Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis
Present address: Centro de Biotecnología, Instituto de Estudios Avanzados, Caracas, Venezuela;
Escuela de Bioanálisis, Universidad Central de Venezuela, Caracas, Venezuela.
These authors contributed equally to this work.
Summary
The Leishmania major aquaglyceroporin, LmAQP1, is responsible for the transport of trivalent metalloids, arsenite and antimonite. We have earlier shown that downregulation of LmAQP1 provides resistance to trivalent antimony compounds whereas increased expression of LmAQP1 in drug-resistant parasites can reverse the resistance. In this paper we describe the biochemical characterization of LmAQP1. Expression of LmAQP1 in Xenopus oocytes rendered them permeable to water, glycerol, methylglyoxal, dihydroxyacetone and sugar alcohols. The transport property of LmAQP1 was severely affected when a critical Arg230, located inside the pore of the channel, was altered to either alanine or lysine. Immunofluorescence and immuno-electron microscopy revealed LmAQP1 to be localized to the flagellum of Leishmania promastigotes and in the flagellar pocket membrane and contractile vacuole/spongiome complex of amastigotes. This is the first report of an aquaglyceroporin being localized to the flagellum of any microbe. Leishmania promastigotes and amastigotes expressing LmAQP1 could regulate their volume in response to hypoosmotic stress. Additionally, Leishmania promastigotes overexpressing LmAQP1 were found to migrate faster towards an osmotic gradient. These results taken together suggest that Leishmania LmAQP1 has multiple physiological roles, being involved in solute transport, volume regulation and osmotaxis.
Introduction
The aquaporin (AQP) protein family can be functionally categorized into two subgroups, the orthodox AQPs, which are water-specific channels, and aquaglyceroporins, which allow the transport of water, glycerol, and other small, uncharged solutes. AQPs are widely distributed from Escherichia coli to humans. The physiological functions of human AQPs have been extensively studied (King et al., 2004). Human AQPs play an important role in water homeostasis, lung moistening, tear and saliva secretion, and body glycerol and fat metabolism (Beitz, 2006). Additionally, studies on AQP-knockout mice indicate that AQPs also facilitate cell migration, neural signal transduction, cell volume regulation and organellar physiology (Verkman, 2005). In contrast, the physiological roles of AQPs in the human pathogenic parasites are less well established.
There have been several recent reports on AQPs from parasitic protozoa. A single aquaglyceroporin (PfAQP) has been isolated from the malaria parasite Plasmodium falciparum (Hansen et al., 2002). PfAQP is highly permeable to glycerol, water, urea, and sugar alcohols up to five carbons long. It is expressed in the blood-stage parasite throughout its development and is localized to the parasite plasma membrane (Hansen et al., 2002). PbAQP, an aquaglyceroporin from the rodent malaria parasite Plasmodium berghei, is permeable to both water and glycerol (Promeneur et al., 2007). Studies on PbAQP-null parasites indicate that PbAQP is the major route for glycerol uptake and contributes to the proliferation of the parasite (Promeneur et al., 2007). The protozoan parasite Trypanosoma brucei, causative for African sleeping sickness, expresses three aquaglyceroporins, TbAQP1, 2 and 3 (Uzcategui et al., 2004). TbAQPs allow the transport of water, glycerol and dihydroxyacetone. They have been proposed to be involved in parasite survival under non-aerobic or osmotic stress conditions. Trypanosoma cruzi, the causative agent for Chagas disease, encodes for an aquaporin (TcAQP) that specifically transports water but not glycerol (Montalvetti et al., 2004). TcAQP was shown to localize to the acidocalcisomes and the contractile vacuole complex, and has been proposed to be involved in osmotic adaptations of these parasites. The Toxoplasma gondii AQP has been shown to be a bifunctional membrane pore with intermediate water and high glycerol permeability (Pavlovic-Djuranovic et al., 2003). In many of the above studies, biochemical characterization of the AQPs has been performed after heterologous expression in Xenopus oocytes, and not in the parasite.
Leishmaniasis is a protozoan parasitic disease ranging from self-healing cutaneous lesions to non-healing mucocutaneous and visceral ailments that affects over 12 million people worldwide. The Leishmania parasite exists in two morphologically distinct forms. The promastigote form of the parasite resides in the intestinal tract of the sand fly vector and appears as a slender, spindle shaped structure with an anterior flagellum. The amastigote forms of the parasite are small, oval-shaped structures that reside in macrophages and other mononuclear phagocytes in the mammalian host. The first-line choice of treatment against all forms of leishmaniasis uses drugs containing pentavalent antimony such as sodium stibogluconate (Pentostam) and meglumine antimonate (Glucantime). We have previously identified an aquaglyceroporin, LmAQP1, from Leishmania major. At the amino acid level, LmAQP1 shares between 41% and 45% identity with T. brucei aquaglyceroporins (TbAQP1-3), 30% identity with P. falciparum aquaglyceroporin (PfAQP1), and 40% overall sequence similarity with the mammalian homologues (Gourbal et al., 2004; Beitz, 2005). We have shown that LmAQP1 is responsible for the transport of antimonite [Sb(III)], an activated form of Pentostam or Glucantime (Gourbal et al., 2004). Disruption of one of the two LmAQP1 alleles in L. major conferred a 10-fold increase in resistance to Sb(III) compared with the wild type. Our experiments established that loss of LmAQP1 can produce resistance and conversely increased expression of LmAQP1 in drug-resistant parasites can reverse resistance (Gourbal et al., 2004; Marquis et al., 2005). It should be noted that the Leishmania parasite never encounters metalloids during its natural life cycle. Therefore, besides transporting metalloids, LmAQP1 must serve a physiological function.
In this paper, we present the biochemical characterization of LmAQP1 in greater detail. The transport property of LmAQP1 was characterized by expressing the gene in Xenopus laevis oocytes and was shown to transport water as well as several other non-ionic solutes. LmAQP1 localized exclusively to the flagellum of Leishmania promastigotes and was observed on the flagellar pocket membranes and the contractile vacuole/spongiome complex in amastigotes. Leishmania promastigotes and amastigotes overexpressing LmAQP1 could better regulate their volume in response to a hypoosmotic stress. Additionally, Leishmania promastigotes overexpressing LmAQP1 moved faster towards an osmotic gradient than vector alone control. These results support the role of LmAQP1 in water homeostasis, glycerol transport, volume regulation, and osmotaxis.
