The exceptionally tight affinity of DnaA for ATP/ADP requires a unique aspartic acid residue in the AAA+ sensor 1 motif
Summary
Escherichia coli DnaA, an AAA+ superfamily protein, initiates chromosomal replication in an ATP-binding-dependent manner. Although DnaA has conserved Walker A/B motifs, it binds adenine nucleotides 10- to 100-fold more tightly than do many other AAA+ proteins. This study shows that the DnaA Asp-269 residue, located in the sensor 1 motif, plays a specific role in supporting high-affinity ATP/ADP binding. The affinity of the DnaA D269A mutant for ATP/ADP is at least 10- to 100-fold reduced compared with that of the wild-type and DnaA R270A proteins. In contrast, the abilities of DnaA D269A to bind a typical DnaA box, unwind oriC duplex in the presence of elevated concentrations of ATP, load DnaB onto DNA and support minichromosomal replication in a reconstituted system are retained. Whereas the acidic Asp residue is highly conserved among eubacterial DnaA homologues, the corresponding residue in many other AAA+ proteins is Asn/Thr and in some AAA+ proteins these neutral residues are essential for ATP hydrolysis but not ATP binding. As the intrinsic ATPase activity of DnaA is extremely weak, this study reveals a novel and specific function for the sensor 1 motif in tight ATP/ADP binding, one that depends on the alternate key residue Asp.
Introduction
In Escherichia coli, the initiator protein DnaA directly binds to specific 9-mer DnaA boxes in the chromosomal replication origin, oriC, resulting in the formation of homomultimeric complexes (Kelman and O'Donnell, 1995; Messer, 2002). DnaA has a high affinity for ATP and ADP (Sekimizu et al., 1987). The ATP-bound form, but not the ADP-bound form, conformationally activates the DnaA–oriC complex (Speck and Messer, 2001; McGarry et al., 2004), which triggers site-specific duplex unwinding of the AT-rich region within oriC, creating an open complex. DnaB helicase is then loaded onto the single-stranded (ss) region in a manner dependent on interactions with the DnaC helicase loader and DnaA. DnaG primase then forms primers, which allows loading of the DNA polymerase (pol) III holoenzyme. The pol III holoenzyme consists of the β clamp subunit, which is directly loaded onto the primed site, and the pol III* subassembly, which binds to the β clamp. Pol III* includes the clamp-loader γ complex and the pol III core complex, which includes the catalytic centre of the polymerase.
After Okazaki fragment synthesis, pol III* is released from the DNA, leaving the β clamp bound to the synthesized DNA (Kelman and O'Donnell, 1995; McHenry, 2003; Johnson and O'Donnell, 2005). The DNA-loaded β clamp subunit can form a stable complex with Hda protein (Kurz et al., 2004; Su'etsugu et al., 2004; 2005; Kawakami et al., 2006). This complex promotes DnaA–ATP hydrolysis, which prevents further replication initiation events by converting ATP–DnaA to ADP–DnaA. This negative control system for DnaA activity is termed RIDA (regulatory inactivation of DnaA) (Katayama et al., 1998; Kato and Katayama, 2001). Orthologues of Hda as well as those of DnaC are present only in parts of γ-proteobacteriae species, suggesting the presence of different systems in the replicational initiation process of eubacteria.
DnaA protein consists of four functional domains (Messer, 2002). Domain I is involved in DnaA self-multimerization and interaction with other proteins such as the DnaB helicase, DiaA (a modulator of DnaA) and the Hda–β clamp complex (Sutton et al., 1998; Weigel et al., 1999; Seitz et al., 2000; Ishida et al., 2004; Felczak et al., 2005; Su'etsugu et al., 2005). Domain II is considered to contain a flexible linker. Domain IV is a DNA-binding region that contains a helix–turn–helix motif for specific recognition of the DnaA box (Fujikawa et al., 2003). Domain III has unique ATP recognition motifs that are characteristic of the AAA+ superfamily (Neuwald et al., 1999; Iyer et al., 2004). The amino terminus of DnaA domain III contains a second site for interaction with the DnaB helicase (Sutton et al., 1998; Seitz et al., 2000).
The process and regulation of DNA replication require several AAA+ proteins (Neuwald et al., 1999; Ogura and Wilkinson, 2001; Hanson and Whiteheart, 2005), including Hda protein and certain subunits of the pol III γ complex. In eukaryotes, proteins in the origin recognition complex (ORC), the MCM helicase, Cdc6 (a component of the pre-replication complex) and RFC (the counterpart of the clamp-loader γ complex) also belong to this family. Of these AAA+ replication-related proteins, DnaA and ORC have exceptionally high affinities for ATP, with dissociation constants (KD) reported to be about 30 nM in both cases (Sekimizu et al., 1987; Klemm et al., 1997). The other proteins also bind ATP but with affinities about 10- to 100-fold lower than those of DnaA and ORC (Davey et al., 2002; Johnson and O'Donnell, 2003; Seybert and Wigley, 2004). The mechanical basis of the extremely high affinity of these initiation proteins for ATP remains unexplored.
The AAA+ domain consists of the NTP-binding Walker A and B motifs and other unique motifs (Neuwald et al., 1999; Ogura and Wilkinson, 2001; Iyer et al., 2004). Much evidence indicates that amino acid residues in the Walker A motif (or P-loop) are required for ATP binding. In certain cases, residues in the Walker B motif are required for ATP hydrolysis activity. The Walker B residues have been structurally indicated to support the co-ordination of Mg2+ for interaction with the β-γ phosphates of ATP.
Substitutions of conserved amino acid residues within the sensor 1 motif of the AAA+ (or AAA subgroup) chaperones such as Saccharomyces cerevisiae Hsp104 and the p97/VCP (valosin-containing protein)-like ATPase from Thermoplasma acidophilum (VAT) cause a severe decrease in ATP hydrolysis activity, but not in ATP binding activity (Hattendorf and Lindquist, 2002; Gerega et al., 2005; Hanson and Whiteheart, 2005). Thus, it has been considered that the AAA+ sensor 1 motif plays a specific role in catalysing ATP hydrolysis. The sensor 1 motif is also reported to be important for ATPase and protease activities of E. coli FtsH (Karata et al., 1999).
The Box VIII (or sensor 2) and arginine finger motifs of the AAA+ domain also play unique roles in ATP recognition and hydrolysis (Neuwald et al., 1999; Ogura and Wilkinson, 2001; Hanson and Whiteheart, 2005). We previously showed that the DnaA Box VIII arginine motif is required for its intrinsic ATPase activity and RIDA-dependent ATP hydrolysis (Su'etsugu et al., 2001; Nishida et al., 2002). The arginine finger motif of DnaA, which is required for the ATP-specific conformational activation of the initiation complex, is thought to recognize ATP bound to DnaA in the complex (Kawakami et al., 2005). These two motifs are not required for tight binding of ATP/ADP by DnaA.
Here, we investigate the role of the DnaA sensor 1 motif in interaction with ATP. Homology modelling suggested that the DnaA Asp-269 and Arg-270 residues within the sensor 1 motif would be close to the bound nucleotide as well as to the Walker A and Walker B motifs (Fig. 1A). Indeed, analysis of a crystal structure of Aquifex aeolicus DnaA domain III-IV showed that among the residues comprising sensor 1, an Asp residue corresponding to E. coli DnaA Asp-269 was closest to the phosphate moiety of the nucleotide (Erzberger et al., 2002). We find that the DnaA D269A mutant protein is severely and specifically defective in its affinities for ATP and ADP. This study demonstrates the first evidence indicating a novel mechanical role for the AAA+ sensor 1 motif in tight ATP/ADP binding.
