MazG – a regulator of programmed cell death in Escherichia coli
Summary
We have previously reported that mazEF, the first regulatable chromosomal ‘addiction module’ located on the Escherichia coli chromosome, downstream from the relA gene, plays a crucial role in the programmed cell death in bacteria under stressful conditions. It consists of a pair of genes encoding a stable toxin, MazF, and MazE, a labile antitoxin interacting with MazF to form a complex. The cellular target of MazF toxin was recently described to be cellular mRNA, which is degraded by this toxin. On the same operon, downstream to the mazEF genes, we found another open reading frame, which was called mazG. Recently, it was shown that the MazG protein has a nucleotide pyrophosphohydrolase activity. Here we show that mazG is being transcribed in the same polycistronic mRNA with mazEF. We also show that the enzymatic activity of MazG is inhibited by MazEF proteins. When the complex MazEF was added, the enzymatic activity of MazG was about 70% inhibited. We demonstrate that the enzymatic activity of MazG in vivo causes depletion of guanosine 3′,5′-bispyrophosphate (ppGpp), synthesized by RelA under amino acid starvation conditions. Based on our results, we propose a model in which this third gene, which is unique for chromosomal addiction systems, has a function of limiting the deleterious activity of MazF toxin. In addition, MazG solves a frequently encountered biological problem: how to avoid the persistence of a toxic product beyond the time when its toxicity is useful to the survival of the population.
Introduction
In bacteria, programmed cell death (PCD) is mediated through a unique genetic system. This system consists of a pair of genes, which code for a stable toxin and an unstable antitoxin that prevents the toxic effect of the toxin. This toxin–antitoxin system (TA), first described in Escherichia coli on low copy number plasmids, was reported to be responsible for post-segregational killing effect. Thus, cells that were cured from the genetic element that carries this TA system are killed by the stable toxin because the unstable antitoxin is being degraded. This plasmid-borne pair of genes was called ‘addiction modules’ (Lehnherr et al., 1993; Engelberg-Kulka and Glaser, 1999) because the cells are addicted to the continuous presence of the ‘dispensable’ genetic element (Yarmolinsky, 1995). The mazEF addiction module reported previously (Aizenman et al., 1996; Engelberg-Kulka and Glaser, 1999) belongs to what was described as proteic killer system (Jensen and Gerdes, 1995). In this system, MazE is the unstable antitoxin, degraded in vivo by an ATP-dependent ClpPA serine protease, and antagonizing the toxic effect of the MazF stable toxic protein (Aizenman et al., 1996). MazEF expression is mainly regulated by negative autoregulation, in which MazE has a mild negative effect and the MazEF complex is strongly inhibiting the expression of the mazEF promoter (Marianovsky et al., 2001). The mazEF expression is also negatively regulated by guanosine 3′, 5′-bispyrophosphate (ppGpp), synthesized by RelA under amino acid starvation conditions (Aizenman et al., 1996). In this case, as in other cases, in which the expression of mazEF is inhibited, the inhibition causes reduction in colony-forming units (cfu) of the bacterial culture (Engelberg-Kulka and Glaser, 1999). Certain antibiotics, inhibiting either the transcription of the promoter of mazEF or the translation of its mRNA, were also found to cause mazEF-dependent cell growth inhibition (Sat et al., 2001).
The cellular target of MazF was recently described to be inhibition of translation (Christensen et al., 2003). It was found that MazF is an endoribonuclease, which preferentially cleaves mRNA at specific sites. However, few laboratories have reported contradictory results for the mechanism of cleavage (Christensen et al., 2003; Zhang et al., 2003a; Munoz-Gomez et al., 2004). According to data, published by Christensen et al. (2003), MazF inhibits translation by a ribosome- and codon-dependent manner and cleavage occurs between the second and third bases of a GGU and AAA codons. Zhang et al. (2003a) demonstrated that MazF is a sequence-specific endoribonuclease that cleaves single-stranded mRNAs at ACA sequences in a codon- and ribosome-independent manner, including the mRNA of mazG. This second version was further confirmed by a more recent report from the same laboratory showing that in vitro MazF functions similarly to RNase A, but with different sequence specificity (Zhang et al., 2005). In contrast, Munoz-Gomez et al. (2004) showed that MazF cleavage occurred at the NAC codon between the first and second nucleotides (where N is preferentially U or A), both in single- and in double-stranded mRNA without ribosomes.
A third open reading frame (ORF), described on the mazEF operon, was called mazG. The protein product of mazG was described to be a pyrophosphohydrolase of nucleotides (Zhang and Inouye, 2002). The same authors also described that the MazG protein interacts with the essential gene product of era. These authors show that purified E. coli MazG could hydrolyse all eight ribo and deoxyribonucleoside triphosphates into their corresponding monophosphates and PPi (Zhang and Inouye, 2002). The NTPase activity of MazG was also reported for the Thermotoga maritima MazG, which has both NTPase and pyrophosphatase activities (Zhang et al., 2003b). As MazG was found to be highly conserved among bacteria, it was suspected to be an essential bacterial gene. However, after an E. coli strain carrying a deletion of mazG was found to be viable, it seems that, under laboratory growth conditions, this gene was not essential (Zhang and Inouye, 2002). Hence, until now, the physiological role of mazG remains elusive. Recent research (Moroz et al., 2005), based on structure analysis of Sulfolobus solfataricus MazG and few related protein families, suggested their possible ‘house-cleaning’ function by hydrolysing and elimination of abnormal NTPs that are result of oxidative damage.
Our group is studying the cellular addiction system, which is comprised of the mazE and mazF genes, located upstream to the mazG gene. We speculated that the location of the mazG gene, in vicinity to the mazEF genes, was not random, and that the mazG gene might be connected to the cellular addiction system. Here we show that overexpression of mazG inhibits cell growth. We further demonstrate that mazG is transcribed in the same polycistronic mRNA as the mazEF genes, and that the MazEF proteins are able to inhibit the activity of MazG in vivo and in vitro. Finally, we show that although overexpression of mazG negatively affects (p)ppGpp accumulation in cells starved for amino acids, overexpression of mazG together with mazEF restores the levels of (p)ppGpp. Based on our results, we propose a model in which a third gene, mazG, which is a unique feature of chromosomal addiction systems, acts by limiting the deleterious effect of the MazF toxin.
