Mating type regulation of cellular tolerance to DNA damage is specific to the DNA post-replication repair and mutagenesis pathway
Summary
In order to help further define DNA post-replication repair (PRR), a conditional synthetic lethal screen was employed to identify new genes involved in the PRR pathway. A synthetic lethal screen with the mms2 mutation resulted in the recovery of two suppressor mutations responsible for regulating PRR. The recovered suppressors are the mating type genes and SIR3. Indeed, controlled expression of both mating type genes or deletion of SIR3 rescued the conditional synthetic lethal mutant phenotypes. Furthermore, comprehensive analyses suggest that mating type heterozygosity confers tolerance to a broad range of DNA damage, and that this effect is limited to all PRR pathway mutations, but does not apply to base excision repair, nucleotide excision repair or recombination repair mutants. In addition, the tolerance conferred to PRR mutants as a result of mating type heterozygosity is dependent on a functional homologous recombination but not the non-homologous end-joining pathway. Thus, mating type status appears to be responsible for signalling DNA content and possibly cell cycle stage, allowing the cell to select the most efficient means to repair the DNA damage.
Introduction
Genetic studies in the budding yeast Saccharomyces cerevisiae have been instrumental in identifying the genes involved in post-replication repair (PRR), which is also known as the RAD6 pathway (Prakash et al., 1993). Within this pathway, Rad6 and Rad18 form a complex and are required for PRR (Prakash, 1981). The RAD6 gene encodes a ubiquitin conjugating enzyme (Ubc or E2), Ubc2 (Jentsch et al., 1987), whose active site cysteine residue is essential for all of its known functions (Sung et al., 1990). The rad6 mutants are extremely sensitive to a variety of DNA-damaging agents, defective in sporulation and UV-induced mutagenesis, and exhibit severe growth deficiencies (Prakash et al., 1993). However, unlike rad6, rad18 mutants do not display either defective sporulation or growth retardation (Lawrence, 1982; Jones et al., 1988). Rad18 contains a RING finger motif known to serve as a ubiquitin ligase (Ubl or E3); it also displays a single-stranded DNA-binding activity (Bailly et al., 1994) allowing for the Rad6–Rad18 complex to bind the single-stranded regions that result from the stalling of DNA polymerases. As a result, the E2–E3 activity of Rad6–Rad18 could mediate the ubiquitination of the stalled replication machinery. The MMS2 gene was classified as a member of the error-free PRR pathway (Broomfield et al., 1998; Xiao et al., 1999), and its product has been shown to form a stable complex with Ubc13; this complex is capable of assembling an alternative type of multiubiquitin chain via Lys63 (Hofmann and Pickart, 1999). Rad5, a chromatin-associated RING finger protein, may be responsible for the recruitment of the Mms2–Ubc13 complex to the Rad6–Rad18 complex (Ulrich and Jentsch, 2000). Polζ, encoded by REV3 and REV7, is a non-essential mutagenic polymerase capable of replicating over-damaged regions of DNA with low processivity (Nelson et al., 1996). Epistatic analysis with rev3 and mms2 places REV3 in the error-prone branch of the RAD6-dependent pathway (Broomfield et al., 2001).
The RAD6 pathway is regarded as a cellular DNA damage tolerance pathway as it enhances cell survival with the cost of increased mutations, without actual removal of the replication-blocking lesions (Barbour and Xiao, 2003). Hence, it is expected that the PRR pathway must be tightly regulated to cooperate with other cellular repair pathways and with DNA replication. To help further define the PRR pathway, yeast cells were screened for mutations that are conditionally lethal in the absence of MMS2. By taking advantage of the synergism between error-free PRR and mutagenesis pathway mutations, we used a synthetic lethal screen protocol (Barbour et al., 2000) in the presence of extremely low does of methyl methanesulphonate (MMS) that will not affect the growth of single mutants, but will effectively kill the double mutants. We report here the isolation of mating type genes involved in suppressing the MMS sensitivity of PRR mutants. Haploid S. cerevisiae exists in one of two different mating types determined by the mating type (MAT) locus. The two mating type alleles, MATα and MATa, encode four open reading frames (ORFs) with functions for three gene products identified. The a and α information is present as intact but silent copies of mating type genes at transcriptionally silent domains of chromatin located at the silent mating type loci, HMR and HML. Silencing at the mating type loci is directed by specific cis-acting regulatory silencer sequences, HML-E, HML-I, HMR-E and HMR-I (Laurenson and Rine, 1992). Each of these elements interacts directly or indirectly with a number of trans-acting factors to establish or maintain silencing at the mating type loci. The trans-acting factors include the Silent Information Regulator (Sir) proteins, Rap1, histones H3 and H4 (Aparicio et al., 1991; Kurtz and Shore, 1991; Thompson et al., 1994) and the Origin Recognition Complex (ORC) proteins (Laurenson and Rine, 1992). Deletions of the SIR genes cause MATa or MATα haploid cells to have the non-mating phenotype characteristic of MATa/MATα diploid cells (Haber, 1998). Diploid cells heterozygous for mating type (a/α) show an increased resistance to UV damage and are more recombination-proficient than haploid cells or diploids homozygous for mating type genes (Friis and Roman, 1968; Heude and Fabre, 1993). This difference has been demonstrated between haploids and heterozygous diploids defective in rev3 (Lawrence and Christensen, 1976) or rad18 (Boram and Roman, 1976), but is abolished when combined with mutations involved in recombination repair (Saeki et al., 1980). We performed a comprehensive analysis and demonstrated that mating type heterozygosity can be created in haploid cells by either expressing both mating type loci or interfering with the Sir silencing function, which resulted in enhanced tolerance to a variety of DNA-damaging agents. Surprisingly, the above rescuing effect by mating type heterozygosity is restricted to PRR mutants and was not observed in other DNA repair mutants, and this rescuing effect is dependent on homologous recombination (HR) but not non-homologous end-joining (NHEJ) pathways.
