The yeast penta-EF protein Pef1p is involved in cation-dependent budding and cell polarization
Summary
Penta-EF-hand (PEF) proteins bind calcium and participate in a variety of calcium-dependent processes in vertebrates. In yeast, intracellular cations regulate processes like cell division and polarized growth. This study reports the identification of a unique PEF protein in Saccharomyces cerevisiae encoded by the uncharacterized open reading frame YGR058w. Pef1p has a long and unstructured N-terminal domain conserved in ascomycetes, and a highly conserved C-terminal calcium binding domain homologous to human ALG-2 and sorcin. Pef1p binds calcium and zinc and homodimerizes in vitro and in vivo like vertebrate homologues. Disruption of PEF1 induces defective growth in SDS and cation depletion conditions. Significantly, a critical substitution in the second EF hand (E218A) lowers the in vitro affinity for zinc and phenocopies growth defects. The dissection of protein–protein interactions and the cellular localization of Pef1p analogous to that of RAM pathway components controlling daughter-specific gene expression at the site of bud emergence bring out the importance of this novel protein. Our data suggest that cation homeostasis is involved in the control of polarized growth and in stress response in budding yeast.
Introduction
Calcium is a universal second messenger that regulates a myriad of diverse cellular processes, acting either directly on target proteins or indirectly through calcium-regulated enzymes. In the budding yeast Saccharomyces cerevisiae, the calcium-dependent signal transduction pathways are essentially similar to those present in higher eukaryotes. For example, Ca2+ signalling mediated by the EF-hand protein calmodulin is required under environmental and hyperosmotic stress, and is achieved primarily through the activation of calmodulin-dependent protein kinases and calcineurin, a calmodulin-dependent protein phosphatase (Cyert, 2001). Further, calcineurin, a Ca2+/CaM-dependent phosphatase, is important for coupling of Ca2+ signals to various cellular processes and in cell cycle regulation (Kubler et al., 1994; Miyakawa and Mizunuma, 2007), and many calcineurin-dependent gene promoters become activated by specific environmental conditions, including exposure to Ca2+ and Na+ (Yoshimoto et al., 2002; Viladevall et al., 2004), often required for survival in adverse conditions. In the cell, calcium sensor proteins actively maintain low cytosolic-free Ca2+ concentrations and a steep gradient across the plasma membrane, providing a direct control of physiological responses like muscle contraction. In mice, for example, a decrease of myofilament calcium sensitivity is accompanied by an increase in excitation–contraction coupling with effects on heart rate (Varian and Janssen, 2007).
The penta-EF-hand (PEF) protein family comprises a number of eukaryotic two-domain Ca2+ binding proteins, such as the large and small calpain subunits (Blanchard et al., 1997; Lin et al., 1997; Hosfield et al., 1999), grancalcin (Lollike et al., 2001), ALG-2 (Vito et al., 1996), peflin (Kitaura et al., 1999) and sorcin (Maki et al., 1997; Ilari et al., 2002). Typically, EF-hand proteins contain conserved helix–loop–helix calcium binding motifs coupled in structural pairs. Calcium binding and affinity are dependent on the length of the loop (12 or 11 amino acids), and on the presence of canonical negatively charged residues in the calcium coordination positions. High-affinity EF hands usually contain, in the coordination position defined as –Z, a glutamic acid residue required for establishing a bidentate interaction with the metal (Blumenschein and Reinach, 2000). PEF proteins are stabilized by homo- and heterodimerization through the odd EF5 hand, and contribute to transduction of a variety of Ca2+-mediated signals by interaction with specific protein targets (Meyers et al., 1995; Maki et al., 2002). Sorcin, for example, participates in intracellular Ca2+ cycling and cardiac excitation–contraction coupling and, in particular, contributes to terminating cardiac contraction (Zamparelli et al., 2000; Meyers et al., 2003; Farrell et al., 2004). Indeed, transgenic mice carrying the F112L missense mutation in the critical D helix–EF3 loop calcium binding region display defective excitation–contraction coupling in the heart (Collis et al., 2007).
In the S. cerevisiae genomic database (http://www.yeastgenome.org), a query for PEF proteins retrieved the open reading frame (ORF) YGR058w on chromosome VII. The corresponding protein, Pef1p, was proposed by Maki et al. (1997) to belong to the PEF protein family due to the homology with mammalian ALG-2, sorcin and calpain (≥ 25% identity). However, YGR058w was never characterized, and no specific phenotype is associated with the disruption of the corresponding ORF. As budding yeast is an ideal model for the dissection of the complex calcium-dependent response, we decided to assess whether the hypothetical YGR058w indeed coded for a PEF protein in S. cerevisiae. In yeast, the identification of factors involved in Ca2+ metabolism has been achieved by the isolation of mutants with altered responses to Ca2+ in the growth medium (Cunningham and Fink, 1994) and to hyperosmotic stress.
The role of Pef1p in the yeast cell was assessed by determining the properties of purified recPef1p and the effects of gene disruption and gene mutations. Taken together, our data point to a role for the unique yeast PEF protein Pef1p in the cation-dependent regulation of budding and post mitotic cell abscission.
Results
Yeast Pef1p shows structural and functional homology with known PEF proteins
A query search for PEF calcium binding proteins in yeast retrieved a hypothetical protein corresponding to the ORF YGR058w on chromosome VII of the S. cerevisiae genome (Maki et al., 1997). The corresponding ORF encodes a 335-amino-acid protein of 38.4 kDa that we named Pef1p (Fig. 1A). The sequence shows the characteristic two-domain structure found in every PEF protein so far described: a flexible N-terminal domain and a C-terminal calcium binding domain containing the 5 EF-hand. The Pef1p N-terminus is long (157 amino acids), and conservation is restricted almost exclusively to putative proteins of ascomycetes. It is very rich in prolines and charged amino acids, with a calculated isoelectric point of about 10 (Gasteiger et al., 2005). Alignment of the C-terminal domain, amino acids 158–335 (Fig. 1B), shows a high conservation with vertebrate PEF proteins, in particular human ALG-2 (30% identity, 50% similarity) and sorcin (29% identity). On this basis, the structure of the calcium binding domain can be predicted with confidence (Fig. 1A and B). In Pef1p, the loop of the EF1 calcium binding site is canonical as in ALG-2, being 12 residues long, while sorcin, grancalcin and calpains have an 11-residue-long loop. On the other hand, the BC loop between EF1 and EF2 is shorter and the EF4 loop is longer than in most PEF proteins. The presence of glutamic acid residues in position –Z of EF1, EF2 and EF3 hands suggests a role as calcium binding sites. However, in Pef1p the low number of negatively charged residues in the co-ordinating positions indicates that the calcium affinity might be lower than for most PEF proteins. Tertiary structure prediction of the Pef1 C-terminal domain was carried out on the basis of a global structure superposition with the X-ray crystal structures of ALG-2 (1HQV), sorcin (1GJY, 1JUO), calpain (1DF0, 1ALV, 1DVI) and grancalcin (1F4Q, 1F4O) using the protein structure homology-modelling server Swiss Model. The resulting architecture (Fig. 1C) is almost completely superposable to the ALG-2 structure (Fig. 1D). Noteworthy, Pef1p contains several putative phosphorylation sites, mainly in loops EF1, EF3, EF4 and EF5, that are exposed to solvent in the modelled structure.