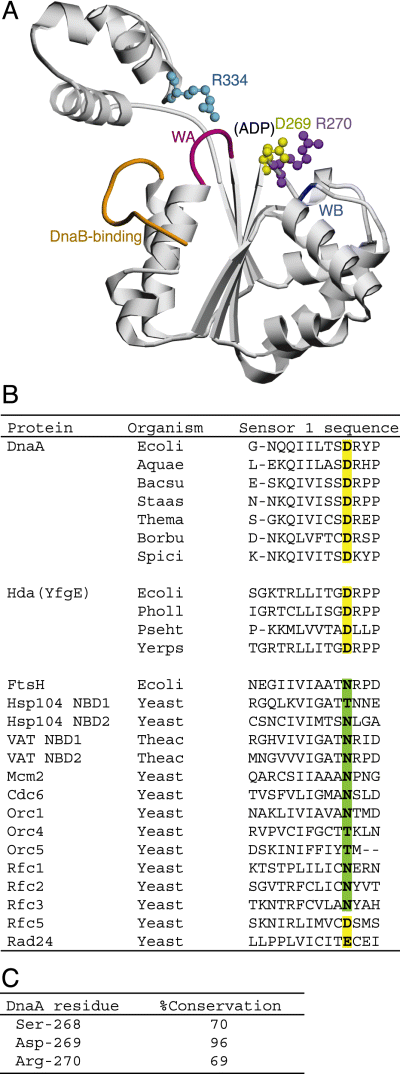
Structural model of the DnaA AAA+ domain and the sensor 1 motif.A. A homology model (ID: P03004–C00001) of DnaA domains III–IV was obtained from the SWISS-MODEL Repository database (Kopp and Schwede, 2004). For clarity, only domain III is shown in this figure, as a ribbon diagram generated by the POVScript+ (Fenn et al., 2003) and POV-Ray programs (Persistence of Vision). As the bound nucleotide was removed during the modelling processes, a probable ADP-binding pocket is indicated (ADP). The DnaB-binding region (DnaB-binding) and Walker A (WA) and B (WB) motifs are coloured orange, red and blue respectively. The side chains of the Asp-269 (yellow), Arg-270 (purple) and Arg-334 (light blue) residues are shown as ball-and-stick models.B. Amino acid residues of the sensor 1 motif of various AAA+ proteins are shown. Where necessary, multiple sequence alignment was performed using the clustal w program (Thompson et al., 1994) and gaps were inserted according to the original AAA+ definition (Neuwald et al., 1999). Amino acid residues corresponding to DnaA Asp-269 are in boldface and classified as acidic (yellow highlighting) or neutral (green highlighting) residues. Organism names are abbreviated as follows: Ecoli, Escherichia coli; Aquae, Aquifex aeolicus; Bacsu, Bacillus subtilis; Staas, Staphylococcus aureus; Thema, Thermotoga maritima; Borbu, Borrelia burgdorferi; Spici, Spiroplasma citri; Pholl, Photorhabdus luminescens; Pseht, Pseudoalteromonas haloplanktis; Yerps, Yersinia pseudotuberculosis; Yeast, Saccharomyces cerevisiae; Theac, Thermoplasma acidophilum. NBD, nucleotide-binding domain.C. Primary sequences of ∼500 DnaA homologues were obtained from the GenBank using the blast program (Altschul et al., 1997). By multiple sequence alignment, the residues corresponding to DnaA Ser-268, Asp-269 and Arg-270 were determined and conservation (%) of each residue is shown.
Results
Purification of DnaA proteins with mutations in the sensor 1 motif
To clarify the role of the DnaA sensor 1 motif, we purified and analysed the DnaA D269A and R270A proteins. Sequence analysis indicates that the DnaA Asp-269 and Arg-270 residues are included in the AAA+ sensor 1 motif (Neuwald et al., 1999). A homology model obtained from the SWISS-MODEL Repository database supports the importance of these residues as they are located near the nucleotide-binding pocket (Fig. 1A). A similar protein structure is shown in our homology model (Nishida et al., 2002) (data not shown). The corresponding residues are highly conserved among DnaA homologues of various eubacterial species (Fig. 1B and C). Especially conservation of Asp-269 is extreme.
The DnaA D269A and R270A proteins were overexpressed in a strain with a chromosomal disruption of dnaA and purified using the same method as that for wild-type DnaA (Fig. 2A). At the final step of purification, DnaA monomers were separated from aggregates by gel filtration. As was the case for wild-type DnaA, both DnaA mutants were readily isolated from aggregates. The final DnaA D269A and R270A fractions were > 90% pure, as determined by SDS-PAGE (Fig. 2A).
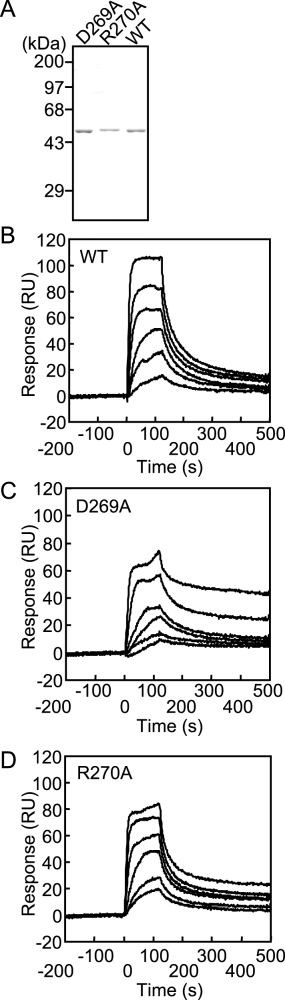
Purification and DnaA box-binding activity of mutant DnaA proteins.A. Mutant DnaA proteins were overproduced in KA450 [ΔoriC dnaA(Am)] and purified as described in Experimental procedures. Purified DnaA D269A (D269A) and DnaA R270A (R270A) proteins (0.5 μg each) were analysed by SDS-10% PAGE and Coomassie brilliant blue R-250 staining. The positions of molecular weight markers are shown. B–D. DnaA box-binding kinetics of wild-type DnaA (B), DnaA D269A (C) and DnaA R270A (D) was assessed by surface plasmon analysis as described in Experimental procedures. The concentrations of DnaA proteins were 2.5, 5.0, 10, 20, 38 and 75 nM for all experiments.
Specific DNA binding activity is retained by mutant DnaA proteins
We next assessed the DNA binding kinetics of the mutant DnaA proteins by surface plasmon resonance analysis using 21 bp DNA fragments containing a typical DnaA box (box R1) or a non-specific sequence that is not recognized by DnaA. The results of this assay indicated that both mutant proteins preserved specific affinities for the DnaA box with binding kinetics similar to that of wild-type DnaA (Fig. 2B–D, Table 1). When each mutant DnaA was assayed at high concentrations, the resonance units slightly increased at the equilibrium binding state and the rates of release were slightly retarded, suggesting that DnaA–DnaA interactions might be modestly enhanced by the amino acid substitutions. Similarly, when a gel mobility-retardation assay was performed using a 15 bp DNA fragment containing the DnaA box R1, DNA-binding activity was observed for the DnaA D269A and R270A proteins at levels similar to that of wild-type DnaA (data not shown).
| DnaA | k a (M−1 s−1) | k d (s−1) | K D (M) |
|---|---|---|---|
| Wild-type | 2.1 × 106 | 1.9 × 10−2 | 9.0 × 10−9 |
| D269A | 6.1 × 105 | 8.4 × 10−3 | 1.4 × 10−8 |
| R270A | 1.9 × 106 | 1.6 × 10−2 | 8.4 × 10−9 |
- Kinetic values were calculated by curve fitting of the sensorgrams shown in Fig. 2B–D to a theoretical 1:1 interaction model (Langmuir binding). ka, association rate constant; kd, dissociation rate constant; KD, dissociation constant.
