Building a bacterial orisome: emergence of new regulatory features for replication origin unwinding
Summary
Triggering new rounds of chromosomal DNA replication during the bacterial cell cycle is exquisitely regulated, ensuring both proper timing and one round per cycle stringency. A critical first step is stable unwinding of oriC, the chromosomal replication origin, by multiprotein orisome complexes comprising the AAA+ initiator DnaA and modulator proteins that bend DNA. Recently identified oriC–DnaA interactions in Escherichia coli raise important questions regarding the molecular mechanisms that regulate origin unwinding in bacteria. We describe staged binding of E. coli origin recognition proteins and suggest an unwinding switch based on interactions between DnaA-ATP and specialized oriC sites that must be filled during orisome assembly. By focusing multiple regulatory pathways on only a few key oriC DNA–protein interactions, this model includes an efficient way to control unwinding followed by orisome inactivation during the cell cycle. Future studies will determine whether this regulatory scheme is correct and whether it is generally applicable to other bacterial types.
Introduction
Bacteria must copy their genomes prior to dividing into two daughter cells. Under normal growth conditions, DNA replication initiates precisely at the same cell mass in each and every cell cycle from a distinct chromosomal region called oriC (Skarstad et al., 1986; Messer, 2002). Failure to start DNA synthesis from oriC at the proper time, or permitting too many starts per cell cycle, leads to suboptimal cellular growth and, in severe cases, cell death. To avoid these problems, bacteria rely on regulatory mechanisms that tightly couple initiation of DNA synthesis to nutritional conditions and absolutely prohibit over-replication. Most of these mechanisms control the critical first step in the DNA replication process by affecting assembly of the orisomes (protein–oriC complexes) that create a small bubble of unwound DNA within the replication origin. While orisomes must exist in all bacteria, most of our information on their assembly and regulation comes from studies of the bacterium, Escherichia coli. This review will cover our current understanding of how orisomes assemble during the cell cycle to unwind oriC, and how they then might be inactivated and disassembled after initiation of DNA replication. We raise the possibility that most of the regulation that promotes or inhibits these assemblies is targeted to only a few weak DNA–protein interactions.
Orisome components
In E. coli, the proteins contributing to the unwound orisome are most easily placed into two classes: (i) the highly conserved AAA+ initiator protein DnaA (reviewed in Skarstad and Boye, 1994; Kaguni, 1997; Messer, 2002) that first mediates DNA strand separation and then recruits helicase, and (ii) histone-like DNA bending proteins, Fis (Gille et al., 1991; Filutowicz et al., 1992; Finkel and Johnson, 1992) and IHF (Polaczek, 1990; Ellenberger and Landy, 1997; Travers, 1997), which modulate DnaA interactions (Messer, 2002; Ryan et al., 2004). A fourth protein, SeqA (Lu et al., 1994), interacts with newly replicated, hemimethylated oriC DNA and helps prevent reinitiation (Torheim and Skarstad, 1999). Additional proteins are known to interact with oriC. Some of these, such as HU, enhance the ability of DnaA to unwind the origin in vitro (Hwang and Kornberg, 1992) while others, such as IciA, repress strand opening (Hwang and Kornberg, 1990). However, their contributions to orisome assembly or disassembly in vivo during the cell cycle remain unclear.
The DNA component of the E. coli orisome, oriC, comprises 245 bp (see Fig. 1). Initial strand separation takes place in a region of helical instability containing three repeats of an AT-rich 13-mer sequence, 5′-GATCTaTTtaTTT (Bramhill and Kornberg, 1988). There is also evidence for DnaA binding within this 13-mer region, most likely at three copies of the sequence 5′-AGATCT, shown in Fig. 1 as S–M sites (Yung and Kornberg, 1989; Speck et al., 1999; Speck and Messer, 2001). Additional binding sites for DnaA are located adjacent to the AT-rich region, and include five copies of the 9-mer consensus DnaA recognition sequence, 5′-TTa/tTNCACA, termed R boxes (R1–R4 and a fifth site referred to in the literature as either R5 or M) (Fuller et al., 1984; Matsui et al., 1985; Messer, 2002), as well as more recently identified DnaA binding sites termed I sites (McGarry et al., 2004), which differ in several bases from the R box consensus sequence (Fig. 1). One distinguishing feature of I sites and 13-mer sites is that they discriminate between the active form of DnaA, DnaA-ATP, and inactive DnaA-ADP, demonstrating strong preference (over threefold higher affinity) for DnaA-ATP (Speck and Messer, 2001; McGarry et al., 2004). This is not the case for R boxes, which bind both active DnaA-ATP and inactive DnaA-ADP with equal affinity (Sekimizu et al., 1987). Mutations that reduce binding to R boxes or I sites diminish in vivo function of oriC plasmids, indicating that DnaA binding to all these sites is important in formation of an optimal orisome that is capable of competing effectively with the wild-type chromosomal oriC (Bates et al., 1995; Langer et al., 1996; McGarry et al., 2004). Mutations that permit I sites to bind DnaA-ADP and DnaA-ATP with equal affinity result in early initiations in the cell cycle and over-replication (K.C. McGarry, J.E. Grimwade and A.C. Leonard, in preparation). Mutations affecting DnaA binding to the 13-mer region have not been studied.
