A potential novel mechanism for the insertion of a membrane protein revealed by a biochemical analysis of the Plasmodium falciparum cytoadherence molecule PfEMP-1
Summary
Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1) is exposed on the surface of infected erythrocytes where it both acts as an important pathogenicity factor in malaria and undergoes antigenic variation as a means of immune evasion. Because the mammalian erythrocyte lacks a protein secretory machinery there has been much interest in elucidating the mechanism whereby this protein is transferred from its site of synthesis within the parasite to its final destination. Current opinion favours a mechanism whereby PfEMP-1 becomes cotranslationally inserted into the endoplasmic reticulum of the parasite and is subsequently transported as an integral part of an erythrocyte cytoplasmic membrane system derived from the parasite. Here we show that the solubility characteristics of this protein during several stages of its transport pathway are inconsistent with this view. Instead we propose that the protein is synthesized as a peripheral membrane protein which only when it arrives at its final destination assumes a transmembrane topology. Even in this state, the extractability of the protein with urea suggest that it is anchored in the membrane by protein-protein rather than by protein–lipid interaction.
Introduction
Inside its host erythrocyte, the human malaria parasite Plasmodium falciparum develops within a parasitophorous vacuole. Although the parasitophorous vacuolar membrane (PVM) which delineates the vacuole forms a barrier between the parasite and the host cell cytosol, several parasite proteins are transported beyond the PVM, into the host cell cytosol and to the erythrocyte plasma membrane. Some of these proteins are known to contribute to the severe pathogenesis of malaria. The P. falciparum erythrocyte membrane protein-1 (PfEMP-1) constitutes a family of proteins encoded by approximately 50 variant genes, called var genes (Baruch et al., 1995; Smith et al., 1995; Su et al., 1995). PfEMP-1 is heterogenous in size, ranging from 200 to 350 kDa, and it is transported to the erythrocyte membrane where it becomes exposed on the exterior face. It binds to endothelial receptors, a phenomenon known as cytoadherence and thereby contributes to the pathology of malaria (see Cooke et al., 2000 for review). The protein is anchored in electron dense protrusions of the erythrocyte membrane, called knobs. The knobs consist of several proteins which integrate into the submembranous cytoskeleton network. Transgenic parasites in which the gene encoding the knob-associated histidine-rich protein (KAHRP) was interrupted were unable to form knobs (Crabb et al., 1997). In erythrocytes infected with these parasites transport of PfEMP-1 to the host cell surface was impaired. Likewise, interruption of the gene encoding another knob-associated protein, PfEMP-3, resulted in reduced transport of PfEMP-1 to the cell surface (Waterkeyn et al., 2000). Collectively, these observations suggest that knob formation is required for efficient insertion of PfEMP-1 into the erythrocyte membrane. Consistent with these data obtained from transgenic parasites are reports that parasites which were selected for a knobless phenotype also showed a reduced cytoadherence (Ruangjirachuporn et al., 1991).
The primary structure of PfEMP-1 consists of a highly variable N-terminus which constitutes the major portion of the protein and which is composed of a variable number of semi-conserved duffy-binding like domains (DBL). This N-terminal portion mediates adherence of infected erythrocytes to various receptors on the vascular endothelium (Smith et al., 1998; Cooke et al., 2000). This is a key event in malaria pathology and therefore PfEMP-1 molecules are the focus of intense research. The acidic C-terminal sequence (ATS domain) is conserved, and it remains at the cytoplasmic face of the erythrocyte membrane. The interaction of the ATS domain with other knob components and with the host cell cytoskeleton has been studied in detail. Specific peptides of the ATS domain interact with specific regions of KAHRP (Waller et al., 1999, 2002), presumably by electrostatic interactions (Voigt et al., 2000). Apart from binding to KAHRP, the ATS domain has been shown to interact with the actin-spectrin-protein 4.1 junction of the erythrocyte cytoskeleton (Oh et al., 2000). Owing to the tight complex formation of PfEMP-1 with other structural proteins extraction with SDS is required to solubilize PfEMP-1 (Baruch et al., 1996). The strong affinity to knob components appears to be a prerequisite to maintain cytoadherence in experiments simulating flow conditions (Crabb et al., 1997). The ATS domain is separated from the N-terminal portion by a hydrophobic domain. In analogy to most integral membrane proteins found in plasma membranes of eukaryotic cells, it is generally believed that PfEMP-1 spans the erythrocyte membrane via this hydrophobic segment (Su et al., 1995; Cooke et al., 2000; Taylor et al., 2000). This view is supported by the type I topology of the protein within the erythrocyte membrane (Gardner et al., 1996) and by morphological studies that show high concentrations of PfEMP-1 associated with endomembranes within the cytosol of infected erythrocytes (Trelka et al., 2000; Wickham et al., 2001; Kriek et al., 2003). Treatment of infected erythrocytes with the fungal metabolite brefeldin A (BFA) which blocks the classical secretory pathway of the parasite (Benting et al., 1994; Ogun and Holder, 1994; Baumgartner et al., 2001) results in the accumulation of PfEMP-1 within the parasite endoplasmic reticulum (ER) (Wickham et al., 2001). Therefore, the following hypothetical pathway involved in biosynthesis and transport of PfEMP-1 has emerged and appears conceivable; the protein is translocated cotranslationally across the parasite ER membrane. In this case the hydrophobic segment would act as a stop-transfer sequence. Transport of the protein would then occur by membrane flow that continues beyond the confines of the parasite plasma membrane, through a parasite derived membrane system within the erythrocyte cytosol, eventually leading to a fusion with the erythrocyte plasma membrane. PfEMP-1 belongs to a group of exported parasite proteins, which do not possess a hydrophobic N-terminal signal sequence which normally directs proteins into the secretory pathway. It is possible that the hydrophobic segment of PfEMP-1 may function both as a transmembrane domain and as an ER targeting signal (Nacer et al., 2001). However, in this case a type I topology of the protein is unlikely because the large N-terminal proportion of the molecule at the cytoplasmic side of the ER membrane would prevent translocation of the N-terminus to the lumenal side. Because of these considerations and in the absence of biochemical evidence that PfEMP-1 is anchored within the lipid bilayer through its relatively short hydrophobic domain we examined the solubility of the protein within the parasite, the erythrocyte cytoplasm, and in the erythrocyte plasma membrane.
