular mass was not influenced upon incubation with the whole bacteria. H. pylori flagellar filaments are known to be sheathed and we showed that the HP0518 protein is located in the cytoplasm. Thus, we could demonstrate that the mutation of the HP0518 gene resulted in elevated levels of Pse on flagellin; however, it remains unclear whether HP0518 affects intracellular Pse levels.
We found that the rHP0518 protein was incapable of changing the molecular mass of the FlaA from the related bacterium C. jejuni. A possible explanation for this observation is that HP0518 recognizes only specific Ser-Thr residues, present in H. pylori FlaA but absent in C. jejuni FlaA. In support of this are the relatively low levels of identity between HP0518 and the corresponding C. jejuni gene products (strains 81-176, NCTC11168, CJJ81176-0915 and Cj0903) ranging at ∼37.5%. Also, since the C. jejuni proteins are predicted to be periplasmic and glycosylated by an N-linked glycosylation system (Young et al., 2002), they probably won't react with cytoplasmic flagellin prior to its secretion. It remains unclear in the present study whether (and if so, where) the Pse-binding sites were modified in the HP0518 mutant. Clarifying this point could provide answers to several questions, for example, why these extra glycosylation sites probably remain unmodified under wild-type conditions. Future work will thus examine the link between flagellin glycosylation and its production/assembly in H. pylori and provide further insights into the mechanisms and biological role underlying H. pylori's FlaA glycosylation.
Bacterial motility and subsequent attachment to the host cell surface are essential factors for successful colonization of H. pylori on gastric epithelia (Eaton et al., 1996; Guruge et al., 1998). Although our work strongly suggests HP0518 promotes pathogen–host cell interactions, it is of course not the only factor influencing cell adherence and motility. H. pylori is known to possess around 20 different adhesins, mostly located on the membrane, which play varying roles in the infection process (Andersen et al., 2007). Despite these adhesins being, in all probability, contained within H. pylori SLS-insoluble protein fractions (Carlsohn et al., 2006), no distinct differences in protein profiles were apparent between the WT and HP0518 mutant strains. Thus, the cell adhesion phenotypes of HP0518 mutant observed here appear to be due to changes in flagellar motility resulting from increased levels of FlaA glycosylation. H. pylori flagellins are not recognized by pattern-recognition receptors (PRRs) such as Toll-like receptor 5 (TLR5) (Andersen-Nissen et al., 2005); therefore, it is conceivable that the pathogen's increased ability to interact with host cells conferred by the inactivation of HP0518 directly promotes rapid cellular responses, including CagA phosphorylation and NF-κB activation. Our in vivo observations suggesting potentially superior colonization features of the mutant lend support to this hypothesis.
In summary, we demonstrate that H. pylori G27 deficient in HP0518 exhibits altered motility and interactions with AGS cells as well as enhanced CagA-phosphorylation/NF-κB activation as a consequence of increased flagellin glycosylation. To our knowledge, this is the first report to demonstrate a functional role of the HP0518 gene during H. pylori infection. Thus, our study provides new insights into the function of flagellar glycosylation in the pathogenic process of this gastric pathogen.
Experimental procedures
Bacteria and cell lines
Bacterial strains and plasmids used in this study are listed in Table 1. H. pylori G27 and its transposon (Tn) mutant library have been described elsewhere (Salama et al., 2004). After receiving the mutant library it was amplified once prior to use. H. pylori was routinely grown on GC agar plates (Oxoid, Wesel, Germany) supplemented with 10% fetal bovine serum (FBS), vancomycin (10 mg l−1), amphotericin B (4 mg l−1) in a CO2 gas jar with a CampyPack (Oxoid) at 37°C for 48 h. The H. pylori G27 flaA mutant (non-motile mutant) was used as a motility-deficient strain. The G27 neuA (HP0326A) mutant was used as a strain that produced unglycosylated FlaA protein (Salama et al., 2004). For infection experiments, bacteria were suspended in cell culture medium consisting of RPMI1640 (Invitrogen, Carlsbad, CA) with 10% FBS. Human gastric epithelial (AGS) cells (ATCC CRL-1739) were cultured in RPMI1640 medium (Invitrogen) supplemented with 10% fetal calf serum (Gibco, Paisley, UK). C. jejuni 81-176 strain was grown on Mueller-Hinton agar (Becton Dickinson) in a CO2 gas jar with CampyPack (Oxoid) at 37°C for 48 h.
