l-galactonate dehydratase is part of the fungal path for d-galacturonic acid catabolism
Summary
An l-galactonate dehydratase and the corresponding gene were identified from the mould Hypocrea jecorina (Trichoderma reesei). This novel enzyme converts l-galactonate to l-threo-3-deoxy-hexulosonate (2-keto-3-deoxy-l-galactonate). The enzyme is part of the fungal pathway for d-galacturonic acid catabolism, a pathway which is only partly known. It is the second enzyme of this pathway after the d-galacturonic acid reductase. l-galactonate dehydratase activity is present in H. jecorina cells grown on d-galacturonic acid but absent when other carbon sources are used for growth. A deletion of the l-galactonate dehydratase gene in H. jecorina results in a strain with no growth on d-galacturonic acid. The active enzyme was produced in the heterologous host Saccharomyces cerevisiae and characterized. It exhibited activity with l-galactonate and d-arabonate where the hydroxyl group of the C2 is in l- and the hydroxyl group of the C3 is in d-configuration in the Fischer projection. However, it did not exhibit activity with d-galactonate, d-gluconate, l-gulonate or d-xylonate where the hydroxyl groups of the C2 and C3 are in different configuration.
Introduction
d-galacturonic acid is the major component of pectin and consequently an important carbon source for microorganisms living on decaying plant material. A bacterial catabolic pathway has been described while a eukaryotic pathway has remained unknown. The bacterial pathway consists of five enzymes converting d-galacturonic acid (d-galacturonate) to pyruvate and d-glyceraldehyde-3-phosphate. The intermediate metabolites are d-tagaturonate, d-altronate, d-erythro-3-deoxy-hexulosonate and d-erythro-3-deoxy-hexulosonate-6-phosphate. The enzymes are uronate isomerase, d-tagaturonate reductase, altronate dehydratase, 2-dehydro-3-deoxy-d-gluconate kinase and 2-dehydro-3-deoxy-d-gluconate-6-phosphate aldolase respectively (Ashwell et al., 1960; Cynkin and Ashwell, 1960; Hickman and Ashwell, 1960; Smiley and Ashwell, 1960; Meloche and Wood, 1964). There are no reports about genes which are similar to the genes of the bacterial d-galacturonic acid pathway in the sequenced genomes of eukaryotic microorganisms. There are also no reports describing these enzyme activities present in bacteria in yeasts or moulds. However, a similar pathway must exist in eukaryotic microorganisms as many species of yeast and mould can utilize and grow on d-galacturonic acid. This suggests that there is a eukaryotic pathway for the catabolism of d-galacturonic acid which is different from the bacterial pathway.
We have previously presented that the first step in the fungal pathway is an NADPH-specific d-galacturonic acid reductase generating l-galactonate. This enzyme activity was induced in the filamentous fungus Hypocrea jecorina (Trichoderma reesei) when grown on d-galacturonic acid and the activity was absent when grown on other carbon sources (Kuorelahti et al., 2005). Information about how l-galactonate is further catabolized is lacking.
There are only a few other studies on d-galacturonic acid catabolism in fungal microorganisms. Uitzetter et al. (1986) mutagenized the filamentous fungus Aspergillus nidulans and found that mutants lacking pyruvate dehydrogenase or pyruvate carboxylase activity were unable to grow on d-galacturonic acid, whereas a pyruvate kinase mutant was able to grow on this carbon source. This was interpreted that d-galacturonic acid is converted to pyruvate but not through phosphoenolpyruvate. It was suggested that in A. nidulansd-galacturonic acid is catabolized via a non-phosphorylating pathway through glyceraldehyde and pyruvate (Visser et al., 1988; Hondmann et al., 1991), and that d-glyceraldehyde is an intermediate (Uitzetter et al., 1986). The non-phosphorylating path was supported by a microarray analysis of genes transcribed in Aspergillus niger grown on d-galacturonic acid. Here genes similar to aldoketo reductase, racemase and aldolase were identified as coexpressed genes (Martens-Uzunova et al., 2005). It was further suggested that d-galacturonic acid is metabolized through glycerol because a glycerol kinase mutant had reduced growth ond-galacturonic acid (Witteveen et al., 1990). It was also suggested that an NADP-dependent glycerol dehydrogenase is involved in the pathway because such an enzyme was induced on d-galacturonic acid (Sealy Lewis and Fairhurst, 1992).
In this study we demonstrate that the second enzyme in the fungal pathway for d-galacturonic acid catabolism is an l-galactonate dehydratase, an enzyme which has not been described previously. We postulate a fungal pathway for the d-galacturonic acid catabolism which results in pyruvate and glycerol and has l-glyceraldehyde as an intermediate.