Results
Trypsin treatment of intact infected erythrocytes allows differentiation between internal and external populations of PfEMP-1
Var gene mRNA can be detected in ring stage parasites as early as 3 h post invasion and levels peak at 12 h post invasion (Chen et al., 1998; Scherf et al., 1998; Kyes et al., 2000). PfEMP-1 proteins appear on the surface of late ring stage infected erythrocytes much later than the first appearance of the mRNA (Gardner et al., 1996; Kriek et al., 2003). Consistent with these observations, high intracellular levels of intraerythrocytic PfEMP-1 are detectable by immunofluorescence microscopy and by immunoelectron microscopy (Trelka et al., 2000; Wickham et al., 2001; Kriek et al., 2003). It appears that translocation of the protein onto the erythrocyte surface occurs during a narrow time window and that a considerable proportion of the molecule remains intracellular (Kriek et al., 2003). Because these results indicate the existence of at least two distinct populations of the protein we undertook experiments to analyse the physical properties of both the internal and the externally exposed populations of PfEMP-1. Erythrocytes from a highly synchronous culture infected with FCBR parasites (Raether et al., 1989) were harvested 20 h post invasion and subjected to trypsin digestion to remove surface exposed PfEMP-1. One aliquot of the cells was lysed by freezing and thawing before the trypsin treatment. Another aliquot was left untreated. The samples were then analysed by immunoblotting using a polyclonal antiserum directed against the conserved C-terminal ATS region of the protein. Full-length PfEMP-1 migrates as a band larger than 200 kDa (Fig. 1A, lane 1). Trypsin treatment of intact erythrocytes generates a characteristic protein fragment of an apparent molecular size of 85 kDa (Waterkeyn et al., 2000) which is recognized by the antiserum (Fig. 1A, lane 2). In addition to the major bands, several immunoreactive bands < 66 kDa were detectable which we attribute to break-down products of intracellular PfEMP-1 or to cross reactivity of the anti-ATS antibody with members of the Pf60 multigene family. No protein bands are detectable when lysates of cells were treated with trypsin (Fig. 1A, lane 3). PfEXP-1, an integral membrane protein of the PVM (Ansorge et al., 1997), was resistant to trypsin digestion in intact erythrocytes but not in lysates (Fig. 1B). Therefore, the full-length protein that is protected from the protease (Fig. 1A, lane 2) represents an internal population of PfEMP-1 rather than a surface exposed population of completely trypsin-resistant protein molecules. It is noteworthy that the relative intensities of the signals obtained for the cleavage product and for the full-length molecule, respectively, appear to vary between different strains of parasites. As described by Waterkeyn et al. (2000) for the 3D7 parasite line, we also found a higher proportion of internal protein when we analysed erythrocytes infected with the clonal A4 parasite line which expresses a single var gene (data not shown).
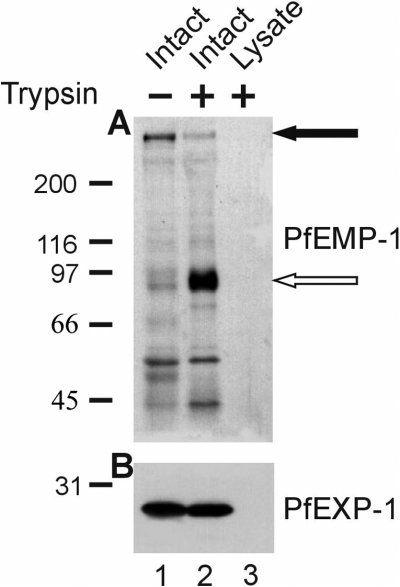
Differentiation between the internal and external population of PfEMP-1. Infected erythrocytes, either untreated (lane 1) or after treatment with trypsin (lane 2), were lysed by freezing and thawing. As a control, a lysate of infected erythrocytes was treated with trypsin (lane 3). A. Samples, each corresponding to 107 cells were separated by 5–7.5% SDS-PAGE and transferred to nitrocellulose filters for the detection of PfEMP-1 using the antiserum against the ATS domain. The closed arrowhead indicates the position of full-length PfEMP-1, the open arrowhead indicates the position of the C-terminal proteolytic fragment. B. In parallel an aliquot of each sample was separated by 12% SDS-PAGE and processed for immunodetection of PfEXP-1. Molecular size markers in kDa are indicated on the left.
PfEMP-1 is synthesized as a carbonate extractable protein
Membrane insertion of secretory proteins that span the lipid bilayer via a segment of hydrophobic amino acids occurs when such proteins are synthesized at the rough ER, i.e. at the initial step in the secretory pathway. The fungal metabolite BFA inhibits the export of proteins from the parasite, including that of PfEMP-1 (Wickham et al., 2001). The following experimental strategy was devised to investigate whether PfEMP-1 is synthesized as an integral membrane protein: (i) Ring stage-infected erythrocytes (12 h post invasion) were treated with BFA to inhibit export of newly synthesized proteins. (ii) Infected cells were then treated with saponin, a procedure that disrupts the erythrocyte plasma membrane and the PVM but largely preserves the integrity of the parasite plasma membrane (Benting et al., 1994; Saliba and Kirk, 1999). (iii) Saponin lysed cells were treated with trypsin to remove proteins that had been transported beyond the confines of the parasite plasma membrane before the treatment with BFA. (iv) After inactivation of the protease, intact parasites were disrupted by repeated freezing and thawing, and solubilized proteins were separated from the membrane fraction by centrifugation. (v) The membrane fraction was extracted with sodium carbonate solution at high pH, a treatment that results in the disintegration of most protein complexes and in the release of proteins associated peripherally with membranes. Under these conditions integral membrane proteins sediment into the membrane fraction (Fujiki et al., 1982; Günther et al., 1991).