Results
Toxic effect of MazG on cell growth and survival
As it was reported that MazG has an in vitro nucleotide pyrophosphohydrolase activity (Zhang and Inouye, 2002), we expected that overexpression of this protein might be toxic to cell growth and survival. To examine this notion, we transformed the strain MC4100ΔmazEFG relA+lacIq either with plasmid pKK-mazG or with a control plasmid pKK-223-3. As can be seen in Fig. 1, overexpression of mazG had an inhibitory effect on cell growth, as well as on the cfu capacity of the cells.
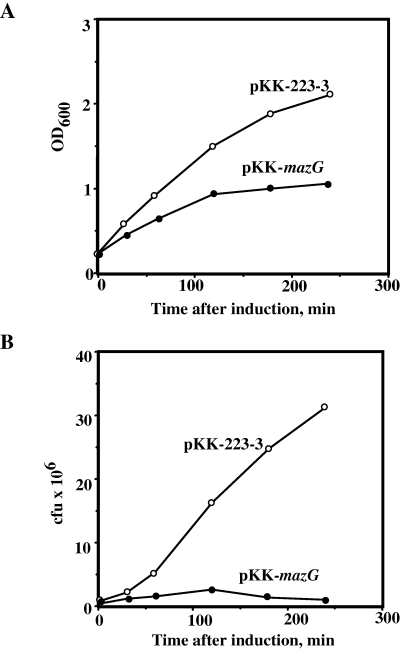
Toxic effect of MazG on cell growth and survival. E. coli strain MC4100ΔmazEFG relA+lacIq was transformed with pKK-223-3 or pKK-mazG plasmids (see Experimental procedures). The transformants were grown to an OD600 of 0.2, when 1 mM IPTG was added for induction. Aliquots from the culture were taken for the measurement of OD600 (A) and for cfu evaluation (B). Experiments were repeated three times, and typical results are presented.
The toxic effect of mazG was observed, when it was overexpressed as a single gene from an external tac promoter. In the E. coli genome the mazG gene is located downstream to the mazE and mazF pair of genes, which comprise the cellular addiction system (Fig. 2A). This system is activated under various stress conditions, including amino acid starvation (Aizenman et al., 1996; Christensen et al., 2003; Hazan et al., 2004).
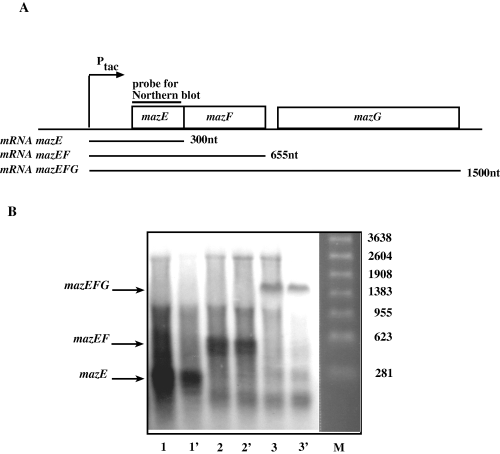
mazG transcript is a part of polycistronic mRNA together with mazE and mazF. A. Schematic representation of mazEFG operon, cloned into pKK-223-3 plasmid. mRNA transcripts, which can be received from this operon, and DNA probe, used for Northern blot, are drawn. B. The Northern blot results. Total RNA was isolated from cells, containing the various plasmids, grown in the M9 medium to early logarithmic phase and induced by 1 mM IPTG. Aliquots of 15 µg RNA were analysed by Northern blot. The used plasmids were: 1,1′- pKK-mazE, 2.2′– pKK-mazEF and 3,3′– pKK-mazEFG. M, molecular weight marker.
MazG transcript is part of a polycistronic mRNA together with mazE and mazF
Next, we investigated whether mazG is transcribed as a single gene or whether it is a component of the mazEF operon, therefore part of a polycistronic message containing the mazEF genes. For this purpose, we transformed strain MC4100ΔmazEFG relA+lacIq with plasmids expressing MazE (pKK-mazE), MazEF (pKK-mazEF) or MazEFG (pKK-mazEFG) from an inducible promoter (see Experimental procedures). Northern blot analysis of the transformed cells, using mazE gene as a probe, resulted in mRNA transcripts that matched the expected sizes for the mazE gene (300 nt) and mazEF genes (655 nt). The cells transformed with pKK-mazEFG gave a single transcript of 1500 nt that corresponded to a polycistronic message containing all three genes (Fig. 2B). The results of the Northern blot analysis revealed that mazG is part of the operon, including the mazE and mazF genes. It should be noted that as we used mazE as a probe, we cannot exclude the possibility that other transcripts of mazG exist.
Protection of cells by MazEF against the toxic effect of MazG
Next, we examined whether overexpression of MazG together with MazE and MazF from a polycistronic message (mazEFG) would also result in inhibition of cells growth.
MC4100ΔmazEFG relA+lacIq cells were transformed with pKK-mazG or pKK-mazEFG. To ascertain that the levels of the MazG protein are similar in both transformed cells, we induced protein expression under various isopropyl β-d-thiogalactopyranoside (IPTG) concentrations and conducted a Western blot analysis using polyclonal antibodies against the MazG protein. We found that induction of pKK-mazG at 0.1 mM IPTG and pKK-mazEFG at 1 mM resulted in similar levels of MazG (Fig. 3A). We therefore used these IPTG concentrations to induce MazG or MazEFG expression in the transformed cells. Surprisingly, we found that the growth rate of cells, overexpressing MazEFG from a polycistronic transcript, was not affected, as opposed to cells that overexpressed MazG (Fig. 3B). These results indicate that when the three proteins were expressed together, MazE and MazF inhibited the harmful effect of MazG on cell survival.
