Continuous periplasm in a filamentous, heterocyst-forming cyanobacterium
Summary
The cyanobacteria bear a Gram-negative type of cell wall that includes a peptidoglycan layer and an outer membrane outside of the cytoplasmic membrane. In filamentous cyanobacteria, the outer membrane appears to be continuous along the filament of cells. In the heterocyst-forming cyanobacteria, two cell types contribute specialized functions for growth: vegetative cells provide reduced carbon to heterocysts, which provide N2-derived fixed nitrogen to vegetative cells. The promoter of the patS gene, which is active specifically in developing proheterocysts and heterocysts of Anabaena sp. PCC 7120, was used to direct the expression of altered versions of the gfp gene. An engineered green fluorescent protein (GFP) that was exported to the periplasm of the proheterocysts through the twin-arginine translocation system was observed also in the periphery of neighbouring vegetative cells. However, if the GFP was anchored to the cytoplasmic membrane, it was observed in the periphery of the producing proheterocysts or heterocysts but not in adjacent vegetative cells. These results show that there is no cytoplasmic membrane continuity between heterocysts and vegetative cells and that the GFP protein can move along the filament in the periplasm, which is functionally continuous and so provides a conduit that can be used for chemical communication between cells.
Introduction
Bacteria are forms of life widely viewed as single-celled organisms, but they are far from being homogeneous from a morphological point of view. In addition to the simplest unicellular bacteria, there are bacteria that exhibit complex structures, including filamentous forms in which the cells are organized in trichomes or strings of cells. An important question is whether cells in these organisms interact, and if they do so, how interactions take place. To better understand this issue, a deeper knowledge of the supracellular structure of the bacterial filament is needed.
The cyanobacteria are a group of bacteria that bear a Gram-negative type of cell wall, which includes a peptidoglycan layer and an outer membrane outside of the cytoplasmic membrane, and perform oxygenic photosynthesis. The heterocyst-forming cyanobacteria constitute an excellent example of multicellular bacteria in which, under certain nutritional conditions, two different cell types are required for growth. When deprived of combined nitrogen, some cells in a filament develop at semi-regular intervals into differentiated N2-fixing heterocysts, which do not fix CO2 by photosynthesis (Wolk et al., 1994; Herrero et al., 2004). Vegetative cells transfer reduced C to the heterocysts and, in turn, heterocysts transfer fixed N to their neighbouring vegetative cells (Wolk, 1968; 1974). Both developing and mature heterocysts also transfer regulatory signals, of which the inhibitor of heterocyst development PatS is thought to be an example (Yoon and Golden, 1998), to vegetative cells. The mechanism for this transfer of compounds between cells is unknown, but it has been suggested that transfer might take place through an extracytoplasmic route (Montesinos et al., 1995; Picossi et al., 2005). Whereas each cell in the filament is surrounded by a cytoplasmic membrane and a peptidoglycan layer, the outer membrane does not enter the septum between cells and is continuous along the filament, suggesting the existence of a structurally continuous periplasmic space (Flores et al., 2006).
We have experimentally addressed the question of whether the periplasm provides a continuous conduit along the cyanobacterial filament. Based on the observation that the green fluorescent protein (GFP) exported through the twin-arginine translocation system is functional in the periplasm of a cyanobacterium (Spence et al., 2003), we sought to produce engineered GFPs in developing or mature heterocysts, with the aim of testing whether those GFPs could be detected at a distance from the cells producing them.