The fractions containing soluble proteins, proteins released by sodium carbonate treatment and integral membrane proteins were analysed by immunoblotting for the distribution of PfEMP-1 and for various marker proteins that had been analysed previously using similar protocols (Fig. 2A). The marker proteins included: (i) PfGBP, a soluble protein that is secreted from the parasite via a BFA sensitive pathway (Benting et al., 1994); (ii) PfBIP, an ER resident protein that is largely soluble (Kumar et al., 1991; Burghaus and Lingelbach, 2001) and (iii) PfEXP-1 an integral membrane protein that is transported to the PVM (Günther et al., 1991).
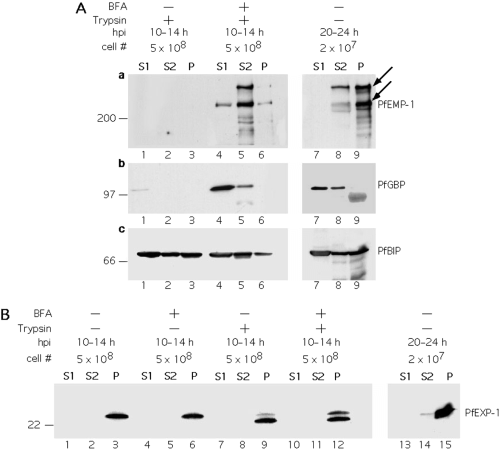
PfEMP-1 is a carbonate extractable protein in the parasite's secretory pathway. Infected erythrocytes (12 h post invasion, hpi) were cultivated in the absence (A, lanes 1–3, 7–9; B, lanes 1–3, 7–9, 13–15) or in the presence of BFA (A, lanes 4–6; B, lanes 4–6, 10–12). Cells were either harvested 2 h (A, lanes 1–6, B, lanes 1–12), or 12 h (i.e. 24 hpi) (A, lanes 7–9; B, lanes 13–15) later. Parasites from saponin lysed erythrocytes were trypsin treated (A, lanes 1–6; B, lanes 7–12) or left untreated (A, lanes 7–9; B, lanes 1–6, 13–15). Parasites and internal membranes were lysed by repeated freezing and thawing in Tris buffered saline and sedimented. The supernatant (S1) was collected and the membranes were extracted with sodium carbonate at high pH resulting in a fraction containing carbonate extractable proteins (S2) and a membrane fraction (P). For the analysis of early developmental stages (A, lanes 1–6; B, lanes 1–12), samples were adjusted to contain equivalents of 5 × 108 infected erythrocytes before loading onto the gels. In the case of the developmentally more advanced stages (A, lanes 7–9; B, lanes 10–12) samples, each corresponding to 2 × 107 infected erythrocytes were analysed. Nitrocellulose filters were probed with antisera against the ATS domain of PfEMP-1 (A, a), GBP (A, b), PfBIP (A. c) and PfEXP-1 (B). The arrows indicate the positions of the major full-length PfEMP-1 proteins.
In the absence of BFA no PfEMP-1 and only minute amounts of PfGBP were detectable in the different fractions (Fig. 2A, lanes 1–3) suggesting that these proteins had been secreted from the parasite and had been degraded in saponin-treated, trypsinized erythrocytes (see Fig. 3 for a schematic interpretation). Under the same conditions the internal protein PfBIP was detectable, thus excluding the possibility that saponin treatment had disintegrated the parasite, allowing access of the protease to the parasite's secretory pathway. When protein export was inhibited, both PfEMP-1 and PfGBP were resistant to tryptic digestion indicating retention of the proteins within the parasite (Fig. 2A, lanes 4–6). These results confirm previous morphological observations based on fluorescence microscopy (Adisa et al., 2001; Wickham et al., 2001) that PfEMP-1 is routed via a BFA sensitive pathway. After fractionation of parasites and extraction of the membrane fraction, PfGBP predominantly segregated into the fraction of proteins which are soluble in buffers of physiological pH, as described previously (Benting et al., 1994). PfEMP-1 was solubilized after treatment of the membranes with high pH buffers which is indicative of a peripheral association of the protein with internal membranes of the parasite. This parasite line expresses several var genes which results in a heterogeneous population of PfEMP-1 polypeptides. Interestingly, one species appears soluble only after carbonate treatment whereas the other major band of lower molecular mass shows partial solubility under physiological conditions. Also, a minor proportion of both protein bands resist extraction with carbonate solution. The majority of PfBIP was soluble in either physiological or alkaline solutions. In other eukaryotes it has been shown that, in the lumen of the ER, BIP tightly associates with the Sec63p subunit of the translocon (Brodsky, 1996; Corsi and Schekman, 1997) which is the most likely reason why a considerable proportion of PfBIP resisted extraction of the membranes with physiological and, to a lesser degree, also with carbonate buffers. PfEXP-1 was included in the analyses as a marker protein that has an experimentally well characterized transmembrane topology. It is synthesized as a type I transmembrane protein (Günther et al., 1991). In trophozoite stage infected cells (24–36 h post invasion) PfEXP-1 is located within the PVM and it spans this membrane via a hydrophobic stop-transfer-sequence such that the N-terminal part of molecule is exposed into the lumen of the vacuole with the C-terminal part being exposed into the erythrocyte cytosol (Ansorge et al., 1997). In both membranes, in the ER membrane and in the PVM, PfEXP-1 is resistant to extraction with sodium carbonate solution (Günther et al., 1991; Johnson et al., 1994). In saponin-treated non-trypsinized cells PfEXP-1 is detected as a single band (Fig. 2B, lanes 3 and 6) that is cleaved to a slightly smaller polypeptide in the presence of the protease (Fig. 2B, lanes 9 and 12). Because in trophozoite infected, saponin-treated cells PfEXP-1 is degraded completely (Ansorge et al., 1997), we anticipate that in ring stage infected cells a substantial proportion of the protein is still located within the parasite plasma membrane (see Fig. 3) and thus is partially protease protected. This view is consistent with the observation that the protected fragment becomes susceptible to trypsin after complete lysis of the parasite (data not shown). More importantly, the amount of uncleaved (i.e. intraparasite) PfEXP-1 is increased after treatment with BFA. In contrast to PfEMP-1 and to the other marker proteins both forms of PfEXP-1 completely resist extraction with carbonate solution. In order to compare the solubility of the respective proteins at their final destinations, infected erythrocytes were cultivated in the absence of BFA until parasites reached the trophozoite stage. Intact parasites were released with saponin and fractionated without prior trypsin treatment. For PfGBP, PfBIP and PfEXP-1 the solubility characteristics were very similar to those observed in earlier stages. In contrast, the solubility of PfEMP-1 decreased notably, but a significant proportion of the protein was still extractable with sodium carbonate solution (Fig. 2Aa, lanes 7–9).