Results
Permeability of LmAQP1 in Xenopus oocytes
We have previously shown that, transfection of LmAQP1 in Leishmania promastigotes and amastigotes renders these cells sensitive to both arsenite and antimonite, and these cells accumulate higher levels of the metalloids than untransfected controls (Gourbal et al., 2004). The metalloid-permeability of LmAQP1-expressing oocytes was examined in this study. Oocytes injected with LmAQP1-cRNA accumulated 40- and 240-fold higher As(III) and Sb(III), respectively, compared with the control set (Fig. 1A). These data reaffirm our previous observation that the Leishmania LmAQP1 serves as a metalloid channel.
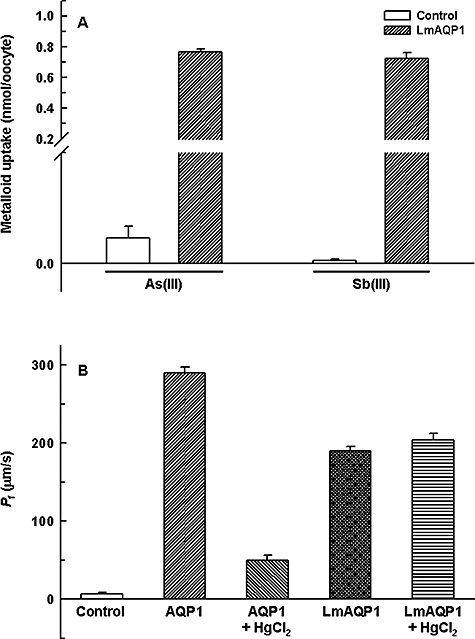
Metalloid and water transport in Xenopus oocytes expressing LmAQP1.A. Uptake of As(III) and Sb(III). Oocytes were injected with water (control) (open bars) and LmAQP1-cRNA (filled bars).B. Water permeability. Oocytes were injected with water (control), AQP1-cRNA or LmAQP1-cRNA. HgCl2 (0.5 mM) was used as an AQP inhibitor.
The water permeability of LmAQP1 was evaluated by expression in Xenopus oocytes and compared with human AQP1. After 3 days of incubation at 16°C, oocytes expressing LmAQP1 or AQP1 were subjected to hypotonic shock in 1:3 diluted ND96 medium, which established an outwardly directed osmotic gradient of 140 mOsm. As shown in Fig. 1B, oocytes injected with LmAQP1-cRNA exhibited a 20-fold (Pf ≈ 180 μm s−1) increase in swelling rate compared with the water-injected control. Under similar conditions, AQP1-cRNA rendered the oocytes 30 times (Pf ≈ 300 μm s−1) more water-permeable than control oocytes. LmAQP1 exhibited a Pf value similar to that of the TbAQPs (Uzcategui et al., 2004), and therefore can be classified as having intermediate water permeability, approximately 60% that of AQP1. Interestingly, treatment with 0.5 mM HgCl2 did not affect the water permeability of oocytes injected with LmAQP1-cRNA, although water transport was inhibited in AQP1-expressing oocytes under similar conditions (Fig. 1B). Thus, in contrast to PbAQP and TcAQP, which are sensitive to HgCl2 (Montalvetti et al., 2004; Promeneur et al., 2007), LmAQP1 is a mercurial-insensitive water channel.
The glycerol permeability of LmAQP1 was determined by an isosmotic swelling assay with a 130 mM inwardly directed gradient of glycerol. This concentration gradient led to an influx of glycerol, resulting in a secondary influx of water, and consequently swelling of the oocytes. As shown in Fig. 2, injection of oocytes with LmAQP1-cRNA increased the glycerol permeability by ∼25-fold when compared with the water-injected control. These results are comparable to that reported for other protozoal aquaglyceroporins (Hansen et al., 2002; Uzcategui et al., 2004; Promeneur et al., 2007). The above studies demonstrate that LmAQP1 is highly permeable to both water and glycerol.
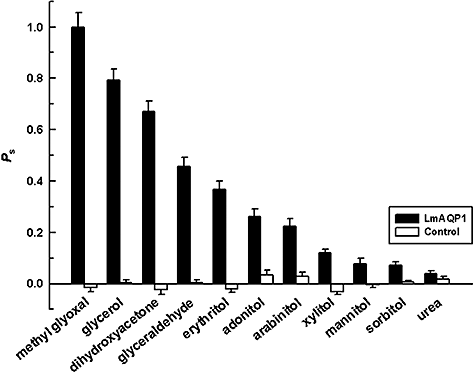
Substrate permeability in Xenopus oocytes expressing LmAQP1. Oocytes were injected with LmAQP1-cRNA (black bars) and water (control) (white bars).
Aquaglyceroporins are able to transport a wide variety of solutes. Human aquaglyceroporin-9 (AQP9), a homologue of LmAQP1, has been shown to be a promiscuous solute channel that, besides water, also allows the passage of a wide variety of non-charged solutes, including carbamides, polyols, purines and pyrimidines (Tsukaguchi et al., 1999). To determine the pore specificity of LmAQP1, we analysed the swelling rates of LmAQP1-expressing oocytes in the presence of a variety of non-ionic solutes (Fig. 2). The solute permeability decreased in the order: methylglyoxal > glycerol > dihydroxyacetone > glyceraldehyde > erythritol (C-4 sugar alcohol) > adonitol (C-5 sugar alcohol). Solute permeability was inversely proportional to the carbon chain length; C3-compounds (methylglyoxal, glycerol, dihydroxyacetone, glyceraldehyde) permeated better than C4 and C5-sugar alcohols, while the C6-sugar alcohols (mannitol and sorbitol) showed negligible transport. Additionally, there was minimal transport of urea through the LmAQP1 channel. This low transport of urea probably helps the parasite to survive within the host liver. The high permeability of methylglyoxal is surprising because methylglyoxal is known to be toxic to cells. This high uptake may explain the antileishmanial activity of methylglyoxal, reported earlier (Sarkar and Bhaduri, 1986). Interestingly, although Xenopus oocytes expressing LmAQP1 showed high uptake of methylglyoxal, Leishmania donovani promastigotes overexpressing LmAQP1 were not hypersensitive to methylglyoxal compared with control cells transfected with vector only (data not shown). This may be because Leishmania can metabolize methylglyoxal to d-lactate (Darling and Blum, 1988). The specific activity of Leishmania methylglyoxal reductase has been found to be the highest of all the catabolic enzymes (Ghoshal et al., 1989).
Expression and localization of LmAQP1
To examine the expression of LmAQP1, a rabbit polyclonal antibody was raised against a synthetic peptide, corresponding to amino acids 139–152 of LmAQP1. Using the affinity-purified LmAQP1 antiserum, Western blot analysis of X. laevis oocytes injected with LmAQP1-cRNA showed a ∼28 kDa protein band (Fig. 3C). In contrast, no such band was detected in oocytes injected with water. Similarly, the pre-immune serum did not give any reaction (data not shown). This 28 kDa band corresponds to the predicted molecular mass of LmAQP1.