Results
For expression of the gfp gene in developing heterocysts (proheterocysts) and mature heterocysts, we used the promoter of the patS gene, which is activated in cells that initiate differentiation when deprived of fixed nitrogen (Yoon and Golden, 1998). Constructs carrying three different versions of the PpatS–gfp fusion were prepared. (i) One carried the native gfp gene, which is translated into a protein that bears no targeting signals and should accumulate in the cytoplasm of the producing cells. (ii) The second one carried a gfp gene encoding a protein fused N-terminally to a signal peptide for export through the twin-arginine translocation system and cleavage by signal peptidase I. The produced GFP will be released as a free protein in the periplasm (Paetzel et al., 2002). (iii) The third version bore a gfp gene whose product was fused N-terminally to a signal peptide for export through the twin-arginine translocation system and cleavage by signal peptidase II. This will position the GFP also in the periplasm but anchored to the cytoplasmic membrane through a lipid covalently attached to the Cys that constitutes the N-terminal residue of the mature protein (Paetzel et al., 2002). Genes putatively encoding signal peptidase I (alr2304, alr2975) and II (alr4577) are present in the Anabaena genome (Kaneko et al., 2001), and they appear to be expressed both in nitrate-grown cultures and at least up to 24 h after nitrogen deprivation (Ehira and Ohmori, 2006). Signal peptides from the periplasmic binding proteins All3333 and Alr0608 of Anabaena sp. PCC 7120 (Kaneko et al., 2001), which are predicted to be cut by signal peptidases I and II, respectively, were used. The three PpatS–gfp constructs were cloned in a mobilizable plasmid and transferred by conjugation into Anabaena sp. PCC 7120. The Anabaena sp. strains CSVM17 (carrying construct i), CSVM18 (construct ii) and CSVM19 (construct iii) had incorporated by single cross-over the corresponding plasmids in the Anabaena genome (see Experimental procedures for details).
The cyanobacterial strains carrying the different PpatS–gfp constructs were incubated in the absence of combined nitrogen to induce the patS promoter, and the localization of GFP fluorescence was analysed by confocal microscopy (Fig. 1). After 10 or 25 h of nitrogen deprivation, the GFP fluorescence in strain CSVM17 was observed confined to individual cells (proheterocysts in the examples shown in Fig. 1A) with an apparent homogeneous distribution in the cell. In strain CSVM19, the GFP fluorescence was also observed in individual cells, proheterocysts or heterocysts, with an especially high signal in the periphery of the cells consistent with anchoring of the GFP to the cytoplasmic membrane (Fig. 1A). The sharp contrast in GFP fluorescence between the producing cells and their neighbouring vegetative cells indicates that the cytoplasmic membrane-anchored GFP does not pass to the vegetative cells. In Fig. 1C, the protrusions of fluorescence at the poles of a mature heterocyst correspond to the heterocyst necks (for electron micrographs of heterocysts, see, for example, Wolk et al., 1994; Flores et al., 2006).
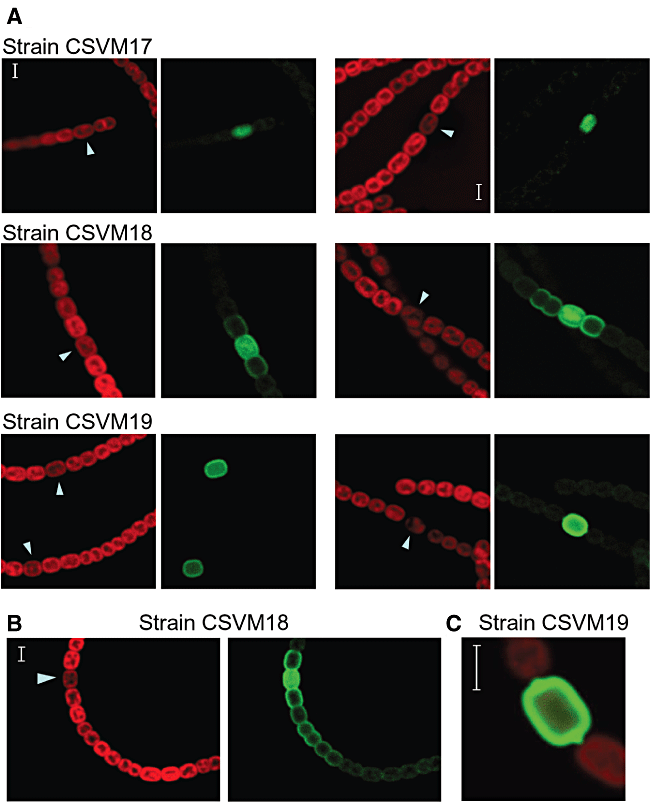
Cyanobacterial autofluorescence (red) and GFP fluorescence (green) of filaments of the indicated Anabaena strains. The filaments were taken from cultures that had been incubated in the absence of combined nitrogen for 10 h (A, left), 25 h (A, right, strains CSVM17 and CSVM19), 26 h (A, right, strain CSVM18), 30 h (B) or 25 h (C). The arrowheads point to developing or mature heterocysts, which show reduced autofluorescence depending on their degree of differentiation and produce the GFP. The figure in (C) shows an overlay of the cyanobacterial autofluorescence and GFP fluorescence. Scale bars, 3 μm.
