Cloning, functional expression and primary characterization of Vibrio parahaemolyticus K+/H+ antiporter genes in Escherichia coli
Summary
The regulation of internal Na+ and K+ concentrations is important for bacterial cells, which, in the absence of Na+ extrusion systems, cannot grow in the presence of high external Na+. Likewise, bacteria require K+ uptake systems when the external K+ concentration becomes too low to support growth. At present, we have little knowledge of K+ toxicity and bacterial outward-directed K+ transport systems. We report here that high external concentrations of K+ at alkaline pH are toxic and that bacteria require K+ efflux and/or extrusion systems to avoid excessive K+ accumulation. We have identified the first example of a bacterial K+(specific)/H+ antiporter, Vp-NhaP2, from Vibrio parahaemolyticus. This protein, a member of the cation : proton antiporter-1 (CPA1) family, was able to mediate K+ extrusion from the cell to provide tolerance to high concentrations of external KCl at alkaline pH. We also report the discovery of two V. parahaemolyticus Na+/H+ antiporters, Vp-NhaA and Vp-NhaB, which also exhibit a novel ion specificity toward K+, implying that they work as Na+(K+)/H+ exchangers. Furthermore, under specific conditions, Escherichia coli was able to mediate K+ extrusion against a K+ chemical gradient, indicating that E. coli also possesses an unidentified K+ extrusion system(s).
Introduction
Vibrio alginolyticus and Vibrio parahaemolyticus are members of the genus Vibrio, which are Gram-negative marine bacteria and human pathogens. The electrical potential across the plasma membrane of V. alginolyticus cells has been approximated at −125 mV at pH 7.8 (Nakamura et al., 1984). This high membrane potential supports the functioning of many electrogenic (membrane potential-consuming) ion transporters, including uniporters, symporters and antiporters (Rosen, 1986; Padan et al., 2001). Different types of efflux systems are involved in returning any excess cations to the external milieu in cases where internal cation concentrations are higher than the maximum concentration tolerated by the cell. Two ions, Na+ and Ca2+, are typical examples of this situation; a large number of efflux systems, including Na+/H+ antiporters (Padan et al., 2001), a respiration-dependent Na+ pump (Tokuda and Unemoto, 1982), Na+/K+-ATPase and Ca2+-ATPase (Levenson et al., 1984; Toyoshima et al., 2000), Na+ decarboxylases (Schwartz et al., 1988) and Na+ oxidoreductases (Krebs et al., 1999), have been described in both prokaryotes and eukaryotes. Because the original habitat of Vibrio strains is the sea, they possess efficient mechanisms for regulating the intracellular Na+ concentration, using for example, primary Na+ pumps and Na+/H+ antiporters (Tokuda and Unemoto, 1982; Nakamura et al., 1995).
In contrast to our knowledge of Na+ and Ca2+ efflux mechanisms, research on K+ efflux has lagged (Stumpe et al., 1996). Because K+ is the most abundant cation in cells and, in most natural environments, the external K+ concentration usually is much lower than the internal concentration, the K+ diffusion potential is not very different from the membrane potential. Exceptions to this situation are bacteria that inhabit seawater, which has comparatively high K+ concentrations, or bacterial pathogens, whose intracellular solute concentrations are dominated by the host components. In these cases, an effective K+ efflux system may be required to overcome the problem of excess K+ (Benito et al., 2002). The most representative example of an indispensable K+ efflux system is in mitochondria (Garlid, 1996), for which Mitchell (1961) predicted the existence of a electroneutral K+/H+ antiporter more than 40 years ago.
Recent functional analysis of Na+ transporters revealed that some could transport both Na+ and K+ cations. For example, Na+- and Ca2+-ATPases from a phylogenetic cluster, named IID (or ENA), may serve as a K+ efflux system for fungi, Leishmania and Trypanosoma (Benito et al., 2002). Kha1, a putative K+/H+ antiporter of yeast, is similar in amino acid sequence to the Enterococcus hirae NapA Na+/H+ antiporter (Ramírez et al., 1998). Transporter LeNHX2 from tomato (Venema et al., 2003) and AtCHX17 from Arabidopsis thaliana (Cellier et al., 2004) are also reported to be K+/H+ antiporters. Their amino acid sequences are also homologous to Na+/H+ antiporters.
Several bacterial potassium efflux systems were discovered during last decade. It was reported that the Pha system in Rhizobium meliloti is a novel K+ efflux system with a role in cell growth (Putnoky et al., 1998), but it is not yet clear that the Pha system is a K+/H+ antiporter. Recent data revealed that multidrug-resistance transporter MdfA catalyses Na+ and K+/H+ antiport activity (Lewinson et al., 2004). Also multifunctional Na+, K+, Li+, Rb+/H+ antiporter gene yvgP have been found in Bacillus subtilis (Fujisawa et al., 2005). However, no gene that functions exclusively as a K+/H+ antiporter has yet been cloned from prokaryotes. Physiological experiments have clearly demonstrated that the marine bacterium V. alginolyticus has a K+/H+ antiporter that functions to regulate internal pH (Nakamura et al., 1984), although the coding gene has not yet been identified. However, recent completion of the V. parahaemolyticus genome sequence (Makino et al., 2003) makes this organism an easier object of study compared with V. alginolyticus. V. parahaemolyticus has 12 putative Na+/H+ antiporters. We searched among these putative Na+/H+ antiporter genes for candidate K+/H+ antiporter gene(s). We found that Vp-NhaA and Vp-NhaB from V. parahaemolyticus have not only Na+/H+ but also K+/H+ antiporter activity. Furthermore, we report that Vp-NhaP2 is the first bacterial K+(specific)/H+ antiporter that functions in uphill K+ extrusion. In addition, we observed that E. coli also has a previously unknown uphill K+ extrusion system(s).
