Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane
Summary
During the maturation of intracellular asexual stages of Plasmodium falciparum parasite-encoded proteins are exported into the erythrocyte cytosol. A number of these parasite proteins attach to the host cell cytoskeleton and facilitate transformation of a disk-shaped erythrocyte into a rounded and more rigid infected erythrocyte able to cytoadhere to the vasculature. Knob formation on the surface of infected erythrocytes is critical for this cytoadherence to the host endothelium. P. falciparum proteins have been identified that localize to the parasite-infected erythrocyte membrane: the variant cytoadherence ligand erythrocyte membrane protein 1 (PfEMP1), the knob-associated histidine-rich protein (KAHRP) and the erythrocyte membrane protein 3 (PfEMP3). In this study, we have generated parasites expressing PfEMP3-green fluorescent protein chimeras and identified domains involved in entry to the secretory pathway, export across the parasitophorous vacuolar membrane and attachment to Maurer's clefts and the erythrocyte membrane. Solubility assays, fluorescence photobleaching experiments and immunogold electron microscopy suggest that the exported chimeric proteins are trafficked in a complex rather than in vesicles. This study characterizes elements involved in the tight but transient binding of PfEMP3 to Maurer's clefts and shows that the same elements are necessary for correct assembly under the erythrocyte membrane.
Introduction
The malaria parasite resides within one of the most highly differentiated cells in the human body, namely the red blood cell (RBC). During the development of the intraerythrocytic asexual stages of Plasmodium falciparum parasites the RBC is dramatically modified leading to alterations in its morphology, deformability and adhesive properties (see for review Cooke et al., 2001). As a result mature P. falciparum-infected RBCs cytoadhere to the microvasculature in a variety of organs, which can cause severe clinical complications (Aikawa et al., 1990). The molecular basis of these structural and functional changes involve interactions between parasite proteins exported into the RBC and the RBC membrane cytoskeleton (Foley et al., 1991; Cooke et al., 2002; Glenister et al., 2002). A group of up to 400 parasite proteins are exported into the host RBC, via a conserved motif termed Plasmodium export element (PEXEL), and together with parasite-induced membrane structures transform a quiescent RBC into a cell capable of protein transport (Hiller et al., 2004; Marti et al., 2004).
The most dramatic change in the infected host cell is the appearance of knobs on the RBC membrane, which are electron dense cup-shaped structures underlying protrusions of the RBC membrane and these are composed predominantly of the knob-associated histidine-rich protein 1 (KAHRP1) (Kilejian, 1979). P. falciparum erythrocyte membrane protein 1 (PfEMP1), the variant cytoadherence-mediating antigen spans the RBC membrane and connects via its cytoplasmic domain to the underlying cytoskeleton and it is concentrated at the knob structure (Baruch et al., 1995; Oh et al., 2000). The extracellular domains of PfEMP1 bind to receptors on endothelial cells such as CD36, ICAM-1 and chondroitin sulphate (Berendt et al., 1989; Rogerson et al., 1995; Baruch et al., 1996). Recent data suggest that KAHRP has a role in the efficiency of PfEMP1 trafficking to the RBC surface (Horrocks et al., 2005).
The kahrp gene is located in the subtelomeric region of chromosome 2 closely linked to a second gene encoding P. falciparum erythrocyte membrane protein 3 (PfEMP3) (Gardner et al., 2002). PfEMP3 is a large, highly charged, protein of about 315 kDa; the N-terminal domain contains a putative hydrophobic signal followed by unique sequence while the C-terminal region is largely comprised of repetitive sequence elements (Pasloske et al., 1993). PfEMP3 is synthesized during ring and trophozoite stages of P. falciparum and transported into the RBC where it associates with membranous structures, known as Maurer's clefts, before relocation to the cytoplasmic surface of the RBC membrane. PfEMP3 can be detected in knob structures but is more broadly distributed under the parasite-infected RBC membrane. Unlike KAHRP, PfEMP3 is not essential for knob formation, but overexpression of a truncated form that fails to translocate from Maurer's clefts to the RBC membrane blocks PfEMP1 trafficking (Waterkeyn et al., 2000).
The molecular machinery involved in trafficking of proteins across the RBC cytosol is poorly understood. It has been suggested that some proteins involved in vesicle-mediated trafficking are associated with Maurer's clefts suggesting that trafficking of parasite proteins to the RBC involves budding and fusion of small vesicle-like structures (Albano et al., 1999; Trelka et al., 2000; Adisa et al., 2001; Hayashi et al., 2001). Indeed, both PfEMP1 and PfEMP3 have been detected associated with vesicle-like structures in the RBC cytosol (Trelka et al., 2000). However, recent data have suggested PfEMP1 may travel as a chaperoned complex through the parasite secretory system and the erythrocyte cytosol and insert into a membrane environment only upon reaching the Maurer's clefts (Knuepfer et al., 2005a; Papakrivos et al., 2005).
In this work, we have generated a series of constructs comprising segments of PfEMP3 fused to GFP, and used the plasmids to transfect P. falciparum-infected RBCs. We have identified a region within PfEMP3 required for tight association with Maurer's clefts and for assembly under the RBC membrane. We have used solubility studies and fluorescence photobleaching to study the physical state of the PfEMP3-GFP chimeras at different stages of the life cycle of these parasites to dissect their routes to their final destinations. These data have shown that association of PfEMP3 with Maurer's clefts is required for trafficking and assembly under the host membrane in P. falciparum-infected RBCs.