In contrast to results obtained with strains CSVM17 and CSVM19, in strain CSVM18 that produces a soluble periplasmic GFP, the GFP fluorescence was observed in clusters or strings of cells. Filaments of CSVM18 from cultures 10 or 26 h after nitrogen step-down are shown in Fig. 1A, and an example of a filament from a culture 30 h after nitrogen step-down is shown in Fig. 1B. Whereas in the presumptive producing cells (proheterocysts in the examples shown in Fig. 1), the GFP fluorescence was observed both internally and in the periphery, in the neighbouring vegetative cells it was observed only in the periphery, with a gradient of decreasing fluorescence away from the proheterocyst. The decrease of fluorescence was stepwise, with consecutive cells (or pairs of cells) generally showing lower fluorescence than the previous cells closer to the producing proheterocyst. These observations suggest that soluble GFP moves in the periplasm along the filament.
Expression of the patS gene appears to be activated in groups of cells immediately after nitrogen step-down, with resolution of these cell clusters into single PatS-producing cells only latter (Yoon and Golden, 2001). To further analyse the expression of our constructs, the number of consecutive cells showing increased GFP florescence in filaments of the three strains, CSVM17, CSVM18 and CSVM19, was counted for a number of fluorescent cell clusters at several time points after nitrogen step-down. Strain Anabaena sp. AMC484, which carries a patS–gfp transcriptional fusion in a plasmid, pAM1951 (Yoon and Golden, 1998; 2001), was used as an additional control. As summarized in Fig. 2, increased fluorescence was observed mostly in groups of cells (mean length of fluorescent cell clusters, 1.6–2.6 cells) 2 h after nitrogen step-down. (We think that the variability observed at 2 h reflects the ease with which the GFP fluorescence is observed in each strain: strain AMC484 seems to have a relatively high expression of the fusion, and the GFP in strain CSVM19 is very clearly observed because it is largely confined to the cytoplasmic membrane.) By 25 h after nitrogen step-down, the mean length of fluorescent cell clusters decreased to close to 1 for strains CSVM17, CSVM19 and AMC484, but increased to more than 3.5 cells for strain CSVM18. The results with strains CSVM17, CSVM19 and AMC484 confirm that, after resolution of the early patS-expressing groups of cells, expression of patS takes place mainly in individual cells (proheterocysts or young mature heterocysts) as previously shown by Yoon and Golden (1998; 2001). Consequently, the results with strain CSVM18 suggest movement of the periplasmic GFP to cells in which the patS promoter is not active.
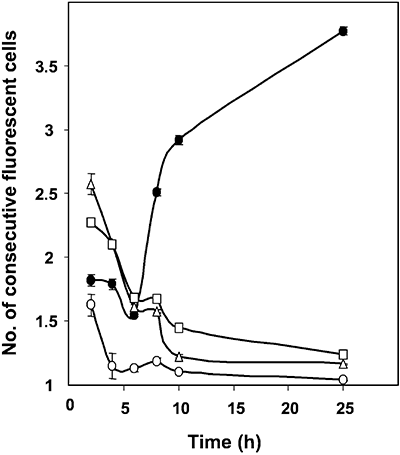
Number of consecutive cells showing increased GFP fluorescence. Nitrate-grown filaments of strain CSVM17 (open circles), CSVM18 (closed circles), CSVM19 (triangles) and AMC484 (squares) were incubated in the absence of combined nitrogen for the indicated times. At each time point, the number of consecutive cells showing increased GFP fluorescence was counted for about 30–100 clusters of fluorescent cells for each strain except CSVM17, for which 8, 7 and 16 clusters were counted at 2, 4 and 6 h of nitrogen deprivation respectively. Data are the mean and standard deviation of the mean.