Results
The filamentous fungus H. jecorina was grown on d-galacturonic acid as a carbon source and the mycelia were disintegrated. The resulting mycelia extract was then tested for different enzyme activities with l-galactonate as a substrate. We tested for NAD(P)H linked reductases, NAD(P) linked dehydrogenase or ATP consuming kinase activity; however, we did not observe such activities. Nevertheless we noticed that the incubation of l-galactonate with the extract resulted in the formation of a reducing sugar. This was followed using the 3,5-dinitrosalicylate (DNS) assay for reducing sugars (Bernfeld, 1955). The formation of a reducing sugar without involvement of a redox cofactor suggested the presence of a dehydratase active with l-galactonate. This activity was observed only when the fungus was grown on d-galacturonic acid. It was absent when the fungus was grown on a different carbon source such as d-galactose, d-glucose, d-xylose, d-fructose, lactose or glycerol. We tested the H. jecorina strains Rut C-30 or QM6a.
In order to clone the corresponding gene for the dehydratase we searched the H. jecorina genome for sequences with homology to other dehydratases. We identified five potential open reading frames with some amino acid similarities. We amplified these open reading frames by PCR using H. jecorina cDNA as a template. The PCR products were then ligated to a yeast expression vector and transformed to Saccharomyces cerevisiae. The S. cerevisiae cell extract was then analysed for the activity of forming a reducing sugar from l-galactonate. S. cerevisiae does not have an l-galactonate dehydratase activity but one open reading frame showed this activity when expressed in S. cerevisiae. We called the gene lgd1 for l-galactonate dehydratase. The cDNA sequence of the open reading frame was deposited in GenBank and has the accession number DQ181420. The open reading frame coded for a protein with 450 amino acids and a calculated molecular mass of 50.049 Da. Comparing the cDNA with the genomic DNA revealed one intron in the genome sequence. The intron was between the nucleotides 156 and 157 of the open reading frame and contained 61 nucleotides.
The yeast extract of the strain expressing the lgd1 was used to convert l-galactonate to the reaction product which was then identified by NMR spectroscopy. The 1H and 13C chemical shifts of the product are given in Table 1. From one dimensional 1H spectrum of the reaction mixture (Fig. 1) the product signals were readily visible, and from two dimensional DQFCOSY and (1H, 13C) HSQC experiments (not shown) it was evident, that the product has a proton spin-system CH2-CH-CH-CH2, in which one of the CH2 functions has typical chemical shifts of a hydroxymethyl group and the second one has quite unique proton chemical shifts typical to a CH2 group close to a keto group or a hemiketal. The DEPT spectrum (Fig. 2) further confirmed that the molecule has two CH2 and two CH type carbon atoms. In addition to these four carbons, the 13C spectrum (Fig. 2) of the product revealed two additional carbon signals. One is on the carboxyl area close to the signal of the carboxyl carbon of the substrate, l-galactonate, and the other one (97.84 ppm) is typical for a quaternary carbon in a hemiketal structure, like C2 signals in sialic acids. Thus NMR results show that the reaction product is l-threo-3-deoxy-hexulonate and that it exists predominantly as a pyranose ring (Fig. 3). The signals of one anomer dominate the spectrum of the product (over 85%), however, it was not possible to determine, which one of two anomers it is.
| δ (ppm)a | δ (ppm)b | ||
|---|---|---|---|
| H3 | 1.789 | C1 | 177.53 |
| H3′ | 2.162 | C2 | 97.84 |
| H4 | 3.859 | C3 | 40.22 |
| H5 | 3.604 | C4 | 70.13 |
| H6 | 3.606 | C5 | 71.92 |
| H6′ | 3.801 | C6 | 64.18 |
- a. Referenced to internal TSP (0 ppm).
- b. Referenced to external acetone (31.5 ppm).
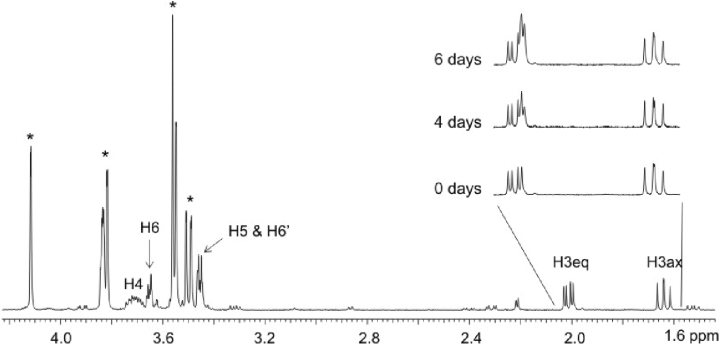
500 MHz 1H NMR spectrum of the reaction mixture. The substrate signals are indicated by asterisks. For numbering of the product hydrogens, see Fig. 3. The insert shows the signals of the two protons at position 3 at different time points of incubation of the reaction mixture in D2O. The signal from the axial proton remained unchanged, while a novel equatorial signal without the geminal coupling appeared on top of the original equatorial signal. This indicates that the enzyme retained activity in the NMR tube and that the hydrogen atom attached to the substrate in the reaction is attached to an axial position.