Results
Expression of PfEMP3-GFP fusion proteins in P. falciparum-infected RBCs
To confirm that the N-terminal hydrophobic signal sequence and the protein export element, PEXEL (Hiller et al., 2004; Marti et al., 2004), of PfEMP3 are sufficient for export across the parasitophorous vacuole (PV) and to identify the sequence elements responsible for attachment to Maurer's clefts and the cytoplasmic face of the RBC membrane, we generated a series of PfEMP3-GFP fusion proteins (Fig. 1). N-terminal segments comprising the first 66 amino acids (E66), 82 amino acids (E82), 120 amino acids (E120) or 500 amino acids (E500) of PfEMP3 were placed upstream of the coding sequence of mut2 eGFP as described previously (Waller et al., 2000; Wickham et al., 2001; Marti et al., 2004) (Fig. 1). These constructs each contained the putative ER entry signal and the PEXEL motif but not the repetitive regions of PfEMP3. The resultant PfEMP3-GFP chimeras were expressed in transfected 3D7 or D10 strains of P. falciparum blood-stage parasites. The 3D7 strain of P. falciparum expresses both KAHRP and PfEMP3 and is able to transport PfEMP1 to the RBC surface on which in mature stages of the asexual life cycle knob structures are formed (Crabb et al., 1997). The D10 strain of P. falciparum used in this study has a truncated chromosome 2 resulting in the loss of KAHRP and PfEMP3 expression and hence the lack of knobs on the infected RBC surface (Corcoran et al., 1986).
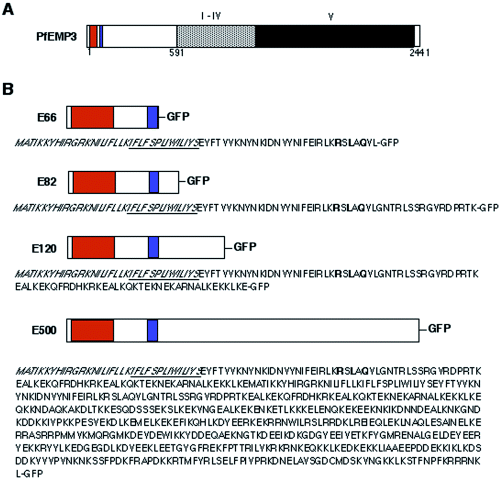
Schematic presentation of PfEMP3 structure and chimeric proteins expressed in P. falciparum. A. PfEMP3 has a putative recessed signal sequence (orange), a PEXEL motif (blue) for export into the host cell and five repeat regions labelled I–V (grey and black). White boxes represent unique, non-repetitive sequences. PfEMP3 is a 2441 amino acid protein with an apparent molecular weight of 315 kDa. PfEMP3 is very hydrophilic and lacks any membrane anchor regions. B. Structures and amino acid sequences of the chimeric proteins expressed in P. falciparum. Colours are the same as in A with the recessed signal peptide in orange and the PEXEL motif in blue. Underlined amino acid sequences represent the hydrophobic core of the putative signal peptide (amino acids in italics). Amino acids of the PEXEL motif are printed bold. E66-GFP represents the fusion protein of the N-terminal 66 amino acids of PfEMP3 attached to GFP; E82-GFP, the 82 N-terminal amino acids of PfEMP3 attached to GFP; E120-GFP, the N-terminal 120 amino acids attached to GFP; E500-GFP, the N-terminal 500 amino acids attached to GFP. Sizes of each domain are not drawn to scale.
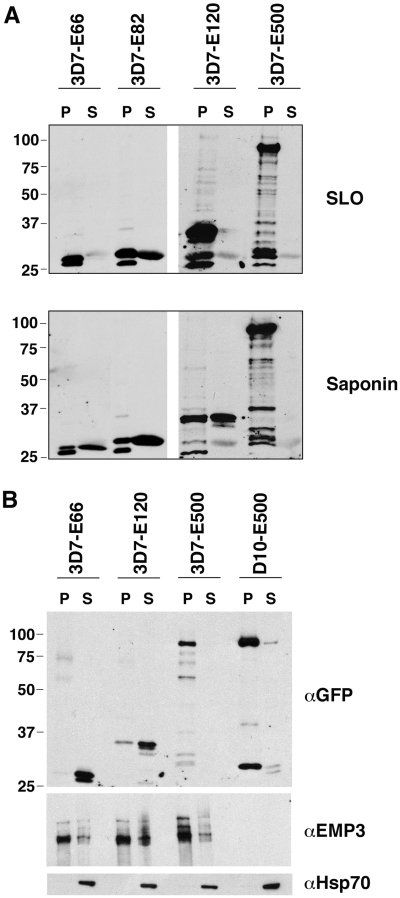
To examine the expression of the GFP chimeras we performed Western blots probed with αGFP antibody. Untransfected 3D7 trophozoite stages showed no reactivity but all transfected parasites expressed GFP fusion proteins (Fig. 2). The upper protein band in E66-GFP-expressing parasites was 30 kDa, in E82-GFP-expressing parasites, 32 kDa, in E120-GFP-expressing parasites, 37 kDa and in E500 transfectants approximately 85 kDa. These sizes correspond to the predicted molecular weights of the fusion proteins minus the putative signal peptide. A lower band of about 26 kDa size was observed in some of the parasite lines; this has been previously ascribed to a degradation product of GFP (Waller et al., 2000; Wickham et al., 2001). Additional degradation products were observed in heavily loaded samples from the D10-E500 transfectants (Fig. 2, right hand lane), 3D7-E500 transfectants (see for example, Fig. 6A) and wild-type PfEMP3 (Pasloske et al., 1993). In summary the data indicate that a range of PfEMP3-GFP fusion proteins can be successfully expressed in transgenic P. falciparum.

Western blot analysis of expression of PfEMP3-GFP chimeric proteins in transgenic P. falciparum. Trophozoite stages of wild-type P. falciparum (3D7 strain) as well as transfected P. falciparum lines E66, E82, E120 and E500 in the 3D7 strain and E500 in the D10 strain were subjected to Western analysis and probed with αGFP antibody (Roche, 1:1000). The upper band represents the full-length chimeric protein. The same immunoblot was stripped and reprobed with αHsp70 antibodies (1:4000) as loading control. Molecular weights are given in kDa on the left.