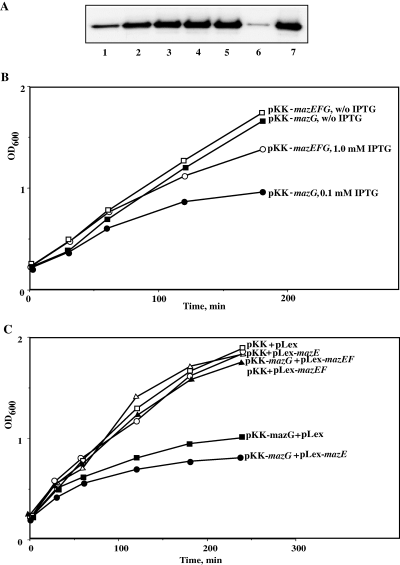
The toxic effect of MazG on cell growth is inhibited by MazEF proteins both in cis and in trans. A. Western blot analysis of amounts of MazG protein, expressed from pKK-mazG and pKK-mazEFG plasmids by different IPTG concentrations. E. coli strain MC4100ΔmazEFG lacIqrelA+ was transformed by the corresponding plasmids, and IPTG was added, when the transformants reached OD600 of 0.2. Samples for Western blot were taken 30 min after induction: lanes 1–5 – pKK-mazG and lanes 6–7 – pKK-mazEFG transformants. Lanes 1 and 6 – without IPTG, lane 2 – 0.01 mM IPTG, lane 3 – 0.025 mM IPTG, lane 4 – 0.05 mM IPTG, lane 5 – 0.1 mM IPTG and lane 7 – 1 mM IPTG. B. The same transformants were grown to OD600 of 0.2. The cells carrying pKK-mazG plasmid were induced by 0.1 mM IPTG, and the cells carrying pKK-mazEFG plasmid were induced by 1 mM IPTG. Samples were withdrawn for OD measurements after certain time intervals. C. The same E. coli strain was transformed by the compatible plasmids: pKK-223-3 + pLex, pKK-223-3 + pLex-mazE, pKK + pLex-mazEF, pKK-mazG + pLex, pKK-mazG + pLex-mazE, and pKK-mazG + pLex-mazEF. All the proteins were induced by the addition of 1 mM IPTG after the transformants reached OD600 of 0.2. Samples were withdrawn for OD600 measurements after certain time intervals.
Next, we investigated whether the protection of MazE and MazF against the inhibitory effect of MazG that was observed, when the three genes were co-transcribed, could also occur, when the MazE and MazF proteins supplied in trans. For this purpose, we cloned the mazE and mazEF genes in compatible plasmids (pLex-mazE and pLex-mazEF respectively). It should be noted that mazF could not be cloned as a single gene because it is toxic to the cells. We then transformed MC4100ΔmazEFG relA+lacIq cells with pKK-mazG together with pLex-mazE, or together with pLex-mazEF, and followed cell growth. As can be seen in Fig. 3C, overexpression of MazE together with MazG did not inhibit the deleterious effect of MazG on cell growth. However, overexpression of both MazE and MazF together with MazG completely restored the cells to a cell growth rate similar to that of control cells. These results demonstrate that the protective effect of MazE and MazF against the toxic effect of MazG could be achieved not only when the three genes were transcribed on the same mRNA transcript, but also when they were expressed separately.
MazEF complex inhibits in vitro nucleotide pyrophosphohydrolase activity of MazG
The protective effect of MazEF against the toxic effect of MazG was observed in vivo. Next, we examined whether this effect could be also demonstrated in vitro with purified proteins. For this purpose, we tested the hydrolytic activity of purified E. coli MazG using radioactively labelled GTP or ATP as substrates. We first optimized MazG concentrations (Fig. 4A) and consequently 1 µg of MazG was used for further experiments. We then confirmed the results of Zhang and Inouye (2002) showing that 1 µg of MazG efficiently hydrolysed both ATP and GTP to their corresponding monophosphates (Fig. 4B). Moreover, MazG hydrolysed GTP more efficiently than ATP (Fig. 4B). Next, we tested the effect of purified MazE, MazF and MazEF proteins on the enzymatic activity of MazG. As can be seen in Fig. 4C, the enzymatic activity of MazG was not affected by the addition of purified MazE or MazF proteins. However, when 20 µg of MazEF was added, a 70% inhibition of the enzymatic activity of MazG was observed. It should be noted that this preparation of MazEF contained 1 µg of MazF, whereas even 2 µg of MazF did not affect the activity of MazG. Thus we conclude that only the MazEF complex was inhibiting the MazG enzymatic activity (Fig. 4C and D).
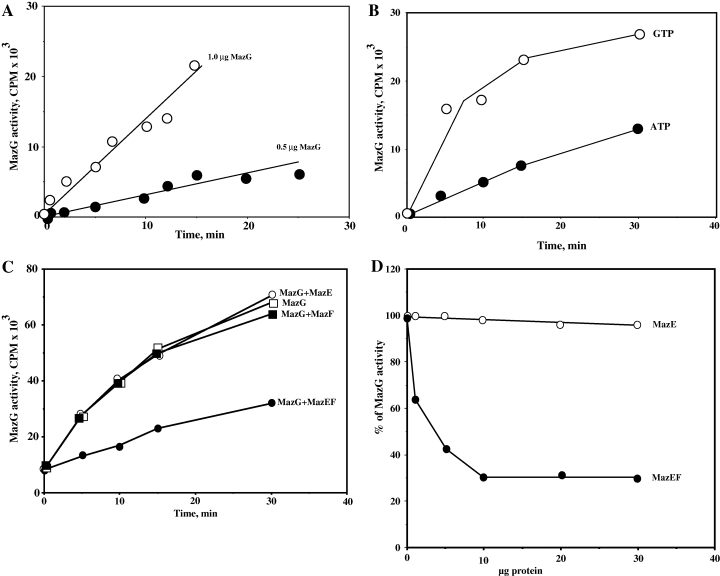
MazE-MazF complex effectively inhibits nucleotide pyrophosphohydrolase activity of MazG. A. Concentration dependence of MazG activity. A total of 0.5 µg and 1.0 µg of MazG was used for hydrolysis of α-[32P]ATP. The reaction conditions are described in Experimental procedures. B. Graphical presentation of time-dependence of ATP and GTP hydrolysis processes. A total of 1.0 µg of MazG was used. C. Influence of MazE, MazF and their complex on enzymatic activity of MazG. The following amounts of purified proteins were used: 1 µg of MazG; 50 µg of MazE; 2 µg of MazF; 20 µg of MazEF complex, contains 1 µg of MazF. D. Dependence of inhibition effect on protein concentrations: empty circles present effect of addition of MazE on hydrolysis of ATP by 1 µg of MazG, and filled in circles are the same reaction in the presence of 30 µg of MazE-MazF complex. One hundred per cent is hydrolysis of ATP by 1 µg of MazG without addition of any proteins.