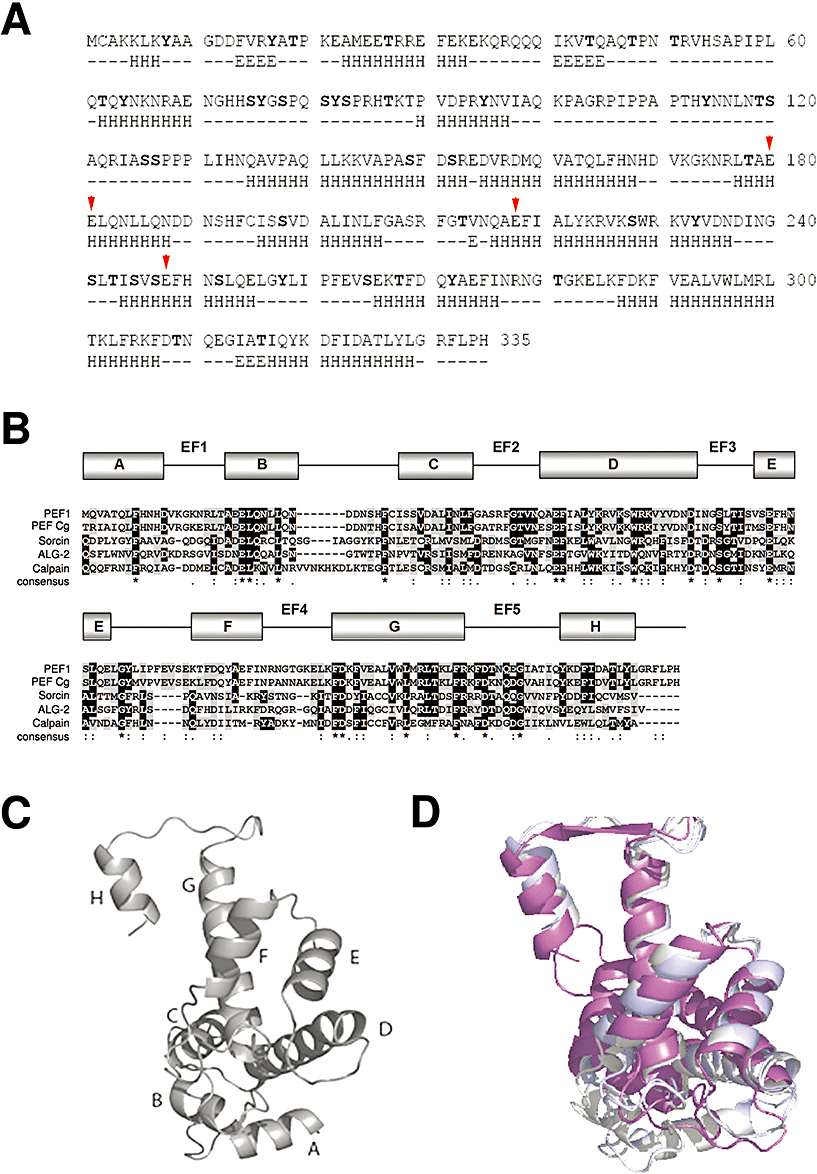
Structural similarity between Pef1p and selected PEF proteins.A. Aminoacidic sequence of Pef1p and prediction of its secondary structure (α-helix, H; beta sheet, E) and of putative phosphorylation sites (bold). N-terminal domain (amino acids 1–157), C-terminal calcium binding domain (amino acids 158–335). Red arrows indicate critical calcium interacting residues E180–E181, E218 and E248.B. Multiple sequence alignment obtained by the clustalw program and comparison of the Pef1p C-terminus (residues 158–335) with the calcium binding domains of those PEF proteins showing the highest degree of sequence conservation: PEF Cg, Candida glabrata putative PEF protein (XP_448418), human sorcin (P30626), mouse ALG-2 (NP_035181) and porcine calpain (P43368). Grey residues indicate conservation and black ones indicate identity. Consensus invariant residues are marked with asterisks at the bottom of the sequences. The predicted α-helices (boxes A to H) and the EF1–EF5 interhelical loops forming the EF hand sites of Sc-Pef1 are shown above the sequences.C. Ribbon representation of the predicted 3D structure of the Pef1p PEF calcium binding domain; the eight α-helices are designated by letters A–H.D. Superposition of the Pef1p (grey) and mouse ALG-2 (magenta) calcium binding domains.
To analyse the evolutionary conservation of this novel protein, we performed a restricted alignment of the Pef1p full-length sequence among fungi and retrieved the cladogram shown in Fig. 2. Interestingly, several uncharacterized ORFs are present in closely related yeasts such as Candida, Kluyveromyces and Debaryomyces, or in more distant filamentous fungi such as Neurospora and Magnaporthe. Most retrieved sequences correspond to uncharacterized, hypothetical proteins similar to known PEF hand proteins like Alg-2. Such strict sequence conservation is suggestive of a common function of these PEF-like proteins in fungi.
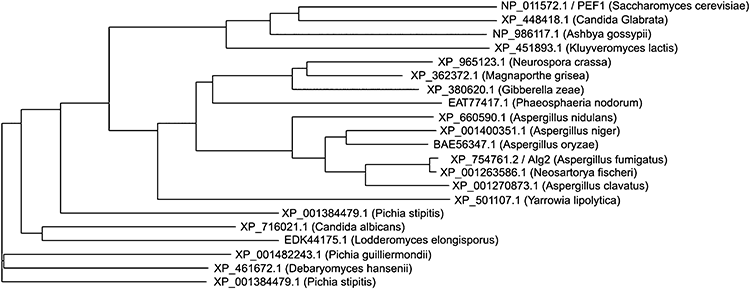
Phylogenetic cladogram of the Pef1p full-length sequence among fungi. Sequences were obtained by NCBI/blast providing the Pef1p full sequence and selecting the most similar sequences. The obtained sequences were clustered using the clustalw program. Each sequence is indicated with the corresponding NCBI accession number and organism.
Disruption of PEF1 induces growth defects in the presence of cation-chelating agents and under conditions of cell wall stress
To investigate whether the function of PEF1 is dependent on cations as for all PEF proteins, we analysed whether PEF1 disruption affects cell growth. Growth of wild-type and pef1Δ cells was tested under a variety of conditions: microtubule-depolymerizing agents (benomyl), high temperature, osmolarity stress, and high CaCl2 or ZnSO4 (Fig. 3A) did not affect growth of pef1Δ cells. Thereafter, cells were serially spotted in the presence of cation-chelating agents EGTA, SDS and Calcofluor (not shown) to identify possible defects in ion response and cell wall integrity (Ram et al., 1994; Shimizu et al., 1994) (Fig. 3A). The slow growth in the presence of EGTA is consistent with a role for Pef1p in response to intracellular cation variations. SDS sensitivity indicated that Pef1p is required for cell wall integrity or endocellular membrane re-assortment (Pruyne et al., 2004). The SDS sensitivity was not suppressed by the addition of sorbitol to the growth medium (data not shown), thus ruling out problems with the homeostasis of cells.