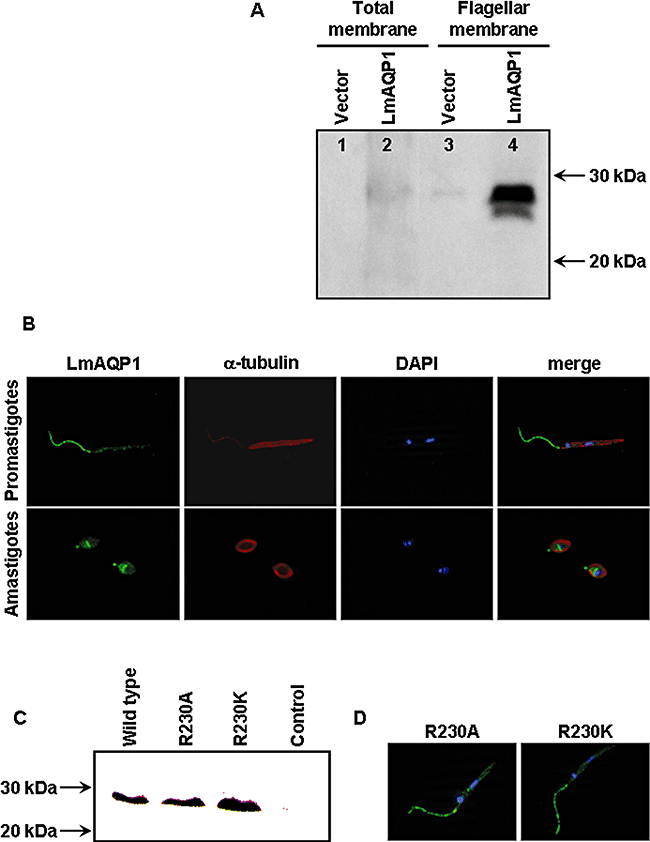
Expression and localization of wild-type and altered LmAQP1 in L. donovani.A. Western blot analysis of membrane protein fractions probed with an LmAQP1 antibody. Lanes 1 and 2, total membrane fraction; lanes 3 and 4, flagellar membrane fraction of L. donovani promastigotes transfected with vector and LmAQP1 respectively.B. Immunofluorescence microscopy of LmAQP1-transfected promastigotes (upper panel) and amastigotes (lower panel). Cells were stained for LmAQP1 (green), α-tubulin (red) and nuclei (blue). The three fluorescence images were finally merged.C. Western blot analysis of total protein extracts from Xenopus oocytes expressing wild-type, R230A and R230K LmAQP1.D. Immunofluorescence microscopy of L. donovani promastigotes expressing either the R230A or R230K LmAQP1. Cells were stained for LmAQP1 (green), α-tubulin (red), nuclei (blue) and the fluorescence images merged together.
Immunoblotting was next performed on plasma membrane-enriched fractions isolated from LmAQP1-transfected L. donovani promastigotes (Fig. 3A). A faint ∼28 kDa band was observed in cells transfected with LmAQP1 but not in vector alone controls. Western blotting was performed on flagellar fraction that was purified by shearing followed by differential centrifugation on sucrose gradients. Immunoblot of the flagellar fraction probed with the LmAQP1 antibody, clearly showed a ∼28 kDa band, suggesting that LmAQP1 was located exclusively to the flagella (Fig. 3A). Immunofluorescence experiments were performed to reveal the localization of LmAQP1 in L. donovani promastigotes and amastigotes (Fig. 3B). Fixed promastigotes, examined by fluorescence microscopy, showed selective immunostaining of the flagella. No staining was visible on the main body of the parasite. In amastigotes, staining was observed in the anterior part of the cell, most likely in the flagellar pocket. Additionally, a prominent medial band of staining was also noted, probably representing the contractile vacuole/spongiome complex (cf. Fig. 4B).
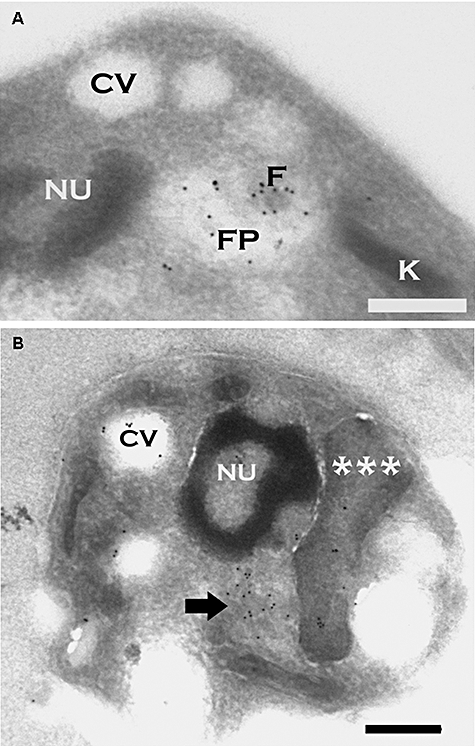
Cryosections from L. donovani amastigotes expressing LmAQP1. The sections were probed with the rabbit LmAQP1 antibody and goat anti-rabbit IgG (10 nm gold).A. Cross-section through the amastigote cell body illustrates probe localization in the remnant flagellar body (F) and surrounding flagellar pocket (FP).B. The contractile vacuole/spongiome (arrow) in longitudinal section is abundantly labelled by particles. The probe is also present on the subtending flagellum/kinetoplast/mitochondrion complex (***) and within the contractile vacuole (CV) complex. Scale bar = 0.5 μm.
To confirm our observations of LmAQP1 localization obtained by fluorescence microscopy, we also performed immuno-electron microscopy on amastigote forms of the LmAQP1-transfected cells. The LmAQP1 primary antibody was detected with a secondary antibody labelled with gold particles. In amastigotes, particles were detected on the flagellar remnant and surrounding flagellar pocket membranes, the kinetoplast-mitochondrion, and the contractile vacuole/spongiome complex (Fig. 4). Identification of the organelles is based upon cellular morphology, as described (Linder and Staehelin, 1979; Attias et al., 1996). Labelling of the exterior plasmalemma was found to be extremely rare in amastigotes.