MazG prevents the induction of the stringent response
As GTP and other ribonucleoside triphosphates were found to be substrates for MazG enzymatic activity (Zhang and Inouye, 2002), we wanted to test whether MazG was also involved in the regulation of the cellular levels of (p)ppGpp, the key regulator of the stringent response. (p)ppGpp is synthesized in cells in response to amino acid starvation by the product of the relA gene. RelA catalyses the pyrophosphoryl group transfer of two phosphates from the ATP donor to GTP (or GDP) to generate (p)ppGpp (Cashel et al., 1996). MazG involvement in the regulation of (p)ppGpp cellular levels might be direct, through hydrolysis of (p)ppGpp, or indirect by hydrolysis of GTP and/or ATP. To examine the effect of MazG on (p)ppGpp cellular levels, we compared the growth of MC4100ΔmazEFG relA+lacIq control cells with the same cells overexpressing MazG (pKK-mazG) on plates containing 15 mM 3-amino-1,2,4-triazole (AT). AT is a histidine analogue, which induces histidine starvation. As (p)ppGpp is a positive regulator of the his biosynthetic operon, only cells with high enough levels of (p)ppGpp can grow in the presence of AT. When we induced MazG expression to a level that did not inhibit growth on minimal plates, cells carrying MazG became AT sensitive, in contrast to control cells, which remained AT resistant (data not shown).
The results of the AT test suggest that cells overexpressing MazG accumulate lower levels of (p)ppGpp upon histidine starvation. However, sensitivity to AT is a qualitative assay that can only distinguish between high and low levels of (p)ppGpp. We therefore performed a quantitative assay of (p)ppGpp accumulation during amino acid starvation, for strain MC4100ΔmazEFG relA+lacIq, which was induced to express MazG (pKK-mazG), or MazEFG (pKK-mazEFG). As can be seen in Fig. 5A, 15 min after the addition of serine hydroxamate (SH), we observed high levels of (p)ppGpp in control cells carrying the vector pKK-223-3 plasmid (data not shown). In contrast, the levels of (p)ppGpp in cells overexpressing MazG were approximately twofold lower than the control cells. Cells overexpressing MazEFG accumulated (p)ppGpp to levels similar to those of control cells. Taken together, the results of the AT test and the measurement of (p)ppGpp levels indicate that overexpression of MazG negatively affects the accumulation of cellular (p)ppGpp, thus leading to inhibition of the activation of the stringent response. However, expression of MazEF together with MazG inhibits the negative effect of MazG on (p)ppGpp accumulation.
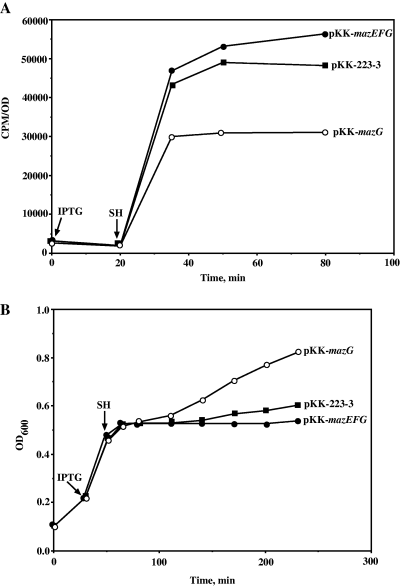
MazG prevents the induction of the stringent response. A. Escherichia coli strain MC4100ΔmazEFG lacIqrelA+ was transformed with pKK-223-3, pKK-mazG or pKK-mazEFG plasmid. The cultures were grown to an OD600 of 0.1. At this point the cells were labelled by the addition of [33P]Na3PO4 for 30 min. We have then added IPTG to a final concentration of 1 mM. Twenty minutes later SH was added to a final concentration of 1 mg ml−1 in order to induce the stringent response. Samples were withdrawn for analysis of ppGpp accumulation. B. In experiment described in (A) samples were also taken for monitoring cell density.
Interestingly, when cell growth was monitored, immediately following addition of SH, we observed a growth arrest in the control cells or in cells expressing MazG or MazEFG. However, cells expressing MazG became released from this inhibition, reverting to their original growth rate before initiation of the amino acid starvation (Fig. 5B). This observation seemed to correlate with the cellular levels of (p)ppGpp. The control cells and the cells transformed by pKK-mazEFG accumulated higher levels of (p)ppGpp, thus inhibiting their growth rate. In contrast, cells overexpressing MazG accumulated lower levels of (p)ppGpp, thus restoring their normal growth rate.
MazG functions as a nutritional stress survival gene
The presence of mazG on the mazEF operon has a positive effect on cell survival. As shown above, MazG can regulate the intracellular levels of (p)ppGpp under amino acid starvation. We have therefore asked: does the presence of mazG in the chromosome affects cell survival under nutritional stress conditions? In order to answer this question, wild-type cells as well as cells deleted for mazG were exposed to limited amino acid starvation. This was done by exposing the cells for 7 h to low concentrations of SH (0.2 mg ml−1). Seven hours after SH addition, the E. coli W3110 and W3110ΔmazG cells were harvested, washed by M9 salts medium and plated on Luria–Bertani (LB) agar plates with serial dilutions. Under these mild starvation conditions, no effect on cfu was observed in wild-type cells. However, as can be seen in Fig. 6, cells deleted to mazG showed two orders of magnitude less cfu. Actually the number of cells in the wild-type control was similar to that of a culture which was not exposed to amino acid limitation at all (data not shown). These results are in line with our model, suggesting that mazG has a protective function on cells under nutritional stress.