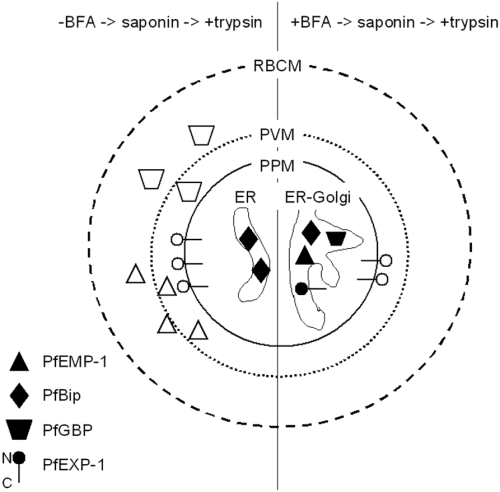
Schematic interpretation of the data from Fig. 2. Infected erythrocytes were cultivated either in the absence or in the presence of brefeldin A (BFA). Cells were subsequently treated with saponin that disintegrates the erythrocyte plasma membrane (RBCM) and the parasitophorous vacuolar membrane (PVM) as indicated by broken lines but which preserves the integrity of the parasite plasma membrane (PPM). Saponin-treated cells were then incubated with trypsin to remove proteins that were either exported into the parasitophorous vacuole before the incubation with BFA or that were exported in the absence of BFA. In the absence of BFA, newly synthesized PfEMP-1 and PfGBP are transported beyond the confines of the PPM and become susceptible to trypsin as indicated by open symbols. In contrast, PfBIP as an ER resident protein is protease protected. In ring stage parasites a transport intermediate of PfEXP-1 is detectable in the PPM. The N-terminal portion of the molecule is exposed at the outer face of the PPM and is trypsin sensitive, while the larger C-terminal portion is protease protected. The treatment with BFA results in the inhibition of protein export and, in other eukaryotes, to an enlarged ER presumably containing Golgi elements. The latter effect has not been analysed systematically in P. falciparum. In BFA treated cells all proteins accumulate in the ER-Golgi compartment and, consequently, resist trypsin treatment as indicated by closed symbols. In contrast to PfEMP-1, PfGBP and PfBIP, PfEXP-1 is a type I integral membrane protein which completely resists extraction of the membrane fraction with sodium carbonate solutions at high pH.
The solubility of PfEMP-1 differs from that of integral membrane proteins
In a recent study we observed that during parasite maturation PfEMP-1 becomes increasingly resistant to extraction with Triton X-100 (Kriek et al., 2003) and that extraction of the protein requires the presence of 2% SDS. An increasing insolubility of PfEMP-1 has also been described in earlier studies (Baruch et al., 1995). Because in advanced developmental stages the protein is present in two locations, namely within the erythrocyte cytosol and on the erythrocyte plasma membrane, we compared the solubilities of both protein populations. As described for Fig. 1, intact, trophozoite stage infected erythrocytes were treated with trypsin to generate the 85 kDa proteolytic fragment as an indicator for the surface exposed population of the protein while the full-length protein represents the internal population. Complete removal of the extracellularly exposed DBL domains of the protein was monitored by FACS analysis (data not shown). Infected erythrocytes were lysed by osmotic shock and a fraction containing all membranes and insoluble particles was collected by centrifugation and extracted either with Triton X-114, with high salt buffer followed by sodium carbonate, or with urea (Fig. 4).

Differential extraction of membranes. Intact infected erythrocytes were either treated with trypsin (A and B; T+ in C) or left untreated (T- in C), and a membrane fraction was prepared by hypotonic lysis. A. Proteins solubilized with Triton X-114 were allowed to partition into an aqueous phase (lane 1) and into the detergent phase (lane 2). Lane 3; Triton X-114 insoluble proteins. B. Membranes were extracted sequentially with high salt buffer and with carbonate buffer. Lane1, proteins solubilized with high salt buffer; lane 2, proteins solubilized by sodium carbonate; lane 3, proteins of the membrane fraction which resisted both extractions. C. Membranes were extracted with urea. Lane 1, urea soluble proteins; lane 2, membrane proteins that resisted extraction with urea. Samples were processed for immunoblotting and analysed for the presence of membrane proteins. For the detection of PfEXP-1 and PfEXP-2, respectively, filters were first processed to detect PfEXP-1 and then reprobed with an antiserum against PfEXP-2, followed by incubation with the appropriate secondary antiserum. The closed arrowhead indicates the position of full-length PfEMP-1, the open arrowhead indicates the position of the C-terminal proteolytic fragment.
Triton X-114 partitioning replaces the lipids from a membrane spanning hydrophobic protein domain and leads to a segregation of these proteins into the detergent phase (Brandt et al., 1990). Peripheral proteins segregate into the aqueous phase whereas high-affinity protein complexes are not disrupted and remain insoluble (Vachon et al., 1991). Proteins of all three fractions (Triton X-114 insoluble, detergent phase, aqueous phase) were analysed by immunoblotting for the distribution of PfEMP-1 and of specific marker proteins (Fig. 4A). Most of the PfEMP-1 was recovered in the Triton X-114 insoluble fraction consistent with previous observations that the protein becomes increasingly insoluble in Triton X-100. Only internal, but no external PfEMP-1 was found to be Triton X-114 soluble. The proportion of the internal protein that was solubilized with Triton X-114 partitioned into the aqueous phase. PfEXP-2 is a protein peripherally associated with the vacuolar membrane (Johnson et al., 1994) which lacks a hydrophobic segment that would qualify as a transmembrane domain (Fischer et al., 1998). It was recovered in the aqueous phase whereas PfEXP-1, owing to its membrane spanning domain, partitioned into the detergent phase.