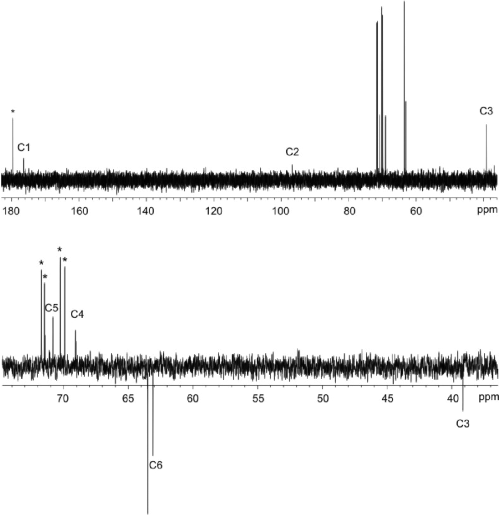
13C (upper) and DEPT135 (lower) NMR spectra of the reaction mixture. The substrate signals are marked with an asterisk and the product signals are numbered according to Fig. 3. The DEPT spectrum indicates that the product has two CH2 groups and the position of the signal of C2 in the upper spectrum is characteristic for a hemiketal structure indicating that the molecule exists predominately in ring form.
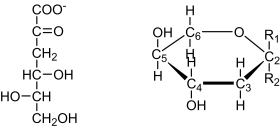
l-threo-3-deoxy-hexulosonate in Fischer-projection (left). The NMR analysis revealed that it predominantly exists in the pyranose form, as shown in Haworth-projection (right). For the pyranose form two anomers are possible with R1 as carboxyl group and R2 the hydroxyl group or vice versa. One anomer was predominant, however, we could not identify which of the two it was.
The enzyme retained activity still in the NMR tube and more product was formed when the sample was incubated in room temperature over several days (Fig. 1). As the reaction took place in deuterium environment (D2O), the hydrogen attached to the molecule was a deuterium atom. We observed that the axial H3 signal did not increase with the product formation, while the equatorial H3 signal from the newly formed product had lost its geminal coupling and experienced a small upfield isotope shift of 0.02 ppm. This indicates that the equatorial hydrogen at carbon 3 must be the one that was bound to the carbon originally and the axial hydrogen at carbon 3 originates from the D2O.
The reducing sugar was then quantified with the thiobarbituric acid assay as described by Buchanan et al. (1999). The activity in the extract of S. cerevisiae expressing the lgd1 was 0.15 nkat mg−1 at an l-galactonate concentration of 7.5 mM. This was similar to the activity in the H. jecorina extract when grown on d-galacturonic acid. There the activity was 0.3 nkat mg−1 using the same l-galactonate concentration.
The LGD1 protein was tagged with a histidine tag and produced in S. cerevisiae. In one construct the C-terminus of the protein was tagged, in another the N-terminal end of the protein. The C-terminal tagging resulted in an inactive protein while the N-terminal tagging resulted in a protein with reduced activity. The activity found in the crude extract of the N-terminally tagged protein was about five times lower than the non-tagged protein.
As the histidine tagged LGD1 protein was either inactive or had reduced activity we used the crude cell extract of the yeast strain expressing the lgd1 to analyse the kinetics of the l-galactonate dehydratase. To study the specificity of the enzyme we used different sugar acids and tested the activity of the enzyme to convert the sugar acid to a reducing sugar using the DNS assay. Activity was observed with the sugar acids l-galactonate and d-arabonate. The activity with d-arabonate was about 50% of the activity with l-galactonate under the conditions as specified in the Experimental procedures. Activity was only observed in the extract of the strain expressing the lgd1. No activity was observed in the control strain. When d-galactonate, d-gluconate, d-xylonate or l-gulonate was used as substrates no activity was observed in the strain expressing the lgd1 or in the control strain.
For the substrate l-galactonate we estimated a Km value of about 5 mM. This was measured at pH 7.0 with yeast extract containing the LGD1 enzyme at a final concentration of 1 g l−1 of extracted protein and following the production of l-threo-3-deoxy-hexulosonate using the thiobarbituric acid assay as described by Buchanan et al. (1999) or measuring the production of a reducing sugar using the DNS assay. The control strain with the empty plasmid showed no activity. The same Km for l-galactonate was found when a H. jecorina extract was analysed. Using the yeast extract, an incubation of 24 h led to a complete conversion when the initial l-galactonate was 20 mM or lower, i.e. the l-threo-3-deoxy-hexulosonate concentration was equal to the initial l-galactonate concentration (data not shown). At l-galactonate concentrations above 20 mM the thiobarbituric acid assay gave values for the product formation which were lower that with the DNS assay. This was interpreted as an interference of the l-galactonic acid with the thiobarbituric acid assay.