Results
Identification of conditional synthetic lethal mutations with mms2
A screen was employed to isolate cells containing mutations that are conditional synthetic lethal with mms2, which resulted in the isolation of mutations known to be synergistic with mms2 with respect to killing by MMS (e.g. rev1 and rev3), as well as novel mutations. One such mutant, SLM-9, displays a synthetic lethal phenotype on plates containing 0.005% MMS when compared with the mms2Δ single mutant (data not shown). To identify the corresponding synthetic lethal mutation, a single-copy and a multicopy yeast genomic library were screened for the functional complementation of the MMS-sensitive phenotype, which resulted in the isolation of three clones from over 20 000 independent transformants.
Mapping the SLM-9 rescuing clones
Two YEp13 multicopy genomic library plasmids were recovered with one containing a 6.6 kb genomic fragment (YEp-SLM9-1) and one having a 4.6 kb fragment (YEp-SLM9-2). To determine which ORF within these clones was responsible for complementing the synthetic lethal mutation in SLM-9, various deletions were constructed and tested for their ability to complement the MMS sensitivity of SLM-9. The insert sequence obtained with YEp-SLM9-1 (Fig. 1A) is located on chromosome 12 and contained three ORFs: YLR440c (SEC39), YLR441c (RPS1A) and YLR442c (SIR3). To determine which ORF was responsible for complementation, two initial deletions were constructed, both inactivating YLR441c but leaving YLR440c or SIR3 intact. YEp-SLM9-1 was digested with either NheI–NcoI or NcoI–PvuII to create YEp-SLM9-1ΔNN and YEp-SLM9-1ΔNP respectively (Fig. 1A). The YEp-SLM9-1ΔNN fragment, containing the SIR3 gene, was still able to complement the MMS sensitivity of SLM-9. Removal of the SIR3 gene in the YEp-SLM9-1ΔNP construct abolished complementation of the MMS sensitivity of SLM-9. As both constructs disrupted the RPS1A ORF, we conclude that SIR3 is responsible for the complementation of the synthetic lethal mutation in SLM-9.
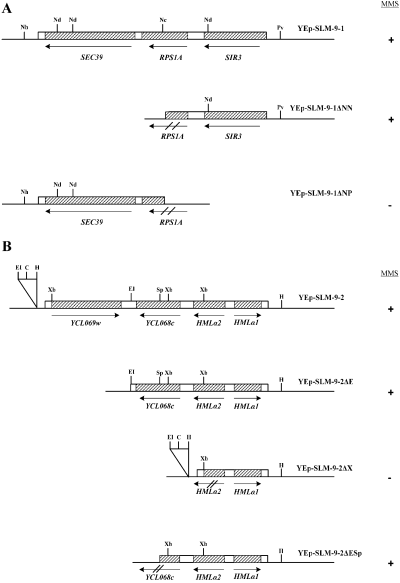
Deletion analysis of SLM-9 suppressors. A. Deletion analysis of YEp-SLM9-1. Schematic diagrams of yeast genomic DNA insert in YEp-SLM9-1, which includes three genes: SEC39, RPS1A and SIR3. B. Deletion analysis of YEp-SLM-9-2, which includes four putative genes, YCLO69w, YCL068c, HMLα2 and HMLα1. Boxed area indicates yeast DNA and hatched boxes indicate ORFs. Restriction sites: C, ClaI; EI, EcoRI; H, HindIII; Nc, NcoI; Nd, NdeI; Nh, NheI; Pv, PvuII; Sp, SphI; Xb, XbaI. Results of complementation of SLM-9 mutation for growth on MMS plates are depicted by +/–.
YEp-SLM9-2 (Fig. 1B) contains a 4.3 kb genomic insert located on chromosome 3. This genomic fragment contains two hypothetical ORFs (YCL069w, YCL068c) and two characterized ORFs (HMLα1 and HMLα2). Two initial constructs were created. YEp-SLM9-2ΔE was created by digesting the genomic library plasmid with EcoRI. This construct lacks the hypothetical ORF YCLO69w and is still able to complement the MMS sensitivity in the SLM-9 mutant (Fig. 1B). The YEp-SLM9-2ΔX was created by digesting the library plasmid with XbaI. This construct deletes all genes except HMLα1, and no longer complements for the MMS sensitivity of the SLM-9 strain. These initial deletions were able to limit the possible complementing genes to HMLα2 or the hypothetical ORF, YCL068c. YEp-SLM9-2ΔE was used for additional deletion analysis to further define which gene is responsible for the complementation of MMS sensitivity. The YEp-SLM9-2ΔE plasmid was digested with EcoRI–SpeI to create the construct YEp-SLM9-2ΔESp (Fig. 1B). This deletion removes the C-terminal half of YCL068c, leaving HMLα1 and HMLα2 intact. The YEp-SLM9-2ΔESp construct complements the MMS sensitivity in the SLM-9 strain. As we already determined that the HMLα1 gene alone is incapable of complementing the MMS sensitivity in SLM-9, we conclude that the HMLα2 gene is either completely responsible or works with HMLα1 in suppressing the MMS sensitivity in SLM-9.
The single-copy plasmid recovered in the library screen contained an approximately 10 kb insert and was named YCpL-SLM9-1. Several ORFs were contained within the genomic insert including MATα1 and MATα2. Although these genes belong to the mating type gene coding region on chromosome 3 and not the silenced HML loci recovered in the multicopy plasmid, the DNA sequences are identical and the genes are not under the control of the cis-acting regulator elements that act to silence the chromosomal HML loci. Therefore, both genomic inserts would act the same when expressed ectopically from a plasmid. Because deletion analysis confirmed the requirement for MATα2 in complementing the MMS sensitivity in SLM-9, further deletion analysis was not carried out on this plasmid.
Deletion of either SIR3 or mating type locus in mms2 does not result in the SLM-9 phenotype
The fact that two different genes are able to rescue the SLM-9 synthetic lethal phenotype suggests that at least one of the genes is an extragenic suppressor. To ask whether the synthetic lethal mutation in SLM-9 belongs to MATα or SIR3, we created mms2ΔmatαΔ and mms2Δsir3Δ double mutants. Gradient plate analysis of the MMS sensitivity revealed that mms2Δ and mms2ΔmatαΔ have the same level of sensitivity to MMS (Fig. 2A). These results suggest that the synthetic lethal mutation in SLM-9 is not in the MATα gene.