Subcellular fractionation experiments show that exported E120-GFP and E500-GFP chimeric protein is not freely diffusible in the RBC cytosol. A. The P. falciparum transgenic lines 3D7-E66, 3D7-E82, 3D7-E120 and 3D7-E500 were treated with SLO (upper panel) and saponin (lower panel) as described in Experimental procedures and analysed by Western blotting. Samples were split into pellet (P) and supernatant (S) fractions and an equivalent of 2 × 106 trophozoites were loaded per lane. SLO causes pore formation in the RBC membrane allowing the release of soluble RBC cytosol components, whereas saponin causes in addition the destruction of the PVM allowing soluble components of the PV to accumulate in the supernatant fraction. PfEMP3-GFP fusion proteins were detected with rabbit αGFP (Roche, 1:1000). B. Triton X100 extraction of trophozoites of the P. falciparum transgenic lines 3D7-E66, 3D7-E120, 3D7-E500 and D10-E500. Isolated trophozoites were extracted with 1% (v/v) Triton X100 as described in Experimental procedures and analysed by Western blotting. Samples were split into Triton X100-insoluble, SDS-soluble pellet fractions (P) and Triton X100-soluble (S) fractions. An equivalent of 5 × 105 trophozoites was loaded per lane. Western blots were probed with rabbit αGFP (Roche, 1:1000), rabbit αPfEMP3 3′ repeats (1:2000) and rabbit αHsp70 (1:4000). Molecular weights are given in kDa on the left.
Trafficking of PfEMP3-GFP fusion proteins
The expression of PfEMP3-GFP chimeras was detected as green fluorescence in well-defined regions within the infected erythrocyte (Fig. 3). 3D7 parasites expressing E66-GFP showed fluorescence within the parasite and at the periphery suggestive of the PV but not within the host cell. By contrast E82-GFP was transported into the RBC cytosol (Fig. 3B). Both constructs contain the putative signal peptide and the PEXEL motif, however, in the E66-GFP construct the GFP sequence was placed within 2 amino acids of the PEXEL motif. In another study we have shown that a spacing element of no defined sequence, but with a minimum length of 10 amino acids is required between the PEXEL motif and GFP for allowing export of the fusion protein into the RBC cytosol (Knuepfer et al., 2005b). This spacing requirement is probably a cause of steric hindrance by GFP to the recognition and/or correct folding of the PEXEL sequence and seems to be the reason why E66-GFP is retained in the parasite and the PV. 3D7 parasites expressing E82-GFP show an even distribution of GFP fluorescence throughout the RBC cytosol in all asexual life cycle stages (Fig. 3B). 3D7 parasites expressing E120-GFP also generally showed an even distribution although there was some apparent concentration at the periphery of the infected RBC (Fig. 3C). In contrast, 3D7-E500 parasites showed very strong peripheral concentration of the GFP chimera under the RBC membrane and associated with punctuate structures within the RBC cytosol (Fig. 3D). The patterns of GFP fluorescence in 3D7 and D10 parasites transfected with E500-GFP are comparable (data not shown). These results show that the N-terminal 82 amino acids of PfEMP3 harbouring a putative signal peptide and a PEXEL motif are sufficient for entry into the RBC cytosol but insufficient for association with the RBC membrane. An additional 38 amino acids of PfEMP3 (in construct E120-GFP) allowed partial association with the periphery of the RBC but not the foci in the RBC cytosol, while the chimera bearing the N-terminal 500 amino acids of PfEMP3 adopted a subcellular distribution that resembled that of native PfEMP3 (Pasloske et al., 1994).
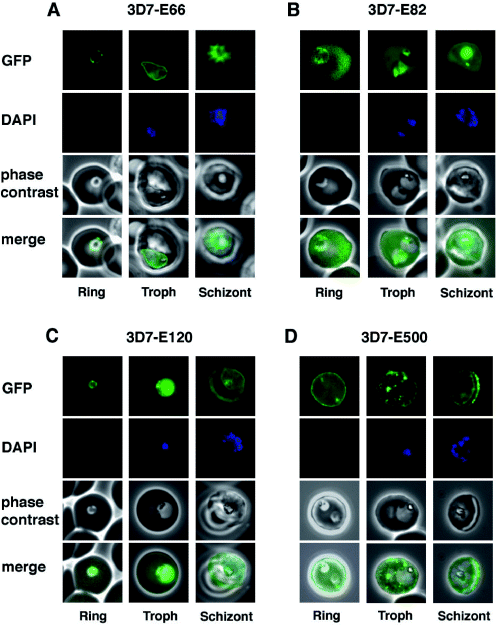
Detection of live GFP fluorescence in the PfEMP3-GFP P. falciparum transgenic lines. 3D7-E66 (A), 3D7-E82 (B), 3D7-E120 (C) and 3D7-E500 (D). Shown for each transgenic line is a representative image of a ring stage-infected RBC, a trophozoite stage-infected RBC and a schizont stage-infected RBC. The first row in each panel depicts the native GFP fluorescence, the second row the nucleus stained with DAPI, the third row the phase contrast image and the forth row the overlays of all three images.
Trafficking of PfEMP3-GFP fusion proteins in transfected parasite lines is brefeldin A-sensitive
Brefeldin A (BFA) is a fungal toxin that blocks secretion of proteins and disrupts Golgi morphology causing the redistribution of Golgi markers to the ER (Lippincott-Schwartz et al., 1991). PfEMP3 trafficking in the parasite is BFA-sensitive (Wickham et al., 2001). To determine whether the PfEMP3-GFP fusion proteins are trafficked through the classical secretory pathway in the transfected parasite we treated early ring-stage E120-GFP transfectants with 5 µg ml−1 BFA for 16 h (Fig. S1). The trafficking pathway of E120-GFP was blocked by the addition of BFA, however, the inhibition was reversible; following removal of the toxin the fusion protein trafficked beyond the ER into the RBC (Fig. S1A). In BFA-treated parasites, the GFP fusion protein was in a perinuclear compartment that colocalized with the ER-resident marker protein, PfBiP (Fig. S1B) (Kumar et al., 1988). Thus, the putative hydrophobic signal peptide seems to direct PfEMP3-GFP chimeras into the secretory pathway within the parasite as is the case for native PfEMP3 (Wickham et al., 2001).
