Regulation of bacterial gene expression by the NTP substrates of transcription initiation
Summary
Many mechanisms of gene regulation in bacteria do not employ repressor or activator proteins. One class of these mechanisms includes those in which the key regulatory element is the control of transcription initiation by the availability of NTP substrates. In this commentary, several distinct examples of initiating NTP-mediated gene regulation are discussed, including a mechanism reported by Krásnýet al. in this issue of Molecular Microbiology. These researchers show that during the stringent response induced by amino acid starvation of Bacillus subtilis, increases in the intracellular level of ATP permit upregulation of promoters with +1A start sites, while concurrent decreases in the intracellular level of GTP cause downregulation of promoters with +1G start sites. This regulation is restricted to stringently controlled promoters.
Although it might not be obvious from current textbooks or most college and graduate school lectures on molecular genetics, transcriptional regulation of gene expression in bacteria involves much more than DNA-binding repressor and activator proteins. The first examples of regulation of transcription without these regulatory proteins were transcription attenuation control mechanisms, and these are now generally known (Landick et al., 1996). The hallmark of these mechanisms is conditional transcription termination at a terminator, usually called the attenuator, located within the DNA (i.e. leader region) between the promoter and first gene of the operon. Although the earliest attenuation control mechanisms described were similar, subsequent studies revealed that a wide assortment of molecules and physiological conditions that affect the secondary structure of the leader transcript (i.e. RNA specified by the leader region) can control transcription termination at the attenuator (Henkin and Yanofsky, 2002; Winkler and Breaker, 2005; Turnbough and Switzer, 2008).
Most other classes of regulatory mechanisms that do not require DNA-binding regulatory proteins are not widely appreciated. One such class includes mechanisms in which gene expression is controlled only by the availability of NTP substrates required for transcription initiation. This regulation is determined by specific sequences in the promoter region. There are many variations of this mechanism, especially if transcription initiation is defined as RNA synthesis from the first nucleotide of the transcript (+1) to the last nucleotide (roughly +9) added to the transcript prior to entry into the elongation phase of transcription. One remarkable mechanism is conditional reiterative transcription, a reaction in which a particular NTP is repetitively added to the 3′ end of a nascent transcript due to slippage between the transcript and DNA template. At some promoters, such as those for the pyrimidine biosynthetic operons carAB and pyrBI of Escherichia coli, reiterative transcription is induced by high intracellular levels of UTP. This reaction produces poly(U)-containing transcripts that are released from the transcription initiation complex, thereby inhibiting the synthesis of full-length transcripts encoding unnecessary pyrimidine biosynthetic enzymes (Liu et al., 1994; Han and Turnbough, 1998).
Another distinct mechanism is NTP-sensitive transcription start-site switching, which produces alternative transcripts with different potentials for translation. For example, transcription initiation at the pyrC promoter of E. coli and Salmonella occurs predominantly at position C7 when intracellular levels of CTP are high and at position G9 when intracellular levels of CTP are low, with start-site designations indicating the non-template strand base and number of bases downstream from the promoter −10 region. Transcripts initiated at position C7 are not translated, however, because they form a stable hairpin at their 5′ ends that blocks ribosome binding to the pyrC Shine–Dalgarno sequence. In contrast, the shorter transcripts initiated at position G9 are unable to form the inhibitory hairpin and are readily translated (Sørensen and Neuhard, 1991; Wilson et al., 1992). This mechanism allows the bacterium to regulate the level of pyrC expression according to its need for pyrimidine nucleotides. Further characterization of transcription initiation at the pyrC promoter and related studies of other E. coli promoters established preferences for selecting transcription start sites that appear to be generally applicable (Liu and Turnbough, 1994; Walker and Osuna, 2002; Lewis and Adhya, 2004). The preferences for the initiating NTP (iNTP) are ATP ≥ GTP > UTP >> CTP and the preferences for the start site position (counting downstream from the −10 region) are 7 > 8 > 6 ≈ 9. These ‘rules’ have been useful in predicting transcription start sites at other promoters and in developing new models for gene regulation (see below).