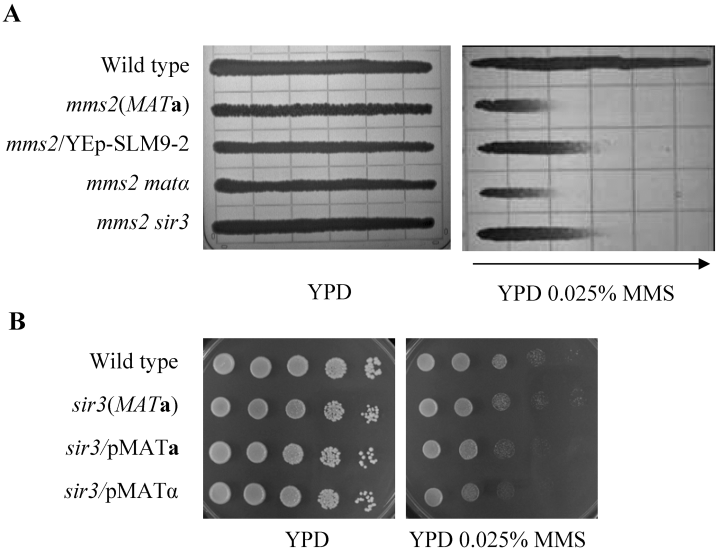
Relative sensitivity of mms2- and sir3-related mutants. A. Rescuing effects of the mms2Δ mutant by various genetic manipulations in a gradient plate assay. Cells cultured overnight were imprinted on YPD and YPD + 0.025% MMS gradient plates, and the plates were incubated at 30°C for 2 days. The arrow points towards the higher MMS concentration. Strains used: HK578-10D (WT); WXY1228 (mms2Δ); WXY1228 transformed with YEp-SLM9-2; WXY1237 (mms2ΔmatαΔ); and WXY1238 (mms2Δsir3Δ). B. Effect of mating type heterozygosity on the sir3Δ mutation by a serial dilution assay. A 10-fold serial dilutions of the cell suspension were spotted onto YPD plates or YPD plates containing MMS. Incubation was carried out at 30°C for 2 days before the plates were photographed. Strains used: HK578-10D (WT); BY4741 sir3 (sir3Δ); and BY4741 sir3 transformed with YCp50-MATa or YCp50-MATα.
To our surprise, deletion of SIR3 from SLM-9 also did not reduce its sensitivity; in contrast, it made the mms2 strain more resistant to MMS (see Fig. 4B). Careful analysis (Fig. 2A) shows that the mms2Δsir3Δ double mutants exhibited the same phenotype as mms2ΔMATa strains expressing MATα (from YEp-SLM9-2). The altered sensitivity is not due to SIR3 deletion per se, as the sir3Δ mutant displays the MMS sensitivity indistinguishable from that of wild-type cells (Fig. 2B). Nevertheless, this result suggests that the synthetic lethal mutation in SLM-9 is not a simple loss-of-function mutation in SIR3.
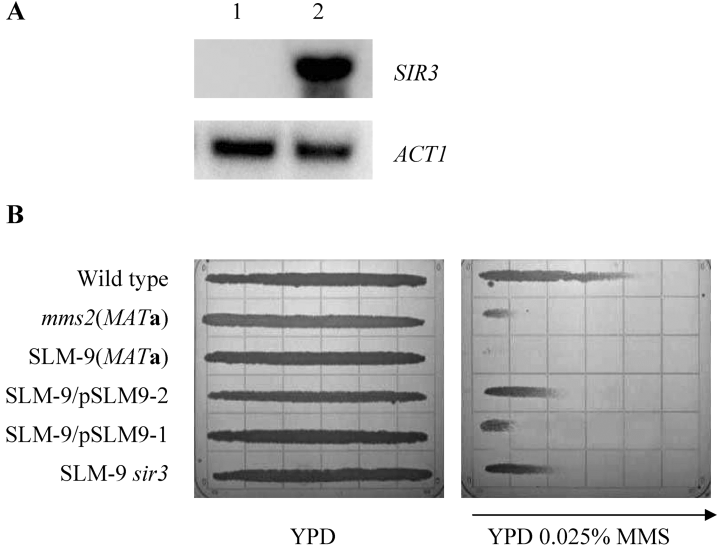
A. Overexpression of truncated SIR3 in YEp-SLM9-1 transformant (lane 2) compared with untransformed cells (lane 1). Total RNA was isolated for Northern hybridization as described in Experimental procedures. The membrane was sequentially hybridized with SIR3 and ACT1 probes. B. Partial rescue of SLM-9 sensitivity to MMS by deregulation of mating type genes. Cells cultured overnight were imprinted on a YPD and a YPD + MMS gradient plate (concentration indicated), and the plates were incubated at 30°C for 2 days. The arrow points towards the higher concentration. Strains used: HK578-10D (WT MATα); WXY1228 (mms2Δ); SLM-9 (MATa); SLM-9 transformed with YEp-SLM9-2; SLM-9 transformed with YEp-SLM9-1; SLM-9 sir3Δ::LEU2.
Mating type heterozygosity as an underlying mechanism of DNA damage tolerance
The observations that either deletion of SIR3 or ectopic expression of the opposite mating type gene partially rescues the mms2 mutants from DNA damage-induced lethality led us to speculate that mating type heterozygosity is an underlying mechanism of DNA damage tolerance. To test our hypothesis, an mms2Δ (MATα) strain was transformed with either plasmid YCp50-MATa or YCp50-MATα and the transformants were examined for MMS sensitivity (Fig. 3). In this case, only YCp50-MATa but not YCp50-MATα was able to rescue mms2 sensitivity. Similar results were also observed with SLM-9 transformants (data not shown). These results indicate that expression of both mating type loci is sufficient to partially rescue mms2 cells from killing by MMS. We ruled out the possibility that the rescuing effect is due to nutritional marker difference (Abdullah and Borts, 2001) in various strains because all the strains in comparison contain the same markers. Furthermore, we have tested three different strain backgrounds with different nutritional markers (Table 1) and observed the same phenomenon.