Effect of Arg230 mutation on LmAQP1 activity
Molecular dynamics simulation of solute permeation through AQP1 and the bacterial glycerol facilitator GlpF demonstrates that the conserved arginine residue (Arg195 in AQP1 and Arg206 in GlpF) functions as a filter for protons and other positive ions (de Groot and Grubmuller, 2001). The corresponding residue in LmAQP1 is Arg230. The role of Arg230 in solute permeation was examined by altering it to lysine, which retains the positive charge, and to alanine, which has lost the charge and is also smaller in size. The effect of mutagenesis was examined in both Leishmania promastigotes and Xenopus oocytes. Expression of the altered proteins was detected by Western blotting. Both R230A and R230K mutants were expressed on oocyte membranes at levels similar to that of wild-type LmAQP1 (Fig. 3C). Additionally, immunofluorescence staining shows that either R230A or R230K LmAQP1 is localized to the flagellum of promastigotes (Fig. 3D).
The effect of the mutations on water permeability was examined using the swelling assays in Xenopus oocytes. The R230A mutant was non-functional while the R230K mutant had only 20% of the wild-type water transport activity (Fig. 5A). The permeability of other solutes was also examined. The R230A mutant showed zero to negligible transport of glycerol, dihydroxyacetone, glyceraldehyde and adonitol (Fig. 5B). In contrast, although the R230K mutant was impermeable to adonitol, it still showed 5–10% of the wild-type activity with respect to the transport of glycerol, dihydroxyacetone and glyceraldehyde (Fig. 5B). Interestingly, both R230A and R230K mutant transported methylglyoxal at ∼40% of wild-type levels (Fig. 5B).
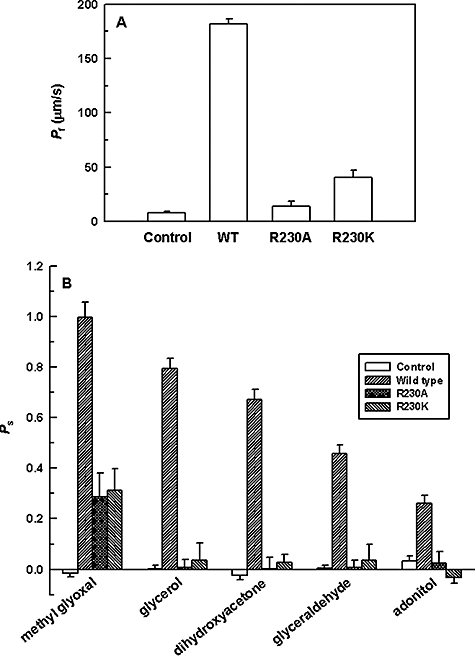
Substrate permeability in Xenopus oocytes expressing altered LmAQP1.A. Osmotic water permeability.B. Solute permeability of oocytes, injected with water (control), wild-type, R230A and R230K LmAQP1-cRNA.
The effect of Arg230 mutation on metalloid sensitivity and transport was also examined in both L. donovani promastigotes and amastigotes. Promastigotes transfected with the wild-type LmAQP1 gene exhibited 60- and 360-fold higher sensitivity to As(III) and Sb(III) respectively (Fig. 6A and B), and accumulated high levels of both metalloids (Fig. 6C and D). In contrast, cells expressing either the R230A or R230K LmAQP1 were resistant to both As(III) and Sb(III) (Fig. 6A and B), and accumulated low levels of the metalloids, similar to the vector alone control (Fig. 6C and D). Similar observations were made when either the wild-type or mutant LmAQP1 was expressed in axenic amastigotes and Xenopus oocytes. Axenic amastigotes, overexpressing wild-type LmAQP1, accumulated higher amounts of metalloids compared with R230A and R230K mutants and the vector alone control (Fig. 6E and F). Oocytes expressing R230A LmAQP1 did not accumulate either As(III) or Sb(III) (Fig. 6G and H). However, oocytes injected with R230K LmAQP1-cRNA transported low levels of As(III) but not Sb(III) (Fig. 6G and H).
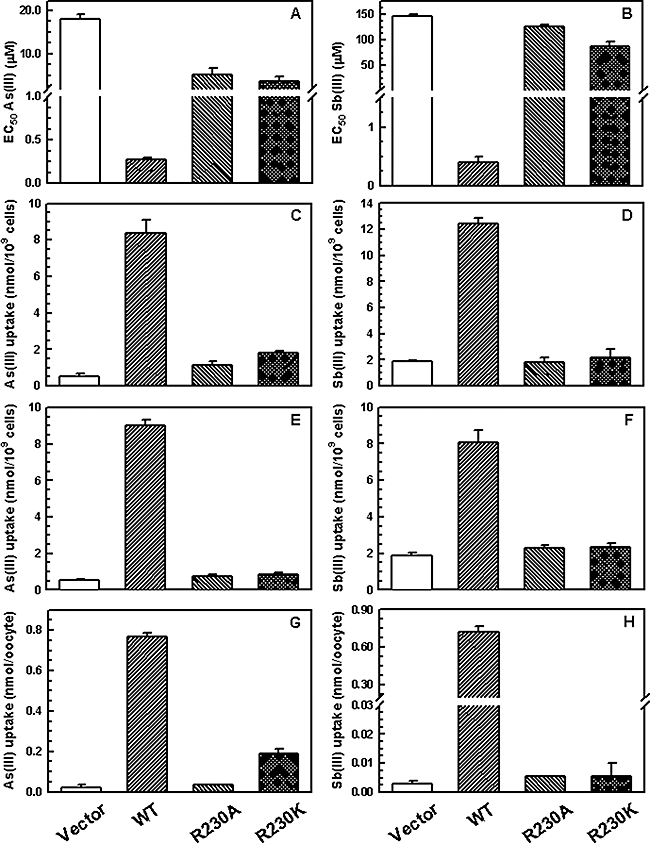
Metalloid sensitivity and uptake of cells expressing wild-type or altered LmAQP1. (A) EC50 for As(III); (B) EC50 for Sb(III); (C, E and G) uptake of As(III); (D, F and H) uptake of Sb(III). A–D represent experiments performed on L. donovani promastigotes; E and F, L. donovani amastigotes; G and H, Xenopus oocytes. Cells were transfected with wild-type, R230A, R230K LmAQP1 or vector control.