The N-terminal 500 amino acids of PfEMP3 allow association with Maurer's clefts
We used immunofluorescence microscopy to determine whether the punctate pattern of GFP fluorescence detected within the RBC cytosol in trophozoite and schizont stages of 3D7 parasites transfected with E500-GFP represents labelled Maurer's clefts. Colocalization experiments were performed with PfSBP1, an integral membrane protein of Maurer's clefts, parasite-induced membranous structures within the RBC (Fig. 4A) (Blisnick et al., 2000). Firstly, we show in Fig. 4A (upper panels) that E120-GFP does not associate with Maurer's clefts. E500-GFP and PfSBP1, however, colocalize in Maurer's cleft structures in 3D7-E500 (Fig. 4A, middle panels); interestingly, not all Maurer's clefts were positive for E500-GFP. The presence of native KAHRP and PfEMP3 are not required for the association of the fusion protein with Maurer's clefts as colocalization was also detected in transfected D10 parasites (Fig. 4A, lower panels).
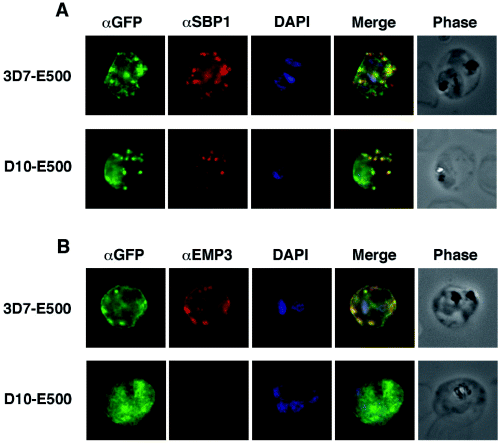
Colocalization of E500-GFP chimeric protein with a Maurer's clefts marker and native PfEMP3. Indirect immunofluorescence assays were performed on trophozoite stages of E120-GFP-expressing transgenic 3D7 and E500-GFP-expressing transgenic 3D7 and D10 P. falciparum lines. A. Formaldehyde fixed trophozoite stages were labelled with rabbit αGFP (first column; green), mouse αPfSBP1, a Maurer's clefts localized protein (second column; red) and DAPI for nuclear staining (third column; blue). The forth column represents the overlay of all three images and the fifth column the phase contrast image of the infected RBC. B. E500-GFP-expressing parasites were fixed and labelled with rabbit αGFP (first column; green), mouse αPfEMP3 (second column; red), an antibody raised against a part of the 3′ repeat region V and DAPI for nuclear staining (third column; blue). The forth column shows the overlays of the three images and the fifth column the phase contrast image of the infected erythrocyte.
To determine whether the PfEMP3-GFP fusion proteins are located in the same compartments as native PfEMP3 in 3D7 transfectants we used αPfEMP3 antibodies raised against a section of the 3′ repeat region V (Fig. 1A) that do not react with the chimera (Fig. 4B). In 3D7 E120-GFP transfectants no substantial colocalization between fusion protein and native PfEMP3 can be detected (Fig. 4B, upper panels). However, complete colocalization between E500-GFP fusion protein and wild-type PfEMP3 can be shown (Fig. 4B, middle panels). No native PfEMP3 fluorescence could be detected in the D10 parasite line as expected due to the lack of the pfemp3 gene. In summary, E500-GFP fusion protein shows an identical localization to full length PfEMP3 both in the presence and absence of KAHRP and native PfEMP3.
Electron microscopic analysis confirms PfEMP3-GFP trafficking to different compartments in the RBC cytosol
Immuno-electron microscopy using αGFP antibodies was used to further examine the cellular locations of the fusion proteins in 3D7 transfectants. We observed a number of gold particles associated with the cytoplasmic face of the RBC membrane and associated with knob structures in the 3D7-E120 transfectants (Fig. 5A, top left panel, closed and open arrows respectively). We also detected patches of gold particles associated with filamentous electron dense material in the RBC cytoplasm possibly en route to the RBC membrane (Fig. 5A, top right panel). However, no gold particles were detected on Maurer's clefts confirming our suggestion that the first 120 amino acids of PfEMP3 are sufficient to direct trafficking to the RBC membrane but not sufficient for Maurer's cleft association. By contrast, in the 3D7-E500 transfectant, numerous gold particles are associated with the RBC membrane (Fig. 5B, left panel), electron dense material within the RBC cytosol (Fig. 5B left panel, inset) and Maurer's clefts (Fig. 5B, right panel). PfEMP3 has previously been found in association with small vesicles within the RBC cytosol (Trelka et al., 2000). We found no evidence for vesicle-mediated trafficking of the chimera under the conditions of this experiment. Indeed the aggregates of fusion protein associated with filamentous electron dense material are reminiscent of aggregates described for KAHRP and MESA in the RBC cytosol (Howard et al., 1987) or more recently PfEMP1 (Papakrivos et al., 2005) and PfEMP1-GFP chimeras en route to the RBC membrane (Knuepfer et al., 2005a) and suggest that PfEMP3 is trafficked independently of vesicles.
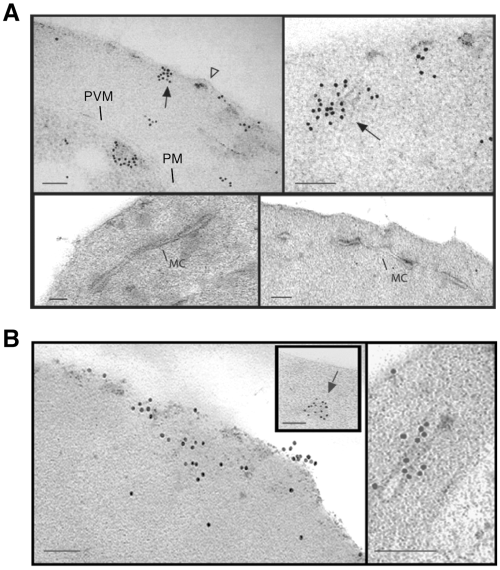
Subcellular localization of E120-GFP and E500-GFP in transgenic 3D7 parasite lines. Immuno-electron microscopy studies of 3D7-E120 (A) and 3D7-E500 (B) P. falciparum transgenic parasites labelled with rabbit αGFP antibody. In both cases gold particles were detected in association with the RBC membrane and electron-dense material within the RBC cytoplasm (closed arrows). In 3D7-E120 transfectants the GFP chimera seems to associate underneath knob structures (open arrow) similar to wild-type PfEMP3. However, whereas E500-GFP can be seen associated with Maurer's clefts, E120-GFP does not associate with these structures. Bars represent 100 nm.