Partial rescue of mms2Δ sensitivity to MMS by mating type heterozygosity. The 10-fold serial dilutions of the cell suspension were spotted onto YPD plates or YPD plates containing MMS. Incubation was carried out at 30°C for 2 days before the plates were photographed. A. Strains used: HK578-10D (WT MATα); WXY902 (mms2Δ) transformed with YCp50; WXY902 transformed with YCp50-MATa or YCp50-MATα. B. Strains used: HK578-10A (WT); WXY1238 (mms2Δsir3Δ); WXY1238 transformed with YCp50-MATa or YCp50-MATα.
| Strain | Genotype | Source |
|---|---|---|
| BY4741 nej1 | MAT a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 nej1::KanR | ResGen |
| BY4741 sir3 | BY4741 with sir3::KanR | ResGen |
| BY4741 yku70 | BY4741 with yku70::KanR | ResGen |
| BY4741 yku80 | BY4741 with yku80::KanR | ResGen |
| WXY1239 | BY4741 yku70 with mms2Δ::LEU2 | This study |
| WXY1240 | BY4741 yku80 with mms2Δ::LEU2 | This study |
| WXY1241 | BY4741 nej1 with mms2Δ::LEU2 | This study |
| DBY747 | MAT a his3-Δ1 leu2-3112 trp1-289 ura3-52 | D. Botstein |
| WXY1243 | DBY747 with apn1Δ::HIS3 mms2Δ::LEU2 | This study |
| WXY1244 | DBY747 with mag1Δ::hisG mms2Δ::LEU2 | This study |
| WXY1245 | DBY747 with rad1Δ::LEU2 mms2Δ::HIS3 | This study |
| WXY642 | DBY747 with mms2Δ::HIS3 | Laboratory stock |
| WXY9216 | DBY747 with mag1Δ::hisG | Laboratory stock |
| HK578-10A | MAT a ade2-1 can1-100 his3-11,15 leu2-3112 trp1-1 ura3-1 | H. Klein |
| HK578-10D | MATαade2-1 can1-100 his3-11,15 leu2-3112 trp1-1 ura3-1 | H. Klein |
| HK615–1 A | HK578-10A with rad1Δ::LEU2 | H. Klein |
| WXY902 | HK578-10D with mms2Δ::HIS3 | This study |
| WXY906 | HK578-10D with ubc13Δ::LEU2 | This study |
| WXY1228 | HK578-10A with mms2Δ::TRP1 ade3Δ::hisG | This study |
| SLM-9 | WXY1228 with synthetic lethal mutation | This study |
| SLM-9 sir3 | SLM-9 with sir3Δ::LEU2 | This study |
| WXY1236 | HK578-10D with matαΔ::LEU2 | This study |
| WXY1237 | HK578-10D with matαΔ::LEU2 mms2Δ::TRP1 | This study |
| WXY1238 | HK578-10D with mms2Δ::TRP1 sir3Δ::LEU2 | This study |
| WXY930 | HK578-10D with rad18Δ::LEU2 | Laboratory stock |
| WXY1242 | WXY902 with rad52Δ::LEU2 | This study |
| LSY387 | Same as HK578-10D with rad52Δ::TRP1 | L. Symington |
| LSY404 | Same as HK578-10D with rad54Δ::LEU2 | L. Symington |
| PY39-46 | PY39-0 with [pBL230-46 (TRP1 pol30-46)] | Laboratory stock |
| WXY858 | PY39-46 with rad5Δ::HisG-URA3-hisG | Laboratory stock |
It is known that disruption of SIR3 also results in the loss of silencing at both HML and HMR loci (Herskowitz and Oshima, 1981), thus allowing expression of both MATa and MATα. If deletion of SIR3 alone is also sufficient to rescue the mms2 sensitivity due to mating type heterozygosity, a critical prediction of our hypothesis is that coexpression of both MATa and MATα should not further enhance DNA damage tolerance in the sir3Δ strain. Indeed, both YCp50-MATa and YCp50-MATα transformants of the mms2Δsir3Δ strain showed the same level of MMS sensitivity as mms2Δsir3Δ and mms2Δ (MATα)/YCp50-MATa strains (Fig. 3), whereas expression of both mating type genes in a sir3Δ strain does not alter its response to MMS (Fig. 2B). Hence, we conclude that the rescuing effects in both mms2Δ and SLM-9 cells by SIR3 deletion or opposite mating type gene expression are due to simultaneous expression of both mating type genes.
A 5′-truncated SIR3 gene plays a dominant-negative role
It remains to be explained why expression of SIR3 from the YEp-SLM9-1 library plasmid rescues the SLM-9 MMS sensitivity. Sequencing of YEp-SLM9-1 indicates that the 5′-terminus of the SIR3 ORF is truncated and the promoter region of the gene is absent. If a cryptic promoter is available within the remaining SIR3 5′ region or the adjacent vector sequence, one would expect an overexpression of a truncated Sir3 protein missing N-terminal 99 amino acids. Northern analysis indeed shows that a truncated SIR3 mRNA is produced en mass in YEp-SLM9-1-transformed cells (Fig. 4A). As Sir3 is required for the functional Sir complex formation (Ivy et al., 1986), we suspect that the overproduced N-terminally truncated Sir3 (Sir3ΔN) may serve as a dominant-negative competitor with the endogenous Sir3, which is reflected in the partial rescue of SLM-9 cells below the level of mating type heterozygosity (Fig. 4B). Our hypothesis predicts that deletion of SIR3 from SLM-9 cells will override the requirement of YEp-SLM9-1 and result in complete suppression of the SLM-9 synthetic lethal mutation to the level of mating type heterozygosity, which is indeed the case (Fig. 4B).
Mating type heterozygosity confers increased DNA damage tolerance to all PRR mutants
It has been previously shown that a/αrad18 homozygous diploids have an increased resistance to the lethal effects of both UV and γ-irradiation as compared with isogenic haploids or MAT homozygous diploids. It was also demonstrated that the sensitivity of subpopulation corresponding to the G2 phase cells of rad18 haploids could be reversed by the expression of both MATa and MATα (Heude and Fabre, 1993). We wanted to further investigate the involvement of the mating status in haploid PRR mutants. Several different mutants representing the error-free and error-prone branches of PRR were transformed with the YCp50-MATa and YCp50-MATα plasmids to determine the response of theses transformants after exposure to MMS. All mutants surveyed within the PRR pathway, including mms2Δ, ubc13Δ (Fig. 5), pol32Δ, rev3Δ, rad18Δ, rad5Δ and pol30-46 (data not shown), displayed an increase in resistance to DNA-damaging agents when expressing both mating type genes. This effect is independent of the initial mating type of the mutant (Fig. 5A and B).