Pef1p disruption, C-terminus deletion and the single substitution E218A cause sensitivity to the cation chelator EGTA and SDS.A. Fivefold serial dilutions of WT (BY4741) and pef1Δ (YGR058w) were spotted on selective media supplemented with the indicated cation, EGTA and SDS concentrations and grown for 2 days at 25°C.B. pef1Δ strains carrying pCEN-LEU-PEF1, pCEN-LEU-PEF1E218 A and pCEN-LEU-PEF1:1–158 plasmids respectively, were spotted and grown as in panel A.C. Sequence logo (WebLogo; Berkeley) representation of the EF2 hand motif of Pef1p, which contains the functionally critical E218 residue.
The role of the C-terminal domain was assessed by expressing a mutated version of Pef1, PEF11−158, in yeast (Fig. S1). PEF11−158, carrying the deletion of the C-terminal PEF domain, displayed a stronger growth defect compared with a PEF1-disrupted strain (Fig. 3B). This result suggested not only that the C-terminal EF-hand domain is essential for protein function, but also that expression of a truncated product has a dominant negative effect and worsens growth in the presence of chelating agents. In addition, to directly assess whether the observed defects were related to the metal binding properties of Pef1p, different mutants with single-residue substitutions in key amino acidic positions were obtained and analysed. Substitutions EE180–181AA, E218A and E248A at the critical –Z coordination position of EF1, EF2 and EF3 respectively, were obtained and tested. No defects were induced by mutating EF1 (EE180–181AA) and EF3 (E248A) (data not shown), while substitution E218A in the EF2 hand induced EGTA and SDS sensitivity (Fig. 3B). The extreme conservation of the critical calcium-interacting glutamic acid E218A in the PEF protein family is represented as sequence logo (Fig. 3C), obtained by global sequence comparison of the Pef1p carboxy-terminal domain with the whole PEF sequence database.
Binding of calcium and zinc to recombinant Pef1p and to site-specific mutants
Cation binding to Pef1p was assessed by means of in vitro assays based on the spectroscopic properties of purified recombinant Pef1p. Binding of calcium or zinc results in a decrease of fluorescence emission intensity at 343 nm, accompanied by a slight shift (about 1 nm) of the emission peak towards lower wavelengths (Fig. 4A), which is indicative of a decreased exposure to solvent in particular of the two tryptophan residues W229 and W296, located on the D and G helix respectively. The cation-induced change in fluorescence was used to determine the calcium and zinc affinity (Fig. 4A and B) according to André and Linse (2002). Ca2+ binding to Pef1 (P) can be described with a single binding site per monomer: P + Cafree = PCa and KD = ([P] × [Cafree])/[PCa], where [Cafree] = [Catotal] − [PCa]. The data can be fitted with an apparent dissociation constant of 201 ± 18 μM. Binding of Zn2+ to Pef1p (Fig. 4B) can be fitted best with two binding sites per monomer with widely different dissociation constants, 0.6 ± 0.3 and 185 ± 25 μM. Binding of Zn2+ to PEF proteins has not been reported previously. The mutation E218A decreases markedly the affinity of the higher-affinity site for zinc (K1 = 4.2 ± 0.9 μM, K2 = 198 ± 18 μM), but affects calcium affinity only slightly (KD = 238 ± 21 μM).

Binding of calcium and zinc to recombinant Pef1p and to Pef1p-E218A.A. Titrations of 4 μM Pef1p (●) and Pef1p-E218A (□) with CaCl2: change in the intensity of fluorescence emission at 343 nm upon addition of CaCl2 for each experimental set was fitted with the program CaLigator (Andrè and Linse, 2002). Ca2+ binding to Pef1 can be described with a single binding site model. KD is the half-maximal Cafree concentration; for each point of the titration, the concentration of calcium added (total Ca2+ concentration) is the sum of [Cafree] and calcium bound to Pef1 [Pef1-Ca]. Three independent experiments are reported. In the inset, the decrease in the intrinsic fluorescence emission spectrum upon addition of calcium is shown.B. Titrations of 4 μM Pef1p (●) and of Pef1p-E218A (□) with ZnCl2: the change in the intensity of fluorescence emission at 343 nm upon addition of ZnCl2 was best fitted according to a two-sites model. Three independent experiments are reported.C. Circular dichroism spectrum of Pef1p in the far UV region. The spectra were measured at 20°C in 10 mM Tris-HCl at pH 7.5; protein concentration, 1 μM. Continuous line: apo-Pef1p + 100 μM EGTA; dashed line: Pef1p + 1.0 mM CaCl2; dotted line: Pef1p + 1.0 mM ZnCl2.
Far UV circular dichroism (CD) spectroscopy was used to estimate the possible effect of cations on the secondary structure. The far UV CD spectrum of Pef1p shows a slight increase in the overall α-helical content upon binding of calcium and zinc (from ∼48% to ∼53%, Fig. 4C).CD spectra were also measured for Pef1-E218A, to assess whether the mutation determines structural alterations or folding differences with respect to the wild-type protein. No differences were observed between the mutant and wild-type protein, both in the presence of EGTA and of 1 mM Ca2+ or Zn2+ (data not shown), thus ruling out important effects of the site-specific mutation on the overall protein folding.
Pef1 is present in a homodimeric form in vitro and in vivo
PEF proteins, such as calpain, ALG-2, grancalcin, sorcin and peflin, undergo dimerization through their unpaired C-terminal EF5 site (Blanchard et al., 1996; Lo et al., 1999; Sorimachi and Suzuki, 2001; Ilari et al., 2002; Maki et al., 2002; Ravulapalli et al., 2005). ALG-2, sorcin and grancalcin were reported to undergo also heterodimerization with other PEF proteins (Kitaura et al., 2001; Hansen et al., 2003). The fifth EF hand is required for these interactions, thus contributing to stabilization of the dimer (Kitaura et al., 2002). A computer graphic model of the Pef1p homodimer was generated by superposing two monomers on the basis of sequence conservation (Fig. 5A). The resulting dimeric structure is very similar to that of ALG-2, in particular the monomer–monomer interface, and the relative positions of the EF hands in the dimer are alike. To assess whether Pef1p is able to homodimerize in solution, the recombinant protein was expressed in Escherichia coli, purified and subjected to sedimentation velocity analysis (Fig. 5B). Pef1p sedimented as a single band with s20,w = 4.2 ± 0.1 S in 20 mM Tris-HCl (pH 7.5), both in the presence and in the absence of 2 mM CaCl2 or 0.5 mM ZnCl2. The s20,w values correspond to a molecular mass of about 80 kDa assuming a spherical shape and indicate that Pef1p is dimeric under the experimental conditions tested, and that neither calcium nor zinc alters the state of association significantly. To investigate the occurrence of Pef1p homodimerization in vivo, a two-hybrid interaction assay was carried out. Positive interaction was obtained in several experiments, as shown in Fig. 5C. These findings prompted us to prove the direct and physical interaction between the two monomers in vivo by coimmunoprecipitation of the homodimer in the diploid strain (SVY076) carrying simultaneously a 9myc- and a GFP-tagged Pef1p version (Fig. 5D). Taken together, the results demonstrate that Pef1p can homodimerize like all other PEF proteins.