In the cytoplasmic membrane, although movement of some protein complexes may be restricted, lateral diffusion of lipids and proteins appears to occur (Chen et al., 2006), as is also the case for proteins in the Escherichia coli periplasm (Mullineaux et al., 2006). To test whether the movement of GFP could take place by diffusion in the periplasm and the cytoplasmic membrane, we examined the recovery of GFP fluorescence after photobleaching. No recovery of fluorescence was observed in minutes when the signal of a whole developing or mature heterocyst was photobleached indicating that no accumulation of newly produced GFP took place (not shown). Within a heterocyst of strain CSVM19 (which bears cytoplasmic membrane-anchored GFP), the GFP signal spread in seconds after photobleaching of a small area of the cell, the time-lapse depending on the extent of the surface area bleached (Fig. 3A and B). Homogenization of the signal took place through the whole heterocyst surface, which implies redistribution of the GFP in all directions by random movement and is indicative of diffusion. Thus, the cytoplasmic membrane-anchored GFP moves in the heterocyst cytoplasmic membrane, but does not pass to the cytoplasmic membrane of the neighbouring vegetative cells.
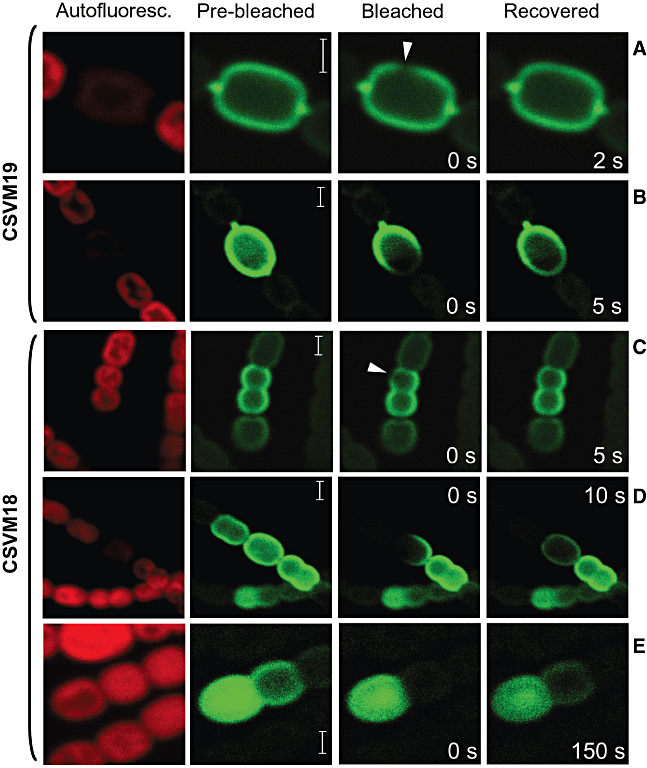
GFP fluorescence recovery after photobleaching. Bleaching was accomplished by increasing the intensity of excitation irradiation over a bleach point (A and C; arrowheads) or over a larger area (B, D and E). Post-bleaching micrographs were taken every second (A–D) or at 5 s intervals (E), and an image is shown from a time when fluorescence has recovered as discussed in the text. Samples were from 4-day N2-grown cultures (A–D) or from a 25 h incubation without combined nitrogen (E). Scale bars, 2 μm.