DnaA D269A is defective in nucleotide binding activity
To determine the affinities of the DnaA D269A and R270A proteins for adenine nucleotides, we performed a filter retention assay (Fig. 3). When incubated at 0°C (Fig. 3A and B) or 30°C (Fig. 3C and D), wild-type DnaA tightly bound ATP and ADP with a KD of 30–90 nM as previously reported. However, under the same conditions, the nucleotide binding of DnaA D269A at 0°C was almost undetectable (Fig. 3A and B). Also, at 30°C it was severely impaired (Fig. 3C and D). In contrast, nucleotide binding by DnaA R270A was detected by this assay even at 0°C, although the binding stoichiometry of DnaA R270A for ATP and ADP decreased by > 50% compared with that of wild-type DnaA (Fig. 3A and B). This result suggests that the binding of DnaA R270A to nucleotides is moderately unstable. The intrinsic ATPase activity and the sensitivity to RIDA in vitro of DnaA R270A were not significantly inhibited (Supplementary material).
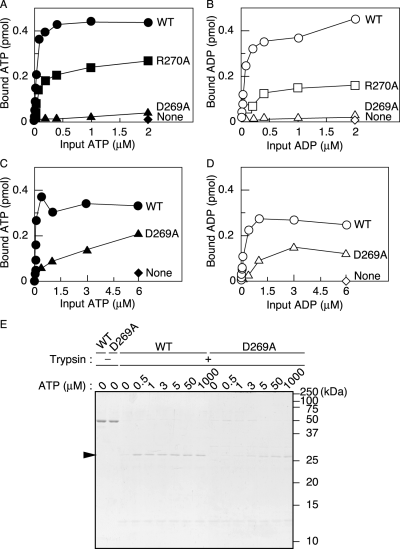
Adenine nucleotide-binding activity assays.A–D. DnaA (1.9 pmol) was incubated for 15 min at 0°C (A and B) or 30°C (C and D) in buffer containing various concentrations of [α-32P]-ATP (A and C) or [3H]-ADP (B and D), and the amount of bound nucleotide was determined by retention on a membrane. Circles, wild-type DnaA (WT); triangles, DnaA D269A; boxes, DnaA R270A; diamonds, no protein (None).E. ATP-induced conformational change of DnaA (52 kDa). Wild-type DnaA (WT) or DnaA D269A (D269A) was incubated for 20 min at 30°C in buffer containing concentrations of ATP ranging from 0 to 1 mM, followed by incubation in the presence (+) or absence (–) of trypsin. Digestion products were analysed by SDS-15% PAGE and Coomassie brilliant blue R-350 staining (see Experimental procedures). An arrowhead indicates the 30 kDa digestion product. The positions of molecular weight markers are shown. A faint signal seen at about 13 kDa is a trypsin-self digestion product.
To more quantitatively assess the affinity of DnaA D269A for ATP, we investigated ATP binding-dependent conformational changes with a trypsinolysis assay at 30°C. Digestion of the ATP-bound form has been shown to produce a 30 kDa trypsin-resistant peptide (Garner and Crooke, 1996; Carr and Kaguni, 2002). This fragment was clearly detected for digested wild-type DnaA, even in the presence of 0.5 μM ATP (Fig. 3E). In contrast, treatment of DnaA D269A produced the 30 kDa fragment at significant levels only in the presence of concentrations of ATP of at least 3 μM at 30°C (Fig. 3E). At lower concentrations (0.5 or 1 μM), the level of the DnaA D269A digestion fragment was comparable to that of wild-type DnaA incubated without ATP. These observations indicate that DnaA D269A binds ATP at concentrations of 3 μM or higher at 30°C, consistent with the data obtained with the filter retention assay. Even at elevated concentrations of ATP, the intensity of the 30 kDa DnaA D269A fragment was about 50% that of the wild-type DnaA fragment (Fig. 3E). This difference indicates that the binding of nucleotides by DnaA D269A is unstable under these conditions.
Association and dissociation rates of ATP
To further investigate the role for DnaA Asp-269 in ATP binding, we assessed the time-course of ATP association/dissociation rates at 30°C. First, wild-type DnaA or DnaA D269A was incubated at 0°C in buffer containing [32P]-ATP, followed by temperature shift up to 30°C. At intervals, the samples were assessed by the filter retention assay. The results indicated that the level of ATP bound to DnaA D269A increased slowly, taking 10 min to reach the saturation level even at 30°C (Fig. 4A). ATP binding to wild-type DnaA occurs rapidly at 0°C.
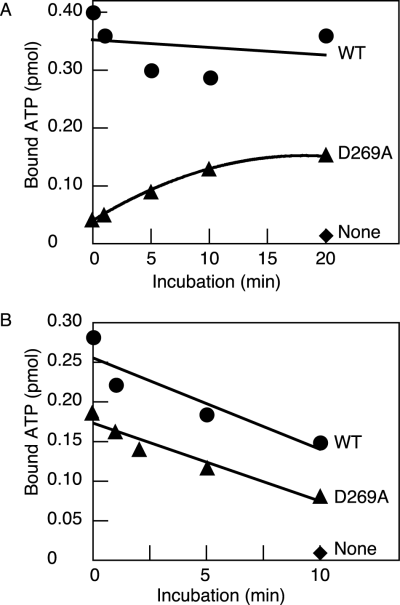
Time-course of ATP association and dissociation.A. ATP-binding time-course of DnaA. DnaA (1.9 pmol) was included in buffer containing 3 μM [α-32P]-ATP and incubated at 30°C for the indicated time (0, 1, 5, 10, or 20 min), followed by retention on a membrane. Circles, wild-type DnaA (WT); triangles, DnaA D269A; diamonds, no protein (None).B. Dissociation rate of DnaA-bound ATP. DnaA (1.9 pmol) was pre-incubated at 30°C for 15 min in buffer containing 3 μM [α-32P]-ATP. The reactions were further incubated at the same temperature for the indicated time (0, 1, 2, 5, or 10 min) in the presence of 5 mM non-radiolabelled ATP, followed by retention on a membrane. Symbols used are the same as the above.
Next, we investigated stability of ATP–DnaA D269A complex. Dissociation of [32P]-ATP bound to wild-type DnaA or DnaA D269A was assessed by incubation at 30°C in the presence of 5 mM ATP and a filter retention assay. The results indicated that the time taking to dissociate 50% bound ATP was 8–10 min for both proteins (Fig. 4B). When 5 mM ATP was excluded, no significant dissociation of bound ATP was observed for both proteins under the similar conditions (data not shown). Taken together, these results suggest that DnaA Asp-269 plays an important role in the initial events required for association with ATP and is less important in stability of the ATP-bound complex.
oriC DNA unwinding activity
To assess the oriC unwinding activity of the mutant DnaA proteins, we performed a P1 nuclease assay (Fig. 5). Unlike the ADP-bound form, the ATP-bound form of wild-type DnaA unwinds the AT-rich 13-mer region within oriC in a process that depends on a superhelical form of the oriC plasmid, under appropriate conditions of temperature and mM levels of ATP. In this assay, DnaA is incubated at 38°C in the presence of the oriC plasmid pBSoriC (3.6 kb), 5 mM ATP and P1 nuclease, which cleaves DNA at single-stranded sites, followed by digestion with ScaI. Digestion of pBSoriC specifically unwound by DnaA produces fragments of 1.6 and 2.1 kb. The results of this experiment indicated that the DnaA mutants exhibit unwinding activity (Fig. 5).