Results
Cloning of 12 putative Na + /H + antiporter genes and their expression as recombinant proteins in E. coli
Twelve genes from the V. parahaemolyticus RIMD2210633 genome were isolated by a polymerase chain reaction (PCR) method and sequenced as described in the Experimental procedures (see Table 1). All of the cloned genes belong to a family of putative Na+/H+ antiporters (designated VPA0051, VPA0464, VP0618, VP0632, VP1134, VP1228, VP1782, VP2072, VP2125, VP2718, VP2785 and VP2867). The deduced amino acid sequences of VP0618 (Vp-NhaC4), VP0632 (Vp-NhaC2), VP1134 (Vp-NhaC3), VP1782 (Vp-NhaC5) and VP2125 (Vp-NhaC1) are similar to the NhaC protein product of Bacillus firmus (57%, 60%, 57%, 55% and 71% protein similarity to Bf-NhaC respectively) (Ivey et al., 1991). The predicted proteins of VPA0464 (Vp-NapA2) and VP2785 (Vp-NapA1) are 37% and 49% similar, respectively, to NapA of E. hirae (Eh-NapA) (Waser et al., 1992). The predicted proteins of VP2718 (Vp-NhaP1) and VP2867 (Vp-NhaP2) are 72% and 61% similar, respectively, to Pseudomonas aeruginosa NhaP (Pa-NhaP) (Utsugi et al., 1998). The protein products of VP1228 (Vp-NhaA) and VP2072 (Vp-NhaB) of V. parahaemolyticus correspond to NhaA (Taglicht et al., 1991) and NhaB (Pinner et al., 1992) protein products of E. coli respectively. The deduced amino acid sequence of VPA0051 (Vp-NhaD) is highly similar to NhaD of V. parahaemolyticus AQ3334 (Nozaki et al., 1998). Similarity was defined with the help of program blas two sequences (http://www.ncbi.nlm.nih.gov/blast). Table 1 presents detailed descriptions of the 12 cloned V. parahaemolyticus genes.
| No. | EBI data bank gene namea | Gene type | Gene size (bp) | Protein MW, (kDa) | Recombinant plasmidb of expression |
|---|---|---|---|---|---|
| 1 | VPA0051 | nhaD | 1266 | 46.128 | pTVPA0051 |
| 2 | VPA0464 | napA2 | 1959 | 71.436 | pTVPA0464 |
| 3 | VP0618 | nhaC4 | 1521 | 54.039 | pTVP0618 |
| 4 | VP0632 | nhaC2 | 1431 | 49.674 | pTVP0632 |
| 5 | VP1134 | nhaC3 | 1602 | 56.510 | pTVP1134 |
| 6 | VP1228 | nhaA | 1152 | 40.431 | pTVP1228 |
| 7 | VP1782 | nhaC5 | 1485 | 52.977 | pTVP1782 |
| 8 | VP2072 | nhaB | 1587 | 57.160 | pTVP2072 |
| 9 | VP2125 | nhaC1 | 1434 | 50.896 | pTVP2125 |
| 10 | VP2718 | nhaP1 | 1335 | 49.039 | pTVP2718 |
| 11 | VP2785 | napA1 | 1797 | 65.079 | pTVP2785 |
| 12 | VP2867 | nhaP2 | 1746 | 62.634 | pTVP2867 |
To study the expression and roles of the 12 putative Na+/H+ antiporters, we cloned each individual gene into a His-tagged expression vector. The recombinant plasmids were transformed into the Na+-sensitive E. coli strain, TO114 (Ohyama et al., 1994) and the K+ uptake-deficient E. coli strain, LB650 (Nakamura et al., 1998). Protein expression levels were estimated using Western blotting. In all cases, we observed well-expressed immunoreactive bands corresponding to the approximate molecular weight of the recombinant proteins indicated in Table 1(Fig. 1). E. coli cells transformed with a control pTrcHis2C vector did not express immunoreactive proteins (data not shown). These results indicate that all 12 recombinant proteins were expressed in E. coli. Because membrane fractions were used for this experiment, we concluded that the recombinant V. parahaemolyticus proteins were successfully assembled in the E. coli membrane.
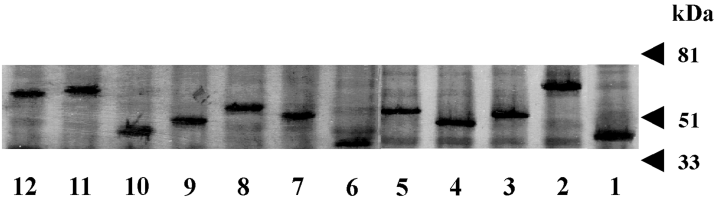
Expression of V. parahaemolyticus genes in E. coli strain TO114. Membrane fractions of recombinant strains were prepared, and proteins were detected with an antibody against the 6×-His tag. The number label for each lane corresponds to the number assigned to each gene in Table 1.
Na + tolerance phenotype of putative V. parahaemolyticus Na+/H+antiporters in E. coli
First, we examined whether any of the 12 cloned genes of V. parahaemolyticus could complement the Na+-sensitive phenotype of E. coli mutant TO114. Due to the absence of the genes, nhaA, nhaB and chaA, TO114 cells cannot grow in medium containing 200 mM NaCl (Ohyama et al., 1994; Nakamura et al., 1996). At pH 7.0, only recombinant strains expressing Vp-nhaA and Vp-nhaB were able to complement the Na+-sensitive phenotype of the TO114 mutants both in liquid and on solid LB media containing 200 mM NaCl (Fig. 2A). At alkaline pH 8.5 or higher, only Vp-nhaA was able to complement the TO114 mutant (Fig. 2B). Moreover, the growth pattern in alkaline conditions was the same as at neutral pH (Fig. 2A). While there was no difference in complementation ability between Vp-nhaA and Vp-nhaB at neutral pH, Vp-nhaB was unable to complement the TO114 defect at alkaline pH (data not shown).