Sensitivity of mms2Δ or ubc13Δ mutants to MMS or UV is dependent on the mating type status. Yeast cells were cultured in YPD at 30°C until they reached log-phase. The 10-fold serial dilutions of the cell suspension were spotted onto YPD plates or YPD plates containing MMS, or on a YPD plate followed by exposure to UV as indicated. Incubation was carried out at 30°C for 2 days before the plates were photographed. The mms2Δ or ubc13Δ mutant cells were transformed with plasmids YCp50, YCp50-MATa or YCp50-MATα and compared with their respective isogenic wild-type cells. A. WXY642 (MATamms2Δ). B. WXY902 (MATαmms2Δ). C and D. WXY906 (MATαubc13Δ).
It was argued that plate-based assays such as the gradient plate assay and series dilution assay represent chronic exposure of cells to the DNA-damaging agents and are semi-quantitative. To address these concerns, we performed series dilution experiments using UV as a DNA-damaging agent, in which cells were spotted, briefly exposed to UV and the plates were incubated in the absence of DNA damage source. As shown by a representative ubc13 mutant (Fig. 5D), cells carrying both mating type alleles are more resistant to killing by UV than those carrying a single or two copies of the same mating type allele. We also performed liquid killing experiments with several selected PRR mutants and the results (Fig. 6) indicate that mating type heterozygosity could confer up to 100-fold resistance to MMS or UV in certain PRR mutants, and they correlate well with plate-based assays. Furthermore, experiments with γ-ray as a DNA-damaging agent showed similar results (data not shown), indicating that the rescuing effect by mating type heterozygosity on PRR mutants is independent of source of DNA damage and the method of assay.
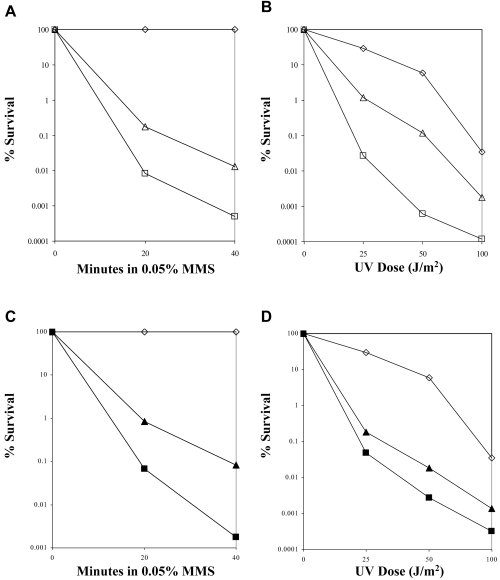
Sensitivity of PRR mutants to MMS or UV is not dependent on chronic treatment. For the MMS-induced liquid killing, the wild-type strain and its isogenic derivatives were treated with MMS for the given time and plated onto YPD plates to score for cell survival and compared with untreated cells. For the UV-induced killing, the wild-type strain and its isogenic derivatives were diluted and plated onto YPD plates and treated with the indicated dose of UV. The cells were incubated in the dark at 30°C for 3 days, scored for cell survival and compared with untreated cells. These results show a typical experiment. Each experiment has been repeated at least three times. A and B. (◊) HK578-10D (WT); (□) WXY930 (MATαrad18Δ); (▵) WXY930 transformed with YCp50-MATa. C and D. (◊) HK578-10D (WT); (▪) WXY858 (MATα pol30-46 rad5Δ); (▴) WXY858 transformed with YCp50-MATa.
The mating type rescuing effect is dependent on functional HR but not NHEJ
It has been reported that diploid cells heterozygous for mating type (a/α) show an increased resistance to DNA-damaging agents and are more recombination proficient than haploid cells and diploids homozygous for mating type genes (a/a and α/α) (Friis and Roman, 1968; Heude and Fabre, 1993). It was thought that the expression of both mating type genes in haploids causes cells to behave as if they were diploid and to activate a recombination repair pathway. In the yeast S. cerevisiae, this repair pathway is largely dependent on the RAD52 epistasis group gene products to repair double-strand breaks (DSBs) (Resnick, 1976; Orr-Weaver and Szostak, 1983). In the absence of a homologous template or when the RAD52 pathway is inactivated, yeast can repair DSBs using a NHEJ pathway that depends on YKU70, YKU80, MRE11, RAD50, XRS2, DNL4, LIF1 and NEJ1 (Tsukamoto et al., 1996a,b; Lewis et al., 1998; Lewis and Resnick, 2000; Teo and Jackson, 2000; Valencia et al., 2001; Dudasova et al., 2004). To determine whether the increased resistance seen in the a/α haploid cells is dependent on HR, we compared cell survival in the presence and absence of the HR pathway. As reported before (Broomfield et al., 1998), the rad52 mutant is much more sensitive to MMS than the mms2 mutant, and the double mutation is additive. Deletion of RAD52 abolishes the tolerance conferred to PRR mutants by mating type heterozygosity (Fig. 7A). This result suggests that expression of both mating type genes allows for the channelling of lesions into a DSB repair pathway; in the absence of HR, the increased resistance to DNA damage seen in PRR mutants is abolished.