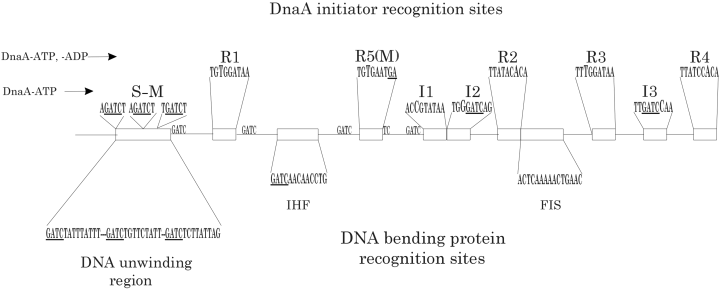
Nucleotide sequence features of the E. coli replication origin, oriC. Wild-type oriC contains 245 base pairs. The sequences shown are all derived from the top DNA strand and are in their correct orientation on that strand. Position 3 of each DnaA recognition site (based on the orientation of R1) is shown in larger font. S–M denotes the DnaA recognition sites described by Speck and Messer (2001). The conserved 3′ recognition sequence shown for IHF is based on the study by Goodrich et al. (1990). Fis recognition site is based on compiled consensus recognition sequences described by Finkel and Johnson (1992). The entire nucleotide sequence of oriC can be viewed in the study by Cassler et al. (1995). See text for details.
DnaA has differing affinities for its oriC binding sites, such that R4 ≥ R1 > R2 > R5 (M), I2, I3 > I1 > R3 (Margulies and Kaguni, 1996; Grimwade et al., 2000). Double-stranded 13-mer sites have very low affinity for DnaA protein, and they bind DnaA better when the AT-rich region is unwound (Speck and Messer, 2001). For simplicity, we will group the sites into three classes based on affinity: (i) the strong sites R1, R2 and R4; (ii) the weak sites R5 (M), R3, I1, I2 and I3; and (iii) the single-stranded sites in the 13-mer region. It was previously proposed that filling of low affinity DnaA binding sites triggered initiation of DNA replication (Hansen et al., 1991; Messer, 2002), and the DnaA-ATP preferring I sites and 13-mer sites are intriguing candidates for this role.
Primary recognition sites for IHF and Fis are located in the left and right half of oriC, respectively (Fig. 1), and additional weaker binding sites for Fis have also been mapped within oriC (Polaczek, 1990; Filutowicz et al., 1992). OriC plasmids carrying mutations that reduce binding of Fis and IHF have decreased function in vivo, although they can replicate in an in vitro assay (Roth et al., 1994; Wold et al., 1996). Strains lacking Fis or IHF also have perturbed initiation timing (Boye et al., 1993). Thus, while it is possible to form orisomes lacking Fis or IHF, such complexes do not function optimally.
The nucleotide sequence GATC is repeated at 11 separate locations within oriC, with 7 of these locations at weak DnaA binding sites in the 13-mer region and positioned within or adjacent to R5 (M), I1, I2 and I3 (Fig. 1). GATC is the recognition site for deoxyadenosine methyltransferase (Palmer and Marinus, 1994), and also for the interaction of SeqA with DNA (Lu et al., 1994). Although there are data to suggest that it functions both before and after initiation (Slater et al., 1995), the best understood role for SeqA is to prevent over-replication from oriC. Mutations changing the nucleotide sequence within oriC GATC sites cause over-initiations and subsequently altered chromosome segregation (Bach and Skarstad, 2004).