Role of LmAQP1 in volume regulation
Upon exposure to hypoosmotic stress, several mammalian cell types as well as a number of protozoa, swell initially but later return to their normal volume. This phenomenon, dubbed as the regulatory volume decrease, is accomplished by the efflux of various osmolytes to the extracellular environment followed by passive water flow (Rohloff et al., 2003). L. donovani promastigotes and amastigotes transfected with either the wild-type or R230A LmAQP1 were subjected to a 50% reduction in osmolarity (from 300 to 150 mOsm). As cell swelling leads to a decrease in the absorbance, the process of volume recovery was monitored by following the absorbance of the cell suspension at 550 nm. Subsequent to a hypoosmotic shock, L. donovani promastigotes expressing wild-type LmAQP1 showed a drop in absorbance, indicating cell swelling, followed by a steady rise in absorbance to near original levels, signifying volume recovery (Fig. 7A). The volume recovery process was essentially complete over a 3 min period. In contrast, promastigotes transfected with either the R230A LmAQP1 gene or the vector control showed increased cell swelling, as reflected by a higher drop in absorbance, and slower volume recovery than cells expressing the wild-type protein (Fig. 7A). Similar results were observed with axenic amastigotes. Figure 7B shows that following a hypoosmotic stress, amastigotes overexpressing wild-type LmAQP1 swelled up as reflected by a decrease in absorbance, and recovered their volumes much faster than the R230A mutant or the vector alone control. A similar decline in volume regulation was also observed in cells expressing R230K LmAQP1 (data not shown). Although in all cases, the volume recovered cells were virtually identical in terms of motility and morphology from control cells maintained under osmotic conditions, cells expressing wild-type LmAQP1 seem to be better equipped in combating abrupt osmotic changes in the environment.
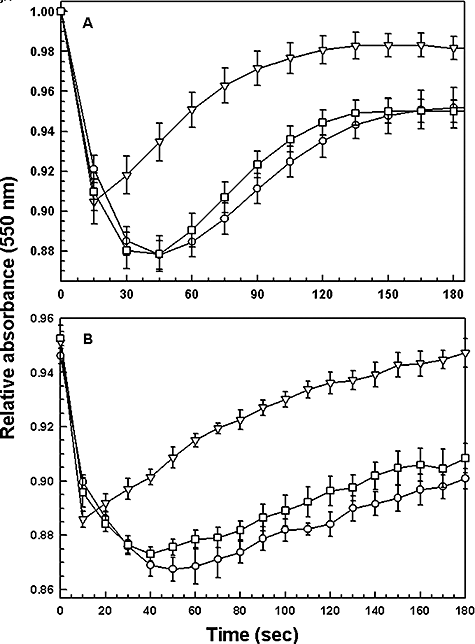
Volume regulation in the two life-cycle stages of Leishmania. (A) L. donovani promastigotes, and (B) L. donovani amastigotes expressing the vector (○); wild-type LmAQP1 (▿); R230A LmAQP1 (□); were subjected to a hypoosmotic shock, and the relative changes in cell volume were followed by monitoring the absorbance at 550 nm, as described in Experimental procedures.
Role of LmAQP1 in osmotaxis response of promastigotes
Leslie et al. (2002) have shown that Leishmania promastigotes move towards concentrations of sugar substrates and this taxis requires the presence of an osmotic gradient. This phenomenon of osmotaxis has been proposed to be involved in the migration of Leishmania promastigotes from the midgut to the proboscis of the sandfly, during the insect phase of the parasite life cycle, which is essential for successful transmission of the parasite to the vertebrate host (Oliveira et al., 2000; Leslie et al., 2002). The exclusive flagellar localization of LmAQP1 in Leishmania promastigotes prompted us to examine the osmotaxis response in cells overexpressing LmAQP1. Leishmania promastigotes transfected with vector, wild-type LmAQP1, R230A LmAQP1 and R230K LmAQP1 were subjected to osmotaxis assay as described in Experimental procedures. In this assay, a capillary tube is filled with agarose gel containing 100 mM glucose, while leaving 1 cm of the end of the tube filled with buffer only. The diffusion of glucose from the agarose filled to the open end of the capillary tube establishes a concentration gradient that simulates the solute gradient within the insect gut. The number of promastigotes that move over a certain interval from a buffered cell suspension into the open end of a capillary tube was determined. A significant higher number of promastigotes, overexpressing wild-type LmAQP1, were isolated from the capillary indicating that these cells moved faster towards higher concentrations of glucose, compared with the vector alone control (Fig. 8). Thus, promastigotes expressing wild-type LmAQP1 exhibited faster osmotaxis than the vector control. In contrast, promastigotes expressing altered R230A and R230K LmAQP1 showed similar osmotaxis response as the vector control. The low level of osmotaxis observed in Leishmania promastigotes transfected with either the vector or altered LmAQP1 is most likely due to expression of the chromosomal copy of LmAQP1 in these cells. These results clearly indicate that LmAQP1 plays a role in osmotaxis.
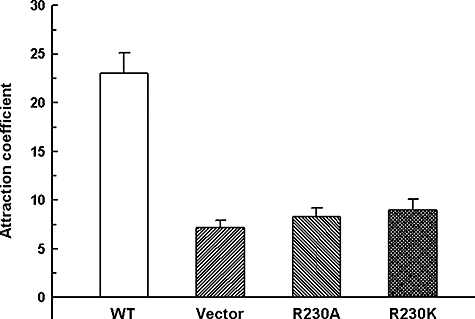
Movement of L. donovani promastigotes in response to an osmotic gradient. Cells transfected with wild-type, R230A, R230K LmAQP1 or vector control were counted for their ability to move into capillary tubes containing ±100 mM glucose.
Discussion
The L. major genome encodes for five AQPs: LmAQP1, LmAQPα, LmAQPβ, LmAQPγ and LmAQPδ. While LmAQP1 shows strong similarity to bacterial AQPs the other L. major aquaporins (LmAQP α–δ) are closer to plant AQPs (Beitz, 2005). This is a unique peculiarity of LmAQPs, because other parasitic AQPs known to date are either bacteria- or plant-like, and not a mixed population (Beitz, 2005). In this paper, we report the biochemical characterization of LmAQP1. The roles of the other LmAQPs have not yet been determined. LmAQP1 was expressed in X. laevis oocytes, and was able to function as a water channel (Fig. 1B). LmAQP1 belongs to the intermediate class of water channels; its water transport capacity is 65% that of AQP1, which is a classical water transporter. Interestingly, in contrast to Plasmodium and Trypanosoma AQPs that are inhibited by mercurials, LmAQP1 water transport was not inhibited by HgCl2, thereby classifying LmAQP1 as a mercurial independent water channel.
The uptake of glycerol by LmAQP1 was also demonstrated (Fig. 2). Very likely, the glycerol so imported is converted to dihydroxyacetone phosphate by the action of glycerol kinase (Hart and Opperdoes, 1984) and glycerol 3-phosphate dehydrogenase (Choe et al., 2003) that can serve as an intermediate in both the glycolytic (Michels et al., 2006) and gluconeogenic pathways (Naderer et al., 2006). Carbrey et al. (2003) have shown that, upon fasting there is an upregulation of AQP9 (homologue of LmAQP1 in humans), along with an increase in blood glycerol levels. It has been proposed that upregulation of AQP9 serves to maximize glycerol influx to sustain gluconeogenesis during starvation (Carbrey et al., 2003). Thus, the high blood glycerol levels in patients suffering from malnutrition (often a scenario in developing countries), may support the Leishmania amastigotes to thrive within the host. We have also shown that LmAQP1 allows the transport of dihydroxyacetone (Fig. 2). The L. major genome encodes for a single dihydroxyacetone kinase 1-like protein (LmjF10.0420; GeneDB). It is very likely that the dihydroxyacetone transported by LmAQP1 is converted to dihydroxyacetone phosphate by dihydroxyacetone kinase for entry into either the glycolytic or the gluconeogenic pathways.