Pef1p homodimerizes both in vitro and in vivo.A. 3D modelling of Pef1p homodimer.B. Sedimentation velocity analysis of recombinant Pef1p at a concentration of 20 μM. Continuous line: Pef1p + 100 μM EGTA; broken line: Pef1p + 2 mM CaCl2 + 0.5 mM ZnCl2. The protein sediments as a single band with s20,w = 4.2±0.1 S in 20 mM Tris-HCl buffer at pH 7.5.C. Two-hybrid analysis: MATa and MATα yeast strains were crossed to obtain diploid strains carrying the indicated combination of empty vector or Pef1p respectively. Yeast colonies were plated on selective SC medium and grown for 2 days at 28°C. The ability of fusion proteins to interact is visualized by expression of LacZ reporter producing yeast colonies stained in blue.D. Coimmunoprecipitation of PEF1–GFP and PEF1–9myc in diploid strain (SVY076). Cell lysates expressing PEF1–GFP (SVY053), PEF1–9myc (SVY059) and PEF1–GFP/PEF1–9myc (SVY076) are shown, respectively, in lanes 1, 2 and 3. Immunoprecipitation with monoclonal mouse anti-myc antibody was performed on diploid strain SVY076. Coimmunoprecipitated complexes were analysed by Western blotting with anti-GFP and anti-myc antibodies.
Pef1 accumulates at the site of polarized growth
The cellular localization of yeast Pef1p was assessed by fluorescence microscopy using a chromosomally integrated PEF1–GFP gene fusion, which was expressed under its own promoter. PEF1–GFP fully complemented the growth defects of pef1Δ cells (Fig. 6A). Protein localization (Fig. 6B) was evaluated in asynchronous growing cultures, and representative pictures were chosen on the basis of the quantitative observation of cells (n = 400). Pef1p–GFP invariably accumulated at the site of bud emergence in G1 cells. In small budded G1 cells, Pef1p–GFP was asymmetrically localized on the tip of the daughter cell and in the nucleus. At a later stage of the G1 phase, Pef1p–GFP decorated the emerging bud cortex and localized in the nucleus. In large budded G2/M cells, Pef1p–GFP stained preferentially the bud neck between the dividing mother and daughter cells, with very weak signals in the nucleus. Because the Pef1–GFP signal frequently appeared dot-like as is the case for proteins that are associated with the spindle pole body (SPB), we finally investigated localization of Pef1p respect to the SPB marker Spc42-eqFP (Pereira and Schiebel, 2001). The Pef1–GFP signal did not colocalize with the Spc42-eqFP signal (Fig. 6C), indicating that Pef1p is not a SPB component.
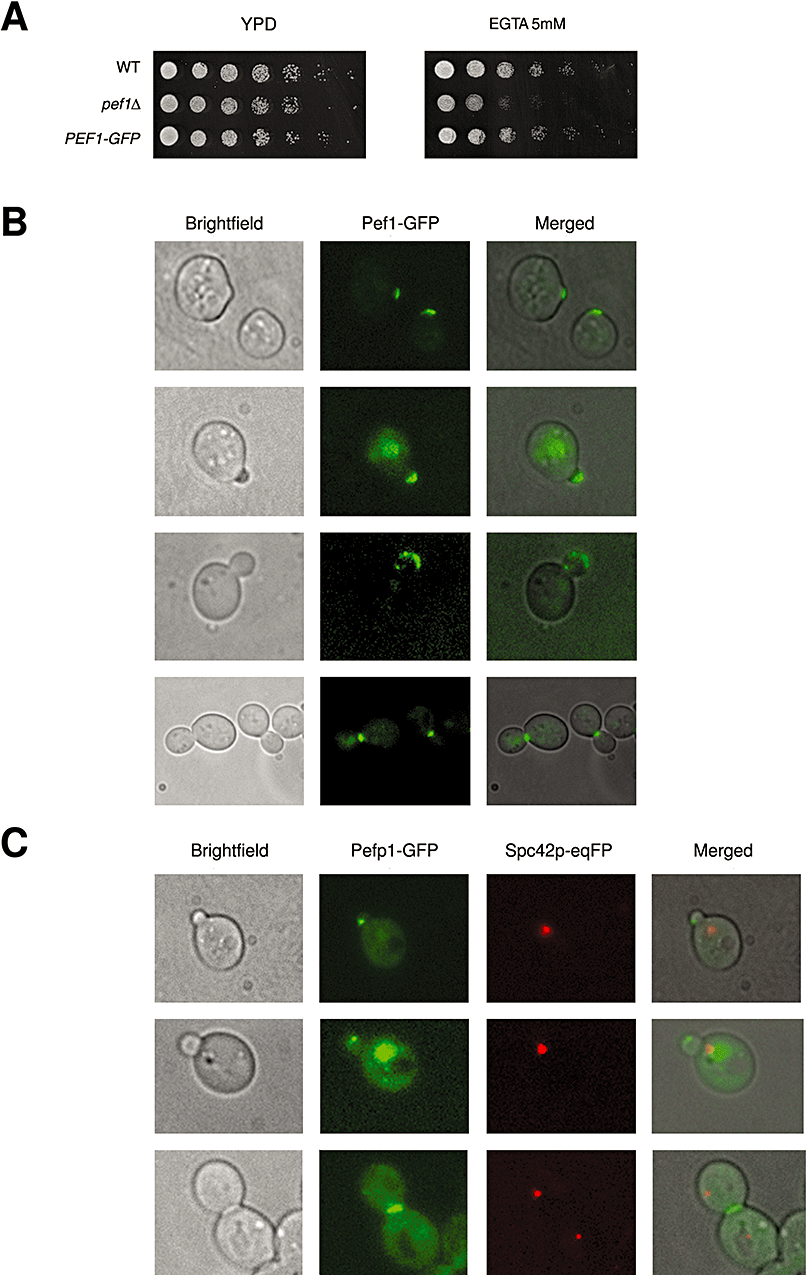
Cellular localization of Pef1p in S. cerevisiae.A. Functional complementation of pef1Δ strain (SVY008) grown on EGTA with chromosomally integrated fusion Pef1p–GFP cells (SVY015). Yeast cell dilutions were spotted and grown for 2 days at 28°C.B. Pef1p–GFP cells were analysed by fluorescence microscopy; cells expressing Pef1p–GFP are reported. G1; round cells showed one Pef1p signal invariably localized at the bud tip (87% of total G1 cells counted). Late G1, G1/S; the signal stained the nucleus of the mother cell and was concomitantly revealed in the emerging small bud (90% of total G1/S cells). Later, the signal became prevalently localized along the bud cortex of the growing daughter bud. G2 large budded cells; Pef1p–GFP marked clearly the bud neck, future bud tip of the next cycle (91% of total G2/M cells); only a 40% cells showed persistent staining in the nucleus.C. Colocalization of Pef1p–GFP (green) with the spindle pole component SPC42-eqFP (red) clearly demonstrates the protein behaviour with respect to the SPB position (SVY033).