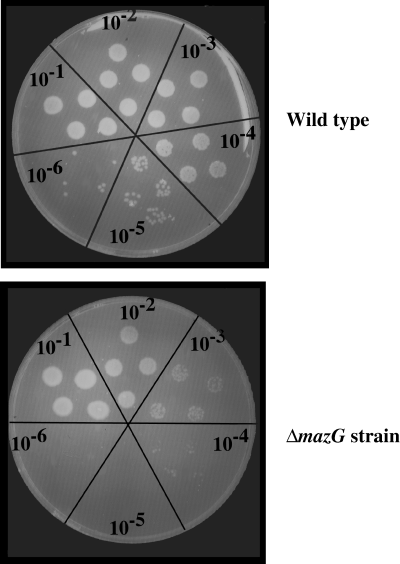
MazG causes increased cell survival at starvation. Wild-type of E. coli W3110 strain and its ΔmazG derivative were grown on M9 medium to an OD600 of 0.2. At this point 0.2 mg ml−1 (final concentration) SH was added, and cells were exposed to this concentration for 7 h. Treated cells were washed with minimal M9 medium in order to remove SH, and dropped on LB plates with serial dilutions. Control plates, which were not exposed to SH, are not shown. Colony-forming unit of both of control plates, wild-type and ΔmazG cells, was the same as that of E. coli wild-type cells exposed to SH.
Discussion
Toxin–antitoxin modules (TA) are usually carried on low copy plasmids to ensure that missegregated cells will not survive. However, it was found that while bacterial chromosomes also harbour TA cassettes that are homologous to those identified on plasmids, they apparently fulfil a different function (Engelberg-Kulka and Glaser, 1999; Gerdes, 2000; Christensen et al., 2003; Pandey and Gerdes, 2005; Gerdes et al., 2005). These TA elements seem to be involved in PCD of the bacteria. PCD is a process that occurs in multicellular, eukaryotic organisms during development. As bacteria were considered to function as unicellular organisms, they were not considered to have PCD. However, this concept was recently challenged and it was suggested that bacteria also possess PCD mechanisms involved in regulating their multicellular organization (Jensen and Gerdes, 1995; Yarmolinsky, 1995; Engelberg-Kulka and Glaser, 1999). Thus, several chromosomal TA elements were described on the E. coli chromosome, and it was suggested that they are either PCD genes or mediators of reversible cell cycle arrest (Pedersen et al., 2002; Amitai et al., 2004; Gerdes et al., 2005; Pandey and Gerdes, 2005). These processes in bacteria might allow surviving cells to scavenge nutrients from dead siblings. Acting as cell cycle arrest factors, these TA elements allow cells to enter a dormant or semidormant state as a protective mechanism against severe nutrient limitation, and then revive when environmental conditions improve (Pedersen et al., 2002; Gerdes et al., 2005). The mazEF TA locus is a very good example of these kinds of processes. Thus, MazE acts as an antidote to the toxin MazF. Transcriptional or translational inhibition of mazEF expression results in the rapid degradation of the antitoxin MazE and thus in activation of the toxin MazF and to cell death or cell growth arrest. Blockage of mazEF expression was found to be triggered by 3′, 5′-guanosine bispyrophosphate (ppGpp) (Aizenman et al., 1996), the product of RelA protein under amino acid starvation conditions (Cashel et al., 1996). Thus, this TA element seems to be triggered under nutritional stress.
Here we have described a third gene in the mazEF module of E. coli. This third ORF, mazG, codes for a protein of about 30 kD. We found that it is a stable protein (Fig. 7), and have also confirmed its enzymatic activity described by Zhang and Inouye (2002) as a nucleoside triphosphate pyrophosphohydrolase. Due to its enzymatic activity, we expected that overproduction of this protein would be toxic to cell growth. We cloned the gene under an inducible promoter and showed that when we overproduced the protein, cell growth was inhibited with a negative effect on cfu. We have also shown that MazG affects the cellular levels of (p)ppGpp, as shown by decreased resistance to AT. Thus, our data support the notion that the presence of MazG contradicts the phenomena observed upon amino acid limitation.
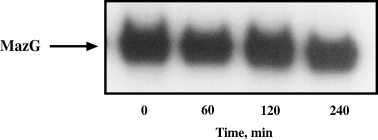
MazG is a stable protein. Western blot analysis of E. coli strain MC4100ΔmazEFG relA+lacIq, transformed with pKK-mazG, which was grown to an OD600 of 0.2, induced by 1 mM IPTG for 30 min and treated with 1 mg ml−1 rifampicin for breaking off the further protein synthesis. The samples were taken at 0, 1, 2 and 4 h after rifampicin addition.
Northern blot analysis of this same mazEFG clone has shown that a polycistronic message was being generated containing all three ORFs –mazE, mazF and mazG. Having this TA system being transcribed as a three-gene polycistronic message suggests that these proteins may either interact or at least their function must somehow be related. As for MazG, which like MazF is a stable protein, it seems that the amounts of protein attained are twice those of MazE (data not shown). Thus, we were surprised to see that when mazEFG operon was overexpressed from the cloned plasmid, we could not observe any toxic effect of MazG, although the amounts that were synthesized, were the same as when we expressed mazG alone, and witnessed its toxic effect. This immediately suggested that when MazE and MazF are present, they somehow mask the activity of MazG. Thus, as can be seen in Fig. 3B, when mazE and mazF were induced in trans to the expression of mazG, again, we could not observe any effect on cell growth or cell survival; in fact, cells continue to grow and the number of cfu was not affected.
As we expected that the toxic effect of MazG results from its pyrophosphohydrolase activity, we assayed this activity in an in vitro purified system. As can be seen in Fig. 4C, the addition of either MazE alone or MazF alone did not affect the enzymatic activity of MazG. However, when the complex MazEF was added, the enzymatic activity of MazG was about 70% inhibited. These results are in line with our in vivo results concerning the protective effect of MazEF against the toxic effect of MazG.