Regulation of DnaA loading onto oriC– a model for assembly of the unwound orisome
During rapid growth, in vivo footprinting studies have revealed that orisome structure is dynamic, changing in stages as E. coli progresses through the cell cycle. For most of the cell cycle, R1, R2 and R4 are occupied by DnaA, and Fis is bound to its primary site, as shown in Fig. 2, part 1 (Samitt et al., 1989; Cassler et al., 1995). At the onset of DNA synthesis, Fis binding is reduced (Fig. 2, part 2), IHF becomes bound, and DnaA binding increases to include the remaining R boxes, R5 (M) and R3, as well as the I sites (Cassler et al., 1995; Ryan et al., 2002). Based on data from in vitro studies, the AT-rich region of oriC is unwound at this stage (Hwang and Kornberg, 1992; Ryan et al., 2002) and the complex includes oligomeric DnaA, some of which is bound to single-stranded 13-mer sites (Speck and Messer, 2001) (Fig. 2, part 3).
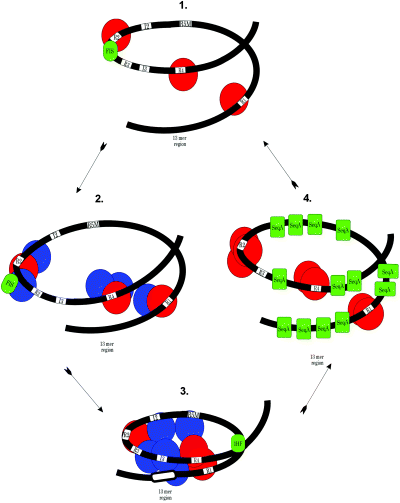
Model for the assembly and disassembly of the E. coli orisome required to unwind oriC. DnaA is shown as red (ADP- or ATP-bound form retained from previous cell cycle) or blue (newly synthesized ATP-bound form) spheres. Modulators of DnaA binding: IHF, Fis and SeqA are shown in green. See text for details.
Multiple factors contribute to the staged assembly of the unwound complex. Clearly, the affinity of the binding sites plays a role, as in vivo DnaA binds to strong sites before weaker sites. Furthermore, the presence of binding sites that discriminate between nucleotide forms of DnaA ensures that oriC unwinding during the cell cycle is co-ordinated with accumulation of DnaA-ATP. Total DnaA levels are constant throughout the cell cycle (Sakakibara and Yuasa, 1982), but DnaA-ATP levels have been reported to fluctuate, peaking near the time of initiation (Kurokawa et al., 1999). Increased levels of DnaA-ATP are attributed to new synthesis (newly made DnaA is in the ATP form) and regeneration of DnaA-ATP from DnaA-ADP by an exchange reaction catalysed by membrane acidic phospholipids (Crooke et al., 1992). Filling the weak I sites and 13-mer sites with DnaA-ATP appears to be required for the last stage of orisome assembly leading to a stable unwound complex (Speck and Messer, 2001; McGarry et al., 2004). Thus, the unwound complex cannot completely assemble until the cell makes enough DnaA-ATP to fill the discriminatory sites. It remains to be determined if orisomes are usually assembled entirely from DnaA-ATP or assemble as chimeras of DnaA-ADP and DnaA-ATP (for example, DnaA-ADP might remain bound to stronger sites during early stages of assembly, with DnaA-ATP being added only in late stages). Previous studies suggest that just a small amount of DnaA-ATP is required for functional orisome assembly (Yung et al., 1990) and we propose that this DnaA-ATP is required to interact with I sites and 13-mer sites.
Both Fis and IHF directly modulate the interaction of DnaA-ATP with the weaker oriC binding sites during orisome assembly. Purified Fis inhibits in vitro open complex formation and replication of oriC plasmids (Hiasa and Marians, 1994; Wold et al., 1996) by reducing binding of DnaA to lower affinity sites (Ryan et al., 2004). Fis also inhibits IHF binding to oriC (Ryan et al., 2004). In contrast, purified IHF stimulates open complex formation (Hwang and Kornberg, 1992), and this stimulation was shown to accompany increased DnaA interaction with weaker R boxes and I sites (I sites were so-named because of the increased binding observed in the presence of IHF) in the absence of any additional DnaA (Grimwade et al., 2000).