| Strain or plasmid | Description | Reference/source | Strain collection no. |
|---|---|---|---|
| Strain | |||
| G27 Tn library | G27 transposon mutant library | Salama et al. (2004) | |
| G27 | Human clinical strain | Laboratory stock | |
| G27 Tn-518 | G27 transposon mutant of HP0518 | This study | P410 |
| 518− | G27 HP0518 deletion mutant | This study | P411 |
| 518−/518+ | 518-strain complemented with pHel3-518 | This study | P412 |
| flaA− | G27 ΔflaA (HP0601) | This study | P413 |
| neuA− | G27 ΔneuA (HP0326A) | Salama et al. (2004) | P422 |
| E. coli BL21 (DE3) | Expression strain | Invitrogen | |
| C. jejuni 81-176 | Human clinical strain | Laboratory stock | |
| Plasmid | |||
| pHel3-518 | pHel3 harbouring HP0518 gene | This study | H3879 |
| pGEM-518-Cm | pGEM harbouring HP0518-Cm | This study | H3880 |
| pET-HP0518 | pET100D-Topo harbouring HP0518 | This study | L325 |
| pGEM-518-HA-Km | pGEM harbouring HP0518-HA-Km | This study | L362 |
Selection of Tn-mutants with increased motility
GC soft agar (containing 0.3% agar) was prewarmed to 37°C. The plates were inoculated with 10 µl of the H. pylori Tn mutant library containing 1010 cells ml−1 in RPMI by stabbing the agar with a pipette tip and plating the cells horizontally along the centre for 30 mm. After incubation at 37°C for 48 h, bacterial growth at the outer edge was transferred to the centre of a fresh soft agar plate. After five passages on the soft agar, highly motile bacteria were transferred onto fresh GC agar plates for single colony isolation. Genomic DNA was purified from each H. pylori clones and the DNA sequence of the Tn-insertional loci obtained using primers corresponding to the Tn cassette-flanking regions (primers N and S, Table S1; Salama et al., 2004).
Construction of gene knockout cassettes
Gene disruption cassettes were constructed according to the method of Chalker et al. (2001). Briefly, N-terminal (−495 base to +19 base from N-terminus) and C-terminal (−28 base to +541 base from C-terminus) fragments of the HP0518 gene were amplified using upstream and downstream primers, designed for the integration of a second copy of the gene combined with gene-internal primers, to obtain fragments of approximately 500 bp in length (518-s, 518-BIas, 518-Bis and 518-as, Table S1). Amplicons contained BamHI recognition sites facilitating ligation with the pGEM-T easy vector for cloning (Promega, Madison, WI, USA). Plasmid DNA from positive clones was extracted, cut with BamHI, and the E. coli CAT gene (chrolamphenicol-acetyltransferase resistant cassette) was introduced into the BamHI site. Ligations were purified, confirmed by sequencing and 12 µg of plasmid DNA was then naturally transformed into H. pylori (Heuermann and Haas, 1998). Because the complete genome sequence of strain G27 was published during the preparation of this manuscript (Baltrus et al., 2009), we use HP0518 instead of the homologue gene name HPG27-477 in G27.
Complementation assays
The HP0518 gene was PCR-amplified with the 518c-s and 518c-as primers (Table S1) and the BamHI/NdeI-cleaved products ligated into the shuttle vector plasmid pHel3 (Km-resistance). The plasmid DNA was then introduced into H. pylori strains by natural transformation.
Motility assays
Swimming motility assays were performed as described previously (Worku et al., 2004), with some modifications. Briefly, approximately 1010H. pylori cells were spotted onto GC soft agar plates (containing 0.4% agar) at a final volume of 5 µl and incubated for three days under microaerobic conditions. The diameter of the swimming colonies was then measured.