The crystal structures of bovine AQP1 and E. coli GlpF show a conserved arginine residue (Arg195 in bovine AQP1, Arg206 in GlpF) as a filter for proton and other positive ions (Fu et al., 2000; Sui et al., 2001). Alteration of the homologous residue Arg219 in rat AQP9 to lysine did not affect either As(III) or glycerol transport, whereas the transport was highly reduced for the R219A mutant (Liu et al., 2004). It was therefore proposed that Arg219 is not required for activity in rat AQP9. The homologous residue in L. major LmAQP1 is Arg230. Oocytes expressing either the R230A or R230K LmAQP1 showed low to negligible transport of water, glycerol, dihydroxyacetone and As(III) (Fig. 5). The transport data for metalloid are also supported by their sensitivity studies in L. donovani. Thus, cells expressing either the R230A or R230K mutant showed negligible to low metalloid transport, and consequently were more resistant than cells expressing wild-type LmAQP1 (Fig. 6). These experiments clearly demonstrate that, unlike Arg219 in rat AQP9, Arg230 is required for LmAQP1 activity, and functions as a selectivity filter for the passage of solutes.
The specific localization of LmAQP1 to the flagellum of Leishmania promastigotes was displayed by Western blotting and immunofluorescence microscopy (Fig. 3). To the best of our knowledge this is the first report of an aquaglyceroporin being localized to the flagellum of any protozoa. The trypanosomatid flagellum is necessary for parasite migration and invasion of host tissues. Furthermore, the flagellum has also been speculated to regulate processes such as secretion and endocytosis in the flagellar pocket, and may be enriched in proteins involved in environmental or nutritional sensing (Gull, 1999; Bastin et al., 2000; Landfear and Ignatushchenko, 2001; Hill, 2003; Vaughan and Gull, 2003). Leishmania promastigotes face acute changes in osmolarity while thriving inside the sandfly gut or metacyclics swimming towards the proboscis of the fly. Leslie et al. (2002) have shown that Leishmania mexicana promastigotes move in response to an osmotic gradient. The possibility of LmAQP1 being involved in osmotaxis was therefore investigated. Our experiments clearly indicate that L. donovani promastigotes expressing wild-type LmAQP1 move towards an osmotic gradient while this property is lost in either the R230A or R230K mutants (Fig. 8). Thus LmAQP1 is the first aquaglyceroporin that is shown to be directly involved in osmotaxis.
In amastigotes, LmAQP1 is localized to the flagellar pocket, rudimentary flagellum, the kinetoplast-mitochondrion, and the contractile vacuole/spongiome complex (Fig. 4). TcAQP has been shown to be localized to the acidocalcisomes and contractile vacuole complex, and these organelles have been proposed to be involved in osmotic adaptations for these parasites (Montalvetti et al., 2004). Leishmania amastigotes face significant osmotic stress within the mammalian host. Our experiments indicate that both Leishmania promastigotes and amastigotes expressing the LmAQP1 gene respond efficiently to osmotic changes by volume regulation (Fig. 7). Additionally, both promastigotes and amastigotes, expressing either the R230A or R230K mutants, showed slower and incomplete volume recovery than cells transfected with wild-type LmAQP1 gene (Fig. 7). We therefore propose that LmAQP1 allows both the life cycle stages of the parasite to respond efficiently to osmotic changes by volume regulation.
The above experiments indicate that LmAQP1 plays an important physiological role in water and solute transport, volume regulation and osmotaxis. We also find that LmAQP1 allows the transport of toxic compounds, such as methylglyoxal, arsenite and antimonite. Each of these compounds has been shown to have antileishmanial activities (Sarkar and Bhaduri, 1986; Gourbal et al., 2004). Therefore, understanding the physiological role of LmAQP1 may aid in the development of new class of antileishmanial agents, which could significantly affect the parasites metabolic machinery as well as its osmotic resilience.
Experimental procedures
Strains and media
Leishmania donovani strain LdBob was a kind gift from Professor Stephen M. Beverley, Washington University School of Medicine. LdBob was cycled between the promastigote and axenic amastigote forms using an established protocol (Goyard et al., 2003). Leishmania promastigotes and amastigotes were grown in culture media as described earlier (Goyard et al., 2003). Promastigotes were grown at 25°C while the axenic amastigotes were cultivated at 37°C with 5% CO2. X. laevis were maintained in our animal facility and oocytes were harvested periodically.
Oligonucleotide-directed mutagenesis
The cloning of wild-type LmAQP1 into pGEM-T Easy vector (LmAQP1/pGEMEasy) has been described previously (Gourbal et al., 2004). Mutations in LmAQP1 were introduced by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis Procedure (Stratagene), as described previously (Mukhopadhyay et al., 2003). The mutagenic oligonucleotides used for both strands and the respective changes introduced (underlined) are as follows: R230A, 5′-GC CTC AAC CCC GCA GCC GAT TTG TCA CCT C-3′ (sense) and 5′-G AGG TGA CAA ATC GGC TGC GGG GTT GAG GC-3′ (antisense); R230K, 5′-GGC CTC AAC CCC GCA AAG ATT TGT CAC CTC GC-3′ (sense) and 5′-GCG AGG TGA CAA ATC CTT TGC GGG GTT GAG GCC-3′ (antisense). Each mutation was confirmed by sequencing the entire gene using a CEQ2000 DNA sequencer (Beckman Coulter).
Expression of LmAQP1 in oocytes
For expression in Xenopus oocytes, wild-type and mutant LmAQP1 in pGEM-T Easy vector were subcloned into the EcoRI site of the pL2-5 vector (Seyfang et al., 1997) (a generous gift from Professor Scott M. Landfear, Oregon Health Sciences University). Following linearization with SmaI, cRNAs were transcribed in vitro using the mMessage mMachine® kit (Ambion). Stage V and VI defolliculated X. laevis oocytes were injected with 50 nl of water (control), 50 nl of water containing 10 ng of cRNA (wild-type, R230A or R230K LmAQP1), 50 nl of water containing 5 ng of AQP1-cRNA, or combination of 10 ng (wild-type, R230A or R230K LmAQP1) and 5 ng of AQP1-cRNA. The oocytes were maintained at 16°C for 3 days in ND96 buffer containing 5 mM HEPES, pH 7.4, 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 2.5 mM sodium pyruvate, 0.5 mM theophylline and 2 μg ml−1 of gentamicin sulphate.