The E500-GFP chimera is tightly associated with structures in the RBC cytosol
To investigate the physical state of GFP fusion proteins in the RBC cytosol we treated trophozoite stages of 3D7 transfectants with streptolysin-O (SLO) or saponin. SLO treatment forms pores of about 30 nm in diameter in the RBC membrane (Bhakdi et al., 1985) and releases soluble proteins from the RBC cytosol. Saponin on the other hand disrupts both the RBC membrane and the PVM causing the release of soluble proteins from both the PV and RBC compartments. E66-GFP is not released by SLO but is released upon saponin treatment consistent with its PV location (Fig. 6A). E82-GFP is partially released by SLO and saponin treatment consistent with the fact that part of the E82-GFP population is exported into the RBC cytosol where it is present as a soluble protein. E120-GFP is not released by SLO but partially released upon saponin treatment (Fig. 6A). However, our immunofluorescence and Immuno-electron microscopy data have shown that E120-GFP was exported to the RBC cytosol. This suggests that E120-GFP was present as a large complex too large to pass through the SLO pores or the protein was associated with filamentous structures via weak interactions in the RBC cytosol. The partial release upon saponin treatment indicates that the PV-located chimera was soluble and/or that saponin was able to disrupt the weak interactions of the chimera located in the RBC cytosol. The E500-GFP fusion protein was neither released by SLO nor saponin treatment only explainable by its tight association with structures in the RBC cytosol. Microscopic analysis of SLO-treated E500-GFP transfectants confirms this tight association with Maurer's clefts and the RBC membrane (Fig. S2).
PfEMP3 in ring-stage parasites has been shown to be partially soluble in the non-ionic detergent, Triton X100, but becomes more insoluble as the parasite matures (Handunnetti et al., 1992; Pasloske et al., 1993). This insolubility is assumed to be due to binding of PfEMP3 to the host cell cytoskeleton. We examined the Triton X100 solubility of different fusion proteins in mature-stage transfectants. E66-GFP and E120-GFP were found largely in the Triton X100-soluble fraction (Fig. 6B). By contrast, E500-GFP was largely Triton X100-insoluble in both D10 and 3D7 transfected parasite lines (Fig. 6B). Similarly, the majority of the endogenous PfEMP3 in 3D7 parasites was Triton-insoluble under the conditions of this experiment (Fig. 6B, middle panel). No PfEMP3 was detected in the D10 transfected parasite line as the PfEMP3 antibodies were raised to the 3′ repeat region. The cytoplasmic parasite protein, Hsp70, which was used to monitor the efficiency of the extraction procedure, was completely Triton X100-soluble (Fig. 6B, lower panel).
From these experiments we conclude that a region within amino acids 120–500 of PfEMP3 is responsible for the tight association with Maurer's clefts and the cytoskeleton underlying the RBC membrane. This tight association might involve electrostatic interactions that are not disrupted by non-ionic detergent. E500-GFP chimeras in 3D7 parasites have trafficking and binding properties similar to native PfEMP3. Furthermore, the association with the RBC membrane in infected RBCs seems to be independent of KAHRP or native PfEMP3 as E500-GFP chimeras behave identical in 3D7 and D10 transfectants.
Photobleaching analysis of the physical organization of the E120-GFP and E500-GFP chimeras
Florescence recovery after photobleaching (FRAP) can be used to provide information on the organization and dynamics of GFP chimeras in transfected malaria parasites (Wickham et al., 2001; Klonis et al., 2002). We have used confocal microscope-based photobleaching to probe the physical organization of E120-GFP and E500-GFP in the cytosol of infected RBCs. As noted above, in mature-stage parasites, E120-GFP is largely located in the RBC cytosol with little concentration of the fluorescence signal at the rim of the erythrocyte membrane (Fig. 7A, panel a). Application of a high intensity laser pulse for a period of 1 s to a region within the host cell cytosol resulted in a decrease in fluorescence throughout the entire cytosol (including the RBC membrane, blue arrows), although the loss of signal is more marked at the bleach site (Fig. 7A, panel a, 0 time). This is particularly evident in the colour-coded bleach (B) image (constructed from the pre- and post-bleach images) that illustrates the higher relative levels of bleaching (orange colour) in regions proximal to the bleach site (Fig. 7A, panel a). Repeated bleaching of the population of E120-GFP in the RBC cytosol (5 × 2 s bleach pulses) resulted in loss of fluorescence throughout the cytosol (Fig. 7A, panel d) but did not affect the parasite-associated fluorescence indicating that these are separate compartments.