In strain CSVM18 (that expresses a soluble periplasmic GFP), the GFP signal also spread in seconds after photobleaching of a small area of the cell (Fig. 3C). However, when a complete vegetative cell and part of a heterocyst were photobleached, fluorescence recovered in 10 s in the heterocyst, but not in the vegetative cell (Fig. 3D). In Fig. 3E, where a complete vegetative cell adjacent to a proheterocyst was photobleached, the GFP fluorescence took about 150 s to recover in the vegetative cell. Recovery of GFP fluorescence in the vegetative cell was accompanied by a decrease of fluorescence in the proheterocyst, which again suggests movement of the GFP. These results show that movement is quicker within the periplasm of one cell, either a vegetative cell or a heterocyst, than between cells.
Discussion
We have taken advantage of the cell specificity of expression of patS (Yoon and Golden, 1998; 2001) to use the patS promoter to drive expression of engineered versions of the gfp gene in proheterocysts and heterocysts of Anabaena sp. PCC 7120. Although the patS promoter was activated in clusters of cells upon nitrogen step-down, these clusters resolved to mostly single cells after a few hours of nitrogen deprivation (Fig. 2), which is consistent with previously reported data (Yoon and Golden, 1998; 2001). Adding a signal peptide for translocation through the twin-arginine translocation system and cleavage by signal peptidase I (Paetzel et al., 2002), as in the construct in strain CSVM18, permits production of GFP as a soluble protein in the periplasm. The twin-arginine translocation system is known to translocate folded proteins across the cytoplasmic membrane (Robinson and Bolhuis, 2004), and we detected intracellular GFP fluorescence in the presumptive producing cells in the filaments of strain CSVM18 (Fig. 1), which suggests intracellular production of the GFP previous to its translocation to the periplasm. Consistent with the observed structural continuity of the outer membrane along the cyanobacterial filament (Flores et al., 2006), which implies the existence of a continuous periplasm, we have observed GFP fluorescence in the periplasm of vegetative cells beyond the producing proheterocysts in strain CSVM18. This result indicates that the GFP moves in the periplasm, which is therefore not only structurally but also functionally continuous along the filament. A continuous periplasm can represent a communication conduit through which traffic of metabolites and/or regulatory molecules could take place. Because diffusion is inversely proportional to particle size, diffusion of small molecules can be quicker than that observed here for the relatively large GFP (about 27 kDa). Our current results therefore are consistent with the hypothesis of a periplamic transfer of metabolites between cells in the diazotrophic cyanobacterial filament (Montesinos et al., 1995; Picossi et al., 2005).
Whereas diffusion within one cell's periplasm takes place quickly, consistent with data available for E. coli (Mullineaux et al., 2006), movement of the GFP between cells is slower and therefore appears to encounter a barrier (Fig. 3). Possible barriers to protein diffusion have been identified in the periplasm of E. coli, where they have been related to cell division sites (Foley et al., 1989). For globular proteins that do not bind to peptidoglycan, the murein sacculus of E. coli and Bacillus subtilis has been shown to have an exclusion limit of about 25–50 kDa (Demchick and Koch, 1996). If similar figures are true for the Anabaena murein sacculus, the peptidoglycan layer, which surrounds each cell in the filament, could represent the barrier that slows down diffusion of GFP between cells resulting in the stepwise decrease in fluorescence. Establishment of a pattern of distribution of heterocysts along the cyanobacterial filament has been consistently modelled assuming a gradient of a diffusible regulatory compound, which is produced by the heterocyst and inhibits nearby cells from differentiating (Wolk and Quine, 1975). If the barrier to free diffusion between cells is effective for physiologically relevant regulatory compounds, it could have the role of helping to create a spatial gradient, as free diffusion could homogenize concentration along the filament too quickly.