Disruption of PEF1 induces bibudded cell morphology
Cell growth in budding yeast is a polarized phenomenon, and a specific bud site is selectively chosen. Wild-type haploid cells bud from only one pole in an axial fashion, and mutations in proteins involved in polarization give rise to a randomized budding pattern (Pruyne et al., 2004). In contrast, diploid (MATa/MATα) cells exhibit a bipolar budding pattern, and the daughter cell buds at the distal pole, while the mother cell buds either distal or proximal to the birth site (Ni and Snyder, 2001). Because of the specific localization of Pef1p at the site of polarized growth, at the bud tip in G1 and at the bud neck in G2, we decided to assess whether lack of Pef1p could affect cell morphology and impair the polarization of budding under physiological conditions or in the presence of cation-chelating agents. Asynchronous, actively dividing cell populations of wild-type and pef1Δ strains were analysed by microscopy (Fig. 7). Wild-type haploid cells showed the expected distribution of unbudded (G1), small budded (S/G2) and large budded (G2/M) cells. In contrast, pef1Δ cells displayed about 10% bibudded cells (Fig. 7A and B), which are completely absent in the wild-type population. This phenotype is further enhanced by a 15 h treatment with the cation chelator EGTA (27.4%). DAPI staining (Fig. 7C) showed a normal segregation pattern of nuclei both in the presence and in the absence of EGTA, ruling out a nucleus segregation defect.
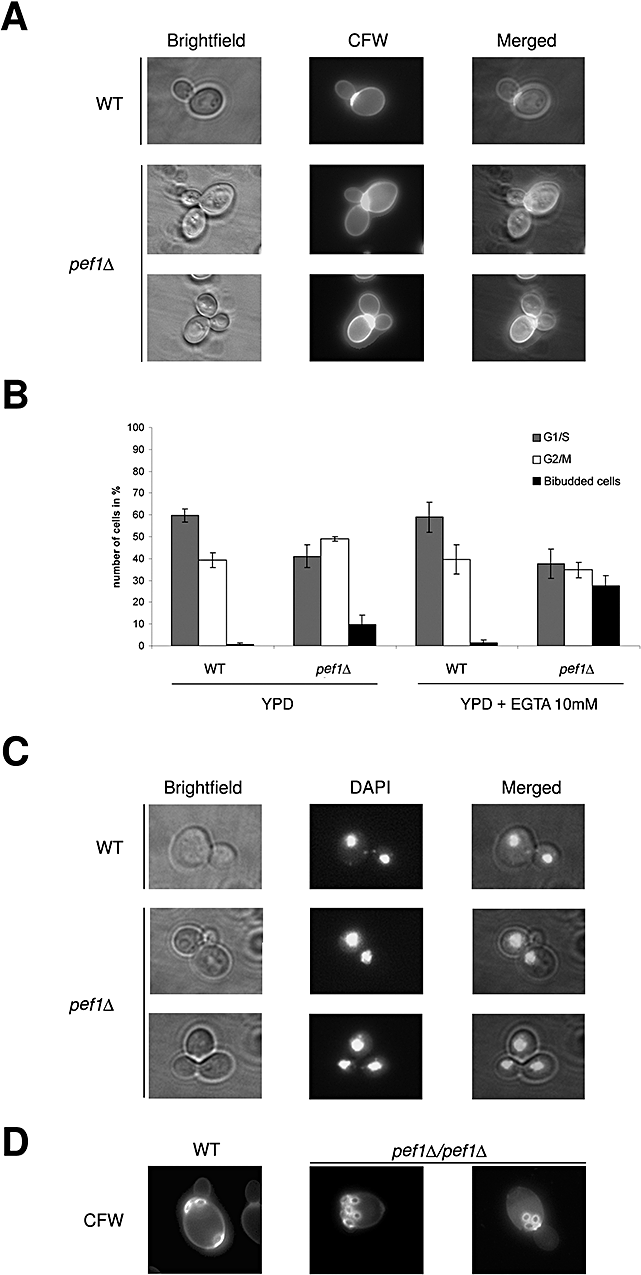
Pef1p is required for monopolar axial budding.A. Morphological traits of haploid wild-type (wt; BY4741) and pef1Δ. Representative budding morphologies in brightfield and after Calcofluor staining.B. Collected data were plotted in the histogram, G1/S round-shaped cells (grey), G2/M large budded (white) and bibudded (black), grown in YPD and YPD supplemented with 10 mM EGTA for 15 h at 28°C.C. DAPI staining of normally segregated nuclei in bibudded pef1Δ; a small, yet anucleated emerging bud is also shown.D. Characterization of bipolar budding pattern in diploid WT (SVY054) and pef1Δ/pef1Δ (SVY055) strains stained with Calcofluor.
In order to establish whether Pef1p is required for cytokinesis or for the completion of cell wall abscission, haploid cells were treated with zymolyase (Norden et al., 2006). The ensuing digestion of cell wall leads to complete resolution of the bibudded cells (n > 300), providing the demonstration that Pef1p is involved in the abscission of cell wall after cytokinesis. To further characterize the budding defects observed in haploid pef1Δ cells, the bipolar budding in diploid cells was investigated. Diploid WT (SVY054) and homozygous pef1Δ/pef1Δ (SVY055) strains were stained with Calcofluor to visualize bud scars. Cells with more than three scars were examined and phenotypes were classified according to Ni and Snyder (2001). The wild-type cells showed the typical bipolar scar localization (Fig. 7D). None of the wild-type cells showed a unipolar budding pattern. In contrast, almost 10% of homozigous pef1Δ/pef1Δ showed a proximal unipolar budding pattern with bud scars laying at one pole. This defect was not worsened by addition of EGTA into the growth medium.
Pef1 protein network
In order to define the protein interaction network of Pef1 in budding yeast, we performed a targeted two-hybrid screening with 125 clones of proteins involved in cell cycle and cytokinesis. The positive interactions obtained, which include Pef1 homodimerization, are shown in Fig. 8A. The global interaction map of Pef1, schematized in Fig. 8B, reports our results together with literature data as available on the SGD site (http://db.yeastgenome.org/cgi-bin/locus.pl?locus=pef1). Positive signals were obtained with RAM (Bogomolnaya et al., 2006) components Cbk1 and Mob2 (Nelson et al., 2003) associated to the Ace2-dependent daughter-specific genetic programme that includes genes involved in cell separation and resolution of the septum (Bidlingmaier et al., 2001). Additional interactions with the GTPase Tem1, a member of the mitotic exit network required at the mother–daughter bud site for the initiation of septum formation (Hwa et al., 2003), suggested once again a role for Pef1 in mitosis exit. Literature data taken together with the interactome database identified Tpk1 of the RAS pathway as an additional interacting partner. The RAS pathway, acting in parallel with RAM, is required for bud site selection and cell cycle progression (Schneper et al., 2004). The interactions with Tem1 and Tpk1, which localize at the mother–daughter bud neck similarly to Pef1, directly connect this novel cation binding protein with bud site determination and cell wall abscission after mitosis. Remarkably, other interactors, such as Lsb1 and SLM2, a calcineurin substrate, are linked to calcium osmoregulation (Bultynck et al., 2006), while HOG1 kinase, belonging to the HOG pathway, is involved in the response to external osmolarity (Alonso-Monge et al., 2001). Overall, the latter interactions with proteins related to yeast survival during environmental stress point to Pef1 as a novel player in cation osmoregulation.