Perhaps the best-known example of regulation by iNTPs is the mechanism controlling (in part) transcription initiation at ribosomal RNA promoters in E. coli (Gaal et al., 1997; Murray et al., 2003). This bacterium contains seven rRNA (rrn) operons, each of which possesses two promoters designated P1 and P2. At moderate to high rates of growth, most transcription of rrn operons is initiated at promoter P1. Six of the P1 promoters initiate at an A residue, while the seventh starts at a G residue. Each start site is located nine bases downstream from the −10 region. This atypical position for initiation (apparently dictated by C residues at positions 7 and 8) is one of several promoter elements that cause each P1 promoter to have an unusually high Km for its iNTP (Gralla, 2005; Haugen et al., 2006). Consequently, transcription initiation at the P1 promoters – along with production of rRNA and ribosomes – changes in proportion to the intracellular levels of ATP and GTP, the energy sources for protein synthesis. This regulation appears to be most important during periods of outgrowth from stationary phase, when ATP and GTP levels increase and more protein synthesis is required, and during stationary phase, when ATP and GTP levels and the need for protein synthesis are low (Murray et al., 2003). Interestingly, regulation of transcription initiation of the E. coli fis operon, which encodes the nucleoid-associated protein Fis, occurs by a mechanism analogous to that described for the rrn P1 promoters. At the fis promoter, however, CTP is used as the iNTP (Walker et al., 2004). This mechanism regulates fis transcription according to nutritional conditions and phase of cell growth.
In E. coli, transcription initiation at the rrn P1 (and P2) promoters is also regulated during the exponential phase of cell growth by the small-molecule effector guanosine-3′,5′-(bis)pyrophosphate (ppGpp), which functions by binding to RNA polymerase (Murray et al., 2003). Regulation of transcription initiation mediated by ppGpp (and also by the iNTP) requires the transcription factor DksA, which gains access to the RNA polymerase active site through the secondary channel of the enzyme (Paul et al., 2004; Perederina et al., 2004). In many bacteria, the intracellular levels of ppGpp rapidly increase upon amino acid starvation and mediate a global response called the stringent response. The hallmark of this response is the inhibition of cellular activities necessary for protein synthesis in conjunction with the stimulation of activities capable of relieving the amino acid limitation. A central component of the stringent response is inhibition of rRNA synthesis, which can occur by direct binding of ppGpp to RNA polymerase, as described for E. coli, or by other mechanisms.
One such alternative mechanism is used during the stringent response in Bacillus subtilis (Krásný and Gourse, 2004). B. subtilis contains 10 rrn operons; apparently, six possess tandem P1 and P2 promoters, and four contain a single promoter. Transcription initiation at all 16 rrn promoters apparently initiates with GTP at sites located seven or eight bases downstream from the promoter −10 region. Transcription initiation at selected rrn promoters requires high concentrations of the iNTP and is insensitive to ppGpp in vitro. In addition, the level of transcription initiation at these promoters in vivo correlates with changes in the concentration of GTP, but not that of ppGpp. The synthesis of ppGpp is required for stringent control of rrn promoter activity, however. Interestingly, a +1G to A mutation at an rrn promoter eliminates inhibition of transcription initiation during the stringent response. These results indicate that the inhibitory effect of ppGpp on transcription initiation at rrn promoters is indirect and suggest that inhibition is through ppGpp-mediated decreases in the intracellular concentration of GTP. Such decreases could be due to GTP consumption during ppGpp biosynthesis and to documented ppGpp-mediated inhibition of IMP dehydrogenase, the first enzyme in GTP biosynthesis (Fig. 1). A similar mechanism for stringent control of rRNA synthesis has been proposed for Thermus thermophilus (Kasai et al., 2006).
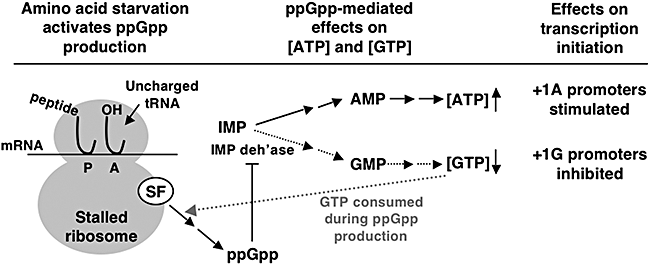
Regulation of transcription initiation by iNTPs during the stringent response in B. subtilis. Amino acid starvation causes translating ribosomes to stall with an uncharged tRNA in the ribosomal A site, leading to activation of a ribosome-associated enzyme called stringent factor (SF). SF produces pppGpp, which is rapidly converted to ppGpp. The resulting high level of ppGpp inhibits IMP dehydrogenase, the first enzyme in the pathway that converts IMP to GTP. This inhibition results in a reduced level of GTP and an increased level of ATP in cells. (The consumption of GTP during the production of pppGpp also contributes to the reduction in the GTP level.) The increased level – or at least the maintenance of a high level – of ATP is required to stimulate transcription initiation at stringently controlled promoters that contain a +1A start site. On the other hand, the decreased level of GTP inhibits transcription initiation at stringently controlled promoters that contain a +1G start site.