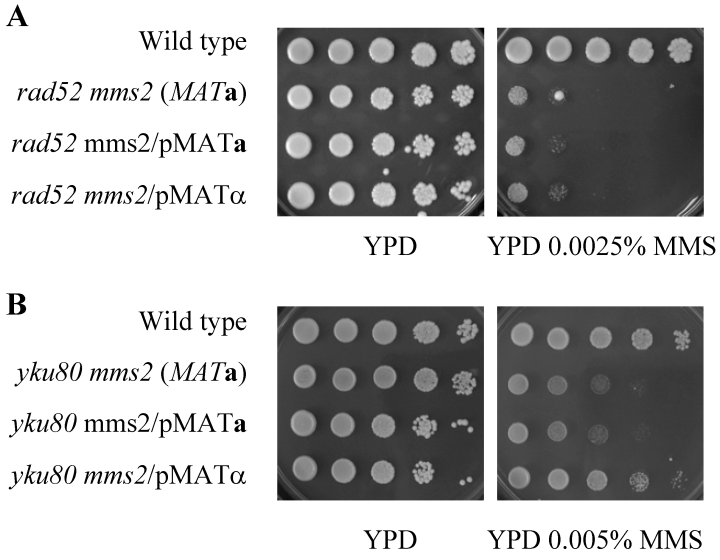
Mating type resistance of PRR mutants is dependent on HR but not NHEJ. A 10-fold serial dilution assay was performed as described and the plates were incubated at 30°C for either 3 days (A) or 2 days (B). A. Strains used: HK578-10D (WT); WXY1228 (mms2Δ); LSY387 (rad52Δ); WXY1242 (MATamms2Δrad52Δ); WXY1242 transformed with YCp50-MATα. B. Strains used: HK578-10A (WT); WXY1240 (MATamms2Δyku80Δ); WXY1240 transformed with YCp50-MATa or YCp50-MATα.
In order to determine whether inactivation of NHEJ has the same effect as HR inactivation, we combined mms2Δ with yku70Δ, yku80Δ or nej1Δ mutation. All three nhej single mutants showed minor if any sensitivity to MMS compared with the wild-type cells, and the mms2 nhej double mutations appear to be additive. Nevertheless, the rescuing effect of mms2 mutant by mating type heterozygosity was still observed when a NHEJ pathway is inactivated (Fig. 7B and Fig. S1 in Supplementary material ), suggesting that NHEJ is not absolutely required to repair lesions channelled from the PRR pathway.
It remains possible that any of the other DNA repair mutations can also abolish the mating type effect. To address this issue, we combined mms2Δ with apn1Δ, mag1Δ or rad1Δ representing defects in base excision repair or nucleotide excision repair. In each case the double mutants expressing only MATa showed a characteristic sensitive phenotype. In contrast, the double mutants expressing both mating types showed an increased resistance to MMS compared with that expressing only one mating type (Fig. S2 in Supplementary material ). These results confirm that the mating type heterozygosity effect seen in the PRR mutants is only dependent on Rad52-mediated DSB repair.
Cellular tolerance to DNA damage in response to mating type is specific to the PRR pathway mutants
To determine the involvement of mating type status with respect to enhanced DNA repair and tolerance, we surveyed genes involved in other DNA repair pathways. Strains with mutations in recombination repair genes, including rad52Δ (Fig. 8A) and rad54Δ (Fig. 8B), the BER mutant mag1Δ (Fig. 8C) and the NER mutant rad1Δ (Fig. 8D), were transformed with YCp50-MATa and YCp50-MATα. The sensitivity of these mutants to MMS or UV was determined. There was no noteworthy difference in sensitivity to all DNA-damaging agents tested when either recombination or excision repair mutants were heterozygous for mating type (Fig. 8 and data not shown). These results suggest that the effect of mating type status is specific to the PRR pathway mutants. The benefit of protection by mating type heterozygosity is also limited to PRR-defective mutant cells, as wild-type cells do not display enhanced resistance when harbouring both active mating type genes (data not shown).
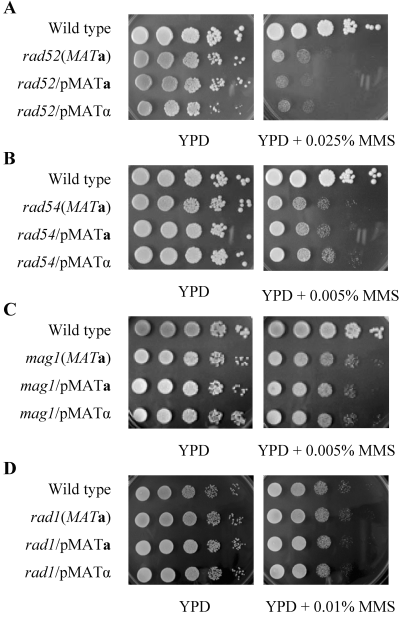
Sensitivity of recombination repair, BER and NER mutants to MMS is independent of mating type status. Cells were cultured in YPD at 30°C until they reached log-phase. A 10-fold serial dilution assay was performed as described and the plates were incubated at 30°C for 2 days before being photographed. A. LSY387 (rad52Δ) with YCp50, YCp50-MATa or YCp50-MATα. B. LSY404 (rad54Δ) with YCp50, YCp50-MATa or YCp50-MATα. C. WXY9216 (mag1Δ) with YCp50-MATa or YCp50-MATα. D. HK615-1A (rad1Δ) with YCp50, YCp50-MATa or YCp50-MATα.
Discussion
In this report, we demonstrate that simultaneous expression of both MATa and MATα alleles, either by introducing active mating type genes or by derepressing the silent mating type loci HML and HMR, increases cellular resistance to DNA damage in PRR mutants. In addition, a truncated SIR3 gene is capable of partially rescuing SLM-9 as well as mms2 cells from killing by MMS. As the SIR genes are required for silencing at the HML and HMR loci, it is not surprising that a truncated Sir3 protein could serve as a dominant-negative suppressor of Sir function and allow for partial derepression of the silencing at the HML and HMR loci, resulting in mating type heterozygosity. We also demonstrate that the increased resistance to DNA damage conferred to PRR mutants heterozygous for mating type is independent of types of DNA damage but dependent on HR; inactivation of RAD52 abolishes the protective effect of mating type heterozygosity. Furthermore, this genetic interaction is limited to PRR and DSB repair as mutants defective in other DNA repair pathways are not affected by mating type heterozygosity, neither do they influence the mating status-specific effect of PRR mutants.