The in vitro data on Fis, IHF and DnaA, combined with the observed stages of assembly during the cell cycle, lead to the following testable model of complex assembly in vivo. First, as cells progress towards initiation, an origin that is strongly interacting with DnaA at R1, R2 and R4 is also associated with Fis, which prevents IHF from binding. This complex promotes accumulation of DnaA oligomers (containing DnaA-ATP) at the strong sites in preference to binding to the weak sites (Fig. 2, parts 1 and 2). The close proximity of the Fis site and R2 leads to the attractive hypothesis that Fis is displaced by a build-up of DnaA oligomers binding at R2 (Fig. 2, part 2). Displacement of Fis allows IHF to bind, and the newly positioned bend places DnaA-ATP molecules oligomerized around the stronger sites close to each other where they can interact. The change in protein–protein interactions induced by IHF binding is proposed to bring DnaA-ATP in close proximity to the weak R boxes and I sites, which then become bound. The remodelled complex generates sufficient torsional stress to trigger DNA strand separation in the AT-rich region, stabilized by DnaA interaction with 13-mer sites (Fig. 2, part 3). With the opposing activities of Fis and IHF, a rapid and precise switch in orisome assembly is brought into play, driven and stabilized by accumulation of DnaA-ATP, to convert a non-functional, partially assembled orisome into a fully assembled unwound complex, thereby triggering a new round of DNA synthesis.
The potential interaction between I sites and 13-mer DnaA-ATP binding sites suggests structures for the stably unwound orisome (Fig. 2, part 3). We propose that the bending and wrapping of oriC around DnaA could produce interactions among I sites, R boxes and 13-mer sites by placing three double-stranded DNA regions in close proximity, with the discriminatory I sites and 13-mer sites linked by DnaA-ATP oligomers. DnaA oligomerization was recently shown to be required for initiation of DNA replication (Simmons et al., 2003), and cooperativity among bound DnaA molecules is further indicated by mutational analysis of oriC showing that the relative positions and helical facings of DnaA binding sites are important for function (Woelker and Messer, 1992). Although the exact arrangement of DnaA within the structure is not known, it is proposed that DnaA could form either a closed ring or a helical filament (Erzberger et al., 2002).
Preventing reinitiation by disassembly of the complex
The unwound orisome must complete the initiation process by loading helicase and then polymerase holoenzyme, but the resulting replication forks must form only once per cell cycle. Therefore, when DNA replication is successfully initiated, the complex must be inactivated and disassembled, resetting the orisome so that it can reassemble in the next cell cycle. The movement of the helicase or polymerase may play a role in the disassembly by displacing IHF (J.E. Grimwade, unpubl. obs.), thus removing the bend that helped place DnaA molecules clustered around R1, R2 and R4, near the weak sites. However, further inactivation is needed to prevent the complex from immediately reforming. At least four negative regulatory paths appear to be involved in this inactivation, reviewed in the study by Katayama (2001): one that inactivates DnaA-ATP, one that prevents DnaA binding to oriC by physically blocking recognition sites, one that reduces DnaA-ATP synthesis and one that titrates DnaA away from oriC. We propose that all four mechanisms work in concert to reduce DnaA-ATP binding to the discriminatory 13-mer and I sites.
Following initiation, the negative regulators act sequentially. It is likely that, first, a process termed RIDA (regulatory inactivation of DnaA) inactivates bound DnaA-ATP after the DNA polymerase III beta-subunit sliding clamp (with a cofactor Hda) is loaded onto single-stranded DNA in the oriC region (Katayama and Sekimizu, 1999). RIDA stimulates the low intrinsic ATPase activity of DnaA, and it is likely that, initially, hydrolysis is localized to DnaA-ATP bound to oriC, resulting in loss of DnaA-ATP binding to I sites and 13-mer sites, with subsequent partial disassembly of the complex. Hydrolysis of DnaA-ATP will continue as forks move and Hda encounters more bound DnaA outside oriC. Second, interaction between DnaA and its weaker binding sites is also affected when oriC becomes hemimethylated after DNA replication commences. Remethylation is transiently blocked (for 1/3 of the cell cycle), most likely because of strong binding of SeqA to hemimethylated GATC sites (Campbell and Kleckner, 1990). Although SeqA does not appear to displace DnaA from the orisome in vitro (Torheim and Skarstad, 1999), binding of DnaA-ATP to I2, I3 and R5 (M) sites is prohibited when equimolar levels of SeqA are preincubated with a hemimethylated template (C. Nievera, J.E. Grimwade and A.C. Leonard, in preparation). In these first steps to prevent reinitiation, we propose that RIDA and SeqA act together to form a ‘post-initiation’ orisome that is similar to the preinitiation Fis-bound orisome but with DnaA-ADP oligomers bound to R1, R2 and R4, and SeqA bound to hemimethylated GATC sites (Fig. 2, part 4). Thus, SeqA would protect the 13-mer and I sites from any available DnaA-ATP until DnaA-ATP levels are reduced by transcriptional repression and titration, as described below.