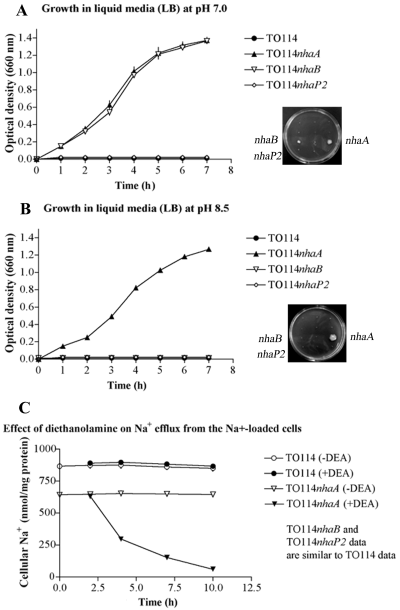
A. Complementation of E. coli TO114 NaCl sensitivity by V. parahaemolyticus genes Vp-nhaA and Vp-nhaB in LB medium containing 200 mM NaCl at pH 7.0. Growth in liquid medium is shown in the graph on the left and growth on solid medium is shown on the right. Values for growth in liquid medium are the means ± SD of three determinations. The growth patterns of recombinant strains carrying the control vector or the remaining putative antiporter genes were similar to TO114 and therefore are not displayed. B. Complementation of E. coli TO114 NaCl sensitivity by Vp-nhaA in LB medium containing 200 mM NaCl at pH 8.5. Growth in liquid medium is shown on the left and growth on solid medium is shown on the right. Values for growth in liquid medium are the means ± SD of three determinations. The growth patterns of recombinant strains carrying the control vector or the remaining putative antiporter genes were similar to TO114 and therefore are not displayed. C. Effect of diethanolamine on Na+ efflux from the Na+-loaded recombinant Vp-nhaA TO114 cells. Cells were suspended in buffer containing 0.15 M NaCl, 20 mM [EPPS]-KOH, 0.2% glucose at pH 8.5 (open symbols); 50 mM diethanolamine-HCl (pH 8.5) was added to the buffer after 2 min (closed symbols).
Extrusion of Na + from the Na + -loaded E. coli cells induced by diethanolamine
Continuous and accurate regulation of local H+ is crucial to the maintenance of many cellular functions. When membrane permeable amine is added to the buffer, it passes bacterial membrane in neutral form and consumes internal cellular H+, thus causing cytoplasmic alkalinization by becoming positively charged (Nakamura et al., 1995). Typically, bacterial Na+/H+ and probably K+/H+ antiporters catalyse the exchange of intracellular cations (Na+ or K+) for extracellular H+, thereby counteracting the tendency of the cytosol to become alkaline (Nakamura et al., 1984; Padan et al., 2001). If the stoichiometry of the exchange process is one Na+ (or K+) for nH+ (where n > 1), then internal concentration of H+ becomes higher than external concentration. Thus, balance between neutral and protonated forms of amine inside of cell shifts toward protonated form. This event prompts exchange activity of electrogenic antiporters and we are able to detect it as a cation extrusion.
Electrogenic antiporters are active at external alkaline pH. Therefore, amount of extruded cations under diethanolamine pressure is high. At neutral external pH the activity of antiporters is neglectable and it is difficult to detect extrusion of cations under diethanolamine pressure (Nakamura et al., 1995). Because the E. coli TO114 mutant does not possess the capacity to extrude Na+ at alkaline pH, it presents an ideal environment to measure the activity of the 12 putative V. parahaemolyticus Na+/H+ antiporters. We studied Na+ exit from TO114 recombinant strains carrying V. parahaemolyticus Na+/H+ antiporter genes and compared it to Na+ exit from TO114 mutant cells. E. coli TO114 cells transformed with Vp-nhaA were loaded with 600 nmol Na+ per mg of protein. The remaining recombinant TO114 strains, TO114 cells transformed with control vector, and untransformed TO114 cells were loaded with 800–1000 nmol Na+ per mg of protein. Following the addition of 50 mM diethanolamine-HCl at pH 8.5, E. coli TO114 carrying Vp-nhaA released approximately 550 Na+ nmol per mg of protein. The cellular Na+ decreased from 600 nmol Na+ per mg of protein to 50 nmol Na+ per mg of protein (Fig. 2C). On the other hand, untransformed E. coli TO114 cells did not undergo Na+ efflux (Fig. 2C). The function of TO114 cells transformed with the control vector and strains carrying the other recombinant plasmids closely resembled that of the untransformed TO114 strain (data not shown).
Vp-NhaA, Vp-NhaB and Vp-NhaP2 mediate K+ efflux
In addition to Na+ extrusion, K+ extrusion may be an important component of cellular ionic homeostasis (Stumpe et al., 1996). The data shown in Fig. 3A demonstrate that V. parahaemolyticus cells were able to mediate downhill K+ efflux following the addition of diethanolamine to NaCl buffer. The K+ exit observed at alkaline pH can be explained by the existence of a K+/H+ antiporter, similar to the case of V. alginolyticus (Nakamura et al., 1982; 1984). We hypothesized that some of the 12 putative Na+/H+ antiporters from V. parahaemolyticus could efficiently mediate this extensive release of K+, thus working as K+/H+ antiporters.
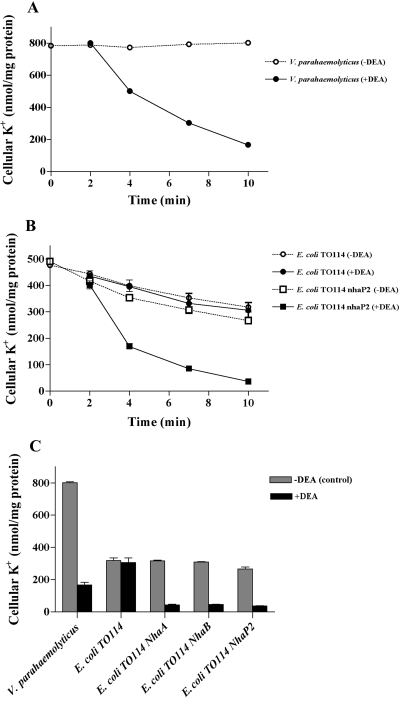
Effect of diethanolamine on K+ efflux from V. parahaemolyticus cells, E. coli TO114, and recombinant strains. A. V. parahaemolyticus cells were suspended in 0.4 M NaCl and 50 mM Tris-HCl at pH 9.0 (open symbols); 50 mM diethanolamine-HCl (pH 8.5) was added to the buffer after 2 min (closed symbols). B. E. coli TO114 and recombinant Vp-nhaP2 cells were suspended in 0.4 M NaCl and 50 mM Tris-HCl at pH 9.0 (open symbols); 50 mM diethanolamine-HCl (pH 8.5) was added to the buffer after 2 min (closed symbols). C. Final extent of efflux in strains under the same experimental conditions. When diethanolamine was added to the buffer, effluxes were reduced to 20.8% (V. parahaemolyticus), 96.3% (E. coli TO114), 13.6% (E. coli TO114 carrying Vp-nhaA), 16.5% (E. coli TO114 carrying Vp-nhaB), and 13.7% (E. coli TO114 carrying Vp-nhaP2) of the respective control value. Data in A, B and C are the means ± SD of three determinations. Using two-way anova of the data in C for the diethanolamine effect [–DEA (control) vs. +DEA], the differences were considered significant at P < 0.0001.