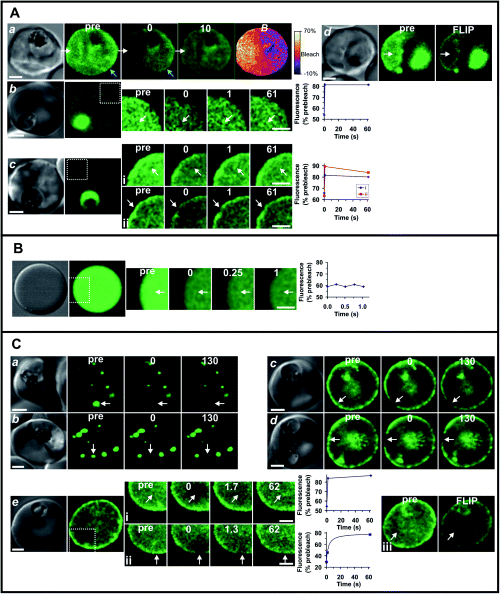
Photobleaching analysis to determine the molecular organization of E120-GFP and E500-GFP chimeras in infected RBCs. The dynamics of chimeras E120-GFP (A) and E500-GFP (C) in infected RBCs were compared with fluorescein-BSA in resealed RBCs (B). In each case, the first panel shows the differential interference contrast image of the cell. The fluorescence images (green) comprise a pre-bleach image (pre) and post-bleach images obtained at the times (in seconds, indicated in the figures) following the bleach pulse or following a fluorescence loss in photobleaching (FLIP) treatment. (The first post-bleach image is defined as zero time.) In cases where time resolution was important, only a small section of the cell was imaged in the photobleaching series (indicated by the dotted white line in the fluorescence image of the whole cell). The position of the bleach pulse is indicated by the white arrows. The graphs associated with several of the panels show the temporal dependence of the fluorescence intensity in the bleached region following the bleach event, relative to the pre-bleach intensity. Scale bars = 2 µm. A. E120-GFP in the RBC cytosol of 3D7 transfectants was subjected to a 2 s bleach pulse and imaged immediately and after 10 s (row Aa). The B image was calculated from the pre-bleach and post-bleach images as described in Experimental procedures and illustrates the localized nature of the bleach profile. The kinetics of fluorescence recovery into a bleached region of the cytosol was examined for a young-stage E120-GFP transfectant (row Ab; bleach time, 1 s) and for cytosol and rim fluorescence for a more mature parasite (Aci, Acii, 0.5 s bleach). For FLIP analysis (row d), the region indicated by the arrow was subjected to five 1 s bleach pulses with 10 s time intervals prior to the acquisition of the final image. B. Bleach and recovery profile for fluorescein-BSA in resealed RBCs subjected to a 50 ms bleach pulse. C. Photobleaching of transfectants expressing E500-GFP at the Maurer's clefts (a, b) and the RBC rim (c, d). Measurements were performed using 3D7 (a, c) and D10 (b, d) transfectants. Bleach times were 200 and 500 ms in the Maurer's clefts and rim regions respectively. Row Ce shows a young D10 transfectant expressing E500-GFP in the RBC cytosol. Photobleaching of the cytosol (i) and rim (ii) utilized a 0.5 s bleach pulse. A FLIP measurement of the cytosol (Ceiii) was performed as described for row Ad.
In younger-stage parasites, E120-GFP was more extensively associated with the parasite and only relatively weak fluorescence was observed in the RBC cytosol (Fig. 7A, panels b and c). However, this population could be readily visualized by increasing the photomultiplier gain. Using a decreased pixel resolution and bidirectional line scanning, we were able to undertake a semi-quantitative analysis of the rate of recovery of the E120-GFP in the RBC cytosol of these transfectants. We examined both very young transfectants exhibiting no rim fluorescence (Fig. 7A, panel b) and more mature parasite exhibiting some rim fluorescence (Fig. 7A, panel c and i). In both cases, a recovery time of hundreds of milliseconds was observed. Photobleaching of the fluorescence associated with the RBC rim produced some localized bleaching and subsequent recovery of fluorescence with similar kinetics to that observed for the cytosolic population (Fig. 7A, panel cii). This indicates that the RBC cytosol- and rim-associated E120-GFP is in rapid equilibrium. While the rate of diffusion appears rapid relative to the time domain that can be readily accessed using confocal-based FRAP, recovery times of a few hundred milliseconds are two orders of magnitude longer than expected for a protein monomer (Klonis et al., 2002).
For comparison, we examined resealed erythrocytes containing fluorescein-BSA in the cytosol (Fig. 7B). For these experiments, we used a short bleach pulse (50 ms) and a short image acquisition time (250 ms) in an effort to capture the bleach process. Nonetheless, we observed an even bleaching of the entire cytosol and no subsequent recovery of signal (Fig. 7B, and data not shown). Freely diffusing proteins are predicted to diffuse very rapidly, moving into and out of the bleach region during the time taken for bleaching and image acquisition. This leads to rapid and even bleaching of the entire compartment in which the molecule resides. Thus, fluorescein-BSA equilibrates throughout the RBC cytoplasm with a half time of significantly less than 300 ms.
Thus, the recovery time for E120-GFP of a few hundred milliseconds indicates that the chimera may be present as part of a larger complex with itself and/or with other proteins. This is consistent with electron microscopy data that show PfEMP3-GFP associated with filamentous material in the RBC cytosol as well as the data on release of the chimeras during SLO treatment. In a separate study, we have also found that transfectants expressing a KAHRP-GFP chimera and a PfEMP1-GFP chimera have a RBC cytosol-located population of the chimeras that display similar diffusion kinetics (Knuepfer et al., 2005a). Thus, passage of exported proteins across the RBC cytosol as vesicle-free aggregates may be the major mode of transport.
The E500-GFP chimera is located in punctate structures in the RBC cytosol that appear to be Maurer's clefts (Fig. 7C, panels a and b), before redistribution to the RBC membrane (Fig. 7C, panels c–e). The apparent organization of the E500-GFP was similar in 3D7 and D10 transfectants. When the fluorescence signal associated with the Maurer's clefts was ablated with a short bleach pulse there was no recovery of fluorescence over a period of minutes (Fig. 7C, panels a and b). Similarly, there was no recovery of signal following bleaching of the RBC membrane-associated E500-GFP. This suggests that the chimera is very tightly associated with these structures. The data confirm that a region of PfEMP3 between amino acids 120 and 500 contains a motif that permits very tight binding to the Maurer's clefts. In mature-stage transfectants, there was no evidence for a population of RBC cytosol-located E500-GFP. However, in some erythrocytes infected with very young parasites, a faint cytosolic and/or rim fluorescence could be detected (Fig. 7C, panel e). Application of a laser pulse produced localized bleaching of the cytosol with subsequent recovery of fluorescence on a time-scale similar to that observed for E120-GFP (Fig. 7C, panel ei). Interestingly, bleaching of the membrane-associated fluorescence of this parasite was also associated with some recovery of fluorescence (Fig. 7C, panel eii). Indeed, repeated bleaching of a region of the cytosol of this cell decreased both the cytosol and rim fluorescence (Fig. 7C, panel eiii). This indicates that E500-GFP is present in a rapidly exchanging pool in very-young-stage parasites. It is possible that an additional parasite component that is produced in more-mature-stage parasites is responsible for tightly attaching E500-GFP to the Maurer's clefts and the inner surface of the RBC membrane.