The oriC unwinding activity of mutant DnaA proteins. Wild-type DnaA (WT) and DnaA D269A (D269A) were pre-incubated for 15 min at 30°C in the presence of 3 μM ATP or ADP (A and C). Wild-type DnaA (WT) and DnaA R270A (R270A) were pre-incubated for 15 min at 0°C in the presence of 3 μM ATP or ADP (NTP) (B and D). The mixtures, including the indicated amounts of DnaA, were further incubated for 3 min at 38°C in the presence of pBSoriC (400 ng) and 5 mM ATP, followed by digestion with P1 nuclease and ScaI (A–D). Samples were analysed by 1% agarose gel electrophoresis with molecular markers and stained with ethidium bromide (A and B). The DNA fragments in A and B were quantified by densitometric scanning, and values were determined with an image-analysing program. The total amounts of the 2.1 kb and 1.6 kb fragments were normalized to total DNA and plotted as open complex (%) (C and D).
Because DnaA D269A binds ATP at 30°C in the presence of 3 μM ATP (Fig. 3), the wild-type DnaA and DnaA D269A proteins were incubated for 15 min at 30°C in buffer containing 3 μM ATP or ADP. P1 nuclease analysis of these samples indicated that DnaA D269A exhibits ATP form-specific oriC unwinding activity at a level comparable to that of wild-type DnaA (Fig. 5A and C). Despite the presence of 5 mM ATP in the unwinding reaction and the decreased affinity for the nucleotides, DnaA D269A that was pre-incubated at 30°C with 3 μM ADP was inactive. Most likely, this mutant DnaA was irreversibly inactivated by pre-incubation with ADP.
The wild-type DnaA and DnaA R270A proteins were incubated for 15 min at 0°C in buffer containing 3 μM ATP or ADP, followed by a P1 nuclease assay (Fig. 5B and D). The extent of oriC duplex unwinding by the ATP form of DnaA R270A was about 30% that of wild-type DnaA. The slight inhibition might be due to labile conformation of DnaA R270A by unstable ATP binding. The ADP form of this mutant was totally inactive, as was observed for wild-type DnaA. The unwinding activity of wild-type ATP–DnaA pre-incubated at 0°C was slightly higher than that of the protein pre-incubated at 30°C (Fig. 5C and D), which might be due to slight denaturation that occurred during pre-incubation at 30°C.
DnaB loading activity of the DnaA D269A and DnaA R270A proteins
To investigate the activities of DnaA D269A and DnaA R270A for DnaB loading, we used an in vitro ABC-primosome system that replicates a circular ssDNA template containing a typical DnaA box hairpin structure (Masai et al., 1990; Kawakami et al., 2005). DnaA tightly binds to this hairpin and the DnaB helicase is loaded onto single-strand binding protein (SSB)-coated ssDNA by direct interaction with the bound DnaA. Then, DnaG primase is loaded by direct interaction with DnaB and pol III holoenzyme carries out replication of the ssDNA template. In the case of wild-type DnaA, the identity of the bound nucleotide is irrelevant to DnaB-loading activity in this system. Domain I and the domain III amino terminus (amino acids 130–148) of DnaA have been reported to contain DnaB-interacting sites (Sutton et al., 1998; Seitz et al., 2000).
As ATP–DnaA in the oriC–DnaA complex interacts with DnaB during the initiation of replication, we assessed the ABC primosome activity of mutant DnaA proteins that were pre-incubated in the presence of ATP at 30°C or 0°C as described earlier. The results of this analysis indicated that both DnaA D269A and R270A exhibited DnaB loading activity (Fig. 6A). The activity of DnaA D269A was comparable to that of wild-type DnaA, and the activity of DnaA R270A was about 30% that of wild-type DnaA. This slight inhibition might be due to indirect conformational changes affecting the DnaB-interacting sites, as Arg-270 is predicted to be spatially distant from the DnaB binding region in the protein structure (Fig. 1A).
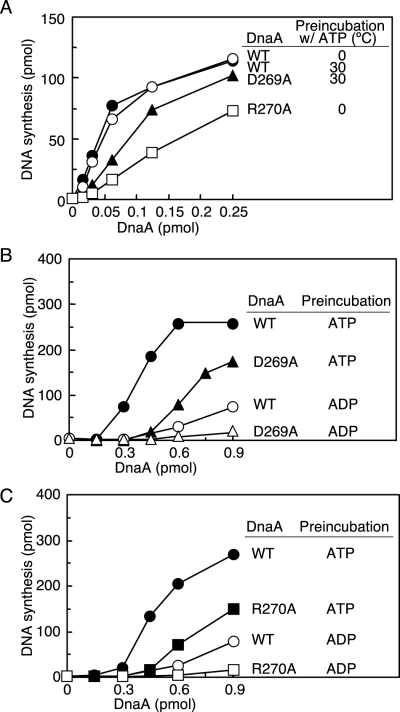
DNA replication activity in in vitro systems reconstituted with purified proteins.A. DnaA activities were assessed with the ABC primosome system. Wild-type DnaA (WT), DnaA D269A (D269A) and DnaA R270A (R270A) were pre-incubated at 0°C or 30°C in buffer containing 3 μM ATP as described in the legend to Fig. 5 (Pre-incubation w/ATP). The indicated amounts of the pre-incubated DnaA samples were further incubated for 10 min at 30°C in reactions containing M13-A site ssDNA, DnaB helicase, DnaC helicase-loader, DnaG primase, SSB and the pol III holoenzyme (see Experimental procedures).B and C. DnaA activities were assessed using a minichromosome replication system reconstituted with purified protein. As described in the legend for Fig. 5, in one experiment wild-type DnaA (WT) and DnaA D269A (D269A) were pre-incubated at 30°C (B) and in another experiment wild-type DnaA (WT) and DnaA R270A (R270A) were pre-incubated at 0°C (C) in buffer containing 3 μM ATP or ADP (Pre-incubation). The indicated amounts of each pre-incubated DnaA sample were further incubated for 30 min at 30°C in the minichromosome replication system (see Experimental procedures). Circles, wild-type DnaA; triangles, DnaA D269A; squares, DnaA R270A.
Minichromosome replication in an in vitro reconstituted system
We next assessed the minichromosome replication activity of the mutant DnaA proteins by using an in vitro system reconstituted with purified proteins (Fig. 6B and C). In this system, initiation is promoted by ATP–DnaA but not ADP–DnaA. DnaA proteins were pre-incubated in the presence of ATP or ADP at 30°C or 0°C as described earlier and added to the replication reaction at 30°C. The results of this experiment indicated that the specific activities of the DnaA D269A and R270A proteins pre-incubated with ATP were 45–50% that of the wild-type DnaA (Fig. 6B and C). Thus, the ATP forms of the mutant DnaA proteins exhibit considerable initiation activity, which correlates well with their duplex opening and DnaB helicase-loading activities (5, 6).
When mutant DnaA proteins were pre-incubated in the presence of ADP, initiation activity was completely repressed (Fig. 6B and C). As seen in the P1 nuclease assay (Fig. 5), DnaA D269A may have been irreversibly inactivated during pre-incubation with ADP at 30°C.