To confirm our hypothesis, we studied K+ efflux from control TO114 cells and those expressing the V. parahaemolyticus antiporter genes. Figure 3B shows the effect of diethanolamine on K+ exit for one of the recombinants, Vp-NhaP2. In 0.4 M NaCl at pH 9.0, all recombinant TO114 strains contained about 480 K+ nmol per mg of protein. Only a slow release of cellular K+ was observed, resulting in the release of about 180 K+ nmol per mg of protein. Upon the addition of 50 mM diethanolamine at pH 9.0, TO114 cells expressing Vp-NhaA, Vp-NhaB, or Vp-NhaP2 released approximately 300 K+ nmol per mg of protein, whereas the remaining recombinants, including TO114 cells carrying a control vector, released about 10 K+ nmol per mg of protein (Fig. 3B and C). Thus, the introduction of Vp-nhaA, Vp-nhaB, or Vp-nhaP2 into TO114 mutant cells led to the extensive release of K+ (Fig. 3C). We propose that the K+ efflux observed here was mediated by K+/H+ antiporters.
The tests described above were performed in the absence of external K+ and thus did not address whether Vp-NhaA, Vp-NhaB, or Vp-NhaP2 can mediate K+ extrusion against a concentration gradient. To address this point, we prepared K+-loaded cells and measured the net K+ loss from control TO114 mutant cells and the recombinant strains in the presence of extracellular 0.4 M KCl (data not shown). We did not observe any K+ extrusion following the addition of 50 mM diethanolamine-HCl (pH 8.5). This result probably reflects a strong K+-uptake system of E. coli TO114.
To study the ability of the V. parahaemolyticus K+/H+ antiporters to extrude K+ without the confounding effects of a strong uptake system, we introduced the recombinant antiporter genes into the E. coli K+-uptake mutant, LB650, which has defects in the genes kdp, kup, trkH and trkG (Nakamura et al., 1998). Figure 4A and B show the effect of diethanolamine on K+ exit from E. coli LB650 and its recombinant strains containing approximately 350 K+ nmol per mg of protein. When the K+-loaded cells were preincubated for 5 min in buffer containing 0.4 M KCl, no release of cellular K+ was observed. The addition of 50 mM diethanolamine-HCl (pH 8.5) induced K+ exit (Fig. 4A). At the new steady state, cellular K+ reached about 50 nmol per mg of protein in K+-loaded cells expressing Vp-NhaA, Vp-NhaB, or Vp-NhaP2, demonstrating that they had released about 300 K+ nmol per mg of protein. The remaining recombinant strains, including LB650 cells transformed with control vector, released about 180 K+ nmol per mg of protein (Fig. 4A and B). Thus, E. coli itself possesses some K+ extrusion system, presumably an electrogenic K+/H+ antiporter, as it is able to mediate a K+ efflux against its chemical gradient in the presence of a membrane-permeable amine (Fig. 4B). However, expression of the genes Vp-nhaA, Vp-nhaB or Vp-nhaP2 led to a distinct increase in K+ extrusion.
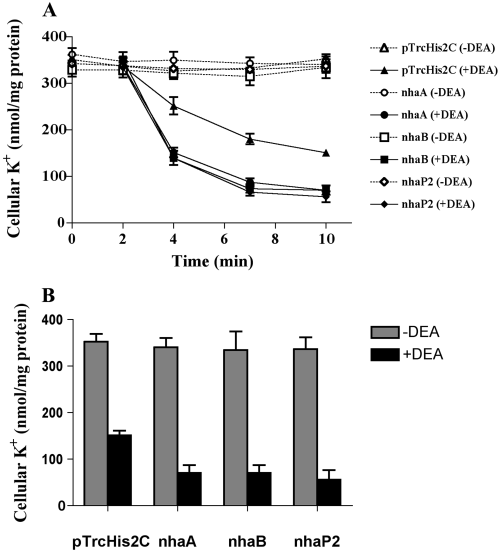
Effect of diethanolamine on K+ efflux from the K+-loaded cells of E. coli having a defect in K+ uptake (LB650 strains) carrying V. parahaemolyticus antiporter genes. A. Cells were suspended in 0.4 M KCl, 20 mM [EPPS]-KOH, 0.2% glucose at pH 8.5 (open symbols), and diethanolamine was added to the buffer after 2 min (closed symbols). B. Final extent of efflux in different recombinant strains of E. coli LB650 under the same experimental conditions. When diethanolamine was added to buffer, effluxes were reduced to 42.7% (E. coli LB650 transformed with pTrcHis2C), 20.6% (E. coli LB650 carrying Vp-nhaA), 21.0% (E. coli LB650 carrying Vp-nhaB), and 16.5% (E. coli LB650 carrying Vp-nhaP2) of the respective control value. Data in A and B are the means ± SD of three determinations. Using two-way anova of the data in B for diethanolamine effect [–DEA (control) vs. +DEA], the differences were considered significant at P < 0.0001.