The enzyme has an essential requirement for bivalent cations. In the presence of 5 mM EDTA we observed a complete loss of activity.
The transcription of the lgd1 gene on different carbon sources was examined by a Northern blot analysis. The carbon sources d-galacturonate, d-glucose, glycerol, lactose and d-xylose were tested. The gene was transcribed on all these carbon sources. The transcription levels of the lgd1 gene were normalized with the transcription levels of actin. The ratio of these two transcription levels, l-galactonic acid dehydratase over actin was the same on d-galacturonic acid, glycerol and lactose. It was three times higher on d-glucose and d-xylose.
To check if the lgd1 gene is essential for the d-galacturonic acid metabolism we deleted the gene in a Rut C-30 strain of H. jecorina. For that purpose we constructed a deletion cassette which contained the hygromycin B phosphotransferase gene (hph), which was transformed and selected for hygromycin B resistance. A PCR of these strains showed that only 10% had the deletion cassette in the intended locus. These strains were further analysed by Southern hybridization to verify that only one copy of the deletion cassette had integrated into the correct position. In the Southern hybridizations the lgd1 and the hph were probed. The lgd1 gene was only seen in the control strain while the hph gene which replaced the lgd1 gene was only in the deletion strain (not shown). The deletion strain (VTT D-05369) had the same growth rate on lactose as the host strain Rut C-30 on plate or in liquid culture but only residual growth on d-galacturonic acid while the host strain grew normal. When the lgd1 gene was transformed back to the deletion strain the growth on d-galacturonic acid was restored. The growth rate of all the strains including the wild-type strain QM6a was compared in liquid medium containing 20 g l−1d-galacturonic acid and 0.5 g l−1 proteose peptone or only 0.5 g l−1 proteose peptone as a carbon source. The residual growth of the deletion strain is due to growth on proteose peptone because all the strains produced the same amount of biomass also on proteose peptone only (Table 2).
| H. jecorina strain | Lactose + peptone dry mass (g l−1) | d-galacturonic acid + peptone dry mass (g l−1) | Peptone dry mass (g l−1) |
|---|---|---|---|
| QM6a | 0.7 ± 0.1 | 3.4 ± 1.3 | 0.1 ± 0.0 |
| RutC30 | 4.2 ± 0.1 | 2.4 ± 0.7 | 0.1 ± 0.0 |
| RutC30-Δlgd1 A | 3.3 ± 1.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| RutC30-Δlgd1 B | 3.9 ± 0.8 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| RutC30-Δlgd1-lgd1 AA | 4.2 ± 0.1 | 2.5 ± 0.6 | 0.1 ± 0.0 |
| RutC30-Δlgd1-lgd1 AB | 4.1 ± 0.7 | 3.2 ± 0.5 | 0.1 ± 0.0 |
- Dry mass (g l−1) after 5 days of cultivation on 0.05% peptone and 2% of another carbon source if indicated. Values are averages of triplicates. Two parallel deletion strains were studied (A and B) and also two parallel retransformed strains (AA and AB) that were constructed by transforming lgd1 into the deletion strain A.
Discussion
In this manuscript we describe the identification of a dehydratase that is active on the sugar acids l-galactonate and d-arabonate. These two sugar acids have in common that the hydroxyl groups of the C2 and the C3 in the Fischer projection are in l- and d-configuration respectively. Other sugar acids with such a configuration were not tested because they were not commercially available. The dehydratase was neither active with sugar acids where the hydroxyl groups of C2 were in d- and C3 in l-configuration as in d-galactonate, d-gluconate and d-xylonate, nor with a sugar acid with the hydroxyl groups of C2 and C3 in d-configuration as in l-gulonate.
Dehydratases and their corresponding genes, active on sugar acids with the hydroxyl groups C2 in D- and C3 in l-configuration have been described previously. A dehydratase active with d-xylonate and l-arabonate was described by Niu et al. (2003) and a dehydratase active with d-gluconate in the non-phosphorylated Entner–Doudoroff pathway was described in Sulfolobus solfataricus by Buchanan et al. (1999).
A dehydratase which is similar to the enzyme described in this manuscript is the d-altronate dehydratase E.C. 4.2.1.7 (Smiley and Ashwell, 1960). d-altronate has the hydroxyl groups of C2 and C3 in l- and d-configuration like l-galactonate. The enzyme is part of the bacterial d-galacturonic acid pathway and the purified d-altronate dehydratase from Escherichia coli has been characterized (Smiley and Ashwell, 1960; Dreyer, 1987). A gene coding for the d-altronate dehydratase had been inferred by electronic annotation (GenBank accession number AAC76126). A clustalw alignment of the l-galactonate dehydratase with d-altronate dehydratase revealed a 13% identity in the amino acid sequences. The low degree of homology suggests that l-galactonate dehydratase and d-altronate dehydratase belong to different protein families.