Mating type heterozygosity in haploid PRR mutants has a similar effect to that seen in diploid cells that are more resistant to γ-irradiation than their haploid counterparts, partly due to the heterozygosity at the mating type locus. This correlation led to the proposal that the a1–α2 complex promotes channelling of some DNA lesions (e.g. DSBs) from a mutagenic DNA repair pathway into the recombination repair process; however, the subsequent events leading to the activation of recombination repair pathway remain to be elucidated. DSBs in diploid cell have a homologous partner that can be used to repair the damage, but haploid cells in the G1 phase of the cell cycle lack homologues and so rely on NHEJ. With recombination repair under the control of the a1/α2 repressor, the haploid yeast cell can adapt the repair process, possibly by upregulation of HR in late S-G2 and by using the NHEJ pathway primarily when homology-driven repair is not feasible. Our findings potentially broaden the scope of a1/α2 regulation to include PRR. For example, when the cells encounter damage during S-phase the cells can employ PRR to bypass the damage and ensure successful replication. Although this repair pathway is highly desired in haploid cells to bypass the damage to ensure survival, the result from this process can result in increased mutations. However, if the cell is expressing the a1/α2 repressor, indicative of a diploid state, the cell could override the PRR pathway and channel lesions into the more pristine HR pathway. This model is consistent with our findings that the increased resistance conferred to PRR mutants is dependent on HR, and is reminiscent of roles of SRS2 (Schiestl et al., 1990) and PCNA sumoylation (Papouli et al., 2005; Pfander et al., 2005) in the regulation between PRR and HR, which are also dependent on functional HR.
It is not surprising to observe that mating type rescuing effect is independent of NHEJ, given the fact that NEJ1 expression is suppressed in the a/α cells (Frank-Vaillant and Marcand, 2001; Valencia et al., 2001), resulting in the loss of NHEJ activity and increased sensitivity to DNA-damaging agents in a rad52 mutant background (Tsukamoto et al., 1997; Astrom et al., 1999). The opposite effects of mating type heterozygosity in PRR and HR mutants are intriguing and suggest that the mating status plays an important role in channelling lesions into different repair pathways. Indeed, the extreme sensitivity to DNA-damaging agents seen in PRR mutants suggests that once the cells commit to the PRR pathway, the process is difficult to reverse, which causes problems when the PRR pathway is inactivated. Expressing both mating type genes in PRR mutants may prevent the commitment into the defunct PRR pathway and instead allows the cells to use HR and/or NHEJ. Our results also indicate that inactivation of PRR primarily results in increased DSBs.
DNA repair pathways and their regulation have not been extensively characterized in diploid cells. We show here that one of the regulatory activities unique to diploid cells can be studied in haploid cells by experimentally creating mating type heterozygosity. The resistant effect in a/α diploids is reproducible in G2 haploids expressing both mating type genes, indicating that the mating status, and hence the expression of both the a1 and α2 products, provide an intracellular signal for HR. The results from this study suggest that several factors determine the most appropriate repair mechanism to use. Expression of both mating type genes is indicative of a 2n content of DNA and would suggest the presence of a sister chromatid to use for HR. This is also substantiated by the evidence that only the PRR pathway, which is proposed to use either a TLS or sister chromatid exchange mechanism, depending on the use of the error-prone or error-free branch, respectively, is influenced by mating type heterozygosity. It can be further argued that as G1 haploid cells have no homologue with which to repair damage through recombination mechanisms, whereas diploid cells contain homologous chromosomes, cells may sense their mating status to favour recombination in diploid cells while favouring NHEJ in G1 and PRR in S-phase haploid cells. Our results suggest that cells contain several mechanisms to ensure that the appropriate type of repair is carried out during the cell cycle. For example, in G1 the cell would benefit from excision repairs and NHEJ, whereas during late S-phase and G2 the cells would benefit from either PRR or recombination, depending on the nature of lesions and availability of a sister chromatid as a homologous template. Creating such mating type heterozygosity in haploid yeast cells allows us to investigate the regulatory cascade involved in genetic switch and cooperation among different DNA repair/tolerance pathways. Results obtained from this study are potentially applicable to diploid yeast cells as well as higher eukaryotic cells.
Experimental procedures
Yeast strains and cell culture
The yeast strains used in this study are listed in Table 1. All the strains are isogenic derivatives of either DBY747, originally obtained from Dr Botstein (Stanford University), HK-578-10A and HK-580-10D obtained from Dr H. Klein (New York University), PY39-46 obtained from Dr P. Burgers (Washington University, St Louis, MI) and L.S. Symington (Columbia University), or BY4741, purchased from ResGen. Construction and use of MMS2 and UBC13 disruption cassettes were as previously described (Xiao et al., 1999; 2000). Plasmid pJH318 containing the matαΔ disruption cassette was obtained from Dr J. Haber (Brandeis University, Waltham, MA) and cleaved with PvuII and HindIII before transformation. Plasmid pDP91 (Bennett et al., 2001) containing the sir3Δ disruption cassette was received from Dr M.A. Resnick (NIEHS, Research Triangle Park, NC) and treated with EcoRI before transformation. The YCp50-MATa and YCp50-MATα plasmids were obtained from Dr F. Fabre. The mms2Δ::TRP1 disruption cassette was produced using a polymerase chain reaction (PCR) method. The plasmid pJJ280 (MT148) was amplified using the primers YGL87 (5′-TTCTTATTCTGTATATGCAACGTAGAAGAAGCAGCCGTTTA CACAAACAGCTATGACCATG-3′) and YGL88 (5′-GTGGCT TGGAATGCTGCAAATACTGTTTAGGAAAAAGTAGATAACG TTTTCCCAGTCACGAC-3′). The PCR product contained the 5′ and 3′ terminus of the MMS2 (underlined) coding sequence disrupted by the TRP1 coding sequence.
Yeast cells were cultured at 30°C in either a rich YPD medium or a synthetic glucose (SD) medium supplemented with various nutrients as instructed (Sherman et al., 1983). In the YPGal medium, glucose is replaced with galactose. Intact yeast cells were transformed by a modified lithium-acetate method (Ito et al., 1983). For one-step targeted gene disruption (Rothstein, 1983), plasmid DNA containing the desired disruption cassette was cleaved with restriction enzymes before yeast transformation.