Antiporter activities in the everted membrane vesicles of E. coli cells expressing putative V. parahaemolyticus cation/proton antiporters
To measure the antiporters activity differently, everted membrane vesicles were prepared from E. coli TO114 carrying vector pTrcHis2C (control), Vp-nhaA, Vp-nhaB or Vp-nhaP2. The exchange activities were monitored by measuring the dequenching of acridine orange fluorescence upon addition of KCl, NaCl or LiCl at a pH range of 6.0–9.0 (Fig. 5, data considering Li+/H+ antiporter activity not shown). The E. coli TO114 pTrcHis2C, and mutants carrying Vp-nhaA, Vp-nhaB, or Vp-nhaP2 exhibited very low Na+/H+ or K+/H+ exchange activities at pH lower than 8.0 (Fig. 5B). The activities increased with increasing pH and prominent activities were observed at pH 9.0 (Fig. 5A and B). The dequenching of fluorescence was observed upon the addition of NaCl (Fig. 5A and B) or LiCl (data not shown) in the cells expressing Vp-NhaA or Vp-NhaB expressing cells, but not in the control (pTrcHis2C) and negligible in the Vp-NhaP2 expressing cells, indicating that Vp-NhaA and Vp-NhaB have Na+/H+ and Li+/H+ exchange activities. Antiporter Vp-NhaP2 did not reveal Na+/H+ or Li+/H+ activities in present experiment. Figure 5A and B reveals that all three antiporters Vp-NhaA, Vp-NhaB and Vp-NhaP2 have K+/H+ activity. However, Vp-NhaA and Vp-NhaB show lower K+/H+ activity when compared with Vp-NhaP2 (Fig. 5A and B).
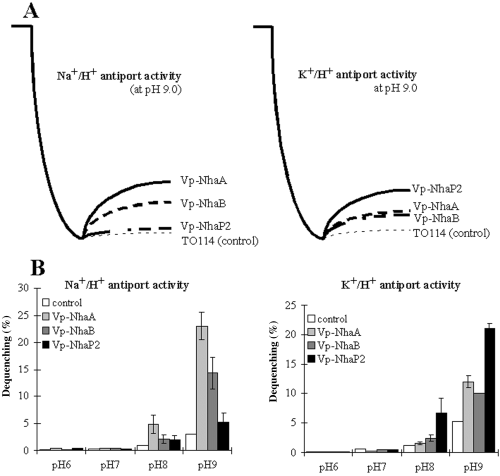
A. Na+/H+, K+/H+ activities of Vp-NhaA, Vp-NhaB and Vp-NhaP2 antiporters measured at pH 9.0 by the acridine orange fluorescence quenching method as described in Experimental procedures. B. Efffects of pH on the 5 mM each of NaCl-, LiCl-, KCl-induced dequenching (%). All data are the means ± SD of three independent repetitions.
Effects of KCl concentrations on the K+/H+ antiporter activity of the Vp-NhaP2
Next, we examined the effects of KCl concentration on the K+/H+ antiporter activity of the Vp-NhaP2 (data not shown). The exchange activity increased with increasing the concentration of KCl. Thus, 5 mM KCl yielded in 7% dequenching, 10 mM KCl yielded in 37% dequenching, whereas 31.25 mM and 62.5 mM KCl yielded in 40% dequenching. This result probably indicates that antiporter functions more efficiently in K+ concentrations higher than 10 mM.
K + tolerance phenotype of putative V. parahaemolyticus cation/proton antiporters in E. coli
To assess the benefits of Vp-NhaA, Vp-NhaB and Vp-NhaP2 antiporters on cell viability at high external K+ conditions, we tested the growth of TO114 recombinant strains in liquid media in which Na+ was replaced with K+ (10–800 mM) over a pH range of 6.5–8.5. At pH 6.5, E. coli TO114 carrying vector alone (pTrcHis2C), Vp-nhaA, Vp-nhaB, or Vp-nhaP2 started to show the growth inhibition at 500 mM of KCl (Fig. 6A). Inhibition exponentially increased with increasing concentrations of KCl. Experiment indicates on K+ toxicity when high concentrations (600 mM, 700 mM, 800 mM) of KCl are present, furthermore strain E. coli TO114 cannot grow in media containing 1 M KCl. More prominent differences in growth were observed after 9 h of incubation at pH 8.5 (Fig. 6B). Experiment showed that E. coli TO114 couldn’t survive in medium containing less then 100 mM KCl, but also was unable to survive in medium containing more then 500 mM KCl. Thus, under described conditions growth limit of E. coli TO114 was determined by KCl concentration, and laid in range from 100 mM to 500 mM of KCl. Growth limits determined by concentration of KCl were wider for recombinant strains: from 10 mM to 700 mM of KCl for the recombinant carrying Vp-nhaA or Vp-nhaB, and from 30 mM to 700 mM of KCl for the Vp-nhaP2 recombinant (Fig. 6B).
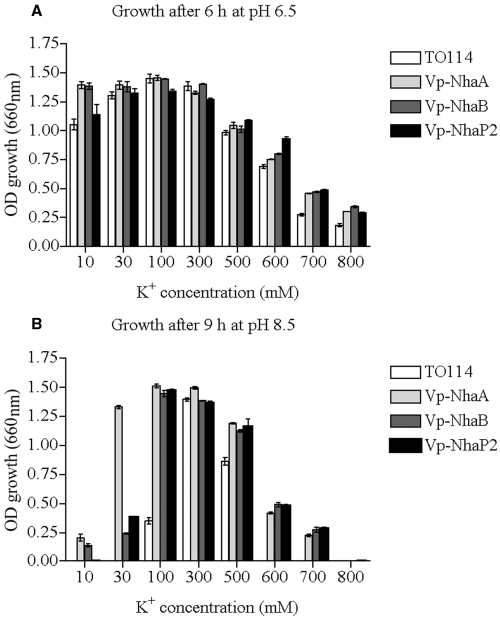
Effects of KCl concentrations and pH on growth of E. coli TO114 (control) and it recombinants carrying Vp-nhaA, Vp-nhaB, or Vp-nhaP2. Cultures were grown as described in Experimental procedures at pH 6.5 (A) or 8.5 (B). Data are the means ± SD of three independent repetitions.
Discussion
It is commonly known that K+ is essential for bacterial growth and is not considered toxic, even at high concentrations. Based on this fact it is easy to assume that K+ efflux systems should not be essential for cell growth. Surprisingly, in this study we observed that high concentrations of K+ are toxic for bacterial cells, especially at alkaline conditions (Fig. 6A and B). Therefore, to avoid excessive internal K+ accumulation under alkaline conditions, bacterial cells seem to use K+ efflux systems. Despite a report that V. alginolyticus has K+/H+ antiporter(s) (Nakamura et al., 1984), the bacterial genes encoding them have not yet been identified. Therefore, the main goal of this study was to identify the first bacterial K+/H+ antiporter gene(s). Twelve genes were cloned and studied for Na+/H+ and K+/H+ antiporter activity. We tested K+ release from V. parahaemolyticus cells using an experimental design similar to the one we used for V. alginolyticus (Nakamura et al., 1984). We were able to verify K+/H+ antiporter activity in V. parahaemolyticus (Fig. 3).