The homology of l-galactonate dehydratase with annotated enzymes was highest with the d-galactonate dehydratase (E.C. 4.2.1.6) of E. coli and the mandelate racemase (E.C. 5.1.2.2) of Pseudomonas putida (Ransom et al., 1988). Their Swiss-Protein accession codes are Q6BF17 and P11444 respectively. Both these enzymes have only 20% identity in amino acid sequences with l-galactonate dehydratase in a clustalw alignment. Nevertheless, the conserved residues such as the active site and the metal binding residues of mandelate racemase subgroup of enolase superfamily suggested by Babbitt et al. (1996) are present in the sequence of l-galactonate dehydratase. These conserved residues were not found in the d-altronate dehydratase of E. coli. The reaction of each enzyme in the enolase superfamily is initiated by an abstraction of the α-proton of a carboxylic acid to form an enolic intermediate.
The reaction product of the l-galactonate dehydratase was identified as a reducing sugar. Reducing sugars are commonly measured with the DNS assay. In order to define the reaction product it was analysed by NMR. To generate a sufficient amount of reaction product l-galactonate was incubated in the yeast extract of the strain expressing the l-galactonate dehydratase gene. In this extract the reaction product did not react further, which facilitated the NMR analysis. In the H. jecorina mycelia extract the reaction product was degraded, making the NMR analysis more difficult. The NMR analysis showed that erythro- or threo-3-deoxy-hexulosonate was formed. Knowing the substrate of the dehydratase reaction we concluded that it was l-threo-3-deoxy-hexulosonate. The NMR analysis also revealed that it is predominantly in the pyranose form (Fig. 3). For the pyranose form two anomers are possible; the carboxyl group in R1 and the hydroxyl group in R2 or vice versa (Fig. 3). The NMR suggested that one anomer is predominant but it did not allow determining which of the two anomers it is. The NMR analysis revealed also that the axial hydrogen at the carbon 3 is the hydrogen that was added during the reaction. However, as there are two possible chair conformations of the pyranose ring it remains unclear which of the two protons is in axial position.
Knowing that the reaction product is l-threo-3-deoxy-hexulosonate enabled us to measure its concentration. An assay where the 2-keto-3-deoxy sugar acid gives a colour reaction with thiobarbituric acid allowed the quantification (Buchanan et al., 1999). The H. jecorina extract produced l-threo-3-deoxy-hexulosonate with a rate of 0.3 nkat mg−1 of extracted protein; in the extract of yeast expressing the lgd1 the rate was 0.15 nkat mg−1. The enzyme activities of the same order of magnitude indicate that the heterologous expression in yeast results in an active enzyme. The accumulation of the reaction product in the H. jecorina extract indicated that at least in the extract it is metabolized only slowly. In the yeast extract l-galactonate was completely converted to l-threo-3-deoxy-hexulosonate demonstrating that the energetic equilibrium is on the side of the reaction product. The irreversibility of the d-galactonate dehydratase reaction was described by Donald et al. (1979). The reaction results in an enolic intermediate that undergoes a spontaneous ketonization. The keto tautomer of the product is greatly favoured relative to the enol tautomer. It was also shown that the d-galactonate dehydratase is unable to enolize the product 2-keto-3-deoxy-D-galactonate.
The l-galactonate dehydratase is part of a eukaryotic pathway for the catabolism of d-galacturonic acid. The first enzyme in this pathway is the d-galacturonic acid reductase converting d-galacturonic acid to l-galactonate (Kuorelahti et al., 2005), and the l-galactonate dehydratase described in this manuscript the second. There are several observations supporting this assumption. First of all the LGD1 protein was active with l-galactonate. Second, the activity was observed in H. jecorina extract only when mycelia were grown on d-galacturonic acid. The activity was not observed when mycelia were grown on other carbon sources. This is already an indication that the l-galactonate dehydratase is related to the d-galacturonic acid catabolic pathway. Another indication is that we did not find any other enzyme activities with l-galactonate such as reductase, dehydrogenase or kinase activities; however, this is not a proof that these activities do not exist. A deletion of the lgd1 gene in H. jecorina gives evidence that the l-galactonate dehydratase is part of the d-galacturonic acid pathway because in the resulting strain growth on d-galacturonic acid is abolished (Table 2).
In the eukaryotic pathway for d-galacturonic acid catabolism the product of this second enzyme, l-threo-3-deoxy-hexulosonate, has to be further catabolized. This part of the pathway is still unknown. A hypothetical pathway is shown in Fig. 4. In this hypothetical pathway l-threo-3-deoxy-hexulosonate is converted to pyruvate and l-glyceraldehyde by the action of an aldolase. l-glyceraldehyde is, unlike pyruvate, a metabolite which is not part of any known metabolic pathway. We hypothesize that l-glyceraldehyde is converted to glycerol. An NADP-dependent glycerol dehydrogenase is induced in filamentous fungi grown on d-galacturonate (Sealy Lewis and Fairhurst, 1992). Such an enzyme might convert l-glyceraldehyde to glycerol. Glycerol is then metabolized through glycerol-3-phosphate and dihydroxyacetone phosphate as described for the mould A. nidulans (Hondmann et al., 1991).