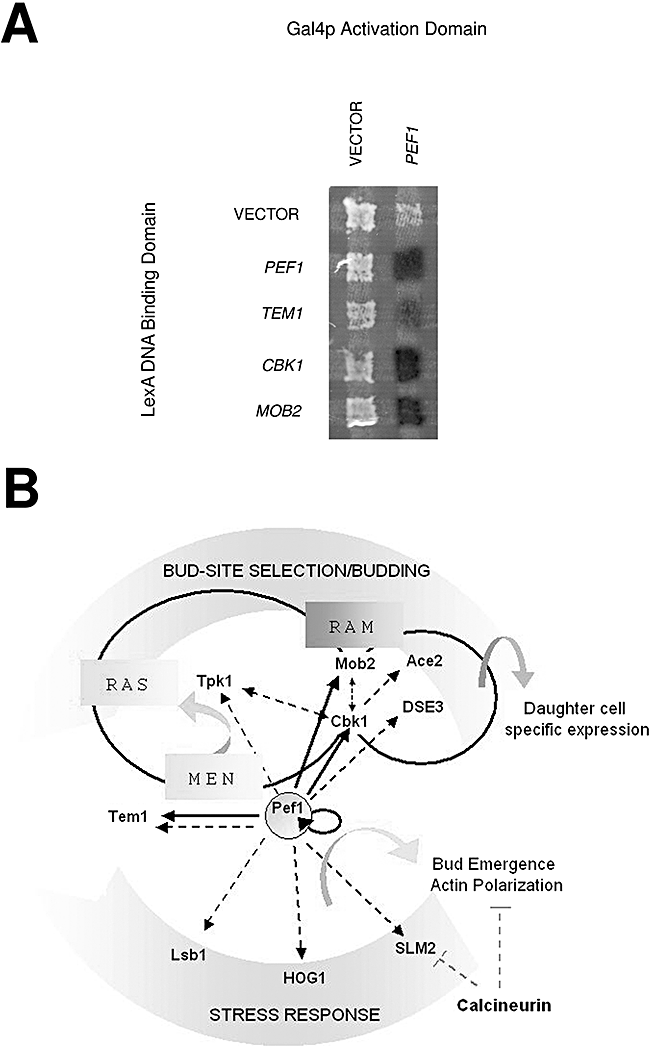
Pef1 protein interaction network.A. Positive Pef1p interactors identified by two-hybrid screening of proteins involved in budding and cytokinesis.B. Protein network of Pef1p: interactions identified in this study (continuous lines), in the literature and in the interactome website (dashed lines). RAM components Cbk1 and Mob2 show the same localization of Pef1 at mother daughter bud neck of dividing G2 cells.
Discussion
In this study, we report the identification and characterization of a unique PEF protein in yeast (Maki et al., 1997): Pef1p binds calcium and zinc with different affinity and is required for polar bud growth and cell wall abscission. Pef1p is localized at the site of bud emergence in early G1 and, later in G2, migrates to the mother–daughter bud neck, providing evidence for a direct correlation between cations and the components involved in the establishment of the cell division plane in budding yeast (Cabib, 2004).
YGR058w encodes a unique PEF protein in S. cerevisiae
Pef1p is encoded by the ORF YGR058w, whose product shows the predicted mass of 38.4 kDa. Overall, S. cerevisiae Pef1p shows high homology among PEF proteins in fungi (Fig. 2). As the proteins reported in the cladogram are mostly hypothetical and not annotated, the cation binding Pef1 may represent a valuable model for the study of their biological function in fungi.
The amino acid sequence of the C-terminal domain displays significant homology (up to 30% identity and 50% homology) to the calcium binding domain of known PEF proteins of lower and higher eukaryotes, such as ALG-2 and sorcin. In addition, the predicted three-dimensional (3D) structure of Pef1p shows a wide structural match with the solved structure of human ALG-2 (Jia et al., 2001). In both domains, several putative phosphorylation sites for enzymes such as calmodulin kinase II, protein kinase C and MAP kinase could be important for protein regulation (Gray et al., 1997; Kahl and Means, 2003). We have shown that Pef1p homodimerizes and aim to assess possible interactions with other partners that may contribute to protein stabilization similarly to mammalian homologues (Hansen et al., 2003).
Pef1p binds calcium in the 100–200 μM range and zinc with higher affinity. Calcium affinity is in the same order of magnitude as in m-calpain, but is lower than in most other PEF proteins (KD between 0.1 and 200 μM), in good agreement with predictions based on the presence/absence of canonical metal ligands in the various EF hands. In PEF proteins, calcium binding determines conformational changes that activate the proteins, and is therefore at the basis of the many different functions of this protein family (Farrell et al., 2004; Shibata et al., 2004; 2007; Katoh et al., 2005; Yamasaki et al., 2006; La Cour et al., 2007). The low calcium affinity of recPef1 measured in vitro, however, does not rule out the possibility that, in vivo, Pef1p may bind calcium in specific environments where the local concentration is higher than in the bulk of the cytoplasm (Iida et al., 1990; Nakajima-Shimada et al., 1991; Halachmi and Eilam, 1993). The high affinity of Pef1p for zinc (KD = 0.7 μM) was unexpected, as to date no other PEF protein was reported to have zinc binding activity. Nonetheless, it is known that PEF proteins may interact with zinc finger proteins, such as Rim-101p (Hayashi et al., 2005). Our results show that mutation E218A, at position –Z of the EF2 hand of Pef1p, lowers in vitro zinc affinity and, to a lesser extent, calcium affinity. Significantly, this mutation phenocopies growth defects of the fully disrupted gene, thus demonstrating that the binding of Ca2+ and/or Zn2+ to EF2 contributes to the function of Pef1p.
Sc-Pef1 is essential for growth when cations are depleted, and is directly involved in polarized cell abscission in haploid strains
The reorganization of the bud site is a morphogenetic function related to endocellular cation homeostasis and to membrane trafficking. In S. cerevisiae, calcium concentration is finely tuned and the vacuoles are devoted to maintain the intracellular calcium level in a physiological range (Miseta et al., 1999). Importantly, calcineurin and a Ca2+-dependent pathway induce formation of a highly polarized bud and a Pkc1p-dependent delay of G2 progression (Mizunuma et al., 2005; Miyakawa and Mizunuma, 2007). We demonstrated that Pef1p is a novel effector of divalent cation-mediated response, required for growth under condition of cation depletion and related to cell wall biogenesis. The cell wall is, in fact, continuously engaged in dynamic re-assortment, mainly concentrated at the bud tip, where a number of proteins soften and remodel the expanding cell wall (Zhang et al., 1998; Pruyne et al., 2004). While the emerging bud is growing, cells duplicate and segregate organelles such as the cortical endoplasmic reticulum, secretory vesicles and mitochondria to provide the necessary energy to this process (Irazoqui and Lew, 2004). Bud emergence is therefore a precisely regulated and polarized phenomenon associated to bud tip formation and growth. Bibudding is induced by mutation of proteins involved in the control of axial budding and in cell abscission; accordingly, we have shown that lack of Pef1p induced bibudded cells in haploid strains, a phenomenon worsened by cation depletion, and mild unipolar budding in diploid strain.