Substitution of the signal peptide for cleavage by signal peptidase I (as in strain CSVM18) by a signal peptide for signal peptidase II (in strain CSVM19) results in the anchoring of the GFP to the cytoplasmic membrane (Paetzel et al., 2002). We have used in this work the signal peptide from Alr0608, a periplasmic nitrate-binding protein (NrtA, also known as the 48 kDa protein) that has been shown to be bound to the cytoplasmic membrane in Synechococcus sp. PCC 7942 (Madueño et al., 1988; Maeda and Omata, 1997) and Synechocystis sp. PCC 6803 (Huang et al., 2006). In strain CSVM19, GFP accumulates in the periphery of the heterocysts, presumably at the cytoplasmic membrane, and does not pass to the adjacent vegetative cells (Fig. 1C). Because the anchored GFP can move in the cytoplasmic membrane (Fig. 3A and B), lack of passage to the neighbouring vegetative cells suggests that there is no cytoplasmic membrane continuity between vegetative cells and heterocysts, which is consistent with lack of plant-like plasmodesmata in filamentous cyanobacteria (Flores et al., 2006).
The finding described here, i.e. movement of a protein along the filament's periplasm in a heterocyst-forming cyanobacterium, illustrates a novel structural feature in bacteria: the presence of a functionally continuous periplasm in a multicellular bacterium. Filamentous cyanobacteria with developmental processes have a long history on Earth, as they were already present about 2 billion years ago (Tomitani et al., 2006). From an evolutionary perspective, a proposed first step in the origins of multicellularity is that the cells remain together after cell division (Bonner, 1998). Our results show that multicellularity in the heterocyst-forming cyanobacteria might be based on a deeper structural innovation, in which the outer membrane became a common structure for the whole filament leading to the creation of a shared compartment for the filament's cells.
Experimental procedures
Cloning and strain construction
Plasmids carrying gfp constructs i to iii (Fig. 4A) were prepared by standard cloning techniques and PCR. Template DNA was isolated from photoautotrophically grown Anabaena sp. PCC 7120 (Cai and Wolk, 1990). Oligonucleotides pPATS-1 (which adds a ClaI site; Table 1) and pPATS-2 (which adds a SpeI site) were used to amplify the PpatS promoter, and the resulting DNA fragment was cloned in pMBL-T (Dominion MBL). Oligonucleotides all3333-4 (which adds a SpeI site) and all3333-5 (which adds a SmaI site), and oligonucleotides alr0608-3 (which adds a SpeI site) and alr0608-4 (which adds a SmaI site) were used to amplify the signal peptide DNA sequences from all3333 and alr0608, respectively (Kaneko et al., 2001), and the resulting DNA fragments were cloned in pMBL-T. Sequences corresponding to signal peptides were then introduced into the PpatS-carrying plasmid digested with SpeI and BamHI. The PpatS sequence and the PpatS-signal peptide sequence fusions, which were corroborated by sequencing, were isolated after ClaI/SmaI digestions and transferred to ClaI/EcoRV-digested pCSEL21 (Olmedo-Verd et al., 2006) rendering fusions to the 5′ end of the gfp-mut 2 gene (Cormack et al., 1996). The resulting fusions were finally transferred to pCSEL24 (Olmedo-Verd et al., 2006) by means of ClaI/PstI digestions (Fig. 4B). This plasmid carries a c. 2 kb fragment from the nucA-nuiA region of the Anabaenaα megaplasmid (Kaneko et al., 2001; Olmedo-Verd et al., 2006). The resulting plasmids were transferred from E. coli to Anabaena sp. PCC 7120 by conjugation, with selection for resistance to spectinomycin and streptomycin, as described (Elhai et al., 1997). Insertion into the nucA-nuiA region was confirmed by PCR analysis with primers 7120-nuc-nui6 and GFP-4 and template DNA isolated from exconjugant clones.