Bacterial tracking assay
Bacteria grown to early-mid log phase on GC agar plates were resuspended at an OD550 of 0.15–0.2 in RPMI medium without phenol red. Bacterial suspensions were 10-fold diluted with the same medium, and aliquots (100 µl) were added immediately into 5 ml of fresh RPMI medium in a 35 mm dish and observed in humidified incubation chambers (5% CO2 at 37°C) using an Olympus IX81 microscope. Images were obtained with the VT-Infinity system (Visitron Systems, Munich, Germany), VT-Infinity Galvo scanner confocal head (Visitron Systems) and Hamamatsu C9100-02 CCD camera (Hamamatsu Photonics K.K., Tokyo, Japan). Bright field images were acquired with a 63× magnification lens and a high-speed shutter system. Digital frames, acquired at 50 frames per second for 5 s, were converted to video sequences and processed using Imaris software (Bitplane, Zurich, Switzerland). The resulting motility pattern of at least 100 bacteria per Helicobacter strain in each experiment was analysed. The determination of single-bacterium velocity was performed using the software MetaMorph (Universal Imaging Corporation, West Chester, PA, USA). At least 20 bacterial tracks per strain (from two independent experiments) were followed in 100 ms steps for 1 s and the respective track velocity was calculated accordingly. To determine the numbers of stops, 25 bacterial tracks per strain (from two independent experiments), each lasting 3 s, were observed for stops connected to direction changes.
Isolation of SLS-insoluble proteins
The SLS-insoluble proteins of either H. pylori or C. jejuni were enriched as described previously (Carlsohn et al., 2006), solubilized in Lamni buffer (62.5 mM Tris, 1% SDS, 5% 2-mercaptoethanol, 12.5% glycerol, 0.05% bromophenol blue) and protein concentrations determined by the Bradford assay using bovine serum albumin as a standard. Proteins were loaded onto the gel and separated by 8% SDS-PAGE (Fig. 2A). The gel was fixed in 40% methanol and 7% acetic acid for 20 min and then stained with Coomassie brilliant blue R-250 for 30 min. The protein bands of interest were excised in pieces of approximately 1 × 3 mm and subjected to mass spectrometry (MS) for identification.
Two-dimensional gel electrophoresis (2-DE)
Helicobacter pylori structure-bound proteins were isolated and subjected to 2-DE as described previously (Backert et al., 2005; see also 2D-PAGE database, http://web.mpiib-berlin.mpg.de/cgi-bin/pdbs/2d-page/extern/index.cgi). Briefly, protein samples were loaded on an IPG strip (pI 3-12) and second dimensional electrophoresis was performed on a 12% acrylamide gel. Proteins were visualized by silver staining.
Detection and deglycosylation of flagellins
For the detection of FlaA glycosylation levels of proteins, SLS-insoluble proteins were separated by 8% SDS-PAGE, followed by staining with GlycoProfile III Fluorescent glycoprotein detection kit (Sigma Aldrich), according to the manufacturer's instructions. After electroblotting onto PVDF membranes, FlaA was detected by probing blots with mouse anti-H. pylori flagellin primary monoclonal antibody (C65516M, Meridian Life Science, Maine, USA), washed and then probed with a horseradish peroxidase-conjugated goat anti-mouse secondary antibody. The blots were washed three times for 15 min each in TBS buffer containing 0.05% Tween 20 between all steps. TfOH is known to remove global types of carbohydrate chains in mammals – the N-linked, O-linked and glycosaminoglycan chains (Edge, 2003), therefore we performed the chemical deglycosylation of FlaA proteins with TfOH as described previously (Edge et al., 1981). Enzymatic deglycosylation assays were performed using a Glycoprotein deglycosylation kit according to the manufacturer's instructions (Calbiochem, San Diego, CA, USA). Treated samples were then subjected to 8% SDS-PAGE and Glycostain/Western blot analysis to determine the FlaA glycosylation level. To test the effect of the presence of HP0518 in H. pylori homogenate on FlaA mass, the SLS-insoluble fraction was treated with water-soluble cell extracts containing no FlaA at 37°C. After incubation overnight, the SLS-insoluble fraction was recollected and FlaA was also detected by Glycostain/Western blot.
Electron microscopy
Bacteria were grown in BHI supplemented with 10% FCS at 37°C for 20 h. After washing with PBS, the bacteria were fixed with 2.5% glutaraldehyde for 30 min at room temperature. Suspension aliquots (25 µl) were applied to 600-mesh carbon-coated copper grids, which were subjected to glow discharge. After absorption for 15 min, the grids were washed three times with water and treated for 30 s with 2% phosphotungsten acid/1% Trehalose. Images were obtained using a transmission electron microscope (Zeiss LEO 906).