Standard oocyte swelling assays
Oocyte swelling assays were performed as described by Hansen et al. (2002). Water permeability was measured by transferring oocytes into 1:3 diluted ND96 medium. Solute permeability was measured in an isoosmotic ND96 in which 65 mM NaCl was substituted with 130 mM of a non-ionic test solute. While determining the permeability of methylglyoxal, NaCl was entirely replaced with 115 mM of methylglyoxal to maintain buffer isoosmoticity. For inhibition experiments, oocytes were pre-incubated in 0.5 mM HgCl2 in ND96 buffer, washed twice in the same buffer, before subjecting to swelling assay. The swelling assays were performed at room temperature and video-monitored. The relative oocyte volume was calculated from the covered area. Osmotic water permeability (Pf, μm s−1) and solute permeability (Ps) were calculated using the following equation:
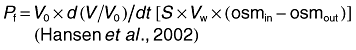 (1)
(1)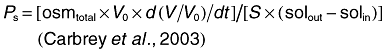 (2)
(2)where, S is the oocyte surface area (= 0.045 cm2), V0 is the initial oocyte volume (= 9 × 10−4 cm3), Vw represents the molecular water volume (= 18 cm3 mol−1), osmin − osmout is the osmotic gradient, osmtotal is the total osmolarity of the system (200 mOsm), solout − solin is the osmotic solute gradient, and d(V/V0)/dt in s−1 is measured from the initial slope of the relative volume increase.
Cloning of LmAQP1 in Leishmania expression vector pSP72αhygroα
pSP72αhygroα was created on the backbone of pSP72 (Promega) and pGEM7αhygroα vectors. pGEM7αhygroα was created as described previously (Papadopoulou et al., 1992; Gourbal et al., 2004). Either the wild-type or mutant LmAQP1/pGEMEasy was digested with XbaI and HindIII and cloned into the same sites of pSP72αhygroα.
Transfection of LmAQP1 in Leishmania
Transfection of pSP72αhygroα bearing either the wild-type or mutant LmAQP1 gene into the Leishmania promastigotes was accomplished as described previously (Ouellette et al., 1990). All transfectants were maintained in the presence of 0.3 mg ml−1 hygromycin (Invitrogen).
Determination of EC50 of metalloids in promastigotes
Metalloid sensitivity of the promastigote transfectants were determined as described previously (Gourbal et al., 2004). Briefly, log-phase promastigote cultures were diluted to 106 cells ml−1 in a culture medium containing various concentrations of As(III) in the form of sodium arsenite, or Sb(III) in the form of potassium antimonyl tartrate. Growth was monitored from the absorbance at 600 nm after 5 days using a microplate reader (Spectramax 340, Molecular Devices). EC50 was calculated from the sigmoidal analysis of percentage live cells versus metalloid concentrations using the Origin Graphing Software. Each assay was performed at least three times. Error bars were calculated from the mean ± SE.
Uptake assays
Log-phase Leishmania promastigotes or amastigotes were washed twice with phosphate-buffered saline (PBS), pH 7.4 (Invitrogen) and suspended in PBS at a density of 108 cells ml−1. The promastigotes were then incubated with 10 μM As(III) or Sb(III) for 10 min at room temperature. A 0.2 ml portion was filtered through a 0.22 μm nitrocellulose filter and the filter washed once with 5 ml of ice-cold PBS. The filters were digested with 0.5 ml of concentrated HNO3 (69–70%) (EM Science) for 1 h at 70°C, allowed to cool to room temperature, and diluted with high pressure liquid chromatography grade water (Sigma) to produce a final concentration of HNO3 of approximately 3%, and then analysed by inductively coupled plasma mass spectrometry (ICP-MS) with a PerkinElmer ELAN 9000. Standard solutions were made in the range of 0.5–10 p.p.b. in 3% HNO3 using arsenic and antimony standards (Ultra Scientific). Each transport experiment was repeated at least three times with duplicate samples. Error bars were calculated from the mean ± SE.
For measurement of metalloid transport in oocytes, five oocytes were incubated with either 1 mM As(III) or Sb(III) in ND96 buffer, for 90 s, at room temperature. The oocytes were washed four times with 1 ml of ice-cold ND96 buffer. Each oocyte was then transferred to a separate tube and digested with 0.1 ml of concentrated HNO3 as described above. Each sample was diluted with high pressure liquid chromatography grade water (Sigma) to produce a final concentration of HNO3 of approximately 3%, and the metalloid concentration was determined by ICP-MS. Each transport experiment was repeated at least three times. Error bars were calculated from the mean ± SE.
Cell volume measurements
Relative changes in cell volume following the induction of hypoosmotic shock were followed as described earlier (Rohloff et al., 2003). Briefly, log-phase promastigotes/amastigotes were washed twice in PBS and resuspended at a density of 109 cells ml−1. One hundred microlitre portions of the cell suspension were transferred to a microtiter plate. Hypoosmotic shock was induced by dilution of the isotonic cell suspension with an equal volume of deionized water and the absorbance at 550 nm was recorded every 15 s for 3 min in a microplate reader (Spectramax 340, Molecular Devices). A decrease in absorbance corresponds to an increase in cell volume. Isosmotic control experiments consisted of dilution of cell suspensions with appropriate volumes of isosmotic buffer. Unless otherwise noted, all hypoosmotic shock experiments were conducted at a final osmolarity of 150 mOsm (1:1 dilution of isosmotic buffer and water). Each experiment was repeated at least three times. Error bars were calculated from the mean ± SE.
Osmotaxis assay
Migration of promastigotes through osmotic gradients was measured as described earlier (Oliveira et al., 2000; Leslie et al., 2002). Glass capillary tubes (75 mm long × 1 mm internal diameter) were loaded with a 1% agarose solution, prepared with either PBS or PBS containing 100 mM d-glucose, such that exactly 1 cm at the end of each tube remained unoccupied by the gel (Leslie et al., 2002). Late-log-phase LdBob promastigotes, overexpressing wild-type LmAQP1 or altered LmAQP1 or vector controls, were washed once with PBS, resuspended in the same buffer (5 ml) at a density of 2.5 × 107 cells ml−1, and placed in a microcentrifuge tube. The open end of each capillary tube was filled with PBS, and the capillary tubes were suspended vertically into the microcentrifuge tube, so that the open end of each capillary was immersed in the promastigote suspension. The capillary tubes were incubated at room temperature for 1 h. The capillary tubes were removed carefully from the cell suspension and the buffer (approximately 6 μl) was collected from the open end of each tube using a fine pipette-tip and mixed with 5 μl of PBS. The density of the promastigotes in each sample was determined using a haemocytometer and the mean density and standard error was calculated for both control and test samples. Interexperimental variability was eliminated by expressing the mean number of cells moving into the capillary tube containing 100 mM glucose relative to the mean number of cells moving towards the capillary tube in absence of glucose (attraction coefficient). An attraction coefficient of 1 thus indicates no specific movement towards glucose. Each experiment was repeated at least three times. Error bars were calculated from the mean ± SE.