In another experiment the membrane fraction of infected erythrocytes was subjected to sequential extraction with high salt buffer and sodium carbonate buffer at high pH. Membrane particles were sedimented at 100 000 g for 1 h and PfEXP-1 and PfEXP-2 were recovered in this fraction (Fig. 4B) as described previously (Johnson et al., 1994). Currently we have no explanation why, despite the lack of an apparent transmembrane domain, PfEXP-2 shows such a tight membrane association. As in the Triton X-114 partitioning, the internal and external populations of PfEMP-1 showed different characteristics. While most of the internal molecules were extracted at pH 11, the external molecules resisted extraction completely. As observed above (Fig. 2), a minor proportion of the internal pool resisted extraction with sodium carbonate.
As a chaotropic reagent urea is capable of denaturing the tertiary structure of many proteins thus disrupting protein complexes. Integral membrane proteins that span the lipid bilayer via a segment of hydrophobic amino acids (transmembrane domain) are resistant to extraction with urea (Gilmore and Blobel, 1985; Borel and Simon, 1996). Membrane fractions obtained either from trypsin treated or untreated (to allow the detection of glycophorins A, B and of surface exposed full-length PfEMP-1) erythrocytes were therefore first extracted with 8 M urea and then dialysed against 0.1 M urea. After centrifugation of the samples proteins were identified in the fraction of soluble and in the fraction of insoluble proteins (Fig. 4C). The glycophorins A and B which are single spanning proteins of the erythrocyte plasma membrane were recovered quantitatively in the fraction of insoluble proteins. Although a considerable proportion of PfEXP-1 was solubilized by urea, the majority of the protein resisted extraction and was recovered with the membrane fraction. In contrast, the internal population of PfEMP-1 was extracted quantitatively, and only a minor proportion of external PfEMP-1 remained membrane associated. The peripheral membrane protein PfEXP-2 which resists extraction with sodium carbonate was released from the membrane fraction. To exclude the possibility that cleavage of the extracellular domain of PfEMP-1 affected the stability of the protein in the erythrocyte membrane, a membrane fraction of erythrocytes not treated with trypsin was analysed. Also in these cells only a minor proportion of PfEMP-1 resisted extraction with urea. In conclusion, these results are consistent with the previous findings that cast doubts on the current model that the hydrophobic domain of PfEMP-1 anchors the protein into the lipid bilayer of internal membranes or of the erythrocyte plasma membrane.
PfEMP-1 is recovered in fractions with low lipid content
The physical properties of PfEMP-1 were analysed by sucrose density centrifugation. Membrane fractions from trypsinized infected erythrocytes were prepared, resuspended in Tris buffer and loaded onto a sucrose gradient. After centrifugation fractions containing 60–20% of sucrose were collected and the proteins were analysed by immunoblotting. Both the intraerythrocytic and the extracellularly exposed PfEMP-1 molecules cosegregated with band 3 and with spectrin (Fig. 5, left panel, A, B and C1). Spectrin is a major component of the erythrocyte cytoskeleton, but the protein itself is not anchored in the lipid bilayer. Band 3 (Fig. 5, left panel, C1) and glycophorins A and B were recovered in the same fractions (data not shown). In contrast, when the membrane fraction was subjected to urea treatment, both populations of PfEMP-1 and spectrin were separated from the glycophorins A and B (Fig. 5, right panel, A, B and C2) which, owing to their single membrane spanning region, are expected to have a less tight association with the lipid bilayer than the multispanning band-3 protein. The recovery of PfEMP-1 from fractions of lower density is consistent with a dissociation of this protein from larger protein complexes which are disrupted by urea. As observed in Fig. 2, the two major internal PfEMP-1 species showed different physical characteristics. The externally exposed protein population, i.e. the tryptic fragment, segregated into the fractions of sucrose density lower than those observed for the intact internal proteins. A lipid analysis of the fractions containing the various marker proteins was carried out (Fig. 6). Fractions 2–6 and 7–11 were pooled and analysed by thin-layer chromatography (TLC). Almost the entire lipid content loaded onto the gradient was recovered in the fractions of the higher sucrose densities which also contained known integral membrane proteins. Consistent with the data described above, this analysis underscores that the majority of PfEMP-1 has no direct interaction with the lipid bilayer.

Density gradient centrifugation of membrane fractions. Following trypsin treatment of intact infected erythrocytes, membrane fractions were prepared by mechanical disruption of the cells. Membranes were resuspended either in Tris buffer or in Tris buffer including 8 M urea. After dialysis of the urea-treated membranes against 0.1 M urea, both fractions were centrifuged through a discontinuous sucrose gradient, either in the absence or in the presence of 0.1 M urea. Fractions were collected from the bottom of the tube and analysed by immunoblot analysis. Filters were probed with antibodies against the ATS region of PfEMP-1 (A), human spectrin (B), human band 3 (C1), glycophorins A and B (C2). The sucrose concentration of each fraction is indicated as the refractory index (RI).
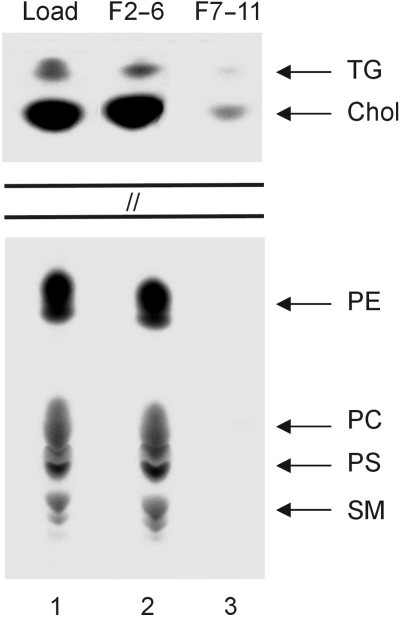
Lipid analysis. The loading material for the sucrose density gradient described in Fig. 6 (lane 1) and the combined fractions 2–6 of the Tris/urea gradient (lane 2) and 7–11 (lane 3) were standardized with respect to equal amounts of protein. The samples were extracted with trichloromethane and methanol, and these lipid extracts were processed for application to TLC plates. Lipids were stained by spraying the plates with cupric sulphate pentahydrate in phosphoric acid. The positions of the following lipid markers are indicated on the right; TG, triglycerols; Chol, cholesterol; PE, phosphatidyl ethanolamine; PC, phosphatidyl choline; PS, phosphatidyl serine; SM, sphingomyelin.