Based on these results, we suggest a model (Fig. 8), which explains the need for a third protein in the mazEF TA module. As MazF is a very toxic protein and very small amounts will cause complete cessation of cell growth, it seems that harbouring a ‘limiting gene’ which can save cells from nutritional stress, is of utmost importance. Pedersen et al. (2002) have shown that the toxic effect of MazF results, for at least few hours, in only bacteriostatic state, like a dormancy of the cells. This situation is reversible, when the antitoxin is overproduced. On the other hand, once the toxic effect of MazF is exerted for a longer period of time, cells cannot be ‘saved’ from the deleterious activity of MazF (Amitai et al., 2004). We have shown that the mazEF TA system can be activated by the product of starvation – (p)ppGpp, which in turn inhibits the expression of the mazEF promoter, and thus stops the continuous synthesis of MazE. MazE is labile, it is rapidly degraded, and enable the toxic activity of MazF. On the other hand, degradation of MazE abolishes the inhibition of MazG by the MazEF complex. As a consequence, MazG is activated and hydrolyses nucleoside triphosphates; this lowers (p)ppGpp levels causing cells to emerge from the nutritional stress they were in. In fact, we find that overproduction of MazG has a strong effect on cellular (p)ppGpp levels, because it changes the sensitivity of wild-type, relA+E. coli, to AT. We also have shown this effect directly, by evaluating the cellular levels of (p)ppGpp upon amino acid starvation (Fig. 5A). It is known that wild-type E. coli can cope with the histidine analogue AT (Cashel et al., 1996). High cellular levels of (p)ppGpp gain resistance to this histidine analogue. MazG were found to lower this resistance. As long as mazEF and mazG are expressed together, MazG enzymatic activity is inhibited. However, under nutritional stress conditions (p)ppGpp is being synthesized and inhibits mazEF promoter. When the labile antitoxin – MazE is degraded, MazF can exert its toxic activity. As we have shown that only MazE and MazF together are inhibiting MazG, once MazE is degraded, this inhibition is released, and MazG is degrading (p)ppGpp and other nucleotides, which in turn will release the mazEF promoter. This will cause therefore – re-synthesis of MazE, which will trigger the cells to emerge from their dormant state. In order to confirm the suggested model, we have performed an experiment, which compared the ability of wild-type E. coli cells and its ΔmazG mutant to survive after long exposure to mild starvation conditions. In accordance with model's predictions, we obtained a significant difference, of two orders of magnitude, in the ability of cells to persist under starvation conditions and to form colonies, between the wild-type cells and ΔmazG mutant cells (Fig. 6). Such a strong protective effect of MazG proves its important role in cell's self-defence processes. First of all, it regulates the concentration of the main stress messenger – (p)ppGpp, which, in turn, changes all cellular metabolism as a response to nutritional stress. Having shown here that the mazEF TA element is activated by (p)ppGpp, as well as the interaction of MazEF with MazG, the role of MazG in this system seems to be crucial. Thus, based on our results, we suggest that MazG is an important element for cell survival under nutritional stress conditions. One of the important consequences of such (p)ppGpp concentration changes was triggering the PCD mechanism. Promoters of at least one of the chromosomal addiction modules, namely mazEF, are inhibited by this messenger (Aizenman et al., 1996). So, decreasing concentration of (p)ppGpp, caused by MazG, allows the synthesis of antitoxin MazE and neutralization of MazF toxin. Thus, the existence of mazG has a protective regulatory effect against the very harmful presence of the toxic mazF on the chromosomal TA module. Therefore, MazG activity results in restraining of death mechanisms and delaying ‘a point of no return’ (Amitai et al., 2004) in the expectation of improving growth conditions.
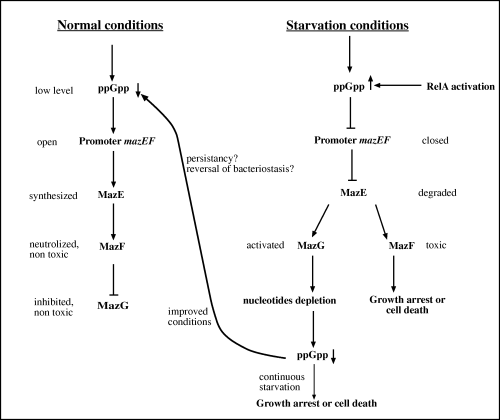
A model, representing possible regulatory role of MazG in programmed bacterial cell death during nutritional stress in E. coli. Nutritional limitation which results in cellular (p)ppGpp accumulation leads to MazF- mediated cell growth arrest or cell death. MazG, which is inhibited by MazEF proteins, is activated when the cellular levels of MazE are decreased as a result of lack of synthesis and degradation. Activation of MazG results in lowering cellular levels of the alarmon (p)ppGpp thus releasing the promoter of the mazEF module enabling synthesis of the anti-toxin MazE which neutralizes the toxin MazF.
Experimental procedures
Media
The media used were LB broth, M9 medium, LB agar (Bio101) or M9 agar supplemented with the appropriate antibiotics at the following final concentrations: 100 µg ml−1 ampicillin, or 34 µg ml−1 chloramphenicol, or 50 µg ml−1 kanamycin.
Bacterial strains
The bacterial strains used in this work are listed in Table 1. The bacterial strain MC4100 and its derivative bearing the mazEFG null allele were described previously (Aizenman et al., 1996). The described mazEF allele is accompanied also by the deletion of the most part of mazG gene. The mazG-deleted derivative of the E. coli W3110 strain (K-12 wild-type) was constructed by the recombineering method (Yu et al., 2003). The mazG gene was exchanged for the kanamycin resistance gene using the primers FKΔG and BKΔG (Table 2).