Identification of proteins by MALDI-MS
The excised protein slices were in-gel trypsinated and the resulting peptides analysed by MALDI-TOFTOF MS, as described previously (Zimny-Arndt et al., 2009), using a 4700 Proteomics Analyzer (Applied Biosystems, Darmstadt, Germany). Proteins were identified by a MASCOT ion search in the complete NCBI database (NCBInr 20080101 with 5815196 sequences) using the combined MS and MS/MS peak lists. A protein was accepted as identified if the Mascot Score was in the confidentiality range of the Mascot Score (P < 0.05), the sequence coverage was > 30% or at least two peptides were confirmed by MS/MS.
Derivatization of pseudaminic acid
Pse was released from the SLS-insoluble proteins of H. pylori using the GlycoProfile β-elimination kit (Sigma Aldrich, St Louis, MO, USA), according to the manufacturer's instructions. The released Pse from 35 µg of protein was labelled with 1,2-diamino-4,5-methylenedioxybenzene (DMB) using the Sialic acid fluorescence-labelling kit (Takara, Tokyo, Japan), and the reaction mixture was applied onto a solid-phase extraction cartridge (Envi-Carb C, Supelco, Bellefonte, PA). After washing the cartridge with 2.5 ml of 5 mM ammonium acetate (pH 9.6) for desalting, the DMB-labelled Pse was eluted with 3.0 ml of 45% acetonitrile/5 mM ammonium acetate (pH 9.6) and the collected fractions freeze dried.
Liquid chromatography/mass spectrometry (LC/MS)
Chromatographic separation of DMB-labelled Pse was performed using Paradigm MS4 HPLC system (Michrom BioResource, Auburn, CA, USA) equipped with a reversed-phase C18 column (L-column Micro, 150 × 0.075 mm, 3 µm, Chemicals Evaluation and Research Institute (CERI, Tokyo, Japan). The DMB-labelled Pse was dissolved in 25 µl of 0.1% formic acid, and 10 µl of the sample solution was injected into a C18 trap column (L-column Micro, 50 × 0.3 mm, 5 µm, CERI). Elution was achieved using 0.1% formic acid/2% acetonitrile (A buffer) and 0.1% formic acid/90% acetonitrile (B buffer) with a linear gradient of 10–90% of B buffer in 30 min at a flow rate of 300 µl min−1. Online mass spectrometric analysis of DMB-labelled Pse was performed using a Fourier transformation ion cyclotron resonance (FT-ICR)/Ion trap (IT) type mass spectrometer (LTQ-FT, ThermoElectron, San Jose, CA, USA) equipped with a nanoelectrospray ion source (AMR, Tokyo, Japan). DMB-labelled Pse was determined by sequential scan comprising selected ion monitoring (SIM, m/z 441-461) using FT-ICR-MS and data-dependent MS/MS-MS/MS/MS/MS (MSn) using IT-MS.
Deglycosylase activity assay
The HP0518 gene was PCR-amplified (primers are listed in Table S1) and cloned into pET100 D-Topo in E. coli TOP10 cells (Invitrogen). His-tagged HP0518 protein was expressed in E. coli BL21(DE3) cells by induction with 1 mM IPTG. After purification with Ni-NTA (Qiagen), the purified HP0518 protein was co-incubated with SLS-insoluble proteins from either H. pylori or C. jejuni, or with whole H. pylori in the reaction buffer [25 mM sodium phosphate (pH 7.0), 0.5% Triton X-100, 0.1% SDS, 50 µM beta-mercaptoethanol] for 6 h. The reaction was then subjected to Western blot to determine the molecular mass of FlaA, as described above. For the detection of C. jejuni FlaA, Western blot was performed using mouse anti-C. jejuni FlaA MAb as a primary antibody, which was obtained by immunization of C. jejuni FlaA protein purified to BALB/c mice, as described previously (Power et al., 1994).