We found that two V. parahaemolyticus gene products, Vp-NhaA and Vp-NhaB, are Na+/H+ antiporters (2, 5). It is already known that NhaA and NhaB from E. coli and V. alginolyticus are Na+/H+ antiporters (Taglicht et al., 1991; Pinner et al., 1992; Nakamura et al., 1994; 1996). Antiporters Vp-NhaA and Vp-NhaB also revealed Na+/H+ exchange activity (Fig. 5A). Furthermore, the antiporter Vp-NhaA mediates rapid Na+ efflux against a Na+ chemical gradient in alkaline conditions (Fig. 2C), whereas antiporter Vp-NhaB was unable to mediate efflux of this type under the same conditions. These data are in agreement with the fact that both antiporters are able to complement the TO114 Na+-sensitive phenotype at neutral pH, but in alkaline conditions Vp-NhaB ceased to function and was unable to complement Na+-sensitive phenotype (Fig. 2B). We consider probability that antiporter Vp-NhaA is electrogenic with respect to Na+ ions, whereas antiporter Vp-NhaB is electroneutral. We also found that, apart from possessing a Na+-specific activity, these proteins play important roles in K+ efflux 3-6), thus allowing for higher K+ tolerance at alkaline pH (Fig. 6B). In this case the antiporters were able to mediate K+ efflux against a K+ chemical gradient, probably suggesting that both Vp-NhaA and Vp-NhaB possess electrogenic activity with respect to K+ cations. Taken together, these data probably suggest that Vp-NhaA and Vp-NhaB primarily function to regulate Na+ homeostasis (2, 5), whereas their secondary function is to maintain K+ homeostasis (3, 6). This probably can be explained by the fact that alkaline pH and toxic concentrations of K+ are not permanent conditions in the life cycle of V. parahaemolyticus. Hence, it appears that this bacterium uses the K+-related function of the Vp-NhaA and Vp-NhaB transport systems only when circumstances warrant, whereas these systems function as constitutive Na+/H+ transporters.
Even more interesting results were observed with respect to the Vp-NhaP2 transporter, because it appeared to have unique K+ ion specificity and functions exclusively as a K+/H+ antiporter (Fig. 5). Vp-NhaP2 is able to mediate rapid K+ extrusion at alkaline pH against a K+ gradient (Fig. 4). The existence of this transporter allows E. coli to tolerate high K+ concentrations at alkaline pH by maintaining K+/H+ antiport activity (5, 6). This is the first report of an apparently K+(specific)/H+ antiporter in bacteria and of an uphill K+ extrusion system from either a prokaryotic or eukaryotic organism.
Our present data also indicate that, at alkaline pH, K+-loaded untransformed E. coli LB650 cells also mediate K+ efflux against an outward K+ concentration gradient in the presence of a membrane-permeable amine (Fig. 4). This result potentially indicates that the cells possess an electrogenic antiporter system involved in K+ homeostasis. Further work will focus on cloning the responsible gene(s).
The functions of the remaining nine cloned putative Na+/H+ antiporters of V. parahaemolyticus are still unknown, because none of them exhibited Na+/H+ or K+/H+ exchange activity under our experimental conditions (2, 3).
Genes encoding components of efflux or extrusion systems with both Na+ and K+ activity have been identified in bacteria, yeast, fungi and plants (Bañuelos et al., 1998; Venema et al., 2002; 2003; Cellier et al., 2004; Lewinson et al., 2004; Fujisawa et al., 2005). To our knowledge, however, there is no report of a gene encoding a protein with K+ efflux and/or extrusion activity against a chemical K+ concentration gradient from prokaryotic or eukaryotic organisms. Identification of novel K+/H+ and Na+(K+)/H+ antiporters of this type is a step forward in better understanding cell physiology and in our ability to elucidate new regulatory features of intracellular pH, cell volume and osmoregulation. Furthermore, the characterization of new bacterial K+ transport systems is significant for current biotechnological challenges, such as allowing crops to grow in the increasing number of the world's areas that have excessive soil salinity and alkalinity.
Experimental procedures
Bacterial strains and growth conditions
Escherichia coli TO114 (nhaA::KmrnhaB::EmrchaA::Cmr) (Ohyama et al., 1994) and E. coli LB650 (ΔkdpFABC5 kup trkH::CmrtrkG::Kmr) (Nakamura et al., 1998) strains were used as the hosts for expression of V. parahaemolyticus genes. V. parahaemolyticus RIMD2210633 was used for gene cloning and was grown aerobically at 37°C in a synthetic medium composed of 0.3 M NaCl, 2 mM Na2HPO4, 15 mM (NH4)2SO4, 10 µM FeSO4, 1% (w/v) glycerol, 50 mM Tris at pH 7.4 adjusted by HCl (the above medium contained about 10 µM K+ as a contaminant) or in VR medium composed of 0.5% tryptone, 0.5% yeast extract, 0.4% K2HPO4, 3% NaCl at pH 7.0 adjusted by KOH. Strains E. coli TO114 and LB650 were grown in LBK100 medium containing 1% trypton, 0.5% yeast extract, 25 mM Tris, 100 mM KCl (final concentration of K+ was determined by atomic absorption spectrophotometry) or in modified synthetic medium (Tokuda et al., 1981) containing 0.2 M KCl, 2 mM K2HPO4, 10 µM FeSO4, 15 mM (NH4)2SO4, 5 mM MgSO4, 1% (w/v) glycerol, 50 mM Tris at pH 7.4 adjusted by HCl.
Escherichia coli DH5α (recA1 gyrA (Nal) Δ(lacIZYA-argF) (φ80 dLacΔ[lacZ]M15), pir RK6) was used for routine cloning procedures and was grown and harvested in LB medium (Sambrook et al., 1989).