Replication activity of crude protein fractions in a minichromosomal system
The initiation activities of the DnaA D269A and R270A proteins were also assessed in a minichromosomal replication system using a crude replicative protein fraction. The wild-type DnaA and DnaA D269A proteins were pre-incubated in the presence of ATP or ADP at 30°C and then incubated in a replicative extract containing the minichromosome (Fig. 7A). When pre-incubated in the presence of ATP, DnaA D269A showed considerable initiation activity: the specific activity for this protein was about 60% that of wild-type DnaA, consistent with the data obtained with the in vitro reconstituted system (Fig. 6B). When the mutant protein was pre-incubated in the presence of ADP, it exhibited initiation activity, unlike what was seen for wild-type DnaA. Most likely, the 2 mM ATP and chaperone proteins present in the reaction re-activated DnaA D269A by dissociating the bound ADP and supporting protein structure. In this crude fraction, mutant proteins such as DnaA46 and DnaA5 have been reported to be activated for initiation due to interaction with chaperone proteins such as DnaK and GrpE (Hwang and Kaguni, 1991; Hupp and Kaguni, 1993).
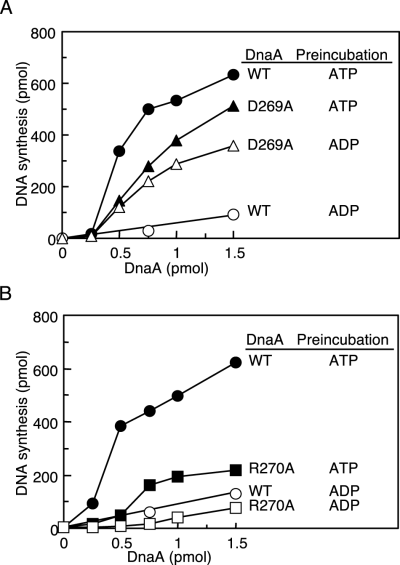
DNA replication activity reconstituted with a crude protein fraction and purified DnaA. As described in the legend for Fig. 5, in one experiment wild-type DnaA (WT) and DnaA D269A (D269A) were pre-incubated at 30°C (A) and in another experiment wild-type DnaA (WT) and DnaA R270A (R270A) were pre-incubated at 0°C (B) in buffer containing 3 μM ATP or ADP (Pre-incubation). The indicated amounts of each pre-incubated DnaA sample were further incubated for 20 min at 30°C in a minichronosome replication system with a crude replicative protein fraction. This fraction was prepared from the strain WM433 (dnaA204) (see Experimental procedures). Symbols used are the same as those in Fig. 6.
ATP–DnaA R270A had an initiation activity that was 25–50% that of wild-type DnaA in this crude system (Fig. 7B), although it exhibited a considerable level of initiation activity in the reconstituted system (Fig. 6C). The interaction of DnaA R270A with chaperone proteins may adversely affect its conformation.
Analysis of regulation for the chromosomal replication in vivo
We analysed using flow cytometry the chromosomal replication in cells bearing wild-type DnaA, DnaA D269A or DnaA R270A (Fig. 8A–C). For this analysis, we introduced a low-copy mini-R vector (pOZ18) bearing the wild-type dnaA and rnhA genes into a strain KA451 (rnhA::cat dnaA::Tn10). In the absence of rnhA gene, the chromosomal replication initiates at alternative origins. Introduction of pOZ18 into KA451 represses the alternative origins and allows initiation at oriC on the chromosome. To deduce the oriC gene dosage in each growing cell, cells were incubated in presence of rifampicin and cephalexin, followed by flow cytometry analysis. The results indicated that KA451 cells bearing pOZ18 predominantly contained four or eight oriCs per cell (Fig. 8A). Under the same conditions, cells of a parental strain KH5402-1 (wild-type dnaA) also contained four or eight oriCs per cell (data not shown). The average cell size of KA451 bearing pOZ18 was only slightly smaller than that of KH5402-1.
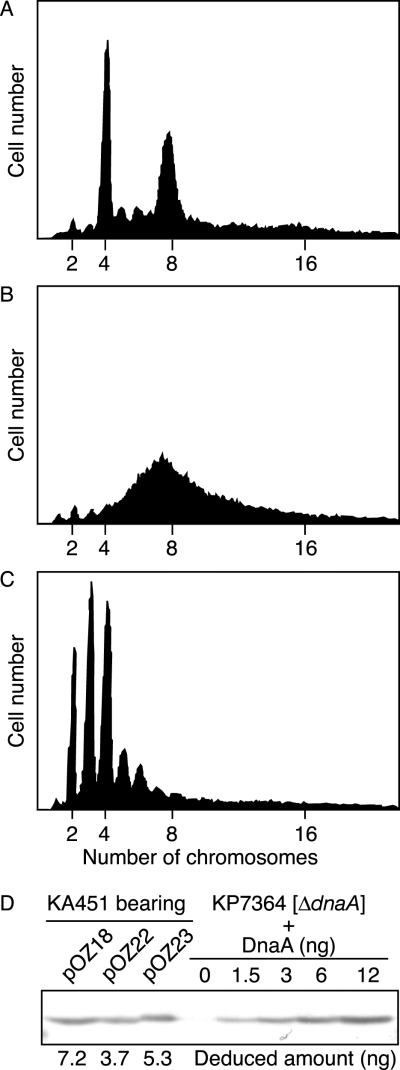
Flow cytometry analysis.A–C. Exponentially growing cells of KA451 (rnhA::cat dnaA::Tn10) bearing mini-R plasmid pOZ18 (wild-type dnaA, rnhA) (A), pOZ22 (dnaA D269A, rnhA) (B), or pOZ23 (dnaA R270A, rnhA) (C) were analysed after incubation in the presence of rifampicin and cephalexin. A total of 30 000 cells are shown in each panel at the same scale.D. The amounts of cellular DnaA were determined by immunoblot analysis. KA451 cells bearing mini-R plasmid pOZ18, pOZ22, or pOZ23 were grown under the same conditions as cells used above. A portion (0.3 ml) of each culture was used for SDS-PAGE, followed by immunoblotting. KP7364 (ΔdnaA) was used as a background control, and the indicated amounts of purified DnaA were mixed with whole cell extract of this strain for a quantitative standard. Deduced amounts of DnaA in the culture analysed are indicated.
We next analysed KA451 cells bearing a pOZ18-derivative pOZ22, which carried the dnaA D269A allele instead of the wild-type allele. Transformation efficiency of pOZ22 into KA451 was similar to that of pOZ18 (data not shown). The results of flow cytometry analysis indicated that KA451 cells bearing pOZ22 have the elevated DNA contents (Fig. 8B). In the comparison of the data of Fig. 8A, the relative number of cells containing four oriCs per cell was significantly decreased and that of cells containing eight or more oriCs was increased although discrete peaks were not formed. These results suggest that the chromosomal replication over-initiated in the cells and the migration rates of ongoing replisomes were retarded. Hda-deficient cells as well as cells that have DnaA molecules in excess also show over-initiation and retardation of replisome migration simultaneously (Atlung et al., 1987; Kato and Katayama, 2001; Morigen et al., 2003; Simmons et al., 2004). The average cell size of KA451 bearing pOZ22 was only slightly smaller than that of KA451 bearing pOZ18 (data not shown), which indicates that cell division is not inhibited.