Discussion
After invasion of P. falciparum into human erythrocytes an essential process of host cell remodelling occurs that is mediated by proteins, such as PfEMP3, that are exported across the parasite membrane and PVM. Once in the infected erythrocyte these proteins must be trafficked to the erythrocyte membrane and assembled on its cytoplasmic face. It has recently been shown that a pentameric motif, called PEXEL, is required for export of proteins through the PVM from the PV (Hiller et al., 2004; Marti et al., 2004); however, the precise mechanism of how these proteins are transported across the PV (released into the PV or sealed within vesicles) and how they reach their final destination once in the erythrocyte is not clear. In this work, we have shown that export of PfEMP3 into the infected-erythrocyte requires an N-terminal hydrophobic signal and a PEXEL motif. Correct assembly of this protein under the erythrocyte membrane requires transient interaction with Maurer's clefts via a specific sequence within the N-terminal region of the protein. Our results show that PfEMP3-GFP chimeras of the N-terminal 500 amino acids are exported into the erythrocyte cytosol and trafficked as a complex to Maurer's clefts. Due to the similar trafficking and physical properties of the E500-GFP chimera and native PfEMP3 we hypothesize that native PfEMP3 behaves like the E500-GFP chimera analysed in this study. Therefore we suggest that the association of PfEMP3 with Maurer's clefts is an important step for trafficking and insertion of this protein under the P. falciparum-infected erythrocyte membrane.
The sequence required for Maurer's cleft association is located within the N-terminal region between amino acids 120–500 of PfEMP3. This region is lysine-rich with a predicted pKi of 9.7 and two predicted coiled-coil regions (coils 2 program) (Lupas, 1996). Coiled-coil motifs are found in a number of proteins such as cytoskeletal proteins, motor proteins and proteins involved in molecular recognition systems (Burkhard et al., 2001). Homology searches using blast showed weak but no significant similarities to all three classes of coiled-coil motif containing proteins. The KAHRP protein also has a short sequence near its N-terminus responsible for binding to Maurer's clefts suggesting that other proteins destined for the erythrocyte membrane may also associate with Maurer's clefts as an interim compartment (Wickham et al., 2001). It is not clear which components of the Maurer's clefts are involved in binding to transit cargo. However, the data presented here show that the same region in PfEMP3 allows attachment to Maurer's clefts and the final tight association with the cytoskeleton underneath the RBC membrane. Whether the binding partner in both locations is the same is unclear. Based on the weak association of E120-GFP with the RBC membrane and a similarly weak association of E500-GFP with the RBC membrane in young transfectants (Fig. 7C, panel e) one can speculate that a cytoskeleton binding site is present in both constructs but only upon expression of one or more parasite proteins is a tight association with Maurer's clefts and the RBC membrane achieved. Although there are no obvious sequence similarities between the N-terminus of KAHRP and amino acids 120–500 of PfEMP3, the basic pKi of both sequences suggests that positively charged domains of both proteins might interact with one or more binding partners by electrostatic interactions a hypothesis supported by the Triton X100 solubility data presented in Fig. 6B. It is interesting to note that the large repeat regions of PfEMP3 have no direct role in the trafficking, localization and tight binding of PfEMP3 to Maurer's clefts and the RBC membrane. It has been previously suggested that repeat elements in P. falciparum proteins may function as immunological smoke screens (Anders, 1986). Similarly, two other parasite proteins, namely RESA and MESA, which are transported into the RBC and bind to host cytoskeleton, have large repeat elements. These proteins bind to spectrin and protein 4.1, respectively, and the regions responsible are also located in short linear amino acid stretches in non-repetitive regions (Bennett et al., 1997; Foley et al., 1994).
The function of PfEMP3 within the asexual life cycle of P. falciparum is not well understood. PfEMP3 appears to contribute to the reduced deformability of parasitized erythrocytes (Glenister et al., 2002). This suggests that its interaction with the host cell cytoskeleton may reduce the flexibility of the spectrin network. Transgenic parasites lacking expression of PfEMP3 still retain cytoadherence although slightly reduced and knob structures suggesting it is not absolutely required for PfEMP1 trafficking (Waterkeyn et al., 2000). Interestingly, however, overexpression of a truncated form of PfEMP3 results in the accumulation of the protein in Maurer's clefts and physical blockage of PfEMP1 translocation to the RBC surface. It is possible that extensive cross-linking of cytoskeleton molecules may prevent efficient transfer of PfEMP1 from the Maurer's clefts to the RBC membrane.
In this study, we expressed the N-terminal 500 amino acids of PfEMP3 linked to GFP in the presence or absence of wild-type PfEMP3 and KAHRP. This PfEMP3-chimeric protein was trafficked normally to Maurer's clefts and assembled under the erythrocyte membrane in a similar manner to endogenous PfEMP3, showing that neither full length PfEMP3 nor KAHRP is required for correct localization. Our experiments suggest that, en route to Maurer's clefts and the RBC membrane, PfEMP3-GFP chimeras are present as aggregates rather than being transported attached to vesicles as has been previously suggested (Trelka et al., 2000). These aggregates are reminiscent of those previously described for KAHRP and PfEMP1 (Wickham et al., 2001; Papakrivos et al., 2005) and smaller in size to aggregates identified in MESA trafficking (Howard et al., 1987). Structures containing actin and myosin have been described in the parasitized RBC cytosol (Taraschi et al., 2003). It is possible that these aggregates of proteins transported to the RBC surface may be transported along a larger cytoskeletal network but only stably associate with the RBC cytoskeleton upon arrival at their final destination, a hypothesis supported by our photobleaching and time-lapse microscopy data. The absence of vesicles in the transport of proteins involved in the knob formation is intriguing in light of the suggested presence of parasite encoded COPII components exported into the RBC cytosol and to Maurer's clefts (see for review Cooke et al., 2004). More recent studies have suggested that PfEMP1 is also trafficked as a complex and not associated with vesicles (Knuepfer et al., 2005a). It is possible that vesicle-mediated events are involved in the formation of the Maurer's clefts and in the trafficking between Maurer's clefts and the RBC but they do not appear to be important for trafficking of cargo to these compartments. Indeed our data support the hypothesis that trafficking of PfEMP3 and PfEMP1 involves a novel vesicle-independent mechanism for protein delivery and assembly at the host cell membrane.