Routine cloning procedures and sequencing
QIAamp DNA Mini Kit (Qiagen Sciences, MD, USA) was used to purify chromosomal DNA of V. parahaemolyticus RIMD2210633. Twelve genes (Table 1) were amplified from it using BamHI site-containing sense primers and EcoRI (or PstI) site-containing antisense primers (Table 2). Stop codons of the genes were deleted in the PCR product. PCR was performed according to the manufacturer's instruction accompanying iTaqTM DNA Polymerase (Bio-Rad). Using DNA Ligation Kit Ver.2.1 (TaKaRa Bio, Shiga, Japan), all PCR products were inserted into pT7Blue T-Vector (Novagen, WI, USA) by TA cloning, and double-stranded dideoxy sequencing of genes was performed (Shimazu Corporation, Kyoto, Japan). PCR products were then released from the pT7Blue T-Vector by digestion with BamHI and EcoRI (or PstI) followed by elution from agarose gels with QFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences, Foster City, CA, USA). Digested and eluted PCR products were inserted into BamHI and EcoRI (or PstI) sites of vector pTrcHis2C (Invitrogen, San Francisco, CA, USA) using DNA Ligation Kit Ver.2.1. The transcriptional orientation of each gene in the expression vector corresponded to that of the corresponding gene in the V. parahaemolyticus RIMD2210633 genome, and genes were fused in-frame to six histidines. The sequences encoding the His-tag were attached to the 3′ end of the genes behind the last codon (the stop codon was deleted). The recombinant expression plasmids were transferred first into E. coli DH5α cells and then into E. coli TO114 and E. coli LB650 cells. Recombinant plasmid DNAs were transformed into fresh competent E. coli cells prepared using calcium chloride (Sambrook et al., 1989). Transformed cells were selected with 100 µg ml−1 ampicillin. Quantum Prep® Plasmid Miniprep Kit (Bio-Rad) was used for plasmid purification.
| Gene | Forward (F) and reverse (R) primers | Base pairs | |
|---|---|---|---|
| VPA0051 | F | 5′-ATGGATCCGATGAGAAAATCCAAA-3′ | 24 |
| R | 5′-AGCTGCAGAGGTAAACAAACCGCC-3′ | 24 | |
| VPA0464 | F | 5′-ATGGATCCGATGACAGGGATATTC-3′ | 24 |
| R | 5′-AGCTGCAGAAATCGCTTCTTCTTT-3′ | 24 | |
| VP0618 | F | 5′-ATGGATCCGATGGAACAGGCTGAC-3′ | 24 |
| R | 5′-GGCGAATTCCTCTGCTGAAACAGA-3′ | 24 | |
| VP0632 | F | 5′-ATGGATCCGTTGACAATAAAAGTC-3′ | 24 |
| R | 5′-AAAGAATTCGTTTTCGGTTTGTAC-3′ | 24 | |
| VP1134 | F | 5′-CGGGATCCGATGAATTTAATAGAT-3′ | 24 |
| R | 5′-AATGAATTCAGCGCTCTGACATGA-3′ | 24 | |
| VP1228 | F | 5′-ATGGATCCGATGAACGATGTCATCC-3′ | 25 |
| R | 5′-TGCGAATTCGTCTTGTGCTTTCTT-3′ | 24 | |
| VP1782 | F | 5′-ATGGATCCGATGCACTTTATGACA-3′ | 24 |
| R | 5′-AGCGAATTCACCGATGATGAAGAA-3′ | 24 | |
| VP2072 | F | 5′-ATGGATCCGATGCCGATATCGCT-3′ | 23 |
| R | 5′-AGCTGCAGAGTGTCCGCCAGACACAA-3′ | 26 | |
| VP2125 | F | 5′-ATGGATCCGATGAAGCAAGTTAC-3′ | 24 |
| R | 5′-TAAGAATTCAGCCGTTTGCTGC-3′ | 22 | |
| VP2718 | F | 5′-ATGGATCCGATGTCGGTCTATCAT-3′ | 24 |
| R | 5′-GCTGAATTCGGCTTCAGTTTGTTC-3′ | 24 | |
| VP2785 | F | 5′-ATGGATCCGATGGCGATAACCAGT-3′ | 24 |
| R | 5′-TGCGAATTCCTTCCAATGATTAGG-3′ | 24 | |
| VP2867 | F | 5′-ATGGATCCGATGGACGCAGACACC-3′ | 24 |
| R | 5′-TCCGAATTCGTCCTCCTCTTCCCC-3′ | 24 | |
- a . The underlined letters indicate the restriction enzyme sites BamHI, EcoRI or PstI.
- b . All genes were cloned from the V. parahaemolyticus RIMD2210633 genome.
Complementation tests
For the complementation tests on agar plates, E. coli TO114 cells transformed with pTrcHis2C or recombinant plasmids (Table 1) were grown 18 h at 37°C in LBK100 (pH 7.0). Cells were then spread on a solid LB medium containing 200 mM NaCl at alkaline or neutral pH 7.0 or 8.5 and incubated 18 h at 37°C. Cell growth was monitored visually.
For the complementation tests in liquid media, 100 µl of overnight cultures of E. coli TO114 cells transformed with pTrcHis2C or recombinant plasmids were transferred into 10 ml of LB medium containing 200 mM NaCl at alkaline or neutral pH (7.0 or 8.5) and incubated 18 h at 37°C. Cell growth was monitored by measuring optical density at 660 nm, using a mini photo 518R spectrophotometer (Taitec, Koshigaya-shi, Saitama-prefecture, Japan).
Phenotypic evaluation of K+/H+ antiporter activities among cloned genes
Cells of E. coli TO114 and transformed cells (Table 1) were grown in LBK media (10 mM KCl, pH 6.5) supplemented with 100 µg ml−1 ampicillin to the end of the exponential phase of growth (OD600 = 1.2), and 5 µl of culture was transferred into 5 ml of medium containing 10 mM K+ (LBK10), 30 mM K+ (LBK30), 100 mM K+ (LBK100), 300 mM K+ (LBK300), 500 mM K+ (LBK500), 600 mM K+ (LBK600), 700 mM K+ (LBK700), or 800 mM K+ (LBK800) (concentrations of K+ in the media were determined by atomic absorption spectrophotometry), at pH 6.5 or 8.5. Changes in pH and K+ concentration of the LBK medium stimulated the cells to rapidly activate one or more of their K+-transport systems, leading to net exit of K+. This resulted in more rapid adaptation and growth of recombinant strains carrying genes encoding K+/H+ transporters. Optical density (660 nm) of the cultures was monitored with a miniphoto 518R photometer.