Post-mitotic resolution of dividing cells brings cytokinesis to completion. The migration of Pef1p at the bud neck in G2 cells accounts for its active role in cytokinesis; this finding, along with morphogenetic data, proves the requirement of Pef1p for polarized cell budding and accounts for a direct link between cation homeostasis and cell polarization. It has been proposed that bud emergence is regulated antagonistically, positively by the HOG pathway, which promotes bud emergence following actin polymerization, and negatively by calcineurin, which regulates actin polarization (Miyakawa and Mizunuma, 2007). The dissection of the protein network of Pef1 highlights that it is not implicated solely in interactions with stress response proteins like HOG1 and SLM2, but is engaged also in interactions with RAM Cbk1 kinase and its interacting partner Mob2. The latter proteins participate in the regulation of cellular asymmetry and of the daughter-specific genetic programme, including products involved in cell wall and septum degradation during cell separation (Colman-Lerner et al., 2001). Accordingly, the complete resolution of bibudded cells obtained after dissolving the cell wall by zymolyase treatment demonstrates unambiguously that PEF1 is involved in cell wall abscission. In this connection, it is of interest that Pef1 interacts with Tpk1, a component of the RAM-parallel RAS pathway, and with Tem1, involved in mitosis exit. Such protein–protein interactions, along with the similarity in localization at mother bud neck, provide both important information on the cellular network of Pef1 and an excellent system for studying the relevance of calcium homeostasis in bud polarization and cell wall abscission.
In conclusion, the involvement of the unique yeast PEF calcium binding protein in the determination of asymmetric cell division and its high evolutionary conservation encourage the utilization of the yeast model for investigating cytokinesis and cell polarization in higher eukaryotes. The properties of Pef1p also pave the path for novel studies in yeast on the regulation of cell morphogenesis by cations, and more specifically on the roles played by calcium and zinc.
Experimental procedures
Strain, media and reagents
The yeast strains used are listed in Table 1. Gene disruption and tagging were performed as described by Janke et al. (2004) and Longtine et al. (1998), and controlled by polymerase chain reaction (PCR) and Western blot analysis (not shown). Plasmid pYM12 (GFP–KanMX6) and pYM20 (9myc–HIS3MX6) (Knop et al., 1999) was used for the construction of the Pef1–GFP and Pef1–9myc cassette. Yeast cells were grown in YPD media (1% yeast extract, 2% bactopeptone and 2% glucose), and in SD minimal medium (0.67% YNB, 2% glucose supplemented with requested amino acids). For cation sensitivity, EGTA (pH 7.3) was added to solid medium. For septum digestion, cells were washed once with phosphate buffer (pH 7.4), 1 M sorbitol, and resuspended in the same buffer. Cell abscission was evaluated after 30 min zymolyase (2 mg ml−1) digestion at 30°C by light microscopy.
| Strain | Relevant genotype | Source |
|---|---|---|
| BY4741 | MATa, his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | Euroscarf |
| YGR058w | MATa, YGR058w::kanMX4, his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | Euroscarf |
| SVY071 | MATa, YGR058w::KanMX4, pGC1(CEN, LEU2, PEF1), his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | This work |
| SVY073 | MATa, YGR058w::KanMX4, pGC3 (CEN, LEU2, PEF1 E218A), his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | This work |
| SVY075 | MATa, YGR058w::KanMX4, pGC5(CEN, LEU2, PEF1 1–158), his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | This work |
| SGY37 | MATa, trp1 Δ63 his 3 Δ200 leu2 Δ1 ura3–52::URA3-lexA-op-LacZ. | Geissler et al. (1996) |
| YPH500 | MATα, trp1 Δ63 his3 Δ200 leu2 Δ 1 ura3–52 lys2–801 ade2–101. | Sikorski and Hieter (1989) |
| SVY053 | MATα, PEF1–GFP–KanMX6, his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | This work |
| SVY059 | MATa, PEF1–9MYC–His3MX6, his3-Δ1, leu2 Δ,met15 Δ, ura3 Δ. | This work |
| SVY076 | MATa/MATα, PEF1-GFP–KanMX6, PEF1–9MYC–His3MX6, his3Δ1/his3Δ1,leu2Δ/leu2 Δ, met1Δ/MET1, lis2Δ0/LYS2, ura3Δ/ura3Δ. | This work |
| ESM356-1 | MATa, ura3–52,trp1Δ63, his3Δ200, leu2Δ1. | Knop and Shiebel (1998) |
| SVY008 | MATa, PEF–KanMX6, ura3–52,trp1Δ63, his3Δ200, leu2Δ1. | This work |
| SVY015 | MATa, PEF1–GFP–KanMX6, ura3–52,trp1Δ63, his3Δ200, leu2Δ1. | This work |
| SVY033 | MATa, PEF1–GFP–KanMX6, SPC42-eqFP-hph, ura3–52,trp1Δ63, his3Δ200, leu2Δ1. | This work |
| SVY054 | MATa/MATα, his3 Δ 1/his3 Δ 1, leu2 Δ/ leu2 Δ, met15 Δ/MET15, lis2Δ0/LYS2, ura3 Δ/ura3 Δ. | This work |
| SVY055 | MATa/MATα, YGR058w::kanMX4/YGR058w::His3MX6. his3Δ 1/his3Δ1,leu2Δ/leu2 Δ, met1Δ/MET1,lis2Δ0/LYS2, ura3Δ/ura3Δ. | This work |
Prediction of structural and functional features of Sc-Pef1
The PEF domain architecture of Pef1p was retrieved by query to Pfam, SMART and Superfamily servers (Finn et al., 2006; Letunic et al., 2006). Homology search of whole sequence and individual domains was carried out using blast and nr (Altschul et al., 1990). Multiple sequence alignments were obtained with clustalw (Higgins et al., 1994). The Pef1p secondary structure was predicted by Prof (as implemented by the Predict Protein Server), SAM-T02 (Ouali and King, 2000; Karplus et al., 2003), and was compared with the structural templates calculated by DSSP (from the RCSB protein databank) according to Kabsch and Sander (1983). The 3D prediction was obtained with SwissModel (Schwede et al., 2003). Prosite Motif Search was used for prediction of phosphorylation sites. Compute pI/MW was employed for calculation of the isoelectric point (Gasteiger et al., 2005; Hulo et al., 2006).