Construction of H. pylori G27 strain producing HA-tagged HP0518
A 457 bp fragment (containing a MluI site), upstream of the HP0518 stop codon, was PCR-amplified from H. pylori G27 chromosomal DNA using the primers 518-s2 and 518-as2-MI. Amplicons were digested with MluI and ligated with the MluI-digested HA-fd-terminator fragment amplified using primers HA-MI and fd-BI (Field et al., 1988). The ligated fragment was PCR-amplified using primers 518-s2 and fd-BI, and then digested with BamHI. The resulting fragment was ligated to a BamHI-digested 534 bp fragment located downstream of the HP0518 stop codon, amplified using primers 519-s-BI (containing a BamHI site) and 519-as. The fragments, including upstream and downstream regions of the HPG27-477 stop codon and HA-fd-terminator, were PCR-amplified using primers 518-s2 and 519-as and cloned into pGEM-T (Promega). The recombinant plasmid was then cleaved with BamHI and ligated with a BamHI-digested Km-cassette. After transformation into E. coli DH5α, the Km-resistant clones were selected and the insert verified by DNA sequencing (designated as pGEM-HP0518-HA-Km, Table 1). All primers used are listed in Table S1. The pGEM-HP0518-HA-Km plasmid DNA was introduced into H. pylori G27 WT strain by natural transformation. Successful transformants were subjected to protein fractionation as described above, and separated on a 12% acrylamide gel. HA-tagged HP0518 was detected by immunoblot with an HA probe (Y-11, Santa Cruz) using the ECL system.
Microarray analysis
Microarray and data analysis were performed as described elsewhere (Wunder et al., 2006). Total RNA was prepared from H. pylori grown on GC agar in mid-logarithmic phase. We excluded spots with intensities less than a cut-off value of two standard deviations below the negative reference samples. The raw data were deposited in Gene Expression Omnibus (GEO) (accession number GSE14752).
Cell adhesion assays
AGS cells (1 × 105) were seeded onto 24-well plates and allowed to attach overnight. H. pylori were added to each well at an moi of 100. After incubations of 30 min, 1 or 2 h in 5% CO2, the wells were gently washed four times with PBS to remove non-adherent bacteria. For centrifuge-assisted adhesion, plates were centrifuged (500 r.p.m. for 3 min) immediately after inoculating bacteria. The cells were then detached with 0.2% saponin for 20 min and the suspension (and respective serial dilutions) spread onto GC agar plates for determining the number of colony-forming units.
Infection experiments, SDS-PAGE and immunoblots
AGS cells were infected with H. pylori strains for 30, 60, 90 or 120 min at an moi of 100. Total cell lysates were subjected to 10% SDS-PAGE and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA), and CagA and phosphorylated-CagA proteins were detected as described previously (Bauer et al., 2005).
NF-κB activation
p65 nuclear translocation in response to H. pylori infection using AGS cells stably expressing p65-GFP (AGS-SIBO2 cells) is described in full elsewhere (Bartfeld et al., 2010). Briefly, this cell line stably expressed GFP-tagged p65, which enabled us to measure the nuclear translocation of p65 by tracing the GFP signal with an automated fluorescent microscopy, ScanR-screening station (Olympus Life Science Europe GmbH, Hamburg, Germany). The number of activated cells was calculated using the ScanR analysis software (Olympus), according to the manufacturer's instructions. The cells were infected with H. pylori at an moi of 100.
In vivo colonization
C57BL/6 female mice aged 6–8 weeks were orally infected with approximately 3.0 × 108 cells of H. pylori G27 WT, HP0518 mutant or flaA mutant strains respectively (n = 5). After 7 days of infection, animals were sacrificed, and gastric tissues were aseptically removed, weighed and homogenized in PBS. Dilutions of the homogenate were plated on GC agar containing H. pylori supplements (Oxoid) and colony-forming units per gram of tissue (cfu g−1) were quantified.
Statistical and bioinformatic analyses
The results of the motility, cell adhesion, cell scattering (hummingbird formation) and mice colonization assays as well as NF-κB activation measurements were expressed as means ± standard deviations of at least three independent observations. Statistical differences between measurements obtained from WT and mutant strains were determined using the two-tailed t-test and P-values of < 0.01 were considered statistically significant.
The predictive software system, SOSUIGramN (Imai et al., 2008) at http://bp.nuap.nagoya-u.ac.jp/sosui/, which assesses the subcellular localization of proteins in Gram-negative bacteria, was used to predict the cellular location of the HP0518 protein.
Acknowledgements
We thank Nina R. Salama from the Fred Hutchinson Cancer Research Center, Seattle, WA, for the kind gift of the H. pylori G27 Transposon mutant library and Lesley Ann Ogilvie for critical editing of the manuscript. We also thank Monika Schmid for her technical assistance in mass spectrometry. This work was supported in part by BMBF through ERA-NET PathoGenoMics (Grant No. 0315435A and 0313938A) and a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS, 22780275).