Experimental procedures
Plasmid constructs, parasite culture and transfection
To generate transgenic GFP-expressing parasites we amplified the sequence encoding 500 N-terminal amino acids of PfEMP3 from cDNA and cloned the products into the pHH2 vector (Reed et al., 2000; Waller et al., 2000) in frame with the 3′ appended mut2 eGFP sequence. The Pfemp3-gfp sequence was subcloned into transfection vector pARL(Marti et al., 2004) to create E66-GFP, E82-GFP, E120-GFP and E500-GFP and the expression of the chimeric proteins was driven by the HSP86 promoter. The sequence of each construct was confirmed by DNA sequencing (Big Dye Terminator cycle sequencing, Perkin-Elmer). 3D7 and D10 P. falciparum parasites were transfected with 100 µg of plasmid DNA (Qiagen) by electroporation and cultured in the presence of WR99210 (20 nM) as described (Wickham et al., 2001).
Subcellular fractionation and Western blotting
Parasites were synchronized by sorbitol, cultured to a parasitaemia of approximately 10% and harvested by gelatine selection as described (Waterkeyn et al., 2001). For extraction of proteins from the RBC cytosol, 2 × 108 infected RBCs were incubated with four haemolytic units of activated SLO (Sigma) for 6 min at room temperature and separated into pellets and supernatants (Ansorge et al., 1996). For microscopic analyses of SLO-lysed infected RBCs the pellet fraction was washed once in phosphate-buffered saline (PBS) before visualization on a Zeiss Axioskop 2 microscope. To extract proteins from the RBC cytosol and the PV, infected RBCs were lysed in 0.09% saponin (Sigma) in RPMI and separated into soluble and pellet fractions (Benting et al., 1994). Pellet fractions were extensively washed in PBS before resuspension in SDS sample buffer.
To determine cytoskeleton association 1 × 107 infected RBCs were sequentially extracted with 150 µl of cold 1% (v/v) Triton X100 in PBS with protease inhibitors (Roche) followed by extraction in 2% SDS (w/v) in SDS sample buffer (Van Schravendijk et al., 1993).
For immunoblot analyses, trophozoites were purified from synchronized parasite cultures by gelatine selection (3D7) or using magnetic cell sorting CS columns (Miltenyi Biotech) for the D10 strain and resuspended in SDS sample buffer. Samples were subjected to SDS-PAGE and transferred to nitrocellulose membrane and probed with mouse αGFP (Roche, 1:1000), rabbit αHsp70 (1:4000) and rabbit αPfEMP3 3′ repeats antibody (Waterkeyn et al., 2000) (1:2000) followed by horseradish peroxidase-conjugated secondary antibodies (Silenius, 1:1000) and visualized using the ECL detection kit (Amersham). Immunoblots were stripped using RestoreTM Western blot stripping buffer (Pierce) for 15 min at 37°C.
Microscopy
Transfected parasites were tightly synchronized using sorbitol and images taken every 6 h at ambient temperature using a Zeiss Axioskop 2 microscope equipped with a PCO SensiCam (12 bit) camera and Axiovison 3 software. For Immuno-electron microscopy trophozoite-infected RBCs were enriched using gelatine and the cell pellet subjected to high pressure freezing using a Leica EM High Pressure Freezer. The samples were processed as described (Rug et al., 2004). Thin-sections were prepared and incubated with a rabbit αGFP antibody (1:500), followed by 10 nm gold-labelled goat anti-rabbit IgG (Aurion). Sections were post-stained with uranyl acetate and lead citrate to enhance contrast before examination in a Philips CM120 transmission electron microscope.
For indirect immunofluorescence assays infected RBCs were smeared on glass slides and fixed with 4% formaldehyde in PBS for 30 min before permeabilization with 0.05% Triton X100 in PBS for 10 min at room temperature. Slides were incubated with antibodies diluted in 3% (w/v) BSA in PBS: rabbit αGFP (1:1000), mouse αPfSBP1 (1:400), mouse αPfEMP3 3′ repeats (1:2000) and rat αPfBiP (1:1000) followed by Alexa Fluor 546 or Alexa Fluor 488-conjugated secondary antibodies (Molecular Probes) and DAPI.
Brefeldin A treatment
Highly synchronized ring-stage E120-GFP-expressing parasites were incubated with 5 µg ml−1 of BFA (Sigma) for 16 h before removal of the drug and culturing of these parasites for a further 12 h to ensure viability (Wickham et al., 2001). BFA-treated parasites were imaged live on a Zeiss Axioskop2 as well as by indirect immunofluoresence of fixed parasites.
Fluorescence recovery after photobleaching
Resealed RBCs containing carboxyfluorescein-labelled bovine serum albumin were prepared as described (Klonis et al., 2002). PfEMP3-GFP chimera expressing transfectants E120 and E500 were subjected to photobleaching experiments using an inverted Leica TCS SP2 confocal microscope with a 100 × oil immersion objective (1.4 NA) as described (Wickham et al., 2001; Klonis et al., 2002). For measurements where it was important to minimize the time delay between the bleach event and the first post-bleach image, the pixel resolution was decreased to 128 × 128 or 64 × 64, and bidirectional line scans were employed. Image processing, including background correction, smoothing and image analysis were performed using NIH ImageJ (http://rsb.info.nih.gov/ij) as described (Adisa et al., 2003). B images showing the spatial distribution of the loss of fluorescence following the bleach event were calculated using the pre-bleach and first post-bleach image were generated as described (Adisa et al., 2003) after conversion to 32-bit images.
Acknowledgements
We thank Drs Braun-Breton and Ryan and the MR4 for antibodies. We thank the Red Cross Blood Service (Melbourne, Australia) for red cells. E.K. was supported by an International Travelling Research Fellowship from the Wellcome Trust. N.K. was supported by an Australian Research Council Fellowship. A.C. was supported by an International Research Fellowship from Howard Hughes Medical Institute. This work was supported by the NIH (RO1 AI44008), the NHMRC of Australia and the Australia Research Council.