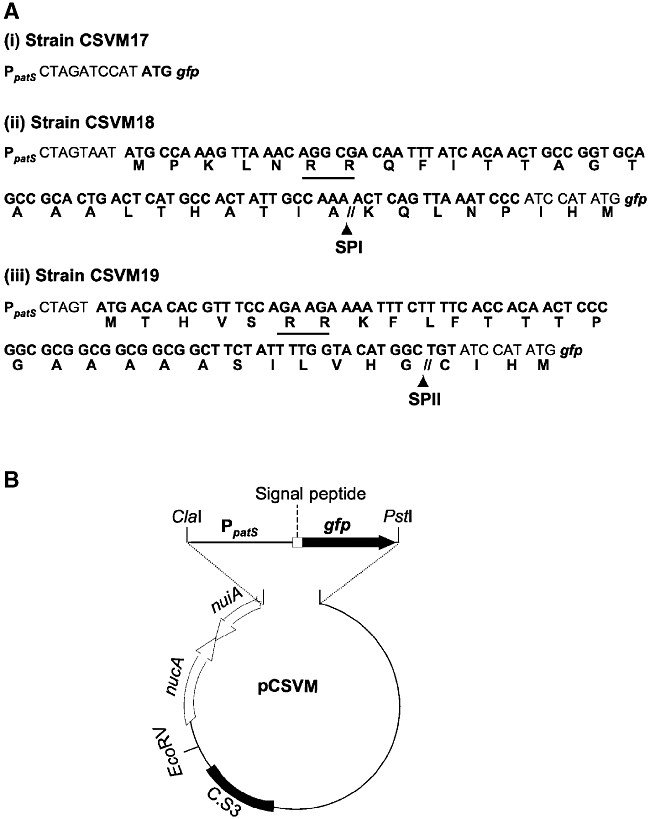
A. Schematic representation of the gfp fusions used in this work. The co-ordinates of the patS promoter in the Anabaena sp. PCC 7120 chromosome are: 2771944–2771056. Signal peptides in CSVM18 and CSVM19 are from open reading frames all3333 and all0608 respectively. The two consecutive Arg residues in the signal peptides of CSVM18 and CSVM19 are underlined. SPI, signal peptidase I cutting site; SPII, signal peptidase II cutting site. ATG-gfp refers to the native gfp gene.B. Schematic representation (not to scale) of the plasmids constructed for transfer to Anabaena sp. PCC 7120; the plasmids differ at the sequences corresponding to the signal peptides, upstream of the gfp gene.
| Name | Sequence (5′−3′) |
|---|---|
| pPATS-1 | ATC GAT GTC AGT ATT GTT TC |
| pPATS-2 | ACT AGT CTT AAA ATC GGT GA |
| all3333-4 | CCA CTA GTA ATA TGC CAA AGT TA |
| all3333-5 | CCC GGG ATT TAA CTG AGT |
| alr0608-3 | ACT AGT ATG ACA CAC GTT TCC AGA AG |
| alr0608-4 | CCC GGG AGT ACA GCC ATG TA |
| 7120-nuc-nui6 | AGC CAA GTT TTA TCA TCT AT |
| GFP-4 | CAA GAA TTG GGA CAA CTC C |
- Letters in boldface indicate introduced restriction endonuclease cleavage sites.
Microscopy
Filaments of Anabaena exconjugants were routinely grown photoautotrophically in BG11 medium (that contains nitrate as the nitrogen source), at 30°C in a shaker, supplemented with 2 μg ml−1 spectinomycin and streptomycin or, for strain AMC484, 10 μg ml−1 neomycin. (For growth on medium solidified with 1% agar, 5 μg ml−1 spectinomycin and streptomycin or 25 μg ml−1 neomycin were added.) After washing with BG110 medium (which lacks any source of combined nitrogen), induction was achieved by incubation of the filaments in this medium under growth conditions for the indicated number of hours. Samples were embedded in 0.5% agarose in BG110 medium and visualized using a Leica HCX PLAN-APO 63X 1.4 NA oil immersion objective attached to a Leica TCS SP2 confocal laser-scanning microscope. GFP was excited using 488 nm irradiation from an argon ion laser. Fluorescent emission was monitored by collection across windows of 500–520 nm (GFP imaging) and 630–700 nm (cyanobacterial autofluorescence).
Acknowledgements
We thank James W. Golden for strain AMC484, Conrad W. Mullineaux and Miguel A. de Pedro for helpful discussion and Alicia M. Muro-Pastor for critically reading the manuscript. This work was supported by Grant BFU2005-07672 from the Ministerio de Educación y Ciencia, Spain.