Immunoblot analysis indicated that the amount of DnaA protein present in KA451 cells bearing pOZ22 was not increased compared with that in KA451 cells bearing pOZ18 (Fig. 8D). Average DnaA content in a single cell was deduced to be ∼3500 molecules for KA451 bearing pOZ18 and 1500–2000 molecules for KA451 bearing pOZ22. Thus, over-initiation in KA451 cells bearing pOZ22 is not attributed to oversupply of the mutant DnaA. These results support the idea that DnaA Asp-269 is required for the regulation for DnaA activity in vivo. Also, these data are consistent with those obtained in in vitro replication system using a crude protein fraction (Fig. 7A).
Similar analyses were also performed for KA451 cells bearing pOZ23, a pOZ18-derivative bearing the dnaA R270A. Transformation efficiency of pOZ23 into KA451 was similar to that of pOZ18 (data not shown). The results of flow cytometry indicated that most cells contain only two, three or four oriCs per cell (Fig. 8C). The average cell size of KA451 bearing pOZ23 was only slightly smaller than that of KA451 bearing pOZ18 (data not shown), which indicates that cell division is not inhibited. The amount of DnaA protein present in KA451 cells bearing pOZ23 was slightly smaller than that in KA451 cells bearing pOZ18 (Fig. 8D). Average DnaA content in a single cell was deduced to be 2000–2500 molecules for KA451 bearing pOZ23. These results are consistent with those of in vitro crude replication system.
ATP binding of DnaA D269N and D269T proteins
Many AAA+ proteins have Asn or Thr residue at the sensor 1 position (Neuwald et al., 1999; Ogura and Wilkinson, 2001). We asked whether these residues can replace DnaA Asp-269 for the optimum ATP binding. We purified DnaA D269N and D269T proteins by the method used for the other DnaA proteins. SDS-PAGE analysis indicated that these protein samples were > 90% pure, similar to the other DnaA samples (Fig. 9A). Activities for DnaA box binding were assessed using a gel shift assay. The results indicated that these mutant DnaA proteins sustained the DnaA box-specific binding activity at a level similar to wild-type DnaA (Fig. 9B). In contrast, when activities for ATP binding were assessed using the filter retention assay, these mutant DnaA proteins have impaired activities compared with wild-type DnaA (Fig. 9C). The binding stoichiometry of these mutant DnaA proteins decreased by ∼50% compared with that of wild-type DnaA, consistent with the idea that the sensor 1 Asp residue is prerequisite to optimize the tight and stable ATP binding of DnaA. Specific activity of DnaA D269N and D269T proteins in RIDA-dependent ATP hydrolysis in vitro was ∼50% that of wild-type DnaA (data not shown).

Affinities of DnaA D269 T/N mutant proteins for DnaA box and ATP.A. Wild-type and mutant DnaA proteins were overproduced in KA450 [ΔoriC dnaA(Am)] and purified as described in Experimental procedures. Purified DnaA D269N and DnaA D269T proteins (0.5 μg each) were analysed by SDS-10% PAGE and Coomassie brilliant blue R-250 staining. The positions of molecular weight markers are shown.B. DnaA box binding activity was determined using gel-mobility retardation analysis. The indicated amounts of DnaA were incubated for 20 min on ice in buffer containing 15 bp DNA (2 pmol) with (R1, open symbols) or without (N, filled symbols) DnaA box R1. The samples were analysed by 8% PAGE at room temperature and Gel-star (Cambrex) staining. The protein-free DNA (Free DNA) was quantified by densitometric scanning and the relative amounts are shown. Circles, wild-type DnaA (WT); triangles, DnaA D269T; squares, DnaA D269N.C. ATP binding activity was determined as described for Fig. 3A. DnaA (1.9 pmol) was incubated for 15 min on ice in buffer containing various concentrations of [α-32P]-ATP. Circles, wild-type DnaA (WT); triangles, DnaA D269T; squares, DnaA D269N; diamonds, no protein (None).
Discussion
Wild-type DnaA stably binds ATP/ADP with a KD of about 30–100 nM at 0°C (Sekimizu et al., 1987). This high binding affinity is a prerequisite for control of initiator activity as well as for transcriptional regulation of the dnaA and nrdAB operons (Speck et al., 1999; Messer, 2002; Gon et al., 2006). In the present study, we show that the DnaA Asp-269 residue, located within a unique AAA+ sensor 1 sequence, plays a specific role in supporting high-affinity ATP/ADP binding. The affinity of DnaA D269A for adenine nucleotides is notably reduced compared with that of the wild-type protein (Fig. 3). In contrast, the specific affinity of this mutant DnaA for a typical DnaA box and its oriC duplex unwinding activity in the presence of high concentrations of ATP are comparable to those of the wild-type protein (2, 5, Table 1). Also, DnaA D269A retains high activities for DnaB loading and minichromosomal replication in a reconstituted system, with only slight reductions relative to the wild-type protein (Fig. 6). These slight changes are most likely consequences of indirect conformational changes in the DnaA AAA+ domain. Although the role of the sensor 1 motif has been analysed for several AAA+ proteins, the present study provides the first evidence that the AAA+ sensor 1 motif has a specific function in tight ATP/ADP binding.
The sensor 1 motif of various AAA+ proteins contains a highly conserved hydrophilic residue, usually Asn or Thr (Neuwald et al., 1999; Ogura and Wilkinson, 2001; Schepers and Diffley, 2001; Hanson and Whiteheart, 2005) (Fig. 1B). These residues are thought to be close to the γ-phosphate moiety of ATP in the protein structure and to participate in ATP binding or to promote ATP hydrolysis. Indeed, previous studies show that mutation of the conserved Asn/Thr residue in AAA+ proteins such as S. cerevisiae Hsp104 (Thr-317 and Asn-728) and T. acidophilum VAT (Asn-334 and Asn-612) reduces their ATPase activity (Hattendorf and Lindquist, 2002; Gerega et al., 2005). In contrast, these mutations in Hsp104 and VAT do not significantly affect their affinity for adenine nucleotides (Hattendorf and Lindquist, 2002; Gerega et al., 2005). In the case of DnaA, the corresponding residue is not neutral Asn/Thr but acidic Asp (amino acid residue 269) (Neuwald et al., 1999). Asp at this position is highly conserved among DnaA homologues in various eubacterial species (Fig. 1B and C). Also, DnaA Asp-269 is essential for supporting tight ATP/ADP-binding as mentioned earlier. These observations imply diversification in the roles of the sensor 1 motif and in the corresponding identity of the amino acid residue near the phosphate moiety of the nucleotide. The ATPase activity of FtsH, Hsp104 and VAT is important for their protease, chaperone and unfoldase activities respectively (Karata et al., 1999; Hattendorf and Lindquist, 2002; Gerega et al., 2005). The intrinsic ATPase activity of DnaA is very weak and it is unnecessary for initiation at oriC (Messer, 2002; Nishida et al., 2002), whereas the tight binding of ATP/ADP to DnaA is indispensable for the regulation of initiation during the cell cycle.
DnaA D269T and D269N are not optimized for the tight ATP binding (Fig. 9), suggesting a unique interaction mode between Asp-269 and ATP. The sensor 1 Asn/Thr residues of typical AAA+ proteins are speculated to interact with the phosphate moiety of ATP/ADP via a hydrogen bond network and a polar contact (Neuwald et al., 1999; Ogura and Wilkinson, 2001). As Asp is acidic, DnaA Asp-269 might form a unique electrostatic interaction in addition to a hydrogen bond network to assist the tight ATP/ADP binding.