| Strain/plasmid | Relevant phenotype/construction | Source/reference |
|---|---|---|
| E. coli strains | ||
| MC4100 | araD139 (argF-lac)205 flb5301 ptsF25 rpsL150 deoC1 relA1 | New England Biolabs |
| MC4100ΔmazEFG | A ΔmazEFG derivative of MC4100, kanR | Aizenman et al. (1996) |
| MC4100ΔmazEFG relA+ | A relA+ derivative of MC4100ΔmazEFG | Engelberg-Kulka et al. (1998) |
| MC4100ΔmazEFG relA+ lacIq | A lacIq (tetR) derivative of MC4100ΔmazEFG relA+ | This work |
| W3110 | Wild-type K-12 | ATCC 27325 |
| W3110ΔmazG | A ΔmazG derivative of W3110, kanR | This work |
| Plasmids | ||
| pKK-223-3 | Expression vector with strong tac promoter, AmpR | Amersham Pharmacia Biotech |
| pKK-mazG | A pKK-223-3 derivative carrying the mazG gene | This work |
| pKK-mazEFG | A pKK-223-3 derivative carrying the mazE, mazF and mazG gene | This work |
| pLex1 | Vector for the construction of plasmid set compatible with pKK-223-3, CamR | Diederich et al. (1994) |
| pLex-mazE | A pLex1 derivative carrying mazE gene under ·the tac promoter, CamR | Marianovsky et al. (2001) |
| pLex-mazEF | A pLex1 derivative carrying mazE and mazF genes under the tac promoter, CamR | Marianovsky et al. (2001) |
| pQE30 | Vector for high-level expression of his-tagged proteins, AmpR | Qiagen |
| pQE30-mazE | A pQE30 derivative carrying mazE gene with his-tag | Loris et al. (2003) |
| pQE30-mazF | A pQE30 derivative carrying mazF gene with his-tag | This work |
| pQE30-mazG | A pQE30 derivative carrying mazG gene with his-tag | This work |
| pQE30-mazEF | A pQE30 derivative carrying mazE gene with his-tag and mazF gene | This work |
| Name | Sequence (5′-3′) | Purpose |
|---|---|---|
| FKΔG | ggaattacaactcattaaagccaaaattaacgtactgattgggtagggggggggggggaaagcc | Exchange of mazG gene for the kanamycin resistance gene |
| BKΔG | catttaaaaaatgacacaaatggcgcttgaccgcgtaattcccttagaaaaactcatcgagcatc | Exchange of mazG gene for the kanamycin resistance gene |
| EE1 | ggaattcgaaggagatatacatatgatccacagtagcgtaaag | Cloning of mazEFG into pKK-223-3 vector |
| M2 | gcgacgaagcttagagatcaatttcctgcc | (i) Cloning of mazEFG or mazG into pKK-223-3 vector; (ii) cloning of mazG into pQE30 vector |
| OEG | ggaattcgaaggagatatacatatgaatcaaatcgaccgtttg | Cloning of mazG into pKK-223-3 vector |
| M3 | cgggatccatgaatcaaatcgaccg | Cloning of mazG into pQE30 vector |
| FUPC | gcgacgagatctgatgacgatgacaaaatggtaagccgatacgtacccgatatg | Cloning of mazF into pQE30 vector |
| FG1 | gcgacgaagcttctacccaatcagtacgttaa | Cloning of mazF or mazEF into pQE30 vector |
| EUPC | gcgacgagatctgatgacgatgacaaaatgatccacagtagcgtaaagcgttgg | Cloning of mazEF into pQE30 vector |
Plasmids bearing the genes of mazEFG operon
The plasmids used in work are listed in Table 1.
Construction of plasmids pKK-mazEFG and pKK-mazG bearing the mazE, mazF and mazG genes or mazG gene alone, correspondingly, was done as described previously for pKK-mazE and pKK-mazEF (Marianovsky et al., 2001). In these plasmids the ORFs of corresponding genes were cloned under the tac promoter present on the expression vector pKK-223-3. The primers (see Table 2), used for polymerase chain reaction (PCR)-synthesis of mazEFG, were: EE1 and M2; and for mazG synthesis were: OEG and the same primer M2 as for mazEFG.
We also previously described the construction of the modified pKK-223-3-compatible plasmids pLex-mazE and pLex-mazEF that bear the chloramphenicol resistance gene, the p15A replication origin, and either mazE or mazEF such that they were under the control of the ptac promoter (Marianovsky et al., 2001).
In order to purify MazG and MazE proteins and MazE-MazF complex, we introduced respective ORFs into the high-level expression vector pQE30 (Qiagen). This vector contains the 6-his tag coding sequence between start codon and multilinker for desired gene cloning. The following primers were used for respective genes cloning (Table 2): for mazG– M3 and M2; for mazF– FUPC and FG1; for mazEF– EUPC and FG1. PCR products were digested with BglII and HindIII, and cloned into BamHI and HindIII sites of the pQE30 vector. Primers FUPC and EUPC include the sequence for enterokinase digestion after BglII site. Cloning of mazE gene into pQE30 was described in previous paper (Loris et al., 2003).
Culture conditions and cfu determination
Escherichia coli strain MC4100ΔmazEFG relA+lacIq was transformed with pKK-mazG or pKK-223-3 control plasmid. Transformants were grown overnight at 37°C in LB medium supplemented with ampicillin and kanamycin. The cells from overnight culture were diluted 1:100 in 25 ml of M9 medium with supplements (0.2% glucose, all amino acids – 20 µg ml−1 each, 0.05 mM thiamine, 2 mM MgSO4, 0.1 mM CaCl2 and ampicillin). The cultures were grown with shaking 300 rpm at 37°C to the early log phase (OD600 approximately 0.2). At this point, 1 mM IPTG was added to the cultures. Samples were collected at the appropriate times, washed in M9 minimal medium, diluted, plated on M9 agar plates, and incubated at 37°C overnight. Monitoring of cultures’ optical density was done at the same time points. Cell survival was evaluated by comparing the colony-forming ability of cells, which contain pKK-mazG plasmid, with that of control cells, which contain empty pKK-223-3.
The same culture conditions were used when MC4100ΔmazEFG relA+lacIq was transformed with pKK-mazG or pKK-mazEFG, which were induced by 0.1 mM and 1.0 mM IPTG respectively.
Escherichia coli strain MC4100ΔmazEFG relA+lacIq was also transformed with pairs of compatible plasmids, supplying MazG and MazE or MazEF genes in trans, in the following combinations: (i) pKK-223-3 and pLex – control combination; (ii) pKK-mazG and pLex; (iii) pKK-mazG and pLex-mazE; (iv) pKK-mazG and pLex-mazEF; (v) pKK-223-3 and pLex-mazE. The transformants were grown, as in previous experiment, in M9 medium, contained besides the noted supplements also chloramphenicol. The induction was carried out by addition of 1 mM IPTG, and cell growth was monitored by the measuring the OD600.
In order to test cell survival under nutritional starvation, both E. coli wild-type and its ΔmazG derivative were grown on M9 medium to an OD600 of 0.2. At this point, 0.2 mg ml−1 (final concentration) of SH was added, and cells were exposed to this concentration for 7 h. Treated cells were washed with minimal M9 medium in order to remove SH, and dropped on LB plates with serial dilutions.