During their investigation of iNTP-mediated regulation of transcription initiation at rrn promoters in B. subtilis, Krásný and Gourse observed that transcription initiation at the +1G to A mutant rrn promoter increased during the stringent response. This increase was accompanied by an increase in the intracellular level of ATP, which occurs as GTP levels fall. These observations suggested the possibility that iNTP-mediated regulation of transcription initiation might occur at many promoters (in addition to the rrn promoters) and might be involved in both activation and repression of operon expression during the stringent response in B. subtilis. This is the hypothesis explored by Krásný and co-workers in a report in this issue of Molecular Microbiology.
Consistent with the hypothesis, Krásný and colleagues have now discovered that (with perhaps rare exceptions) operons that are activated or repressed during the stringent response contain promoters at which initiation begins with ATP or GTP respectively (Fig. 1). Using a small set of DNA templates containing individual promoters of activated and repressed operons, the Krásný group showed that maximal transcription initiation at these promoters in vitro requires atypically high concentrations of the iNTP and that the efficiency of initiation at these promoters is proportional to various physiological concentrations of the iNTP. These results were similar to those described for B. subtilis rrn promoters. Krásnýet al. then used their set of activated and repressed promoters to make mutant promoters in which the +1 position was changed to the other purine (i.e. +1A to G and +1G to A). These promoters were examined in vitro for their dependence on NTP concentrations, and it was found that for maximal transcription initiation +1A to G mutant promoters required high concentrations of GTP, while +1G to A mutant promoters required high concentrations of ATP. Finally, transcription initiation at the mutant promoters (fused to lacZ) was examined in B. subtilis cells following induction of the stringent response. In this case, initiation from +1A to G mutant promoters decreased, while initiation from +1G to A mutant promoters increased. These results provide convincing evidence that the identity of the iNTP determines whether a stringently controlled operon will be activated or repressed during the stringent response in B. subtilis.
In this study, Krásný and co-workers focused on core promoter activity. During the stringent response, activation of the promoters that employ ATP as the iNTP was modest. In the case of the Pilv promoter, a highlighted example, activation of the core promoter was much less than that observed with the promoter containing its normal upstream sequences known to bind regulatory proteins. However, all activation of transcription at the longer Pilv promoter region required initiation with ATP. This requirement was demonstrated by the reduction during the stringent response of transcription initiation at a longer Pilv promoter region carrying a +1A to G mutation. These results indicate that activation of transcription at the normal Pilv promoter requires the maintenance of a high intracellular level of ATP (Fig. 1).
It is important to note that not all promoters with +1A or +1G start sites are regulated during the stringent response in B. subtilis. The critical differences between regulated and unregulated promoters are unknown. Interestingly, all of the promoter elements known to be important for iNTP- and ppGpp-mediated regulation of transcription initiation at rrn P1 promoters in E. coli (e.g. G+C-rich discriminator sequence between the −10 region and +1, suboptimal 16 bp spacing between the −35 and −10 regions) are absent in the iNTP-sensitive B. subtilis promoters. Consistent with this observation, iNTP-regulated promoters of B. subtilis do not require high concentrations of their iNTPs when they are transcribed with RNA polymerase from E. coli. In future experiments, it will be interesting to identify the B. subtilis promoter elements required for iNTP-mediated regulation. It will also be important to identify the differences between E. coli and B. subtilis RNA polymerases that account for their different requirements for transcription initiation.
It is also noteworthy that the control mechanism described by Krásnýet al. is apparently insensitive to the concentration of the second NTP (+2 NTP) added to the transcript. This observation is somewhat surprising because E. coli rrn P1 promoters have high Km values for the +1 (i.e. iNTP as defined here) and +2 NTPs and both NTPs apparently act as effectors of promoter P1 activity (Lew and Gralla, 2004). Actually, significantly higher Km values for the +1 and +2 NTPs compared with those for NTP substrates used during transcription elongation appear to be a general feature of E. coli promoters. This requirement for a high concentration of the +2 NTP – the so-called second nucleotide effect – is a core element in the transcription start-site switching mechanism for regulation of expression of the pyrimidine salvage operons codBA and upp-uraA in E. coli (Qi and Turnbough, 1995; Tu and Turnbough, 1997).
The report by Krásnýet al. accomplishes two important goals. First of all, it adds a number of new examples to the list of operons whose expression is regulated by iNTPs. Second, it draws much needed attention to the entire class of mechanisms of iNTP-mediated gene regulation. Because of the simplicity and flexibility of these mechanisms, it seems likely that many more examples will be discovered – provided more researchers become aware of the prototypes. New examples might be found in other bacteria, or in archaea or eukaryotes. Perhaps another useful contribution to the educational process would be a catchy name. ‘Gene regulation by initiating NTPs’ has not quite done the job.
Acknowledgements
I would like to thank David Bedwell, Robert Switzer, David Schneider, Richard Gourse and Michael Niederweis for reading the manuscript and providing helpful suggestions. I also thank Evvie Allison for editorial assistance.