The DnaA Asp-269 residue is extremely highly conserved among eubacterial DnaA orthologues (Fig. 1B and C). This information supports the crucial role for the sensor 1 Asp residue in DnaA function. A special case seen in Chlamydiae should be noted in that two DnaA orthologues are encoded on the genome (for example, open reading frame No. CTA0272 and CTA0297 in Chlamydia trachomatis A/HAR-13) and only one of the two preserves Asp at the position equivalent to E. coli DnaA Asp-269 (C. Weigel, pers. comm.). These two DnaAs might have different affinities for ATP/ADP. As DnaA is a multifunctional protein that plays roles in oriC duplex unwinding, DnaB loading, transcriptional regulation and so on, we speculate a possibility that the DnaAs in Chlamydiae are functionally differenciated to specialize their roles. Even in initiation complex at oriC, roles for DnaA molecules are specialized in DNA binding modes (Speck and Messer, 2001; McGarry et al., 2004; Kawakami et al., 2005).
DnaA Arg-270, like Asp-269, is highly conserved among DnaA orthologues (Fig. 1B and C). Unlike the DnaA D269A protein, DnaA R270A exhibits considerable affinity for ATP/ADP compared with the wild-type protein (Fig. 3), indicating that the Arg-270 and Asp-269 residues have different roles. Whereas DnaA R270A retains DNA binding and ATPase activities (Figs 2 and S1), its oriC duplex unwinding, DnaB loading and minichromosomal replication activities are slightly impaired (5, 6). This amino acid substitution might induce modest intrinsic conformational change of domain III, leading to these slight defects.
The role of the sensor 1 motif of most AAA+ proteins remains to be experimentally elucidated. The residue corresponding to E. coli DnaA Asp-269 is highly conserved among eubacterial DnaA homologues (Fig. 1B and C). The Thermotoga maritima and Staphylococcus aureus DnaA proteins tightly bind ATP/ADP (Ichihashi et al., 2003; Ozaki et al., 2006), and T. maritima DnaA unwinds the predicted oriC duplex in an ATP-dependent fashion (Ozaki et al., 2006). As these eubacterial species are phylogenically distant from E. coli, the mechanical and structural basis of exceptionally high affinity ATP/ADP binding may be highly conserved among DnaA homologues. The DnaA-type acidic residue is also conserved in the sensor 1 motif of Hda (Fig. 1B). This protein promotes DnaA–ATP hydrolysis in a manner mediated by the DNA–clamp complex (Kato and Katayama, 2001; Su'etsugu et al., 2004; 2005; Kawakami et al., 2006). The role of the Hda sensor 1 motif is under investigation. Also, S. cerevisiae Rfc5 and Rad24 have a similar acidic residue in the sensor 1 motif (Neuwald et al., 1999) (Fig. 1B). Rfc5 is a subunit of the proliferating cell nuclear antigen clamp-loader complex, which consists of Rfc1–5 subunits (Johnson and O'Donnell, 2005). Rad24 and the Rfc2–5 subunits form the checkpoint clamp (Rad17/Mec3/Ddc1) loader complex. Although the Walker A-ATP binding motifs of Rfc5 and Rad24 do not completely match the consensus sequence GK[T/S] (GKK for Rfc5 and SKS for Rad24) (Neuwald et al., 1999), Rfc5 binds nucleotides in the crystal structure, and the Rad24 Walker A motif is essential for ATPase activity and the clamp-loading activity of the Rad24/Rfc2–5 complex (Bowman et al., 2004; Majka et al., 2004). As seen for DnaA, the acidic residues in the sensor 1 motif of these proteins may contribute to a nucleotide-binding mechanism. In contrast, a eukaryotic DnaA homologue, S. cerevisiae ORC, contains subunits of AAA+ proteins that have a typical Asn or Thr residue in the sensor 1 motif (Fig. 1B). Unlike what has been shown for DnaA, hydrolysis of ATP bound to Orc1 (a main subunit of ORC) is essential for the formation of the ORC–Cdc6–DNA pre-initiation complex (Speck et al., 2005). The mechanical basis of the high-affinity binding of ATP by ORC remains to be elucidated. ATP binding and hydrolysis are important events for the regulation of protein structure, complex formation and functional changes. The findings reported here will provide new leads in revealing the ATP-driving mechanisms of many AAA+ proteins.
Experimental procedures
Buffers
Buffer D contained 50 mM HEPES-KOH (pH 7.6 at 1 M), 0.1 mM EDTA, 10 mM magnesium acetate, 20% sucrose, 0.2 M ammonium sulphate and 2 mM dithiothreitol. Buffer N contained 50 mM HEPES-KOH (pH 7.6 at 1 M), 2.5 mM magnesium acetate, 0.3 mM EDTA, 7 mM dithiothreitol, 20% (v/v) glycerol and 0.007% Triton X-100. HKM150′ buffer contained 25 mM HEPES-KOH (pH 7.6 at 1 M), 150 mM potassium acetate, 1 mM magnesium acetate, 0.005% Surfactant P20 (Biacore), 0.025 μg ml−1 poly(dA-dT)-poly(dA-dT), 0.025 μg ml−1 poly(dI-dC)-poly(dI-dC) and 100 μM ATP. Buffer R contained 20 mM Tris-HCl (pH 7.5 at 1 M), 8 mM dithiothreitol, 10% (v/v) glycerol, 0.01% Brij 58, 8 mM magnesium acetate and 0.1 mg ml−1 bovine serum albumin (BSA).
Escherichia coli strains and nucleic acids
Escherichia coli strains KH5402-1 (wild-type), KA450 [ΔoriC1071::Tn10 rnhA199(Am) dnaA17(Am)], KA451 (dnaA::Tn10 rnhA::cat), KP7364 (ΔdnaA::spec rnhA::kan), WM433 [dnaA204(Ts)] (Nishida et al., 2002; Ishida et al., 2004; Kawakami et al., 2005) and BL21(DE3) (Studier and Moffatt, 1986) have been described.
pKA234, pBSoriC, M13KEW101 replicative form (RF) I (supercoiled form) DNA and M13-A site ssDNA were previously described (Kawakami et al., 2005). pSS12-1, pSS13-1, pOA-D269N and pOA-D269T were derivatives of pKA234 (Kawakami et al., 2005) that carried the dnaA D269A, dnaA R270A, dnaA D269N and dnaA D269T alleles, respectively, instead of the wild-type dnaA gene. pOZ18, pOZ22 and pOZ23 were derivatives of a low-copy mini-R vector pKP1673 (Miki et al., 1992) that carried the rnhA gene in addition to wild-type dnaA, dnaA D269A and dnaA R270A alleles respectively.
Construction and purification of mutant DnaA proteins
Base substitutions were introduced into the wild-type dnaA allele carried on pKA234 with a QuikChange site-directed mutagenesis kit according to the manufacturer's instructions (Stratagene). Mutagenic primers used for the construction of pSS12-1 (bearing dnaA D269A) were 5′-CAGATCATTCTCACCTCGGCTCGCTATCCGAAAG-3′ and its complementary strand, those for pSS13-1 (bearing dnaA R270A) were 5′-CTCACCTCGGATGCCTATCCGAAAGAGATCAACGGCG-3′ and its complementary strand, those for pOA-D269N (bearing dnaA D269N) were 5′-CAGATCATTCTCACCTCGAATCGCTATCCGAAAGAG-3′ and its complementary strand, and those for pOA-D269T (bearing dnaA D269T) were 5′-CAGATCATTCTCACCTCGACTCGCTATCCGAAAGAG-3′ and its complementary strand. Mutant DnaA was purified using a method previously described for wild-type and other mutant DnaA proteins (Kawakami et al., 2005).