After the replication forks move through the dnaA gene, transcription of dnaA and consequently synthesis of new DnaA-ATP is repressed for 1/3 of the cell cycle, a process also mediated by SeqA (Campbell and Kleckner, 1990; von Freiesleben et al., 1994). Continued hydrolysis of DnaA-ATP by RIDA combined with SeqA-induced shutdown of DnaA-ATP synthesis would cause intracellular DnaA-ATP levels to fall below that required to overcome the SeqA blockage of discriminatory sites in oriC. In addition to reducing the amount of DnaA-ATP in the cell by RIDA and dnaA repression, the levels of free DnaA are decreased when chromosomal DnaA binding sites outside of oriC are replicated (Hansen et al., 1991; Ogawa et al., 2002). DnaA binds to approximately 300 additional sites on the chromosome (Roth and Messer, 1998). Included among these sites is one of extremely high affinity (datA) near oriC that is capable of binding up to 350 molecules of DnaA (Kitagawa et al., 1996). The reduction of available DnaA by datA would not only augment the existing blocks on the discriminatory sites, but also decrease the number of DnaA oligomers bound at R1, R2 and R4. In particular, reduction of oligomeric DnaA at R2 would allow Fis to rebind oriC, thus resetting the complex to the preinitiation stage.
Beyond E. coli?
The observations reported above for E. coli do not necessarily reflect the rules used by other bacteria for the unwinding of replication origins, but can be used to provide one model for how this process might be achieved. The fact that DnaA is highly conserved suggests that it has a fundamental role in the assembly of the unwound structure. Additionally, most bacterial origins contain multiple DnaA binding sites with varying affinities for DnaA, reviewed in the study by Messer (2002). We suggest that in all bacteria, assembly of the unwound orisome is staged, with weak DnaA sites being filled as a last required step prior to initiation. However, there can be no doubt that the regulatory mechanisms that modulate the assembly of the unwound structure will differ and are likely to reflect the lifestyle of a bacterial type. Bacteria capable of multifork replication possess certain requirements to prevent misinitiations that are unlikely to exist for oligotrophs. Special requirements to control the onset of DNA replication during the cell cycle based on differentiation or environmental cues would likely be different from the regulation found in enteric bacteria. For this reason, it is expected that a variety of interesting regulatory mechanisms await discovery as more information becomes available about DNA–protein complexes in a wider variety of organisms.
It is interesting to note that similarities between bacterial and eukaryotic origin unwinding mechanisms appear as we learn more about these systems. In addition to the structural similarities of origin recognition proteins found in both systems (Giraldo, 2003), the common theme of regulation by ATP binding and ATPase activity has extended to yeast and mammalian origin recognition complexes (ORCs) (Baker and Bell, 1998; Lee and Bell, 2000; Davey et al., 2002; Takenaka et al., 2004). Furthermore, recent studies suggest that single-stranded DNA plays a role in ORC protein binding and stimulation of ATPase regulatory activity (Lee et al., 2000). Eukaryotic orisomes are reported to be stable structures seen associated with replication origins throughout the cell cycle. We suggest that as in bacterial systems, the weakest DNA–protein interactions within the assembled ORC are likely to be the most important regulatory features and also likely to be dependent on ATPase activities of the AAA+ proteins comprising the eukaryotic prereplication complex. Despite the fact that these interactions are difficult to study, it will be interesting to see if this turns out to be the case.
Acknowledgements
We wish to thank our friend and colleague, Charles Helmstetter, for introducing us to the bacterial cell cycle, and for many stimulating discussions regarding initiation of DNA replication.