Synthetic lethal screen
Yeast cells (WXY1228) harbouring the pSLS-based plasmids (Barbour et al., 2000) were grown overnight in 10 ml of SD medium lacking uracil. The cells were harvested by centrifugation, resuspended in the same volume of YPD medium and incubated for another 4 h. The cells were collected, washed twice in 50 mM potassium phosphate buffer (pH 7.5) and resuspended in 10 ml of the same buffer. Ethyl methanesulphonate was added to a final concentration of 3% and the culture was incubated at 30°C for 30 min. Ten per cent (w/v) filter-sterilized sodium thiosulphate was added to stop the reaction. The cells were washed twice, diluted and plated on to YPD or YPGal medium and incubated for 4 days at 30°C. Individual non-sectoring colonies were picked and further characterized by two steps. First, they were streaked onto the same medium to monitor colour segregation. Cells from the non-sectoring colonies were then used to inoculate 2 ml of liquid YPD. After an overnight incubation, cells were diluted and plated onto YPD to record colony–colour segregation.
The single-copy yeast genomic library was purchased from American Type Culture Collection (ATCC; Cat. ♯77162) and utilizes the centromeric YCp50-based plasmid ensuring low copy number and mitotic stability. The library vector, p366, contains a 2.23 kb SalI–XhoI LEU2 fragment instead of the URA3 gene. The multicopy yeast genomic library was purchased from ATCC (Cat. ♯37323) and utilizes the YEp13 plasmid as the vector (Nasmyth and Reed, 1980).
To confirm that the yeast genomic library plasmids were responsible for the MMS resistance, a co-segregation test was performed. The SLM-9 colonies that were unable to grow on SD-Leu plates were sensitive to MMS. The SLM-9 colonies that were Leu+ were resistant to MMS. These results confirm that the genomic library plasmids were indeed responsible for complementing the MMS-sensitive phenotype of SLM-9.
Cell killing by DNA-damaging agents
Methyl methanesulphonate- and UV-induced liquid killing was performed as previously described (Xiao et al., 1996).
The gradient plate assay was performed as a semi-quantitative measurement of relative MMS sensitivity. Molten YPD agar (30 ml) was mixed with the appropriate concentration of MMS to form the bottom layer. The gradient was created by pouring the media into tilted square Petri dishes. After brief solidification for 1 h, the Petri dishes were returned flat and 30 ml of the same molten agar without MMS was poured to form the top layer. A 0.1 ml sample was taken from an overnight culture, mixed with 0.4 ml of sterile water and 0.5 ml of molten YPD agar, and then immediately imprinted onto freshly made gradient plates via a sterile microscope slide. Gradient plates were incubated at 30°C for the indicated time. Each assay was repeated several times and a representative plate was photographed.
Methyl methanesulphonate sensitivity was also determined by a serial dilution assay. Yeast cells were inoculated in 3 ml of YPD medium (or selective medium if required) overnight and subcultured into 3 ml of fresh medium. Cells were incubated at 30°C until a mid-logarithmic phase was reached. The cell density was adjusted to 2 × 106 cells ml−1 as determined by a haemocytometer, and further diluted serially 10-fold with ddH2O. The relative MMS sensitivity was determined using freshly made YPD plates containing the indicated amount of MMS. Aliquots (5 µl) of each dilution were applied onto YPD and YPD + MMS plates. The plates were incubated at 30°C for 2 days and photographed. Each assay was repeated several times and a representative plate was photographed.
RNA isolation and Northern hybridization
One millilitre of overnight culture was used to inoculate 4 ml of fresh medium and was further incubated for another 2 h. RNA was isolated by a glass-bead method (Carlson and Botstein, 1982), separated by gel electrophoresis, blotted onto a GeneScreen Plus nylon membrane (DuPont) and hybridized with an α-32P-labelled DNA probe as instructed. The SIR3 probe was isolated as a 1.6 kb EcoRI fragment from the YEp13 genomic library plasmid. The probe was stripped off the membrane and the membrane was subsequently hybridized to a 1.6 kb ACT1 probe as the internal control. The ACT1 probe was isolated as a 1.6 kb BamHI–HindIII fragment from pAA93 (Dr Sherman, Rochester University, New York).
Acknowledgements
The authors wish to thank Drs Haber and Resnick for plasmids, Dr Klein and Symington for yeast strains, Lindsay Ball for technical assistance and Michelle Hanna for proofreading the manuscript. This research was supported by the Canadian Institutes of Health Research Operating Grant MOP-38104 to W.X. L.B. is the recipient of the College of Medicine Graduate Scholarship and the University of Saskatchewan Graduate Teaching Fellowship.
References
Supplementary material
The following supplementary material is available for this article online:
Fig. S1. Sensitivity of yku70Δmms2Δ and nej1Δmms2Δ mutants to MMS. Cells were cultured in YPD at 30°C until they reached log-phase. The 10-fold serial dilutions of the cell suspension were spotted onto YPD plates or YPD plates containing the indicated concentration of MMS. Incubation was carried out at 30°C for 2 days before the plates were photographed. Strains used: (A) HK578-10A (wild type); WXY1239 (MATayku70Δmms2Δ); WXY1239 transformed with YCp50-MATa or YCp50-MATα. (B) HK578-10A (wild type); WXY1241 (MATanej1Δmms2Δ); WXY1241 transformed with YCp50-MATa or YCp50-MATα.
Fig. S2. Sensitivity of mms2Δapn1Δ, mms2Δmag1Δ and mms2Δrad1Δ mutants to MMS. Yeast cells were cultured in YPD at 30°C until they reached log-phase. The 10-fold serial dilutions of the cell suspension were spotted onto YPD plates or YPD plates containing 0.001% MMS. Incubation was carried out at 30°C for 2 days before the plates were photographed. The mms2Δapn1Δ, mms2Δmag1Δ and mms2Δrad1Δ mutant cells were transformed with plasmids YCp50, YCp50-MATa or YCp50-MATα and compared with their respective isogenic wild-type cells. (A) WXY1243 (MATaapn1Δmms2Δ); (B) WXY1244 (MATamag1Δmms2Δ); (C) WXY1245 (MATarad1Δmms2Δ).
This material is available as part of the online article from http://www.blackwell-synergy.com