DNA binding assays
Kinetic measurements by surface plasmon resonance analysis were performed using a single DnaA box R1-containing dsDNA as previously described (Kawakami et al., 2005) except that DnaA proteins were incubated for 5 min at 25°C in HKM150′ buffer and then injected into the Biacore X flowcells (Biacore) filled with buffer consisting of HKM150′ buffer and buffer D at a ratio of 29.5:1. Gel mobility retardation experiments using 15 bp DNA fragments were performed as previously described (Obita et al., 2002; Kawakami et al., 2005; Ozaki et al., 2006) except that the DNA binding reaction (10 μl) contained 1 mM ATP. The DNA fragment contained 9-mer DnaA box R1 or a non-sense box that has no affinity for DnaA (Obita et al., 2002).
Nucleotide binding assay
Assays to determine the nucleotide-binding and intrinsic ATPase activities of DnaA were performed essentially as previously described (Kawakami et al., 2005; Ozaki et al., 2006). Typically, DnaA (1.9 pmol) was incubated for 15 min at 0°C in buffer N (25 μl) containing various concentrations of [α-32P]-ATP or [3H]-ADP, followed by retention on nitrocellulose membranes and liquid scintillation counting. Alternatively, DnaA (1.9 pmol) was incubated for 15 min at 30°C (for wild-type DnaA and DnaA D269A) or 0°C (for the wild-type DnaA and DnaA R270A) in buffer D (25 μl) containing the nucleotide before the filter retention assay.
Assay for the ATP binding-dependent conformational changes of DnaA using trypsinolysis
This assay was performed essentially as previously described (Garner and Crooke, 1996; Carr and Kaguni, 2002). Briefly, DnaA (5 pmol) was incubated for 20 min at 30°C in buffer (20 μl) containing 50 mM Tris-HCl (pH 7.8 at 1 M), 10 mM magnesium chloride, 50 mM NaCl, 2 mM dithiothreitol, 15% (v/v) glycerol and various concentrations of ATP, followed by incubation for 60 min at 16°C in the presence of 6 μg ml−1 trypsin. Reactions were stopped by the addition of 10% trichloroacetic acid and the products were analysed by SDS-15% PAGE and Coomassie brilliant blue R-350 staining.
Open complex formation assay using P1 nuclease
This assay was performed essentially as previously described (Kawakami et al., 2005; Ozaki et al., 2006). DnaA (2 μM) was incubated for 15 min at 30°C or 0°C in buffer containing 3 μM ATP or ADP as described earlier. The indicated amounts of this DnaA sample were further incubated for 3 min at 38°C in pre-warmed buffer (50 μl) containing 60 mM HEPES-KOH (pH 7.6), 0.1 mM zinc acetate, 8 mM magnesium acetate, 30% (v/v) glycerol, 0.32 mg ml−1 BSA, 16 ng HU protein, 5 mM ATP and pBSoriC RF I (400 ng; 170 fmol), followed by incubation for 50 s at the same temperature in the presence of P1 nuclease (10 units, Yamasa). DNA was purified and a portion (one-tenth volume) was digested with ScaI, followed by electrophoresis in a 1% agarose gel and ethidium bromide staining.
Reconstituted ABC-primosome system
This assay was performed as previously described (Kawakami et al., 2005) with some modifications. Briefly, DnaA (2 μM) was incubated in buffer containing 3 μM ATP as described earlier. The indicated amounts of this DnaA sample were incubated for 10 min at 30°C in a reaction mixture (25 μl) containing M13-A site ssDNA, DnaB, DnaC, SSB, DnaG, the pol III holoenzyme, 1 mM ATP, 0.25 mM each of GTP, CTP and TTP, and 0.1 mM each of deoxyribonucleoside triphosphates including [α-32P]-TTP. SSB was overproduced in BL21(DE3) cells that carry a derivative of pET-21a(+) (Novagen) bearing the native ssb gene and purified using a previously published method (Kinebuchi et al., 1997), except that DE52 (Whatman) replaced phosphocellulose in column chromatography (K. Mazda, T. Morofuji, M. Su'etsugu and T. Katayama, unpubl. results).
In vitro minichromosome replication system
A minichrosomome replication system was reconstituted with purified proteins as previously described (Higuchi et al., 2003; Kawakami et al., 2005), with minor modifications. Briefly, DnaA (2 μM) was incubated in buffer D containing 3 μM ATP/ADP as described earlier. The indicated amounts of this DnaA sample were incubated for 30 min at 30°C in a reaction mixture (25 μl) containing M13KEW101 RF I DNA (200 ng), DnaB, DnaC, DnaG, HU, gyrase, SSB, the pol III holoenzyme, ribonucleoside triphosphates and deoxyribonucleoside triphosphates including [α-32P]-TTP. The minichromosome replication assay using a soluble crude protein fraction was performed as previously described (Ishida et al., 2004; Kawakami et al., 2005).
Construction of low-copy mini-R plasmid bearing dnaA and rnhA
The chromosomal rnhA gene region containing its promoter was amplified by polymerase chain reaction (PCR) using the primers 5′-GGAAGATCTAGCGGTCATTTATGTCAG-3′ and 5′-CCTCCACAGGCTTAAACTTCAACTTGG-3′. The resultant 0.55 kb PCR product was digested with BglII, and ligated to a 7.5 kb BglII-StuI fragment of mini-R plasmid pKP1673, resulting in pRNHA-1. A 2.4 kb EagI (blunted)-XhoI fragment, which contained the dnaA gene and its intact promoter region, was isolated from pHB9 (Obita et al., 2002), and ligated to a 3.7 kb EcoRI (blunted)-SalI fragment of pBR322, resulting in pDNAA-1. A region containing the dnaA and bla genes was amplified by PCR using pDNAA-1 and the primers 5′-CACGCAATTGTTACCAATGCTTAATCAGTGAGGCACC-3′ and 5′-GAATTCTTACGATGACAATGTTCTGATTAAATTTG-3′. The resultant 3.4 kb PCR product was digested with MfeI, and ligated to a rnhA-bearing 4.4 kb EcoRI-StuI fragment of pRNHA-1, resulting in pOZ16 (7.7 kb). The dnaA gene and its promoter region were verified by sequencing. A 6.4 kb EcoRI fragment of pOZ16 was ligated to a 1.5 kb EcoRI fragment of pKA234, pSS12-1 and pSS13-1 respectively. The resultant plasmids were pOZ18 (wild-type dnaA), pOZ22 (dnaA D269A) and pOZ23 (dnaA R270A).
Flow cytometry analysis
This analysis was performed as previously described (Ishida et al., 2004) with minor modifications. Briefly, cells were grown exponentially for about 10 generations at 37°C in M9 medium containing 25 μg ml−1 thymine, 0.2% casamino acids, 25 μg ml−1 tryptophan, 5 μg ml−1 thiamin, 0.2% glucose and 100 μg ml−1 ampicillin until the optical density (A660) reached 0.2. Incubation was further continued in the presence of rifampicin (150 μg ml−1) and cephalexin (10 μg ml−1) for 4 h at the same temperature. Cells were collected by brief centrifugation and suspended in cold 70% ethanol. After washing the cells in cold buffer containing 10 mM Tris-HCl (pH 7.5) and 20 mM magnesium sulphate, chromosomal DNA was stained in the same buffer containing 2 μM SYTOX green (Invitrogen), followed by analysis using a FACS Calibur flow cytometry (Becton Dickinson).
Acknowledgements
We are grateful to Dr T. Ogura for discussion. This work was supported in part by a research grant from the Naito Foundation and by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. H.K. was a recipient of postdoctoral fellowships from the Japan Society for the Promotion of Science.