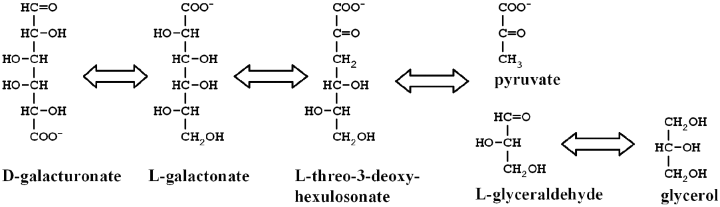
Hypothetical pathway for the catabolism of d-galacturonic acid in eukaryotic microorganisms. The metabolites are represented in Fischer-projection. In this projection the C1 of the d-galacturonic acid is the reduced C6 of the l-galactonate. The first two enzymes of this pathway, the d-galacturonic acid reductase and the l-galactonate dehydratase have been identified. The l-galactonate dehydratase is the subject of this communication. The remaining two enzymes, the l-threo-3-deoxy-hexulosonate aldolase and l-glyceraldehyde reductase are hypothetical.
The catabolic path for d-galacturonic acid in fungi is different from the corresponding pathway in bacteria. For E. coli a pathway was described consisting of five enzymes converting d-galacturonic acid to pyruvic acid and d-glyceraldehyde-3-phosphate (Ashwell et al., 1960; Cynkin and Ashwell, 1960; Hickman and Ashwell, 1960; Smiley and Ashwell, 1960; Meloche and Wood, 1964). The enzymes of this pathway are uronate isomerase, NADH-utilizing d-tagaturonate reductase, altronate dehydratase, d-erythro-3-deoxy-d-hexulosonate kinase and d-erythro-3-deoxy-d-hexulosonate-6-phosphate aldolase. We suggest that in the fungal path only four enzymes are required, NADPH-utilizing d-galacturonate reductase, l-galactonate dehydratase, l-threo-3-deoxy-hexulosonate aldolase and a glycerol dehydrogenase that can convert l-glyceraldehyde in the reverse reaction (Fig. 4). The differences between bacteria and fungi are that in fungi NADPH is the required cofactor, no phosphorylated intermediates are involved and no isomerases are used.
That bacteria and fungal microorganisms use different catabolic pathways is not unique for d-galacturonic acid. Also other abundant carbon sources such as d-xylose and l-arabinose are catabolized differently. In bacteria d-xylose is isomerized to d-xylulose before it is phosphorylated to d-xylulose 5-phosphate (Lawlis et al., 1984), while in fungi the conversion from d-xylose to d-xylulose is a two-step process involving two redox reactions (Wang et al., 1980). Also the l-arabinose catabolic pathway is distinctly different in bacteria and fungi (Lee et al., 1986; Richard et al., 2001; 2002). Common in these examples is that the bacterial pathways have isomerase activities which is absent in their fungal counterparts.
When comparing the sequence of the open reading frame with other sequences deposited in GenBank in a blast search, we identified hypothetical proteins with high homology in other filamentous fungi such as Neurospora crassa and A. nidulans. This suggests that l-galactonate dehydratase activity is not a unique feature of H. jecorina, but common in filamentous fungi. We also found hypothetical proteins with high homology (more than 50% identities in the amino acid sequences) from bacteria and higher eukaryotes indicating that the l-galactonate dehydratase might also be present in organisms other than filamentous fungi.
Experimental procedures
Strains, growth conditions and protein extracts
The E. coli strain DH5α was used in all the cloning procedures except the deletion cassette construction where TOP10 Electrocomp Cells (Invitrogen) were used. Bacteria were grown in Luria–Bertani medium with ampicillin at 37°C. The S. cerevisiae strain CEN.PK2-1D (VW-1B) was the host for the heterologous expression. It was grown in synthetic medium lacking uracil when required for selection at 30°C. We used the H. jecorina (T. reesei) strains Rut C-30 or QM6a. The strains were grown in a liquid medium containing 20 g l−1d-galacturonic acid/sodium d-galacturonate (pH 7.0) or another carbon source when specified, 0.5 g l−1 proteose peptone, 15 g l−1 KH2PO4, 5 g l−1 (NH4)2SO4, 0.6 g l−1 MgSO4·7H2O, 0.6 g l−1 CaCl2·2H2O and trace elements (Mandels and Weber, 1969) at 28°C. The agar plates for the mould contained also 1 ml l−1 Triton X-100 and 20 g l−1 granulated Difco Agar. To make protein extracts of H. jecorina or S. cerevisiae about 100 μg of fresh mycelia or cells were mixed with 300 μl of glass beads (diameter 0.4 mm) and 400 μl of buffer [5 mM sodium phosphate pH 7.0 and Complete, EDTA-free protease inhibitor (Roche)] and disintegrated in a Mini-Bead Beater (Biospec Products) for three times for 30 s. The mixture was then centrifuged in an Eppendorf microcentrifuge at full speed for 25 min at 4°C and the supernatant used for the analysis. For fungal protein extract supernatant a second centrifugation of 10 min was needed. The protein content of the extract was estimated using the Bio-Rad protein assay and BSA as a standard. To assay the l-galactonate dehydratase activity l-galactonate was mixed with the protein extract and formation of reducing sugars detected using the DNS assay for reducing sugars following a standard protocol (Bernfeld, 1955). l-galactonate was derived from l-galactonic acid-γ-lactone as described previously (Kuorelahti et al., 2005).