Cloning, expression and mutagenesis
The ORF YGR058w of S. cerevisiae strain BY4741, i.e. the PEF1 gene (606137–607144: chr.VII), was amplified by PCR. For expression in E. coli, the PCR product obtained from yeast genome using oligonucleotides Pef1NdeI forward and Pef1HindIII reverse was digested with NdeI and HindIII, cloned into plasmid pET22b(+) (Novagen), transformed into E. coli DH5α and sequenced. Recombinant Pef1p was expressed in E. coli BL21(DE3), under T7 lac promoter, after 4 h induction in 1 mM isopropyl β-D-1-thiogalactopyranoside. Cells were harvested by centrifugation, resuspended in lysis buffer [100 mM Tris-HCl (pH 7.4) + 100 mM NaCl +1 mM dithiotreitol +1 mM phenylmethylsulfonylfluoride] and lysed with a MSE Soniprep sonicator. The insoluble fraction, containing the expressed Pef1p, was recovered by centrifugation at 25 000 g for 20 min, resuspended in lysis buffer, treated for 30 min with 1 mg ml−1 DNase I at room temperature, and was washed five times in lysis buffer. Extraction of recombinant Pef1p from the pellet was achieved either by lowering the pH < 5.5 (complete extraction was obtained in 0.1 M sodium acetate at pH 4.4), or by incubating with denaturing buffer (6 M urea in 0.1 M Tris-HCl at pH 7.5). Thereafter, Pef1p was dialysed versus 10 mM sodium acetate buffer (pH 5.5), loaded onto a MonoS column (Pharmacia) and eluted with a gradient of the same sodium acetate +1 M NaCl. The protein concentration was determined spectrophotometrically at 280 nm using a molar extinction coefficient of 34 500 calculated according to Edelhoch (1967). For expression in S. cerevisiae, genomic ORF YGR058w and 5′ sequence from −620 bp were amplified with oligonucleotides Pef1PstI forward and Pef1HindIII reverse. The product was digested with PstI and HindIII and cloned into centromeric pFL36 vector (Bonneau et al., 1991). Mutation E218A was obtained with the Quikchange Mutagenesis kit (Stratagene) using the oligonucleotides: E218A forward and E218A reverse (Table 2).
| Oligonucleotide | Sequence (5′−3′) | Map position from ATG |
|---|---|---|
| Pef1NdeI forward | CCTACACCATTAACATATGTGTGCAAAGAAGC | −16/+16 |
| Pef1HindIII reverse | CCAGAAAGAACGAAGCTTTTTCATCAATGAGG | +1031/+1000 |
| Pef1PstI forward | CATCTATTTCTGCAGCCATTTAAACGCTAAC | −627/−597 |
| E218A forward | CACTGTCAACCAGGCAGCATTCATCGCCCTATACA | +636/+670 |
| E218A reverse | TGTATAGGGCGATGAATGCTGCCTGGTTGACAGTG | +670/+636 |
| Pef1: 1–158 forward | CGTTCGATAGCAGATAAGATGTACGAGACATGC | +446/+478 |
| Pef1: 1–158 reverse | GCATGTCTCGTACATCTTATCTGCTATCGAACG | +478/+446 |
Determination of Ca2+ and Zn2+ affinity
Direct titrations with calcium and zinc were carried out on a Fluoromax spectrofluorimeter in 0.1 M Tris-HCl buffer at pH 7.5 and 25°C. The excitation wavelength was 280 nm (slit width 1.0 nm); fluorescence emission was followed at 300–400 nm. CaCl2 or ZnCl2 was added to 4 μM Pef1p and Pef1p-E218A. Special care was taken to reduce Ca2+ contamination to 0.5–1.0 μM by treating the protein solutions and the glassware with Chelex 100. To estimate the affinity constants of Pef1p, each experimental set was fitted with the program CaLigator (Andrè and Linse, 2002).
Circular dichroism spectra
Circular dichroism spectra were recorded on a Jasco J-710 spectropolarimeter in the far UV (190–250 nm) and in the near UV (250–350 nm) regions. The experiments were carried out at 20°C in 20 mM Tris-HCl buffer at pH 7.5. The spectra shown are the average of 20 accumulations. The α-helical content was calculated from the ellipticity value at 222 nm according to Chou and Fasman (1974).
Analytical ultracentrifugation
Sedimentation velocity analysis was carried out on a Beckman Optima XL-I analytical ultracentrifuge on Pef1p dialysed versus 20 mM Tris-HCl, at pH 7.5, in the absence or in the presence of 0.5 or 2 mM CaCl2 or ZnCl2. The experiments were carried out at 10°C and at 40 000 r.p.m. The movement of the protein towards the bottom of the cell was followed by absorption scans along the centrifugation radius at a wavelength of 280 nm. Sedimentation coefficients were corrected to s20,w using standard procedures.
Two-hybrid assay
The two-hybrid assay was performed and tested as described by Uetz et al. (2000). The PEF1 gene was amplified using the following oligonucleotides: (5′-AGCGAGATCTCAATGTGTGCAAAGAAGCTCAAAT-3′) and (5′-TCCGCTCGAGTCAATGAGGTAGGAAACGACCT-3′), digested with BglII–XhoI, cloned into a BamHI–XhoI site of pMM5 (LexADNA. bait) or pMM6 (Gal4pTA prey vector) (Schramm et al., 2001) and transformed respectively into YPH500 (Sikorski and Hieter, 1989) and SGY37 (Geissler et al., 1996) strains. Interacting proteins shown in Fig. 8A were expressed in pMM5 and pMM6 vectors containing Tem1 (pCL9-2), Mob2 (pSM886-1) and Cbk1 (pSLS015-1) cloned in BamHI–XhoI site (E. Schiebel plasmid collection).
Coimmunoprecipitation
Coimmunoprecipitation was performed as described (Kemp and Sprague, 2003): yeast extract and immunoprecipitates were separated by SDS-PAGE gel 10% and transferred to nitrocellulose. Samples were analysed by immunoblotting and developed by standard ECL system (Amersham Biosciences), using anti-GFP (B-2) and anti-myc (9E10) antibodies (Santa Cruz Biotechnology).
Fluorescence Microscopy
Cells containing Pef1p–GFP were grown to early logarithmic phase in YPD plus adenine (100 mg ml−1), and were resuspended in phosphate-buffered saline (PBS) without fixation. GFP signals were detected by their autofluorescence; DNA was stained with 4′,6-diamidono-2-phenylindole (DAPI; Sigma Chemical, St Louis, MO). Images were collected on a Zeiss Axiophot microscope using a Coolsnap HQ camera (Photometrics, Tucson, AZ) and Metamorph software (Universal-Imaging, West Chester, PA). The budding pattern was analysed by Calcofluor White staining. Cells were grown in complete medium until mid-log phase, collected, washed with PBS and resuspended in the same buffer. Calcofluor White (Sigma, St Louis, MO) was added to a final concentration of 1 mg ml−1 for 10 min at room temperature, washed four times and resuspended in PBS. Cells' bud scars were visualized by fluorescence microscopy with a DAPI filter and with phase-contrast microscopy. In each experiment, more than 400 cells were counted.
Acknowledgements
The authors wish to thank Dr P. Ballario for helpful discussions. This work was funded by RSTL-CNR (2005) to G.C. S.V. was the recipient of EMBO Short-Term Fellowship ASTF 177–2005; his PhD programme was supported by Ministero Italiano dell'Interno, Dip. P.S. We also wish to thank Tullio Riosa and Genesio Ricci for excellent technical support.