Cell lysates and western blots
Plasma membrane-enriched fraction from Leishmania promastigotes was prepared as described previously (Mukhopadhyay et al., 1996). All procedures were performed at 4°C. Briefly, after washing the cells in Buffer A (75 mM Tris-HCl, pH 7.4, 140 mM NaCl, 11 mM KCl), cells were resuspended in Buffer B [10 mM HEPES, pH 7.4, 10 mM KCl, 1 mM MgCl2, 400 mM mannitol, and Complete Protease Inhibitor Cocktail (Roche)] and broken by sonication using five pulses of 10 s each with 50% amplitude. Unbroken cells and cell debris were removed by centrifugation at 3000 g for 10 min. The supernatant was centrifuged at 17 000 g for 40 min to remove organelles and other intracellular membranes followed by centrifugation at 140 000 g for 1 h. The pellet, which is highly enriched in plasma membrane fraction, was washed and suspended in 1 ml of Buffer C (75 mM HEPES, pH 7.4, 150 mM KCl, 2 mM MgCl2).
Flagella were isolated as follows. Promastigotes from a 4-day-old, 100 ml culture were harvested, washed twice in ice-cold Buffer D (25 mM Tris-HCl, pH 7.4, 0.2 mM EDTA, 5 mM MgCl2, 12 mM β-mercaptoethanol, and 0.32 M sucrose) and resuspended in 2 ml of ice cold buffer E [Buffer D plus 1% BSA, 0.1 mM CaCl2 and Complete Protease Inhibitor Cocktail (Roche)]. Flagella were detached without lysing the body of the cells by sonication on ice using three pulses of 1 s each, with 25% amplitude. Flagellar preparation was isolated from the supernatant by centrifuging at 700 g for 15 min followed by pelleting at 6780 g for 20 min. The pellet was resuspended in buffer A and loaded onto 0.8 M sucrose cushion and centrifuged at 1080 g for 20 min. The upper layer was removed and overlaid on a discontinuous gradient of sucrose (1.65 M and 1.85 M) and ultracentrifuged at 130 000 g for 3 h. The interphase flagellar layer was removed and examined by phase-contrast microscopy to confirm that the flagella were largely free of cell bodies. Protein contents of either the plasma membrane enriched or flagellar fractions were determined by the BCA protein assay kit (Pierce).
A polyclonal anti-peptide antibody against the synthetic peptide, SHFDEAEKRLLLNE, corresponding to amino acids 139–152 of L. major, was developed at Covance Immunology Services (Denver, PA) and affinity purified. For Western blot analysis, protein samples in 1× SDS gel-loading buffer (Sambrook and Russell, 2001) were heated to 50°C for 1 h, electrophoresed into a 12% SDS-polyacrylamide gel, and electro-blotted onto nitrocellulose membrane (Whatman). The blots were incubated with affinity-purified LmAQP1 antiserum (1:500) for 1 h at room temperature, and the labelling was visualized with horseradish peroxidase-conjugated goat anti-rabbit antiserum (Sigma) using the Western Lightning Chemiluminescence Reagent Plus system (PerkinElmer).
Immunofluorescence analysis
Late-log-phase promastigotes or amastigotes (∼5 × 105 cells) were fixed with 4% paraformaldehyde/PBS overnight at 4°C. The cells were washed twice with PBS, transferred to Teflon-coated Immunofluorescence slides (Polysciences), and permeabilized with 0.1% Triton X-100, 50 mM Na2PO4, 50 mM glycine, pH 7.2, for 2 min. After blocking with 1% bovine serum albumin in PBS for 30 min, cells were incubated for 1 h at room temperature with LmAQP1 antiserum (1:300 in 1% BSA). The primary antibody was removed by washing three times with 1% BSA followed by incubation with the monoclonal anti-α-tubulin antibody (Sigma) 1:500 for another hour. After three washes with 1% BSA, the slides were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:1000 in 1% BSA) and Alexa Fluor 594-conjugated goat anti-mouse IgG (1:2000 in 1% BSA) for 1 h. Finally, the cell nuclei were stained with DAPI (0.1 μg ml−1 final concentration) (Fluka) for 10 min, the slides washed three times with 1% BSA, and once with sterile water. The slides were mounted in Fluoromount-G (SouthernBiotech) and examined by Zeiss Axiophot Photomicroscope with a 63× water immersion objective lens.
Immuno-electron microscopy
LdBob promastigotes or amastigotes were processed for immuno-electron microscopy as described previously (Russell et al., 1992; Piper et al., 1995). Briefly, cells were fixed in 4% formaldehyde in HEPES saline (30 mM HEPES, 100 mM NaCl, 0.4 mM CaCl2), pH 7.0, and rinsed in HEPES saline alone. They were then pelleted, embedded in gelatin, and infiltrated with 2.3 M sucrose/20% polyvinyl pyrrolidone in PBS. The blocks were trimmed, frozen and sectioned in a Reichert FC4E cryoultramicrotome. The cryosections were placed on carbon-coated formvar grids, blocked with 10% fetal calf serum (FCS) in PBS, incubated with LmAQP1 antiserum (1:150 in 10% FCS in PBS), washed in the same solution without antibody, followed by incubation with gold-conjugated secondary antibody. The grids were then washed sequentially in 10% FCS in PBS, PBS, and double-distilled water, prior to staining with uranyl acetate. All labelling experiments were conducted, in parallel, with controls omitting the primary antibody, and in the presence of non-immune normal rabbit serum or unrelated mouse monoclonal antibody against other Leishmania-specific antigens. These controls were consistently negative.
Acknowledgements
We are grateful to Dr Marco A. Sanchez for help with the initial oocyte experiments and to Professor Scott M. Landfear for critical reading of the manuscript. This research was supported by a grant from the National Institutes of Health to R.M. (AI058170). M.O. is a Burroughs Wellcome Fund Scholar in Molecular Parasitology and holds a Canada Research Chair in Antimicrobial resistance.