Discussion
After invasion, the parasite dramatically alters the physiological properties of its host erythrocyte. This process includes the establishment of a complex parasite derived membrane network in the cytoplasm of the erythrocyte (Hibbs and Saul, 1994; Martinez et al., 1998) and the transport of parasite proteins to both the host cell cytosol and the plasma membrane (see for review van Dooren et al., 2000). Protease protection assays and immunochemical data have shown that the topology of PfEMP-1 within the erythrocyte membrane is such that the N-terminal domain of the protein is exposed and that the C-terminal domain remains at the intracellular face of the membrane (Waterkeyn et al., 2000). Therefore it has been inferred that the protein is integrated into the lipid bilayer by its hydrophobic segment (Trelka et al., 2000; Kriek et al., 2003; Taraschi et al., 2003). By analogy to the well established and highly conserved principles of membrane protein synthesis and trafficking in eukaryotic cell model systems it has been anticipated that also this protein is synthesized and trafficked as an integral protein via a classical secretory pathway which extends beyond the confines of the parasite (Albano et al., 1999; Adisa et al., 2001; Hayashi et al., 2001). However, this hypothesis has not yet been supported by biochemical evidence. We were therefore prompted to examine the biochemical properties of PfEMP-1 at several stages of its synthesis and transport from the parasite to the erythrocyte membrane. Our data are consistent with previously published observations that PfEMP-1 exits from the parasite via a classical BFA sensitive pathway (Wickham et al., 2001). However, when secretion is blocked with BFA, the majority of the protein is solubilized with sodium carbonate suggesting that PfEMP-1 is not inserted into the ER membrane but rather translocated into the ER lumen and secreted as a carbonate-extractable protein. Once outside the parasite, two major populations of PfEMP-1 can be distinguished by their sensitivity to protease digestion. Red cell surface exposed PfEMP-1 is cleaved by trypsin leaving an 85 kDa membrane-associated fragment but protein en route to the surface is protected from digestion. In general terms, the cell surface associated fraction resists extraction by sodium carbonate or non-ionic detergent whereas the intracellular pool is partially solubilized by these detergents and largely solubilized by carbonate extraction. This latter observation is inconsistent with the trafficking of PfEMP-1 across the erythrocyte cytoplasm as an integral membrane protein. Consistently, we observed a minor proportion of the intracellular protein that showed different physical properties, resembling those of the extracellular population. Thus, we can distinguish at least three states of PfEMP-1 exterior to the parasite, namely: (i) red cell surface protein, (ii) intracellular peripheral membrane protein and (iii) intracellular protein with solubility characteristics of the cell surface population. The latter observation is consistent with our previous report that a minor proportion of the molecule within the erythrocyte has its C-terminus protected from protease digestion in permeabilized erythrocytes (Kriek et al., 2003). Together, these data may seem to offer support for a vesicular transport of the protein during the late stages in the trafficking pathway. The extractability of all protein populations with urea, however, argues against a direct interaction of the hydrophobic domain with the lipid bilayer. Moreover when compared by sucrose density gradient centrifugation in the presence of urea, PfEMP-1 populations clearly segregate from true integral membrane proteins such as the glycophorins and from lipids respectively.
Several algorithms exist on the internet as bioinformatic tools to predict transmembrane sequences. In several recent studies the accuracies of these algorithms were compared using sequences of transmembrane proteins for which the topologies are experimentally confirmed. These analyses generally favour algorithms based on the hidden Markov Model (HMM), such as HMMTOP and TMHMM, as the currently most reliable tools (Tusnady and Simon, 1998; Melen et al., 2003; Kim et al., 2003). We carried out a comparative analysis on 10 randomly-selected PfEMP-1 sequences, two sequences of parasite proteins experimentally confirmed to be single spanning transmembrane proteins, and two single spanning host cell proteins of the erythrocyte membrane using five different algorithms (Table 1). All algorithms predicted transmembrane regions within the experimentally confirmed proteins but only TMpred and TopPred predicted transmembrane regions within all PfEMP-1 sequences. The majority of PfEMP-1 molecules were not predicted to be transmembrane proteins using the other algorithms. An alignment of 10 hydrophobic domains shows a conserved tri-lysine motif which indicates the C-terminal boundary of the hydrophobic segment (Fig. 7). An assessment of the N-terminal boundary is less clear. Nine out of the 10 segments contain a hydrophobic isoleucine residue which is preceded by a stretch of hydrophilic residues. Therefore we determined this isoleucine residue as the N-terminal boundary for the following considerations. In comparison to the transmembrane regions of the other proteins with hydrophobic cores of 22–23 amino acids, this hydrophobic segment of PfEMP-1 which consists of 17 contiguous amino acids is relatively short. The overall hydrophobicity of the 22 amino acids N-terminal to the tri-lysine motif is much lower than the hydrophobicity of the experimentally confirmed transmembrane regions (Table 1). These considerations, although not conclusive, support our view that the hydrophobic segment of PfEMP-1 does not primarily act as a transmembrane region. How then do we reconcile the well established topology of surface exposed PfEMP-1 with these data? One possibility is that PfEMP-1 spans the erythrocyte membrane as part of a multimeric protein complex without a direct interaction with the lipid bilayer. This complex is likely to contain proteins already known to either interact directly with PfEMP-1 (Waller et al., 1999; Oh et al., 2000; Voigt et al., 2000) or to modulate its trafficking (Crabb et al., 1997; Waterkeyn et al., 2000). A bioinformatic analysis of all var genes within the 3D7 parasite genome shows a remarkable degree of conservation of the hydrophobic domain (Fig. 7), opening up the possibility that this domain interacts with an additional specific partner. Most likely, PfEMP-1 adopts its transmembrane topology at the late stages of transport pathway, i.e. when the protein comes closely associated with Maurer's clefts located at the cytosolic face of the erythrocyte membrane. This view is consistent with a recent study in which an intraerythrocytic population of PfEMP-1 with an apparent transmembrane topology was detectable (Kriek et al., 2003). Taken together, our data suggest the existence of a novel mechanism whereby parasite proteins are inserted into the plasma membrane of the infected cell. In comparison to the efficiency of protein sorting processes in eukaryotic cells, the inefficiency of this novel mechanism may explain why only a proportion of PfEMP-1 becomes surface exposed.