Cloning of the l -galactonate dehydratase and Northern blot analysis
The H. jecorina genome at JGI database (http://gsphere.lanl.gov/trire1/trire1.home.html) was screened for genes with homology to d-galactonate dehydratases (E.C. 4.2.1.6). This enzyme catalyses reaction of d-galactonate to 2-dehydro-3-deoxy-D-galactonate and water and has a role in d-galactonate catabolism in bacteria (De Ley and Doudoroff, 1957). PCR primers containing BamHI restriction sites were designed to amplify the open reading frames and PCR was run using a cDNA library as a template (Margolles-Clark et al., 1996). The PCR product was ligated to a pCR2.1-TOPO vector (Invitrogen). From the resulting vector the BamHI fragment was released and ligated to the yeast expression vector p2159. This expression vector was a multicopy yeast expression vector with the constitutive TPI1 promoter. It was derived from the pYX212 plasmid (R and D Systems) by digesting it with EcoRI and XhoI to remove the ATG and HA-tag from the multiple cloning site and introducing a BamHI restriction site to the cloning site by inserting a EcoRI and SalI cut fragment from the pUC19 plasmid (Norrander et al., 1983). The resulting vectors were then transformed to the S. cerevisiae strain CEN.PK2-1D (VW-1B). The resulting S. cerevisiae strains were then disintegrated by vortexing with glass beads and the yeast extract analysed for l-galactonate dehydratase activity by monitoring the production of a reducing sugar. Using the primer 5′-GGATCCACCATGTCTGAAGTCACCAT-3′ in sense and the primer 5′-GGATCCTCAGATCTTCTCTCCGTTCA-3′ in antisense direction resulted in an active l-galactonate dehydratase after expression in S. cerevisiae. The gene was called lgd1. To generate a histidine-tagged l-galactonate dehydratase with six histidines at either the N-terminal or the C-terminal end of the protein, primers containing the coding sequences for the histidines were used. For the N-terminal tag an ATG followed by the histidine coding sequence was introduced before the starting ATG-codon of lgd1 and for the C-terminal tag the histidine coding sequence was introduced before the stop codon.
For the Northern blot analysis the H. jecorina strain QM6a was grown on different carbon sources. The mycelium was collected by filtration, frozen in liquid nitrogen and ground with a pestle and mortar. Total RNA was isolated with the Trizol reagent kit (Invitrogen); 15 μg of RNA was glyoxylated, separated in a 1% agarose gel and transferred to Hybond N filter (Amersham). The Northern blot was performed using standard procedures. As a probe we used a 750 bp PCR fragment form the 5′ end of the open reading frame of the lgd1. To normalize the signals a PCR fragment of the gene for actin (act1) was also used as a probe. The intensities of the signals were measured using the Typhoon 8600 instrument (Amersham).
Quantification of the reaction product and testing the specificity of the l -galactonate dehydratase
For the quantification of the reaction product and the Km measurement different concentrations of l-galactonate (pH 7.0) were mixed with the yeast extract of the strain expressing the lgd1 and incubated at 28°C. The reaction mixture contained 10 mM sodium phosphate buffer pH 7.0 or 10 mM Tris-HCl, pH 7.0. The buffer had no effect on the activity. The pH did not change after the addition of l-galactonate. As a control the extract of the yeast strain with the empty p2159 plasmid was treated in a similar way. The reaction product was identified as a 2-keto-3-deoxy sugar acid in a chemical assay using thiobarbituric acid and quantified as described by Buchanan et al. (1999). In this colorimetric assay the absorbance was read at 549 nm and an absorbance coefficient of 67.8 × 103 M−1 cm−1 was used.
To test the specificity of the enzyme, the yeast extract prepared as described before was mixed at a final concentration of 1 g l−1 of extracted protein with 10 mM sugar acids d-gluconate, d-arabonate, d-xylonate, l-gulonate, d-galactonate and l-galactonate in 10 mM sodium phosphate buffer pH 7.0. After incubating 4 h at 28°C the formation of a reducing sugar was verified with the DNS assay as described above.