RNA isolation and Northern blotting
Total RNA was isolated from cells, grown in the M9 medium to early logarithmic phase and induced by 1 mM IPTG, using RNeasy kit (Quiagen). RNA concentrations were determined by measuring the OD values of 260 and 280 nm. Aliquots of 15 µg were subjected to electrophoresis in denaturing morpholinepropane sulphonic acid (MOPS)-formaldehyde 1.2% agarose gel (Sambrook et al., 1989). The RNA was then transferred onto a positively charged nylon membrane and UV-cross-linked. The PCR-generated probes were prepared for mazE gene (the same primers as above). The PCR fragment was labelled by a Renaissance random primer fluorescein labelling kit (NEN Life Science Products). After hybridization, the washed membranes were autoradiographed for 8–12 h on Kodak film.
Western blot
Strains were grown at 37°C in M9 medium as indicated above. At OD600 of 0.2 (early logarithmic growth phase) expression of the mazEFG operon proteins was induced by the addition of IPTG, and after 1 h induction cells were collected by centrifugation (12 500 g, 4 min), resuspended in BugBuster Protein Extraction Reagent (Novagen) and 5 µg of each extract was added to loading buffer [50 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulphate (SDS), 10% glycerol, 2.5%β-mercaptoethanol and 0.1% bromophenol blue]. Protein solutions were fractionated on 12% SDS-polyacrylamide gel (Laemmli, 1970). After electrophoretic transfer of proteins onto nitrocellulose membranes (Towbin et al., 1979), using rabbit primary antibody raised against this protein revealed MazG. The anti-rabbit antibody raised in goat coupled to peroxidase was used as secondary antibody (Zymed Laboratories). ECL chemiluminescent Western Blotting Detection Reagents kit (Amersham Biosciences) was used to visualize the desired proteins on Kodak X-ray film.
His-tagged MazG, MazE and MazF proteins and MazE-MazF complex purification
The E. coli MC4100ΔmazEFG relA+lacIq cells harbouring the pQE30-mazG were grown to midexponential phase in LB medium supplemented with 100 µg of ampicillin per ml, and then the expression of his-MazG was induced in the presence of 1 mM IPTG for 3 h. The cells were harvested by centrifugation, resuspended in buffer A (20 mM Na-phosphate buffer, pH 7.4, 0.5 M NaCl, 10 mM imidazole) supplemented with protease inhibitor cocktail Complete EDTA-free (Roche Diagnostics GmbH) and then sonicated. Cell lysate was centrifuged at 10 000 g for 15 min to remove cell debris and unbroken cells. The supernatant was loaded on a Ni-NTA agarose column (Qiagen). The column was washed with the buffer A containing 20 mM imidazole, and his-MazG was eluted with 0–0.3 M imidazole in buffer A. Fractions containing his-MazG were pooled together and dialysed against buffer B (50 mM Tris-HCl, pH 8.0; 1 mM EDTA; 2 mM DTT; 0.9% NaCl; 50% glycerol). Protein concentrations were measured with the Bio-Rad protein assay dye reagent. Purification of his-MazE protein was described previously (Loris et al., 2003). The his-tagged MazF or MazE-MazF complex were purified in the same fashion from the E. coli transformant bearing pQE30-mazF or pQE30-mazEF plasmid respectively.
MazG activity determination
To identify the hydrolysed products of GTP or ATP, [α-32P]GTP or [α-32P]ATP, respectively, were used for the MazG activity determination. The reaction was carried out in a 20 µl reaction mixture of 50 mM Tris-HCl (pH 7.5) containing 400 mM KCl, 5 mM MgCl2, 1 mM DTT, 10 µM [α-32P]GTP or [α-32P]ATP, and 1 µg of purified MazG protein at 37°C. Transfer of 5 µl aliquots to 10 µl of ice-cold 20 mM EDTA terminated the reaction. Portions of the terminated reaction mixture were spotted onto a polyethyleneimine-cellulose (PEI) thin layer chromatography (TLC) plate, which was developed in 0.75 M KH2PO4 (pH 3.7). The plate was autoradiographed to identify monophosphate nucleotide products. Spots corresponding to GTP and GDP were identified under UV light. Amounts of tri- and monophosphate nucleotides were evaluated on Fijix Bas100 PhosphorImager (Japan) and the results were presented as CPM × 103. Every experiment was repeated at least three times.
In order to study the influence of MazE and MazF on the MazG activity, the same experiments were performed in the presence of corresponding proteins in the following concentrations: 50 µg MazE, 2 µg MazF and 20 µg MazEF complex.
Amino acid starvation experiments and measurements of ppGpp levels
Escherichia coli strain MC4100ΔmazEFG relA+lacIq was transformed with pKK-223-3, pKK-mazG or pKK-mazEFG plasmid. Transformants were grown overnight at 37°C in LB medium supplemented with ampicillin. The overnight cultures were diluted 1:100 in 25 ml of MOPS medium (Niedhardt et al., 1974). The cultures were grown with shaking 300 rpm at 37°C to the early log phase (OD600 approximately 0.1). At this point the cells were labelled by the addition of [33P]Na3PO4 for 30 min. After 20 min induction with 1 mM IPTG, cultures were exposed to SH, an analogue of serine, inducing the starvation condition. Changes in optical density of cultures were monitored and the samples were taken at different time intervals, and stored in ice after formic acid addition. Labelled nucleotides were separated by TLC in 1.5 M KH2PO4, pH 3.7. The levels of (p)ppGpp were evaluated on phosphoimager. Each experiment was repeated at least three times.
Checking the stability of MazG protein
The E. coli strain MC4100ΔmazEFG relA+lacIq containing pKK-mazG plasmid was grown in LB medium to OD600 approximately 0.2. At this time 1 mM IPTG was added for induction of MazG protein. After 30 min we stopped transcription, and as a consequence – protein synthesis, by the addition of 1 mg ml−1 rifampicin. The samples for Western blot were taken at 0, 1, 2 and 4 h after rifampicin addition.
Acknowledgements
We thank Dr Michal Gropp and Mrs Mery Clausen for critical reading of this manuscript. This work was supported by Israel Science Foundation Grant 507/03-16.2 awarded to G.G.