| Protein | HMMTOP | Sousi | TMHMM | TMpred | TopPred | Hydroph. TM |
|---|---|---|---|---|---|---|
| 1. A4 | no | no | no | yes | yes | 44.6 |
| 2. Q8I218 | no | no | no | yes | yes | 38.5 |
| 3. Q8I639 | no | no | no | yes | yes | 34.1 |
| 4. Q25733 | no | no | no | yes | yes | 36 |
| 5. Q8ID09 | no | no | no | yes | yes | 35.7 |
| 6. Q8I098 | no | yes | yes | yes | yes | 42.6 |
| 7. Q8IHM0 | no | yes | yes | yes | yes | 42.6 |
| 8. Q8IIZ4 | yes | yes | yes | yes | yes | 41 |
| 9. Q8IEU9 | yes | yes | no | yes | yes | 37 |
| 10. Q81220 | no | no | no | yes | yes | 36 |
| 11. PfEXP-1 | yes | yes | yes | yes | yes | 48.8 |
| 12. PfSBP-1 | yes | yes | yes | yes | yes | 46.8 |
| 13. Glycophorin A | yes | yes | yes | yes | yes | 52.7 |
| 14. Glycophorin B | yes | yes | yes | yes | yes | 66.4 |
- Ten randomly chosen PfEMP-1 sequences (1–10) and 4 well characterized single-spanning membrane proteins of the parasite (11 and 12) and of the red cell plasma membrane (13 and 14) were subjected to topology prediction using the programs HMMTOP, Sousi, TMHMM, TMpred and TopPred, all freely accessible on the internet. For PfEMP-1, the overall hydrophobicity of 23 amino acids N-terminal to the positively charged residues that determine the C-terminal boundaries (Fig. 7A) were calculated according to Kyte and Doolittle (1982). The same procedure was applied to the hydrophobic cores of the confirmed transmembrane proteins (Fig. 7B).
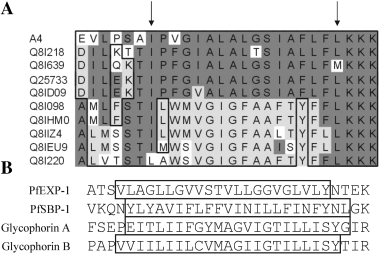
Sequence comparison of hydrophobic segments. A. The hydrophobic segments of 10 randomly chosen PfEMP-1 sequences were aligned by the Clustal method. Arrows indicate the boundaries of the contiguous stretches of hydrophobic amino acids. Identical amino acids in different segments were shaded in dark grey and in light grey respectively. Homologous amino acids are boxed. B. Hydrophobic segments (boxed) of experimentally confirmed single spanning membrane proteins. PfEXP-1 is a parasite protein located in the parasitophorous vacuolar membrane (Günther et al., 1991). PfSBP-1 is an integral membrane protein of Maurer's clefts (Blisnick et al., 2000). The glycophorins A and B are human integral membrane proteins of the erythrocyte plasma membrane.
Experimental procedures
Parasites
Plasmodium falciparum lines A4 and FCBR were used in this study. The A4 laboratory line was initially isolated by micromanipulation from the ITO4 line, which was derived from a parasite isolate from Ituxi in Brazil (Roberts et al., 1992). The FCBR originated from Columbia. Infected erythrocytes of blood group A+ were cultivated in RPMI1640 medium supplemented with 10% human serum (Trager and Jensen, 1976). Ring stage infected erythrocytes at a parasitaemia of 10% were synchronized using l-alanine (Braun-Breton et al., 1988). Trophozoite-stage infected erythrocytes were enriched by plasmagel flotation as described elsewhere (Pasvol et al., 1978).
Antibodies
Mouse monoclonal antibodies against human band 3 and glycophorin A, B, and a rabbit polyclonal antiserum against human spectrin were from Sigma. Rabbit antisera to the C-terminal domain of PfEXP-1 and against PfEXP-2, respectively, were described previously (Ansorge et al., 1997; Fischer et al., 1998). Polyclonal antisera against the ATS domain of PfEMP-1 (Kriek et al., 2003) and against PfBIP (Burghaus and Lingelbach, 2001) were described previously.
Trypsin treatment and fractionation of infected erythrocytes
Erythrocytes infected with tightly synchronized parasites were harvested and washed twice with PBS pH 7.2. For the removal of externally exposed protein domains aliquots of 109 infected erythrocytes were resuspended in 10 vols of PBS, pH 7.2 containing 1 mg ml−1 of trypsin (Sigma) and incubated at 37°C for 15 min The protease reaction was stopped by adding soybean trypsin-inhibitor (Sigma) to a final concentration of 2 mg ml−1 and incubating the cells on ice for 5 min For the preparation of membranes from infected erythrocytes cells were lysed either hypotonically or with saponin.
Hypotonic lysis.
If not stated otherwise infected erythrocytes were suspended in 20 vols of either 10 mM ice-cold HEPES, pH 7.4 or 10 mM Tris-HCl, pH 7.4 containing a cocktail of protease inhibitors (1 mM PMSF, 10 µM pepstatin A, 1 µM leupeptin, 1 mM EDTA) and lysed by three cycles of freezing and thawing in liquid nitrogen. Cells were centrifuged at 10 000 g for 15 min Supernatants were discarded and the pellets were washed three times.
Saponin lysis.