Immunoblotting
Transformed E. coli TO114 or E. coli LB650 cells were grown 18 h in LBK medium containing 100 µg ml−1 ampicillin and 0.05 mM ml−1 IPTG. Bacterial pellets were suspended in 50 mM Tris-HCl. The suspensions containing approximately 100 mg protein ml−1 of fresh cells were disrupted by sonication. Cell debris was removed by centrifugation for 10 min at 16 000 g. Membranes were collected by ultracentrifugation for 30 min at 100 000 g and resuspended in a solution containing equal volumes of milliQ H2O and 2 × SDS loading buffer. Protein concentration was determined using the BCA Protein Assay Reagent (Pierce Chemical Company, Rockford, IL, USA). Protein 20 µg was separated by electrophoresis (Bio-Rad) on 10% SDS-polyacrylamide gels and transferred electrophoretically (ATTO electrophoresis machine and ATTO protocol, ATTO Technology, Buffalo, NY, USA) onto a 0.2 µm pure nitrocellulose membrane (Bio-Rad). Immunoblotting was performed with an anti-His-rabbit polyclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymed Laboratories, CA, USA). The detection of His-fused proteins was carried out with ECL Western blotting detection reagents and HyperfilmTM ECLTM (Amersham Biosciences, UK).
Preparation of Na+-loaded cells and measurement of Na+ effluxes
Na+-loaded/K+-depleted cells were prepared using a membrane-permeable amine as described previously (Nakamura et al., 1982). Briefly, E. coli TO114 cells were suspended in 0.15 M NaCl containing 50 mM diethanolamine-HCl, pH 9.3. The amine-loaded cells were washed with 0.15 M NaCl containing 20 mM N-2-hydroxyethylpiperazine-N′-3-propanesulfonic acid (EPPS)-NaOH buffer, pH 8.5. Immediately after the addition of 0.2% glucose, the cells were transferred to 25°C. The extrusion of Na+ from the cells was induced by the addition of 50 mM diethanolamine-HCl, pH 8.5 (Nakamura et al., 1995). At time intervals, the cells were collected by filtration on 0.45 µm pore size cellulose acetate membrane filter (Advantec, CA, USA) and washed with 1 ml of 0.4 M choline chloride containing 10 mM Tris-HCl, pH 7.2. The filter was immersed in 6 ml of 5% trichloroacetic acid, and Na+ content was determined by flame photometry using an atomic absorption spectrophotometer AA-6200 (Shimazu Corporation, Kyoto, Japan). The intracellular concentrations were calculated as mentioned previously (Nakamura et al., 1982) using the 3.3 µl internal water space per mg of protein value of V. alginolyticus for V. parahaemolyticus and 5.4 µl per mg of cell protein for E. coli.
Measurement of K+ effluxes from normal cells
Harvested cells were washed twice with 0.4 M NaCl. The reaction was started at 25°C by the addition of 2 µl of concentrated cell suspension to 100 µl of 0.4 M NaCl, 50 mM Tris-HCl, pH 9.0. The extrusion of K+ from the cells was induced by the addition of 50 mM diethanolamine-HCl, pH 9.0. The filtration method and flame spectrophotometry were performed as previously described (Nakamura et al., 1982).
Measurement of K+/H+ antiporter activity through detection of K+ movement
Harvested cells were briefly suspended in 0.4 M KCl containing 50 mM diethanolamine-HCl, pH 9.3. The amine-loaded cells were washed with 0.4 M KCl containing 20 mM EPPS-KOH buffer, pH 8.5. Immediately after the addition of 0.2% glucose, the cells were transferred to 25°C. The extrusion of K+ from the cells was induced by the addition of 50 mM diethanolamine-HCl, pH 8.5 (Nakamura et al., 1995). The filtration method and atomic absorption by flame spectrophotometry were performed as previously described (Nakamura et al., 1982).
Measurement of K+/H+, Na+/H+ and Li+/H+ antiporter activities detected by the acridine orange fluorescence quenching method
All activities were examined on everted membrane vesicles prepared from the cells grown in LBK (Rosen, 1986) as described (Hamada et al., 2001). E. coli cells were harvested by centrifugation at 3100 g for 10 min at 4°C, washed and suspended in 10 ml of TCDS buffer containing 10 mM Tris-HCl (pH 7.0), 0.14 M choline chloride, 0.5 mM dithiothreitol, 0.25 M sucrose. The cells were applied to a French Pressure cell (4000 psi). Then the solution was centrifuged at 110 000 g for 60 min at 4°C and suspended in 600 µl of TCDS buffer. The antiporter activity was based upon the establishment of transmembrane pH gradient (ΔpH) by addition of salt to the reaction mixture that contained 10 mM Tris-HCl (titrated with HCl to the indicated pH), 5 mM MgCl2, 0.14 M choline chloride, 1 µM acridine orange and membrane vesicles (50 µg of protein) in a volume of 2 ml. The ΔpH was monitored at 25°C with acridine orange as a probe at an extinction wavelength of 492 nm (band width 1.5 nm) and emission wavelength of 525 nm (band width 3.0 nm) of Shimadzu RF-5300PC spectrophotometer. At the onset of the experiment, Tris-DL-lactate (2 mM) was added and the fluorescence quenching was recorded. KCl, NaCl, or LiCl (5 mM) was then added and the new steady state of fluorescence obtained (dequenching) after each addition was monitored.
Acknowledgements
We thank Mitsuaki Nishibuchi, Hiroshi Kobayashi and Evert P. Bakker for providing us with Vibrio parahaemolyticus strain RIMD221063, E. coli strain TO114 and E. coli strain LB650 respectively. We thank Yasutomo Matsuzaki for technical assistance. We appreciate Evert Bakker and Nobuyuki Uozumi for their helpful discussions. This work was supported by a grand-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan (14572051) and by a grant from the Promotion and Mutual Aid Corporation for Private Schools in Japan.