NMR analysis of the reaction product
l-galactonate at a concentration of 110 mM was incubated with the yeast extract of the strain expressing the lgd1 as described above. The reaction mixture was then analysed by NMR after different time intervals. The reaction product was identified by comparing with the NMR spectrum of pure l-galactonate.
The NMR experiments were carried out at 23°C on a Varian Inova spectrometer operating on a proton frequency of 500 MHz. The spectral widths of the 1D 1H and 13C spectra were 5000 Hz and 30 675 Hz respectively. In DQFCOSY and TOCSY experiments, the spectral width was 3400 Hz and matrices of 1024 × 128 complex data points were acquired. The spinlock time in the TOCSY was 80 ms. In HSQC the spectral widths in 1H and 13C dimensions were 1654 Hz and 10 000 Hz respectively, and a matrix of 1024 × 256 complex data points was acquired. All 2D data matrices were zero-filled once in F1 and a cosine bell weighting function was applied in both dimensions prior to the Fourier transformation.
Deletion of the l -galactonate dehydratase in H. jecorina
For the deletion of the lgd1 gene in H. jecorina a deletion cassette was constructed. For the deletion cassette 1.5 kb of the genomic DNA sequence from both sides of the l-galactonate dehydratase gene were cloned and ligated to the pBluekan7-1.NotI plasmid (obtained from P. J. Punt, TNO Nutrition and Food Research, the Netherlands). The plasmid contains an expression cassette for hygromycin resistance consisting of the A. nidulans gpdA promoter and trpC terminator and the E. coli hph. The part upstream the lgd1 was cloned using the primers 5′-GAGCTCAAGCTTCCACGCAGTTGCTACTTCTA-3′ and 5′-GAGCTCTGGTTATTTGGCAGAGCGAC-3′ introducing SacI and HindIII restriction sites. The SacI fragment was ligated to the SacI cloning site of the pBluekan7-1.NotI. The part downstream of the lgd1 was cloned with the primers 5′-ACTAGTGGGGCAAAGTTGGACATGAT-3′ and 5′-ACTAGTAAGCTTGCAATACCTGGACCAAGCTA-3′ introducing SpeI and HindIII restriction sites. The SpeI fragment was ligated to the SpeI site of the pBluekan7-1.NotI. In the resulting vector it was checked that the orientation of the two DNA fragments relative to each other was not changed, the gene for hygromycin B resistance was placed in between them and HindIII digestion could release the deletion cassette. The deletion cassette released by HindIII digestion was transformed to the H. jecorina Rut C-30 strain as described previously (Penttiläet al., 1987) and selected for hygromycin B resistance (Mach et al., 1994). Strains where the deletion cassette had integrated into the lgd1 locus were identified by PCR. Primers used were from the gpdA promoter sequence of the deletion cassette and from the genomic DNA sequence 1.6 kb downstream from lgd1 gene. Southern analysis was then used to verify that these transformants contained only one copy of the hph gene and that this copy replaced the coding sequence of the lgd1 gene. The Southern hybridization was performed using standard procedures (Sambrook et al., 1989). DNA was isolated from the Rut C-30 and the strain with the lgd1 deletion and the DNA was digested with the restriction enzymes EcoRI and HindIII. The Hybond N filter was then probed with a 800 bp fragment of the hph gene and a 750 bp fragment of the lgd1. The resulting deletion strain was called VTT D-05369.
The lgd1 gene was transformed back into the H. jecorina lgd1 deletion strain to verify that there had not been any other changes in the genome in addition to the lgd1 deletion. An expression plasmid pAN52-1NotI (obtained from P.J. Punt, TNO Nutrition and Food Research, the Netherlands) was modified by PCR so that NcoI restriction site was removed and restriction sites for BamHI, SacI, SpeI, EcoRV, ClaI and ApaI were included in between the A. nidulans gpdA promoter and trpC terminator. The H. jecorina lgd1 gene was cut from TOPO-vector with BamHI and ligated into the BamHI site of the modified pAN52-1NotI plasmid. The resulting plasmid was cotransformed into the lgd1 deletion strain with a selection plasmid pTOC202 that has an acetamidase enzyme encoding A. nidulans amdS gene as a marker. Transformants were selected for acetamide resistance. The retransformation of the lgd1 gene was verified by PCR using oligos from the lgd1 gene and from the gpdA promoter and was also confirmed by growing the strains on plate with d-galacturonic acid and 10 mM acetamide.
Acknowledgements
This work was supported by the Maj and Tor Nessling Foundation. P.R. is an Academy Research Fellow of the Academy of Finland. We thank Dr Ann Westerholm-Parvinen for helping us with the transformation of H. jecorina cells.