Infected erythrocytes were lysed by adding saponin (Serva) to the culture suspension at a final concentration of 0.1%. Lysed erythrocytes containing intact parasites were collected by centrifugation at 2000 g for 5 min and washed three times in PBS to remove residual haemoglobin. For trypsin treatment, cells were resuspended in 10 vols of PBS containing 10 µg ml−1 of trypsin (Sigma) and a protease inhibitor cocktail including pepstatin A, leupeptin and 1 mM EDTA at final concentrations of 10 µM, 1 µM and 1 mM respectively. Trypsin inactivation was carried out by adding an equal volume of PBS containing 20 µg ml−1 of soybean trypsin inhibitor and protease inhibitor cocktail at 4°C for 5 min Cells were sedimented and resuspended in 10 mM Tris-HCl pH 8, containing 140 mM NaCl and protease inhibitor cocktail. The suspension was repeatedly frozen and thawn using liquid nitrogen to disrupt the parasite plasma membrane and all internal membranes. The lysates were then centrifuged at 100 000 g for 1 h to separate the soluble proteins from the insoluble fraction.
Extraction of membrane fractions from infected erythrocytes
Treatment of membranes with high salt and carbonate buffers.
Membrane fractions from 2 × 108 infected erythrocytes were resuspended in 100 µl of a high salt buffer containing 50 mM HEPES, pH 7.5, 0.6 M KCl, 5 mM DTT, 3 mM MgCl2 and left on ice for 30 min Samples were centrifuged at 100 000 g for 1 h at 4°C. The pellet was resuspended in 10 vols of a 0.1 M sodium carbonate buffer adjusted to pH 11. Extraction was carried out on ice for 30 min and samples were centrifuged as above. Proteins from the supernatant fraction were precipitated in 10% TCA. Precipitated proteins and the pellet fraction from the final centrifugation were prepared for SDS-PAGE.
Triton X-114 phase partitioning of proteins.
Membrane fractions corresponding to 108 infected erythrocytes were extracted in a mixture of 200 µl of Tris-buffered saline (TBS), pH 7.4, and 50 µl of Triton X-114 saturated with TBS. After incubation for 30 min on ice samples were centrifuged at 10 000 g for 15 min at 4°C. The supernatant was removed from the pellet fraction containing Triton X-114 insoluble proteins, incubated at 37°C for 5 min and centrifuged at 5000 g at 4°C for 5 min The upper aqueous phase was separated from the lower detergent phase and added to 50 µl of TBS-saturated Triton X-114. To the detergent phase 200 µl of TBS was added. The phase partitioning of both fractions was repeated twice and the proteins of both phases were acetone-precipitated at − 20°C overnight. The fraction containing Triton X-114 insoluble proteins and the acetone precipitated proteins were processed for SDS-PAGE.
Urea extraction of membranes.
Membrane fractions corresponding to 5 × 108 infected erythrocytes were extracted in 10 mM Tris pH 8 containing 8 M urea and 1 mM EDTA for 1 h at room temperature. Samples were then dialysed against 10 mM Tris pH 8 containing 0.1 M urea and 1 mM EDTA at 4°C overnight and centrifuged at 10 000 g for 30 min The proteins from the supernatant were precipitated in 10% TCA. The samples (the membrane pellet and TCA precipitated proteins) were processed for SDS-PAGE.
BFA treatment
Parasites were tightly synchronized by consecutive alanine treatments at the schizont-ring transition phase until the time window of reinvasion was decreased to less than 4 h. Parasites were grown for one more cycle to a parasitaemia of 10–15%. At 12 h post invasion BFA was added to the culture at a final concentration of 5 µg ml−1. After cultivation for 2 h parasites were collected by saponin-lysis and subjected to trypsin treatment. Membrane fractions were then prepared as described above. The post 100 000 g supernatant was centrifuged once more to clear it from contaminating insoluble particles. The sediment fraction containing protein complexes and membranes was treated with sodium carbonate as described above.
Sucrose density gradient centrifugation
Membrane fractions of infected erythrocytes were prepared by lysing aliquots of 109 parasitized cells in 100 vols of 5 mM Tris-HCl, pH 8, containing the protease inhibitor cocktail, using a hand-driven ball-bearing homogenizer. Samples were precleared by centrifugation at 2000 g, at 4°C for 10 min The supernatant was centrifuged at 100 000 g at 4°C for 30 min The pellet fraction, corresponding to 4 × 108 cells, was resuspended in 400 µl of 10 mM Tris-HCl, pH 8, containing the protease inhibitor cocktail. The suspension was layered on the top of a discontinuous sucrose density gradient. The gradient consisted of 800 µl of cushions containing 60, 50, 40, 30 and 20% sucrose, respectively, in 10 mM Tris-HCl, pH 8, and 1 mM EDTA. Centrifugation was carried out at 100 000 g for 12 h in a swing-out rotor. Fractions were collected from the bottom of the tube. Proteins were TCA-precipitated and processed for SDS-PAGE and immunoblotting. For the density gradient centrifugation of samples extracted with urea the same conditions were used except that 0.1 M urea was included in each cushion.
Lipid analysis
Fractions obtained after density centrifugation in urea were subjected to protein quantification according to Bradford. Samples adjusted to identical amounts of protein were diluted fivefold with ice-cold water and membranes were pelleted at 100 000× g for 1 h at 4°C. The pellets were extracted in a mixture of chloromethane, methanol and water at a ratio of 10:10:3 using a water quench sonicator. The samples were centrifuged at 3500× g for 5 min The extraction procedure was repeated once, the supernatants were combined, and dried by nitrogen-aided evaporation. The dried samples were solubilized in 1 ml of n-butanol. After the addition of 1 ml of H2O the samples were shaken vigorously and centrifuged again. The upper organic phase was separated from the aqueous phase and washed with water. The samples were dried by nitrogen-aided evaporation and resolubilized in a running solution containing chloromethane, methanol and 2 M aqueous NH3, at a ratio of 65:25:4. Samples were applied to TLC plates and separated by capillary force. The TLC plates were air-dried over night and stained by spraying the plates with 6.9 mM cupric sulphate pentahydrate in 7.3% phosphoric acid.
Acknowledgements
We thank Nahid Azzouz and Ralph Schwarz for help with the lipid analysis. We also thank Paul Horrocks and Bob Pinches for help with the work on the A4 parasites. We further thank Julius Nyalwidhe and Stefan Charpian for helpful discussion, and Uwe-Gallus Maier and Paul Horrocks for critical reading of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Wellcome Trust.




